Translate this page into:
Bi-verse relationship between gold nanoparticles and intracellular pH
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Gold nanoparticles (AuNPs) are extensively used in biomedicine and as therapeutic agent as they can be drug carriers, imaging agents, gene-regulating agents, and photoresponsive therapeutics. On the other hand, especially when dealing with intracellular activity, fundamental parameters such as the pH must be taken into account and may be conveniently used to direct the action of the AuNPs. Cancer and normal cells, among other things, are characterized by different pH in several intracellular compartments, a difference can be conveniently used to modulate the action inside the cells of AuNPs (possibly functionalized for higher cancer recognition), for instance by regulating the associated drug release, the intrinsic fluorescence properties or the aggregation related aspect-ratio in plasmonic photo-thermal therapy. However, AuNPs may also be employed in a reversed perspective, i.e. for introducing acidifying agents to cause a decrease in pH beyond the tolerance level of cancer cells. In this mini-review the most recent findings are reported on the bi-verse effects of intracellular pH and gold nanoparticles.
Keywords
Gold nanoparticles
Intracellular pH-dependent properties
1 Introduction
Plasmonic nanoparticles, such as silver and gold nanoparticles (AgNPs and AuNPs) have been long studied for their multiple applications in several fields, such as electronics, optics, catalysis and biomedicine (Saji and Ashutosh, 2015; Carbone et al., 2016a). As far as the latter is concerned, an interest has arisen on their therapeutic possibilities. AuNP structures have been developed which behave as drug carriers, imaging agents, gene-regulating agents, and photoresponsive therapeutics. Furthermore, investigations were carried both in the cell environments and in the context of many debilitating diseases. These structures are not simply alternatives to molecule-based systems, but benefit of their peculiar physical and chemical properties, allowing substantial advantages in cellular and medical applications (Giljohann et al., 2010). The application of AuNPs in biomedicine and as theranostic agents compels to deal with pH issues, as the intracellular pH (pHi) is carefully regulated, depending on the type of cell, the cell function, the growth phase, as well as cellular compartments (organelles). The pH in oncological condition is a special case, as cancer cells withstand much lower pH values than normal cells, due to their higher metabolic activity, characterized by large H+ production. As a consequence pH dysregulation was proposed as a possible tool to develop cancer-specific therapeutics (Webb et al., 2011). The resistance to more acidic environments can be conveniently used in regulating the AuNPs and functionalized AuNP properties, in such a way that the moieties crossing the cell membranes are effectively different from those acting inside the cell. Finally, AuNPs can be used as carrier of acidifying agents, for manipulating the intracellular pH, thus changing perspective in intracellular pH-AuNP issue, and rendering the pHi variation the objective rather than a tool for further intracellular manipulations. This can be achieved by the use of properly designed molecules to bind AuNPs, such as, for instance, disulfide photoacids (Sabbatella et al., 2015). In this mini-review, the state-of-art achievements are reported on the bi-verse dependency between AuNP properties and pHi, for cellular manipulation, examining the different related aspects.
2 AuNP properties as a function of the pHi
Cancer and healthy cells, among other differences, are characterized by a different pHi (Gerweck and Seetharaman, 1996) which can be exploited in different ways to achieve target selective carrier, and/or drug delivery by AuNPs. The pHi-related chemical reactions which can be used as trigger for the specific intracellular action are of different types and can have an aggregative or a cleaving nature. For instance, a higher degree of hydrogen bonding is observed as a function of decreasing pH, whereas specific bonds used for connecting the AuNPs to the antitumoral drug such as hydrazine or carbodiimide linkages are labile in acid conditions, causing the separation of the carrier and the drug. Sometimes, the sheer protonation of a compound, consequent to a decreasing pH, turns it from atoxic into cytotoxic; in other cases the protonation changes the electrostatic properties of compound, triggering a variation of the coulombic interactions.
All these pH-related effects were used in tuning the properties of functionalized AuNPs, and their intracellular action. The most recent examples in literature are reported in the following section, whereas type of action, triggering chemical reaction and corresponding reference are summarized in Table 1.
Type of pH-dependency
Materials
Reference
Protonation
pH-responsive alkoxy-phenyl acylsulfonamide functionalized AuNPs
Mizuhara et al. (2015)
Non-covalent interaction between a carboxylic and an amino group
Carboxymethyl chitosans covered AuNPS coupled with DOX
Madhusudhan et al. (2014)
Release by the cleavage of acid−labile hydrazine linkage
Multifunctional nanocomposite AuNP@CD-AD-DOX/RGD
Chen et al. (2015)
Aggregation via coulombic interaction
AuNCs covered with glutathione bonded to polymer poly(allyl amine hydrochloride)
Yahia-Ammar et al. (2016)
Ligand assembling through hydrogen-bonding attractive interactions
PEG-SH functionalized AuNRs with the tip covered by lipoic acid
Ahijado-Guzmán et al. (2016)
Release by the cleavage of acid−labile carbodiimmide linkage
AuNPs covered with a carbodiimmide derivative and coupled with the DOX
Nam et al. (2013)
A way to regulate pH-dependent intracellular uptake of AuNPs is via zwitterionic surfaces. Nanoparticles with zwitterionic surfaces, in general, exhibit low toxicity, a long circulatory half-life and high biocompatibility (Arvizo et al., 2011). If these properties are integrated with a pH-dependent zwitterionic-to-cationic charge conversion system, they become attractive scaffolds for therapeutics. In “healthy” conditions, the cellular uptake of zwitterionic particles is low, whereas in tumor conditions, the uptake upon protonation of the resulting cationic particles is significantly higher. Therefore, the concomitant cytotoxicity resulting from the cationic nanoparticles would occur only in tumor environment. Mizuhara et al. (2015) showed how acylsulfonamide-functionalized zwitterionic gold nanoparticles can be employed to enhance the cellular uptake at tumor pH. In particular, they used switchable (acylsulfonamide) and a non-switchable (alkylsulfonamide) gold nanoparticles with a cancer cell-line, the HeLa, and a non-cancer cell line, the HMEC-1, and probed their viability at different pH values. At pH = 7.4 the AuNPs, uptake was similar for both cell lines. However, the HeLa cell uptake of switchable AuNPs increased by a factor of 4, when decreasing the pH to 6.0. Accordingly, the location of the nanoparticles changed, being on the cell membrane, at pH 7.4 and into the endosomes at pH 6.0. This behavior was not observed for the HMEC-1 or by employing the non-switchable AuNPs. Finally, HeLa cells exposed to the switchable at lower pH also exhibited a diminished viability, thus indicating the zwitterionic functionalized AuNPs as potential tool for cancer therapeutics.
Doxorubicin (DOX), albeit being a majorly used chemotherapeutic drug, lacks tumor-targeting ability, when administered directly, thus leading to poor distribution and therapeutic effects and a series of undesirable side effects such as myelosuppression and cardiotoxicity. It has restricted transport through the cellular membrane, due to its hydrophilicity, with consequent minimal drug internalization. Furthermore, chemoresistance is commonly developed especially in ovarian, colon, and breast cancers mainly due to over expression of a membrane transporter, the p-glycoprotein (pgp) that actively pumps DOX out of the cell (Carvahlo et al., 2009). The conjugation of DOX with nanoparticles circumvents this mode of efflux, as the cellular uptake changes become governed by an efficient endocytosis (Wang et al., 2011). The targeting of cancer cells may, then, be achieved by intrinsic enhanced permeability and retention effect (EPR) which can be further enhanced by nanoparticle decoration with targeting agents. The release of DOX inside the target cell, is then, conveniently modulated by the pHi, if a pH-sensitive linker is added, between the DOX and the AuNPs.
Madhusudhan et al. (2014) employed the carboxymethyl chitosan (CMC) as a reducing as well as capping agent. The carboxylic group of CMC interacts with the amino group of the DOX forming stable, non-covalent interactions on the surface of AuNPs and does not agglomerate as a function of the pH in the range 1.2–12. Cytotoxicity tests on HeLa cells indicate a marked decrease in vitality when using DOX-CMC-AuNPs, as compared to free DOX. Furthermore, the DOX fluorescence intensity (Karukstis et al., 1998) probed by confocal microscopy shows a two step DOX release, when HeLa cells are incubated, with DOX-CMC-AuNPs, i.e. first accumulation in the cytoplasm and, then, its entry in the nuclei. The use of free DOX is more aspecific and enters directly the nuclei. This behavior is compatible with the ionization of the CMC-DOX bond at acidic pH, thereby releasing the drug effectively at acidic pH suitable to target cancer cells. The DOX loaded gold nanoparticles were effectively absorbed by HeLa cells compared to free DOX and their uptake was further increased at acidic conditions induced by nigericin, an ionophore that causes intracellular acidification.
The combination of carrier properties, selective cancer cell recognition and pH-dependent drug release has been achieved by host–guest interaction in a complex gold nanoparticle-based multifunctional nanocomposite, labeled AuNP@CD-AD-DOX/RGD (Chen et al., 2015). In this system, the AuNPs are primarily modified with β-cyclodextrin (AuNP@CD) and, then, decorated with two different purposely designed moieties, one for the selective cancer cell recognition, the other one containing the chemotherapeutic drug. The peptidic derivative adamantane-PEG8-glycine-arginine-glycine-aspartic-serine (AD-PEG8-GRGDS) was employed, as it can target cancer cells with overexpressed αvβ3 integrin (Zitzmann et al., 2002). Doxorubicin was chosen as chemotherapeutic, and conjugated with adamantane via hydrazone bond linkage (AD-Hyd-DOX), which is labile in acidic environment and introduces the pH-dependency in the whole system. The double decoration via host–guest interaction allows the achievement of a multifunctional nanocomposite, the AuNP@CD-AD-DOX/RGD that enters preferentially into cancer cells and markedly enhances the cellular uptake for cancer targeted drug delivery via receptor-mediated endocytosis.
Once internalized in cancer cell, the nanocomposite localizes in endo/lysosomes, which have a pH of 4.0–6.0 (in contrast with 7.4 in healthy cells), and causes a rapid release of the anticancer drug from AuNP@CDAD-DOX/RGD due to the cleavage of acid−labile hydrazine linkage between adamantane and doxorubicin. This system also has the advantage of a pH modulated fluorescence.
The fluorescence intensity of doxorubicin is quenched as a function of functionalization with AuNPs (Raikar et al., 2011), but the intensity is recovered as soon as it is detached in acidic environment. This allows, not only the monitoring of the drug release, but also its localization within the cell (Fig. 1).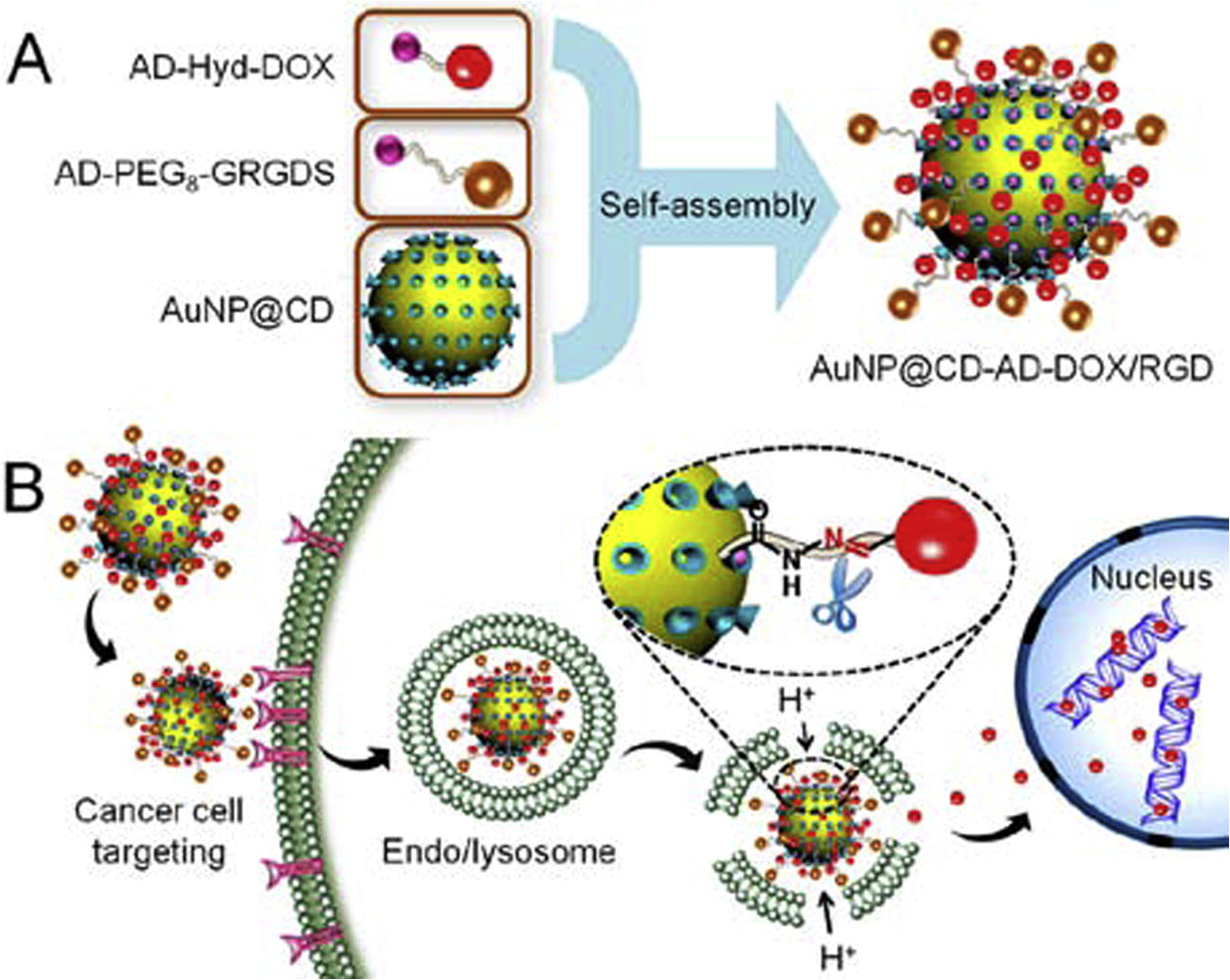
Self-assembly of the AD-PEG8-GRGDS and AD-Hyd-DOX on the surface of AuNP@CD via host–guest interaction. (B) The multifunctional nanocomposite was selectively up taken by cancer cell via the active targeting strategy and the acid microenvironment of endo/lysosomes. Adapted from the paper of Chen et al. (2015), with permission. Copyright (2015) American Chemical Society.
The study was conducted on U87 cancer cells (Human glioblastoma cells) with overexpressed αvβ3 integrin receptor on the cell membrane and αvβ3 integrin receptor-negative COS7 normal cells (African green monkey kidney fibroblast cells) mainly by confocal laser scanning microscopy and showed both an enhanced uptake of the nanocomposite of the cancer vs. normal cells, which also increases as a function of time and a prompt release of the drug selectively in the cancer cells. The pre-treatment with free RGD peptide allows to identify the endocytosis mechanisms as receptor-mediated. Once the drug is released in the cancer cells it induces their apoptosis (Chen et al., 2015).
The pH regulation has a fundamental role in controlling the properties of materials of biomedical interest, such as nanogels, which have shown to deliver high drug content into targeted sites such as tumor tissues (Jiang et al., 2014). The polyelectrolyte-based nanogels are swollen nanosized networks capable of absorbing high water quantities, which can be designed to respond to endogenous as well as exogenous stimuli owing to their swelling behavior. Stimuli-responsive nanogels can be successfully combined to gold nanoclusters (AuNCs) to achieve the stimuli-responsive hybrid nanogels with enhanced induced emission of plasmonic particles. AuNCs which have an ultrasmall size (<2 nm) as they are composed of a few to around 100 atoms and compared to nanoparticles (NPs), exhibit molecular-like properties, long-lifetime fluorescence in the red-near-infrared region, and large two-photon excitation, which makes them highly suitable for in vitro and in vivo imaging (Shang et al., 2011). When they are combined with suitably chosen hydrogels, they keep low toxicity with high renal clearance and long blood circulation times, and exhibit a pH-dependent swelling that causes an aggregation into nanoparticles, which influences their fluorescence properties. Yahia-Ammar et al. (2016) showed that the cationic polymer poly(allyl amine hydrochloride) PAH could be linked to Au NCs protected by glutathione (GSH) (Fig. 2), forming self-assembled particles Au-GSH-PAH with a spherical shape, within a few minutes. Upon an extensive characterization, it was verified that the monodisperse and positively charged NPs exhibit two major properties that make suitable for intracellular use: pH-dependent swelling properties, strong fluorescence enhancement (up to 4 times) upon aggregation (aggregation-induced emission–AIE-). In vitro studies were performed in human monocytic cells that indicate strongly enhanced uptake of the NPs compared to free AuNCs in endocytic compartments.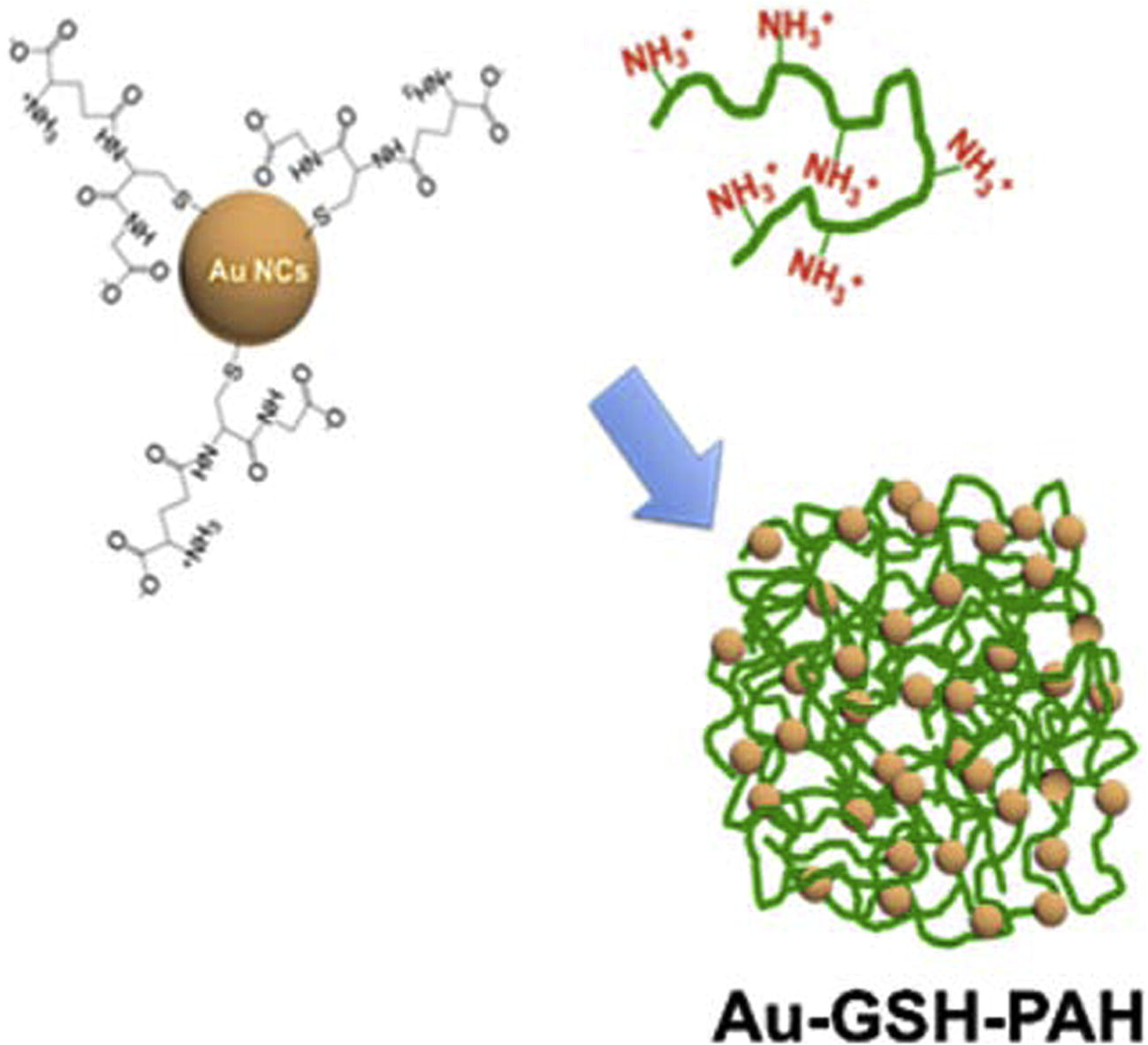
Scheme of the synthesis of self-assembled AuNCs stabilized with GSH, using a cationic polymer (PAH). Adapted from the paper of Yahia-Ammar et al. (2016), with permission. Copyright (2016) American Chemical Society.
The NPs keep their assembly structure with quite low cytotoxicity up to 500 μg Au/mL. Loading peptides or antibodies using NPs one-pot synthesis, demonstrated an enhanced drug delivery.
Intracellular colocalization of the biomolecules and the NP carriers was probed by fluorescence microscopy and flow cytometry, with a respective 1.7-fold and 6.5-fold enhanced cellular uptake of peptides and antibodies compared to the free biomolecules.
The plasmonic photothermal-dynamic therapy is an anticancer therapy that usually employs radiation sensitizers to reduce the irradiation laser power. Gold nanorods (AuNRs) are good sensitizer candidates due to the strong light scattering and absorption at their longitudinal localized surface plasmon resonance (L-LSPR) wavelength. This band is particularly useful for therapy purposes, because it can readily be tuned to the infrared region, which corresponds to the optical window of biological tissues. Furthermore, it can be enhanced by several orders of magnitude, thus creating hot spots, by the generation of controlled AuNR ensembles, which are able to confine light in their interparticle gaps. AuNR tips functionalized with pH-sensitive linking molecules allow the formation tip-to-tip ensembles which respond to intracellular pH conditions. It is worth noticing that, at variance with the examples reported so far, the strategy here aims at a nano-composite agglomeration upon pH variation, rather than in dissociation in different moieties. Ahijado-Guzmán et al. (2016) probed lipoic acid molecules at the tips of the AuNRs as a pH-induced directional intermolecular hydrogen bonding. The AuNRs were obtained by optimized seed-mediated growth synthesis, stabilized cetyltrimethylammonium bromide (CTAB), and, then exchanged with thiol-modified poly(ethylene glycol) (PEG-SH), which imparts AuNR high biological stability and biocompatibility but reduces the AuNR cell uptake levels. This is circumvented by the subsequent specific Au NR tip functionalization with lipoic acid as a pH-sensitive molecular linker that has a high biocompatibility as a cellular micronutrient (synthesized de novo in the mitochondria and/or by uptake) and may guarantee strong binding to the AuR surface and protonation within the acidic cell environments. After penetrating the breast cancer cells (MDA-MB 231 cell line) the functionalized AuNRs accumulate into the lysosomes where they assemble into oligomers through hydrogen-bonding attractive interactions, the key parameters leading the agglomeration, being the low pH of the cancer lysosomes. The femtosecond laser irradiation under low power density conditions, then, increases the temperature at the interparticle gaps of the oligomers enough to induce cell death. The formation of AuNR oligomers with low aspect ratios allows an important reduction in the applied laser power density (down to 0.21 W/cm2) (Becker et al., 2010). Therefore, the pH-induced self-assembly of plasmonic tip-to-tip oligomers with L-LSPR bands close in resonance with the infrared wavelength of the laser was found to be decisive for the plasmonic photo thermal therapy.
A combination of photothermal therapy and chemotherapy was achieved by coupling small sized AuNps (10 nm) and DOX, via pH-sensitive carbodiimide covalent linking.
The hydrodynamic size of gold nanostructures active in photothermal therapy typically reaches ∼100 nm, whereas most molecules based on chemo agents such as DOX are far smaller than that. As the size critically determines the entry of moieties into cells, the size difference inherently limits the concertion of the thermo and chemo agents. Nam et al. (2013) engineered AuNPs with a mixture of N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride and N-hydroxysulfosuccinimide sodium salt, which can covalently link with DOX (named SANDC). The optical properties of the AuNPs remained unchanged after the DOX conjugation, and no noticeable variation in their hydrodynamic size was observed. The lowering of the pH, as in the intracellular environments of cancer cells, triggers the DOX release from the SANDCs and the simultaneous AuNP aggregation to the level of fulfilling the conditions for photothermal therapy, with consequently shifting the absorption to far-red and NIR by creating the collective plasmon modes. The pH-responsive DOX release and aggregate formation of SANDCs was first evaluated in buffer solutions at pH levels of 2.0, 5.5, or 7.4, by monitoring the DOX fluorescence and the surface plasmon resonance. Initially, the conjugated DOX fluorescence was effectively quenched by the proximity to the Au NPs. At pH 2.0 and pH 5.5 the samples showed an initial burst of fluorescence intensity, more rapid in the former than in the latter case, due to the release of DOX, after which the intensity plateaued. No substantial fluorescence variation was observed for the sample at pH 7.4. At the same time, the hydrolysis of the citraconic amide linker in the surface ligand, causes the SANDCs to become positively surface charged, regardless of whether they are DOX conjugated or not. This charge exposure induces rapid aggregation of the Au NPs through the electrostatic interactions between surfaces bearing both positive and negative charges.
The study of SANDCs in B16 F10 mouse melanoma cells reveals their localization in the nuclei, at variance of the separate moieties, which are, instead, localized in the cytoplasm. Furthermore, viability tests indicate that the conjugates exhibit a synergistic effect enhanced by nearly an order of magnitude in cellular level. This allows reductions in both the therapeutically effective drug dosage and the photothermal laser threshold. In vivo tests on mice (four week old female hairless athymic nu/nu mice) bearing B16 F10 melanoma cells grafted onto the flank showed the largest tumor suppression with SANDCs as compared to the blank experiments, exhibiting a complete halt in tumor growth until the last laser irradiation (day 10) and reducing the final tumor to a size.
3 AuNPs for manipulating pHi
One resource to manipulate the pHi is by employing purposely designed proton caged compounds (PPCs). The concept of this method is to obtain compounds which can penetrate the cell membrane and possess photoresponsive capability. The irradiation of the cells exposed to the PCC, at the proper wavelength, then, provokes an intracellular pH decrease. In first instance the 1-(2-nitrophenyl)-ethylhexadecyl sulfonate ester (HDNS) was employed (Carbone et al., 2013), a variant of the PCCs proposed by Barth and Corrie, (2002), which has the advantage of a long aliphatic chain which may facilitate the interaction with the cell membrane. It was tested on NIH-3T3 fibroblasts and probed by synchrotron radiation infrared of single cells. The monitoring of the acidification process was performed by the infrared detection of the CO2 which is produced upon reaction of the photogenerated protons with intracellular HCO3− to give H2CO3, that promptly decomposes. Once established that HDNS is effective in the intracellular acidification process several pathways where followed to enhance the acidification efficacy. The main strategies consisted in enhancing the absorption of the HDNS, either by employing DMSO (Carbone et al., 2016b), an agent that affects the cell membrane permeability, or by modulating the incubation time (Carbone et al., 2016c). In the former case, the DMSO interferes with the acidification process and causes a sort of hyper-acidification characterized with amino acids protonation. In the latter case, the different incubation times are characterized by a different trapping of the HDNS in intracellular vesicles, with an effective different kinetics of metabolization of photogenerated protons. A really efficient way to enhance the PCC acidification power is by associating it with AuNPs (Carbone et al., 2015). In this case purposely PCC functionalized with a S–S bridge were synthesized to bind monodispersed AuNPs of 22 ± 1 nm. Then, separate plates of HEK-293 cell cultures were incubated with PCC bonded and non-bonded to AuNPs. The infrared probing of the cells upon near-UV irradiation gave remarkably different results. The CO2 yield in cells treated with the AuNP bonded PCC was 400 times higher than the non-bonded ones (Fig. 3). This can be related either to larger intracellular concentration of photogenerable protons and/or to more localized.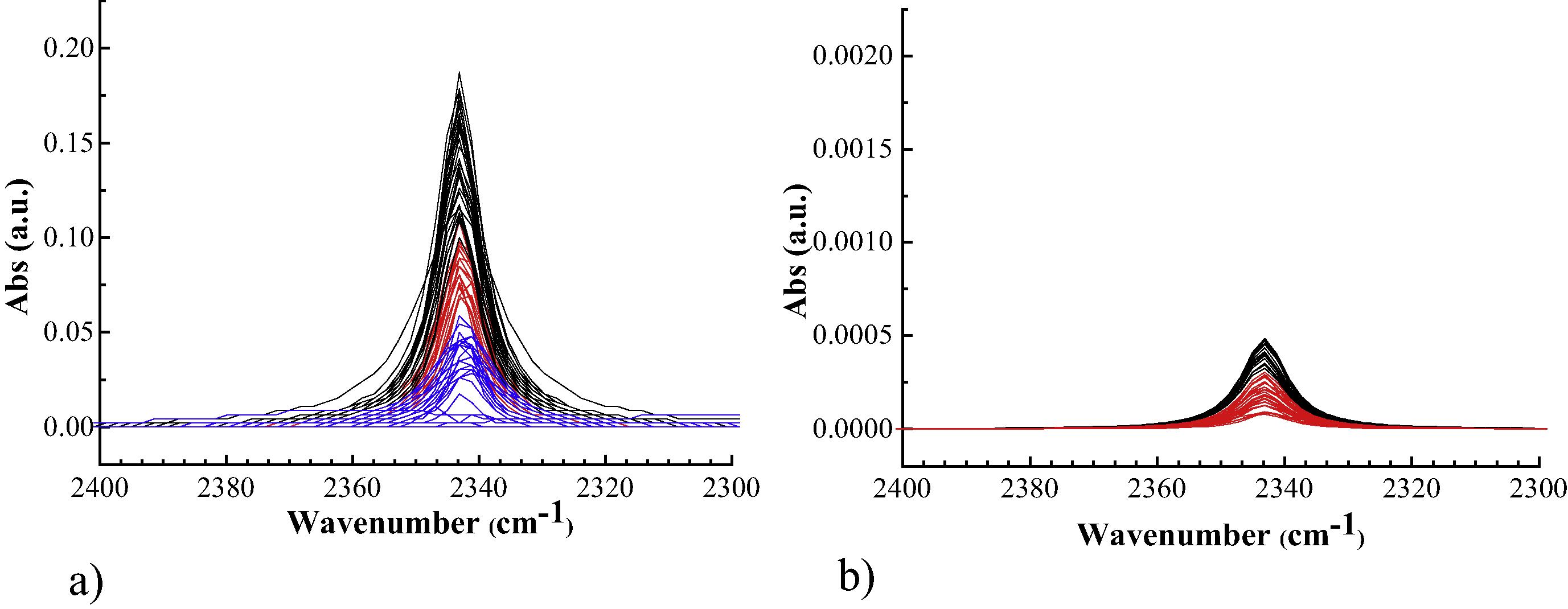
CO2 absorption band after irradiation of dosed HEK-293 cells; (a) cells dosed with a PCC-AuNPs, (b) cells dosed with free PCC. Different colors refer to different irradiation rounds. Note that the scale of the left panel is 100 times larger than the scale of right one. Adapted from the paper of Carbone et al. (2015), with permission. Copyright (2015) Elsevier.
4 Conclusions
AuNPs may play an important role in biomedicine and as therapeutic agent. They are usually sized, shaped and functionalized with coating agents that improve their biocompatibility and circulatory half-life, to make them more suitable as drug carriers as well as imaging agents.
All these parameters can be tuned in such a way that the AuNP properties become pH-dependent. This can be of particular use when acting in cancer cells since, in some intracellular compartments, such as the lysosomes, the pH is lower even by 3 units as compared to normal cells, and the pH-dependency of the AuNPs becomes a selective tool thus minimizing the side effects for normal cells. The AuNP pH-dependency can be aimed at different purposes: using the cancer cells acidic environment as a trigger to reactions such as the selective cleavage of a chemical bond and, for instance, subsequent release of an antitumoral drug or decreasing the pHi beyond the tolerance limit of the cancer cells.
In this sense the pHi can be seen either as the prompt of an action induced by the AuNPs and related moieties or as the limit to overcome through the functionalized-AuNPs.
Several pH-dependent chemical reactions can be exploited to trigger an action of the former type:
Besides the cleavage of labile bonds, protonation, aggregation via Coulomb interaction and assembling through hydrogen bonding can be used, among the others, to direct intracellular action of the AuNPs and/or the associated coating. The most recent systems were reported which are engineered to exploit the intracellular pH to direct the nanoparticle targeted action. In particular, modifications with zwitterionic surfaces, chitosan, polyelectrolyte based nanogels, carbodiimide derivatives, complex aminoacid-adamantane composites as well as lipoic acids were considered.
On the other side, when the perspective is reverted and the pHi is the barrier instead of the trigger, the AuNPs can be conveniently functionalized with acidifying agents for a further intracellular acidification.
References
- Intracellular pH-induced tip-to-tip assembly of gold nanorods for enhanced plasmonic photothermal therapy. ACS Omega. 2016;1:388-395.
- [Google Scholar]
- Modulating pharmacokinetics, tumor uptake and biodistribution by engineered nanoparticles. PLoS One. 2011;6(9):e24374.
- [Google Scholar]
- Characterization of a new caged proton capable of inducing large pH jumps. Biophys. J.. 2002;83(5):2864-2871.
- [Google Scholar]
- The optimal aspect ratio of gold nanorods for plasmonic biosensing. Plasmonics. 2010;5:161-167.
- [Google Scholar]
- Monitoring and manipulation of the pH of single cells using infrared spectromicroscopy and a molecular switch. Biochim. Biophys. Acta Gen. Subj.. 2013;1830:2989-2993.
- [Google Scholar]
- Exogenous control over intracellular acidification: enhancement via proton caged compounds coupled to gold nanoparticles. Biochim. Biophys. Acta Gen. Subj.. 2015;1850:2304-2307.
- [Google Scholar]
- Silver nanoparticles in polymeric matrices for fresh food packaging. J. King Saud Univ. Sci.. 2016;28(4):273-279.
- [Google Scholar]
- Exogenous control over intracellular acidification: enhancement via proton caged compounds coupled to gold nanoparticles and an alternative pathway with DMSO. Data in Brief. 2016;6:745-749.
- [Google Scholar]
- Modulating intracellular acidification by regulating the incubation time of proton caged compounds. Eur. Biophys. J.. 2016;45:565-571.
- [Google Scholar]
- Doxorubicin: the good, the bad and the ugly effect. Curr. Med. Chem.. 2009;16:3267-3285.
- [Google Scholar]
- Rational design of multifunctional gold nanoparticles via host−guest interaction for cancer-targeted therapy. ACS Appl. Mater. Interfaces 2015:17171-17180.
- [Google Scholar]
- Cellular pH gradient in tumor versus normal tissue: potential exploitation for the treatment of cancer. Cancer Res.. 1996;56:1194-1198.
- [Google Scholar]
- Gold nanoparticles for biology and medicine. Angew. Chem. Int. Ed.. 2010;49:3280-3294.
- [Google Scholar]
- Click hydrogels, microgels and nanogels: emerging platforms for drug delivery and tissue engineering. Biomaterials. 2014;2014(35):4969-4985.
- [Google Scholar]
- Deciphering the fluorescence signature of daunomycin and doxorubicin. Biophys. Chem.. 1998;73:249-263.
- [Google Scholar]
- Efficient pH dependent drug delivery to target cancer cells by gold nanoparticles capped with carboxymethyl chitosan. Int. J. Mol. Sci.. 2014;15:8216-8234.
- [Google Scholar]
- Acylsulfonamide-functionalized, zwitterionic gold nanoparticles for enhanced cellular uptake at tumor pH. Ang. Chem. Int. Ed.. 2015;54:6567-6570.
- [Google Scholar]
- pH-responsive assembly of gold nanoparticles and “spatiotemporally concerted” drug release for synergistic cancer therapy. ACS Nano. 2013;7(4):3388-3402.
- [Google Scholar]
- Fluorescence quenching using plasmonic gold nanoparticles. Opt. Commun.. 2011;284:4761-4765.
- [Google Scholar]
- Synthesis of proton caged disulphide compounds for gold nanoparticle functionalization. New J. Chem.. 2015;39:2489-2496.
- [Google Scholar]
- Functionalized gold nanoparticles: synthesis, properties and applications—a review. J. Nanosci. Nanotechnol.. 2015;35:1869-1894.
- [Google Scholar]
- Ultra-Small fluorescent metal nanoclusters: synthesis and biological applications. Nano Today. 2011;6:401-418.
- [Google Scholar]
- Doxorubicin-tethered responsive gold nanoparticles facilitate intracellular drug delivery for overcoming multidrug resistance in cancer cells. ACS Nano. 2011;5:3679-3692.
- [Google Scholar]
- Dysregulated pH: a perfect storm for cancer progression. Nat. Rev. Cancer. 2011;11:671-677.
- [Google Scholar]
- Self-assembled gold nanoclusters for bright fluorescence imaging and enhanced drug delivery. ACS Nano. 2016;10:2591-2599.
- [Google Scholar]
- Arginine-glycine-aspartic acid (RGD)-peptide binds to both tumor and tumor-endothelial cells in vivo. Cancer Res.. 2002;62:5139-5143.
- [Google Scholar]







