Translate this page into:
Breeding behaviour of the Kentish plover (Charadrius alexandrinus) in a salt marsh from the Eastern High Plateaux, northeast Algeria
⁎Corresponding author at: Faculty of Sciences, Department of Biology, Badji Mokhtar University, BP 12, Annaba 23000, Algeria. bensouilah.taqi@yahoo.fr (Taqiyeddine Bensouilah)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
The reproductive biology and nesting site selection of the Kentish plover, Charadrius alexandrinus, were investigated in a semi-arid salt marsh from the Eastern High Plateaux, northeast Algeria. The present study describes for the first time the breeding behaviour of this plover in the Eastern High Plateaux. On the natural ecosystem of Sebkhet Ouled M’Barek, egg-laying occurred from mid-April to late May, with peak in the last week of April. Mean clutch size was 2.71 ± 0.58 (n = 45), and incubation period was 27.0 ± 0.9 days. Hatching success amounted to 69.6% ± 6.4 (45 clutches) and an average of 2.0 ± 0.2 chicks hatched per nest. In this study we observed that incubating plovers usually nested near water edge and very close to a heterospecific nest. Three aspects make this population distinct from most other Kentish plover populations studied to date. It is characterised by a late onset of egg-laying, short egg-laying period and high rate of breeding success. Nevertheless, the present study shows that the mean clutch size, egg volume and incubation period were comparable to those known for other Mediterranean populations. Anthropogenic pressures, habitat loss as well as lack of management plans are major threats of this population.
Keywords
Egg laying date
Clutch size
Nest site selection
Breeding success
Wetlands
Waterbirds
1 Introduction
Among shorebirds, Kentish plover (Charadrius alexandrinus) is a species with a cosmopolitan distribution that breeds in both temperate and subtropical climate zones (Page et al., 1995, 2009; del Hoyo et al., 1996; Delany et al., 2009). This species has an extremely large range and multiple geographical races (Page et al., 1995, 2009; del Hoyo et al., 1996; Isenmann and Moali, 2000; Powell, 2001). Recently, the species has attracted considerable attention in evolutionary and conservation biology because of its flexible breeding system (mating and parental care behaviour) that varies both across and within populations (Lessells, 1984; Warriner et al., 1986; Székely and Williams, 1995; Amat et al., 1999a; Székely and Cuthill, 1999; Kosztolányi and Székely, 2002; Kosztolányi et al., 2006, 2009; Székely et al., 2006; Amat et al., 2008; Delany et al., 2009; Küpper et al., 2009; Page et al., 2009; AlRashidi et al., 2010, 2011; Hanane, 2011) and because many populations are fragmented and declining (Page et al., 1995, 2009; Ruhlen et al., 2006; Scarton et al., 2013). Despite the fact that the population trend appears to be decreasing, the decline is not believed to be sufficiently rapid to approach the thresholds for Vulnerable under the population trend criterion (BirdLife International, 2015).
Extensive studies on the nesting habitat and nest site selection of Kentish plovers have been carried out through their range (Fraga and Amat, 1996; Kosztolányi et al., 2006, 2009; Page et al., 2009; AlRashidi et al., 2010, 2011; Hanane, 2011), as well as detailed studies about nest site selection (Valle and Scarton, 1999; Norte and Ramos, 2004; Scarton et al., 2013). Different aspects of the breeding biology of the species have been investigated in both natural and man-made habitats such as farmland (Székely, 1990; Toral and Figuerola, 2012), alkaline grassland (Székely et al., 1994), marshes (Székely and Cuthill, 1999), salt-pans (Székely, 1996), saline lakes (Fraga and Amat, 1996), shallow coastal lagoon (Scarton et al., 2013), sandy and rocky beaches (Valle and Scarton, 1999; Fojt et al., 2000; Norte and Ramos, 2004; Hanane, 2011) as well as in harsh nesting conditions such as hot environment (Kosztolányi et al., 2009; AlRashidi et al., 2010, 2011). In Algeria, Kouidri (2013) has reported some aspects of the breeding biology of the species in the Desert. However, the breeding phenology and the reproductive success remain poorly known in other localities in Algeria.
Altitude has significant influences on the evolution of bird life history traits (Badyaev, 1997; Lu, 2005; Lu et al., 2008, 2010; Boyle et al., 2015; Hille and Cooper, 2014). Some researchers have suggested that birds breeding at a high altitude may invest less in reproduction (Bears et al., 2009) with a lower breeding pair density, late start of egg laying and short breeding period than birds at a low altitude (Lu, 2004, 2005; Lu et al., 2008, 2010; Boyle et al., 2015; Hille and Cooper, 2014). At a high altitude environment, where the climate is different and the food availability is short in supply and low in quality, birds are expected to adjust their reproductive strategy.
Therefore, the primary objective of this study was to provide baseline information about the breeding behaviour of the Kentish plover in the most important breeding area for the species in the Eastern High Plateau. We expected that: (1) plovers at our study area start egg laying later and have short breeding period than other populations; (2) our population have high breeding success; and we discuss the management plans which may help us to conserve the species in our natural ecosystem.
2 Materials and methods
2.1 Study area
The current study was conducted, during the breeding season 2014, in semi-arid saline wetland situated in the Algerian high plains, wilaya of Khenchela, northeast Algeria (Fig. 1). Sebkhet Ouled M’Barek (35°23′39.60″N, 7°19′53.57″E) is a saline marsh which covers an area of 950 ha and has an elevation of 1062 m a.s.l (Fig. 2). The water level varies from 0.6 m in the dry season to 1.2 m in the wet season. This wetland was supplied continuously by Ounrhal and Gueuntis Rivers. This natural ecosystem has a series of small islets often used by water birds for breeding, resting and as shelter sites. Even though the wetland is comprised of aquatic plants, it is surrounded by cereal crops consisted of Chenopodiaceae (Atriplex halimus, Atriplex patula, Salicornia fruticosa, Salsola fruticosa, Suaeda fruticosa), Brassicaceae (Moricandia arvensis, Matthiola fruticulosa, Diplotaxis erucoides, Capsella bursa pastoris). This wetland harbours an important avifauna. Among the common species there are the greater Flamingo Phoenicopterus roseus roseus, Common Crane Grus grus, Slender-billed Gull Larus genei, Black-winged Stilt Himantopus himantopus, Pied Avocet Recurvirostra avosetta, Common Shelduck Tadorna tadorna, Ruddy Shelduck Tadorna ferruginea and Kentish plover (Saheb, 2009).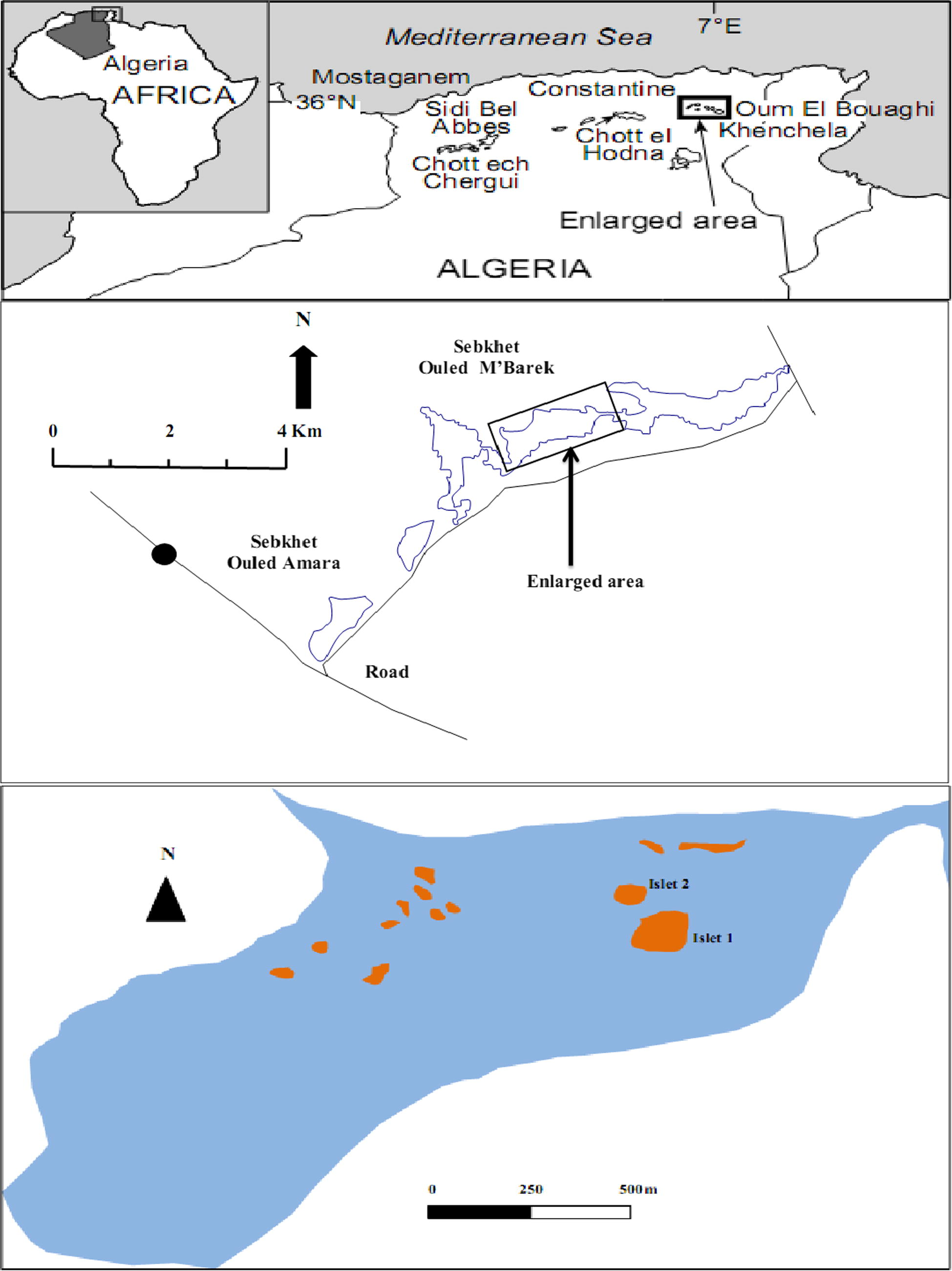
Map of the High Plateaux wetlands, northeast Algeria, showing the study area.

Two views of Sebkhet Ouled M’Barek, an unprotected salt marsh.
2.2 Data collection
We find nests by watching birds with a telescope Konuspot (20 × 60), binoculars (10 × 50) and by searching on foot inside the two islets during the breeding season following Székely et al. (2008). We monitored nests every 3–4 days until the hatching of eggs or the failure of the breeding attempt. The length and width of eggs were measured with a digital calliper (0.01 mm) and weighed with a digital balance (0.1 g) (Székely et al., 2008). We calculated the egg volumes using Douglas’ formula (1990): Ve = kV × L × W2, where kV = 0.5236–(0.5236 × 2 × (L/W)/100), L = the length of egg (cm) and W = the width of egg (cm) (Amat et al., 2001).
We determined the egg laying date, clutch size, egg dimensions and the incubation period for each monitored nest. We collected the following nest site characteristics; nest diameter, cup depth, distance from the nest to mainland, distance from the edge of water to the nest, distance from the nest to the nearest conspecific nest (Kentish plover), and the distance from the nest to the nearest heterospecific nest (other species). We determined the laying date either by observing the date that the first egg was laid or by backdating from known hatching date, supposing that the incubation period lasts for 27 days (Fraga and Amat, 1996; Székely et al., 2008; Kosztolányi et al., 2009; Toral and Figuerola, 2012). The clutch size was recorded for complete clutches only. We calculated the incubation period for 35 nests.
We considered a nest as successful if we find at least one egg hatched. We determined the hatching if we find (1) a hatched chick in the nest or at close proximity, (2) one egg displayed proof of impending hatching (cracked eggshell), or (3) the egg disappeared with the expected hatching date and we don’t find any indication of predation. We considered a nest to as failed if we find (1) remainders of the eggs, (2) the nest was abandoned (the eggs were cold), or (3) the egg disappeared before the expected hatching date. We considered the fate of all other nests as unknown. (Hanane, 2011; Toral and Figuerola, 2012).
If a nest was considered to have failed, it was attempted to identify the cause of nest failure. If the nest was predated it was usually damaged, we find the eggshell fragments nearby. When damaged eggs were found in the nest, with footprints of livestock at the nest or in its vicinity, it was considered as trampled by livestock (Toral and Figuerola, 2012).
2.3 Statistical analysis
Prior to all analyses, all variables were tested for homoscedasticity using Levene’s test and normality using Kolmogorov–Smirnov test. We used linear regression to test for seasonal change in clutch size, incubation period and brood size using the Julian date as an independent variable. Habitat variables were contrasted between the two islets using t tests. Results were considered significant at p < 0.05. Statistical analyses were done using SPSS software Version 19. Means are given ±standard error.
3 Results
3.1 Nesting site
Data were collected for a total of 45 nests. The Kentish plover nests on two small islands at Sebkhet Ouled M’Barek. On the first islet, 26 breeding pairs shared the available nesting sites with two species, the Pied Avocet R. avosetta and the Black-winged Stilt H. himantopus, but in the second one, only the Kentish plover nests (n = 19) were found.
Nests consisted of shallow approximately circular depression in the ground an average of 9.58 mm deep (5.10–18.40) and 72.60 mm of diameter (62.2–83.2). There was neither fresh plant material nor feather within and around the nests, but sometimes a few small dry plants occurred (Fig. 6).
Nest of the Kentish plover with three eggs.
Nests were usually located at 19.89 m ± 1.39 from water’s edge (6–40 m). No difference between the two islets was recorded (t = 0.007, p = 0.994). The distance to the water’s edge increased as the breeding season progressed (linear regression: r2 = 0.895, F1,43 = 367.280, p < 0.0005). The mean distance to the nearest active nest was only 11.47 m ± 0.26. This distance increased as breeding season progressed (linear regression: r2 = 0.357, F1,43 = 23.383, p < 0.0005). Nests were close to each other in both islets (t = −0.952, p = 0.347). The average distance to the nearest heterospecific nests was 9.51 m ±6.94 (see Table 2). Note: SE, standard error.
N
Min
Max
Mean
SE
Nest diameter (cm)
45
6.22
8.32
7.26
0.07
Cup depth (cm)
45
0.51
1.84
0.95
0.04
Distance from the nest to mainland (m)
45
138
307.5
229.05
10.29
Distance from the nest to the edge of water (m)
45
6.7
40.5
19.89
1.39
Distance from the nest to the nearest conspecific nest (m)
37
8.28
15.25
11.47
0.26
Distance from the nest to the nearest heterospecific nest (m)
26
2.2
25.89
9.51
1.36
3.2 Timing of breeding and incubation period
The first pairs were observed in our study area on 16 March. The number of pairs increased in the following weeks till the beginning of April. The maximum length of egg laying (first eggs) was 39 days (14 April–23 May). The distribution of first eggs laying dates showed a seasonal increase with a peak in the mid-season (the last week of April, 35.6%, n = 16) and then a decline (Fig. 3).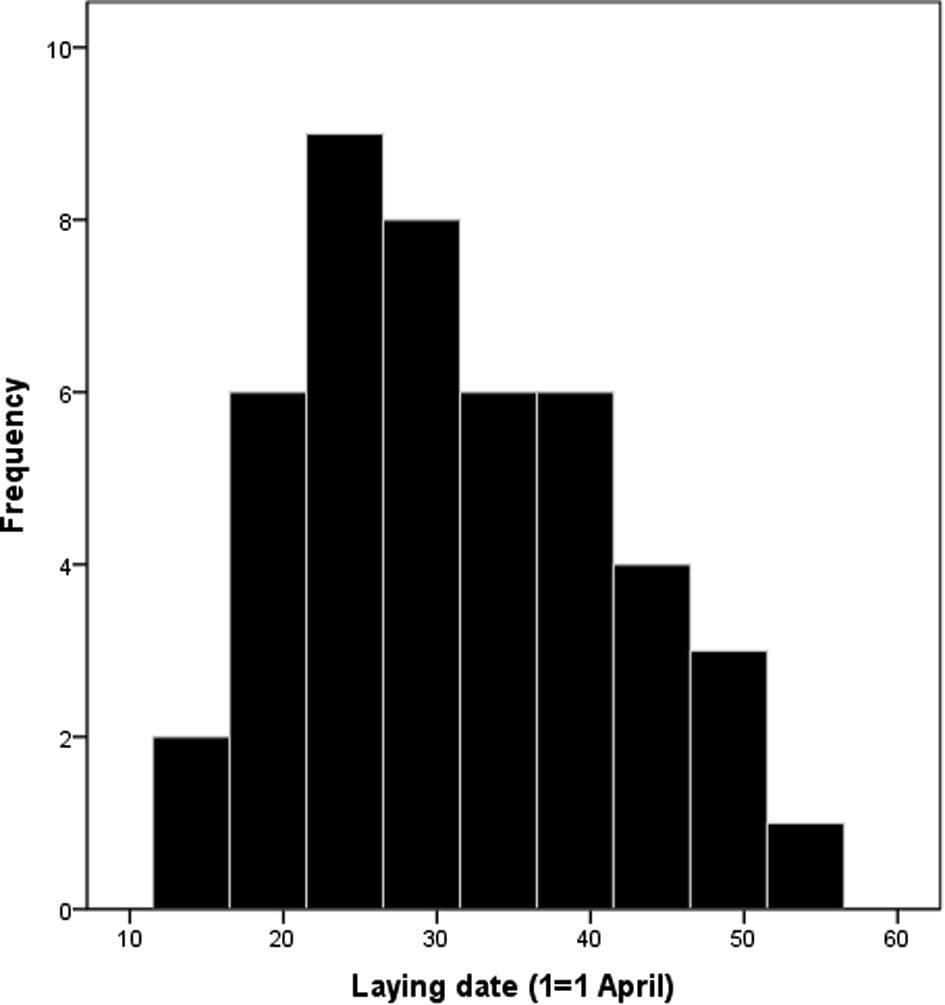
Distribution of egg-laying dates of the Kentish plover.
The mean incubation period for the Kentish plover was 27.0 ± 1.0 (25–28 days, n = 35). Clutches started later in the season had shorter incubation duration than early clutches did (linear regression: r2 = 0.436, F1,32 = 7.496, p = 0.010) (Fig. 4) whereas there was no evident influence of clutch size (linear regression: r2 = 0.0, F1,32 = 0.0, p = 1.0) and egg volume (linear regression: r2 = 0.001, F1,32 = 0.044, p = 0.836) on the incubation period.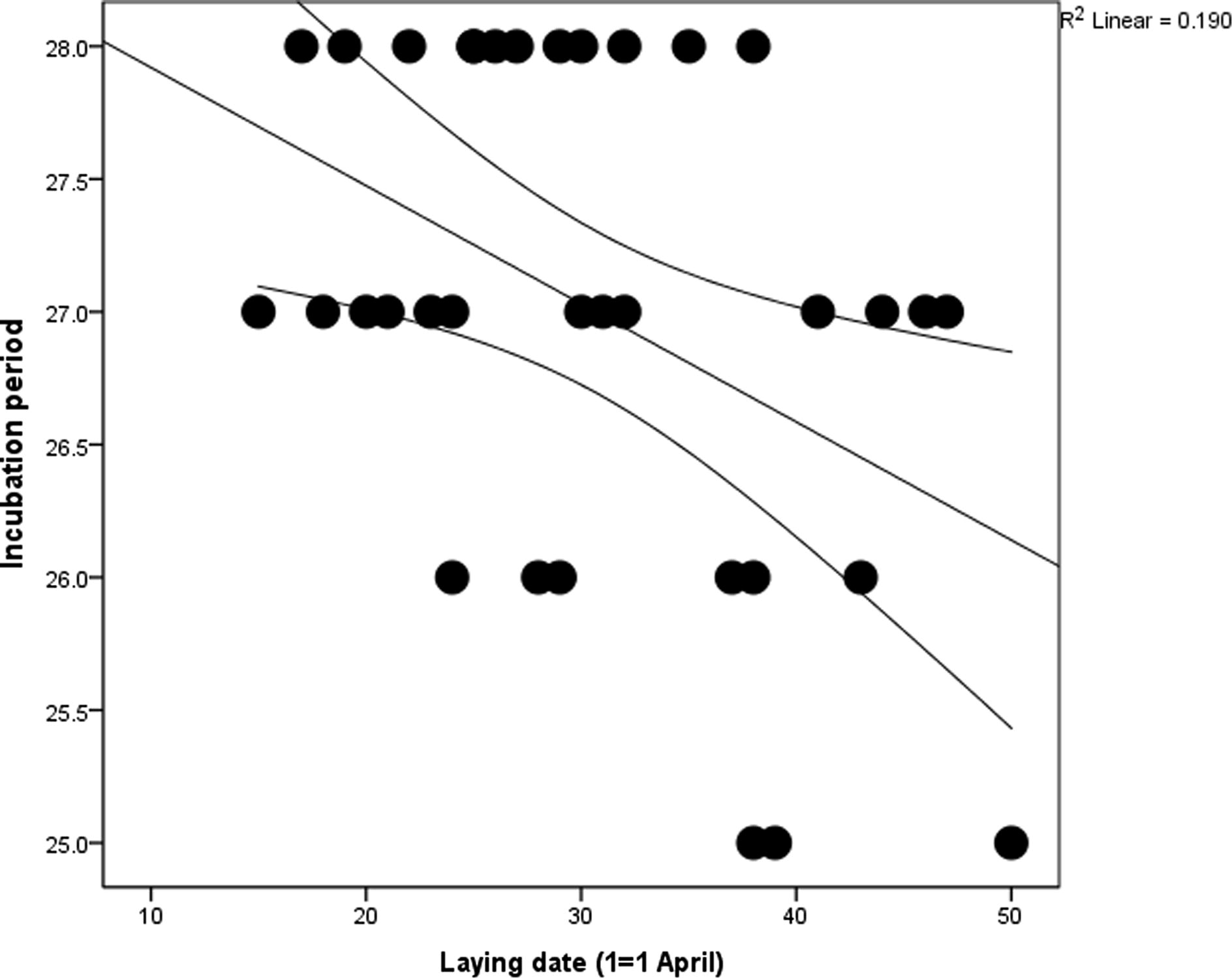
Seasonal change of the incubation period.
3.3 Egg dimensions and clutch size
Mean egg volume, calculated using the mean volume within each of 45 nests, was 8.72 cm3 ±0.68 (n = 122 eggs) and the mean ratio of egg shape (length/width) was 1.39 ± 0.01. There was no relationship between egg volume and both laying date (linear regression: r2 = 0.003, F1,43 = 0.129, p = 0.722) and clutch size (linear regression: r2 = 0.008, F1,43 = 0.354, p = 0.555). Egg mass and dimensions are summarised in Table 1. Note: SE, standard error.
N
Min
Max
Mean
SE
Length (cm)
122
2.86
3.41
3.20
0.01
Width (cm)
122
2.13
2.42
2.30
0.00
Mass (g)
122
6.67
9.56
8.22
0.04
Volume (cm3)
122
6.83
10.03
8.72
0.06
The mean clutch size was 2.71 ± 0.58 (range 1–3, n = 45, median and mode = 3) with 77.8%, 15.6% and 6.7% of clutches having three, two and one egg, respectively. There was no significant change of clutch size over the breeding season (linear regression: r2 = 0.051, F1,43 = 0.011, p = 0.740) (Fig. 5).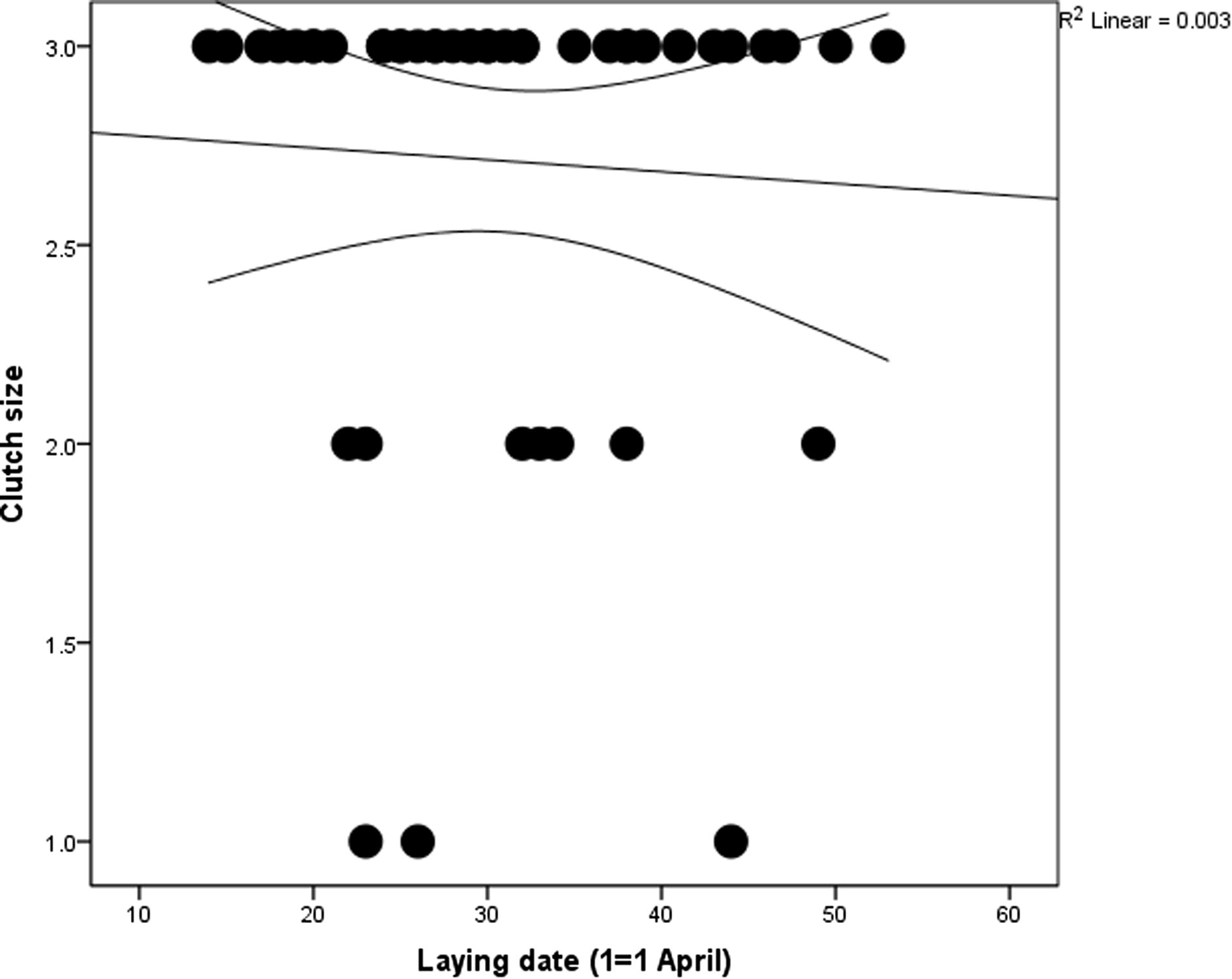
Seasonal change of clutch size.
3.4 Breeding success
The mean number of eggs hatched per successful clutches (with at least one chick produced) was 2.65 ± 0.10 (n = 34). The mean number of nestlings fledged was 2.0 ± 0.2 (n = 45) chick per nesting attempt. The number of hatchlings did not vary as breeding season progressed (linear regression: r2 = 0.006, F1,43 = 0.273, p = 0.604).
The hatching rate (total number of hatchlings/total number of eggs in nests at hatching) for 122 eggs in 45 clutches was 69.6% ± 6.4. Despite the high hatching success in the first islet (76.9% ± 8.0) compared with the second one (59.6% ± 10.4) we found no statistically significant difference between the two localities (χ2 = 3.662, df = 3, p = 0.3). In addition, hatching success did not vary significantly according to egg laying dates (linear regression: r2 = 0.009, F1,43 = 0.381, p = 0.540). Nesting success, as calculated by the percentage of nests produced at last one fledgling, was 71.1%.
The distance to the water’s edge did not affect hatching success (linear regression: r2 = 0.001, F1,43 = 0.040, p = 0.842). There is no significant influence of the nearest active nest on hatching success (linear regression: r2 = 0.001, F1,43 = 0.042, p = 0.839) nor on predation risk (linear regression: r2 = 0.003, F1,43 = 0.110, p = 0.741).
Overall, 13.3% of nests were trampled, 8.9% were predated, 2.2% were deserted and 4.4% failed to hatch. Mortality among chicks was rare, where only one nestling was found dead (Fig. 7). Also, we did not observe any case of nest flooding during the whole breeding season.
Young found dead.
4 Discussion
In spite of the fact that several sites are known as nesting ground for the Kentish plover in Algeria (Heim de Balsac and Mayaud, 1962; Isenmann and Moali, 2000; Samraoui et al., 2011; Kouidri, 2013), this study describes for the first time the breeding behaviour of this wader in the Eastern High Plateaux (Samraoui et al., 2011).
The mean clutch size, egg volume and incubation period compares favourably with the range recorded in many plover populations in America (Warriner et al., 1986; Page et al., 1995; Powell and Collier, 2000; Conway et al., 2005), Europe (Fraga and Amat, 1996; Amat et al., 2001a,b; Norte and Ramos, 2004) and Africa (Heim de Balsac and Mayaud, 1962; Isenmann and Moali, 2000; Zefania et al., 2008; Hanane, 2011; Kouidri, 2013). Although mean clutch size and egg volume did not decrease over the course of breeding season as other authors reported (Wallander and Andersson, 2003; Makrigianni et al., 2008), we found that incubation period decreased as breeding season progressed. This is consistent with the influence of climatic conditions on life history traits of birds (Bensouilah et al., 2014; Bensouilah, 2015).
4.1 Breeding season
The onset of egg-laying date (from mid-April to late May) is relatively late compared with other data published on wader species (Fraga and Amat, 1996; Amat et al., 1999; Powell et al., 2002; Wallander and Andersson, 2003; Colwell et al., 2005; Conway et al., 2005; Ruhlen et al., 2006; Kosztolányi et al., 2009; Hanane, 2011; Kouidri, 2013). Furthermore, the breeding season is markedly shorter than that reported in other localities in Algeria (from early March to mid-June) (Heim de Balsac and Mayaud, 1962; Isenmann and Moali, 2000; Kouidri, 2013) and Europe (Fraga and Amat, 1996; Wallander and Andersson, 2003; Norte and Ramos, 2004; Makrigianni et al., 2008; Székely et al., 2008), suggesting that some factors restrict the timing of breeding in this study population.
Although further surveys, with large sample size and for a long period, are paramount to know why breeding occurs later and for a shorter period of time, there are possible explanations for these differences about timing of breeding, at least between the Sahara (Kouidri, 2013) and the Eastern High Plateaux (this study). First, the short breeding season is probably related to late onset of laying, which may be limited by climatic conditions, mainly the cold temperature (Kouidri, 2013; Bensouilah et al., 2014; Bensouilah, 2015). Second, egg-laying period is a life history trait that depends on habitat conditions (Perrins and Birkhead, 1983; Weggler, 2006; Bensouilah et al., 2014; Bensouilah, 2015). Therefore, a short egg-laying period indicated that Sebkhet Ouled M’Barek might not be an optimum breeding habitat for this plover. Moreover, quality of breeding habitat, as measured by the rate of breeding success, may be affected by a combination of factors, such as food availability, offspring predation and natural disturbance. In contrast, our results, in the light of previous studies, demonstrate that Sebkhet Ouled M’Barek may provide nesting habitat of good quality. Indeed, the occupation of the same territory by Kentish plover, the Pied Avocet and the Black-winged Stilt (Saheb, 2009; authors unpublished data), which found adequate nesting site with large clutch size and high nesting success, is an indication of the suitability of this habitat. Nevertheless, the disturbance by livestock late in the season, which is the main cause of nesting failure, most probably shortened the egg-laying period. The disturbance may affect breeding shorebird populations (Gill et al., 1996; Cuervo, 2005; Montalvo and Figuerola, 2006; Delany et al., 2009; Hanane, 2011; Webber et al., 2013). Alternatively, breeding season may vary both according to climate conditions and altitude (Bensouilah et al., 2014; Bensouilah, 2015). We may speculate that the late start of egg laying and the short-laying period may be a different strategy employed by this population as an adaptation to local conditions which is consistent with the reproductive restriction hypothesis at a high altitude given by Bears et al. (2009). In fact, several investigators have shown that birds in high-elevation had a shorter breeding season (Lu, 2005; Lu et al., 2008, 2010; Bears et al., 2009; Martin et al., 2009; Boyle et al., 2015; Hille and Cooper, 2014). The colder temperatures, one of the most important factors, experienced at higher elevations are associated with a later start of egg laying, resulting in shorter breeding seasons (Weggler, 2006; Bears et al., 2009; Martin et al., 2009).
4.2 Breeding success and nesting site characteristics
Shorebirds are known to incur high rate of clutch loss owing to predation, flooding and trampling, which are the main causes of breeding failure in many ground nesting species (Winton et al., 2000; Baird and Dann, 2003; Wallander and Andersson, 2003; Makrigianni et al., 2008; Zefania et al., 2008; Kosztolányi et al., 2009; AlRashidi et al., 2011; Hanane, 2011).
Surprisingly, in this Kentish plover study population, hatching rate is high, averaging 69.6%. Hatching success reported by this study is much higher than other Kentish plover populations and other wader species, although we did observe a non-significant decline in hatching success as the season progressed (Fraga and Amat, 1996; Powell et al., 2002; Baird and Dann, 2003; Wallander and Andersson, 2003; Norte and Ramos, 2004; Colwell et al., 2005; Makrigianni et al., 2008; Zefania et al., 2008; Kosztolányi et al., 2009; Hanane, 2011; Kouidri, 2013).
Similar to our findings, in many other plovers early clutches have higher success (Fraga and Amat, 1996; Powell and Collier, 2000; Wallander and Andersson, 2003; Kosztolányi et al., 2007, 2009). This is a well-known feature occurring frequently in birds, and it may be due to a declining quality of late breeders or may indicate that nesting conditions affect the reproductive success of birds (Powell and Collier, 2000; Schmidt and Ostfeld, 2003; Neuman et al., 2004; Norte and Ramos, 2004; Colwell et al., 2005; Conway et al., 2005; Lafferty et al., 2006; Pearce-Higgins et al., 2007; Kosztolányi et al., 2009; Bensouilah et al., 2014; Bensouilah, 2015).
In the present study, most cases of nest failure were associated with the trampling by livestock after the decrease in the water level. Thus, we may speculate that water depth early in the season makes the two islets inaccessible for the livestock and mammalian predators which provide better protection for the population and by consequence breeding pairs experience low egg predation pressure.
With regard to nest site characteristics, breeding pairs seem to nest close to each other like other plover populations (Fraga and Amat, 1996; Székely et al., 1999; Valle and Scarton, 1999; Powell and Collier, 2000; Powell, 2001; Norte and Ramos, 2004; Conway et al., 2005). The average distance to nearest heterospecific nest (9.51 m ±6.94) was much lower than the values recorded for some Charadrii species (Powell, 2001; Norte and Ramos, 2004; Makrigianni et al., 2008). Similar to our finding, other investigators found that there was no significant influence of the distance to the nearest heterospecific nest on the reproductive success (Fraga and Amat, 1996). Although we found a higher reproductive success for nests that are in the mixed colony (first islet) comparing with those nests that are in the pure colony (second islet), this result may be explained by the beneficial effect from protection against predators afforded by the aggressive nest defence behaviour of other species (Black-winged stilt and Pied Avocet) nesting on the first islet of Sebkhet Ouled M’Barek. Some plovers seem to benefit substantially from this anti-predator behaviour (Cramp and Simmons, 1983; Valle and Scarton, 1999; Fojt et al., 2000; Powell and Collier, 2000; Schmitz et al., 2001; Powell, 2001; Hernandez-Matias et al., 2003; Nguyen et al., 2003, 2006; Lengyel, 2006; Hanane et al., 2010; Hanane, 2011; Scarton et al., 2013).
In the current study, vegetation cover of nests was very low. We observed that nests were located in microsites with little vegetation cover and in open habitats. Our results are thus consistent with observations on many plover species that prefer open and less vegetated habitat. Therefore, we suggest that individuals nest in exposed sites that minimise as much as possible predation risk, on both nest contents and incubating adults, because such sites facilitate the early detection of predators (Székely, 1990; Page et al., 1995; Valle and Scarton, 1999; Fojt et al., 2000; Winton et al., 2000; Powell, 2001; Powell et al., 2002; Nguyen et al., 2003; Amat and Masero, 2004; Norte and Ramos, 2004; Conway et al., 2005; Makrigianni et al., 2008; Zefania et al., 2008; AlRashidi et al., 2011; Hanane, 2011). Our result of the average distance to the edge of water are in the line with other studies, which suggest that low distance to the edge of water may provide high accessibility to food resources (Powell, 2001; Ruhlen et al., 2006; Makrigianni et al., 2008; Zefania et al., 2008).
5 Management implications
Our study provides fundamental data on the breeding behaviour of a Kentish plover population in Sebkhet Ouled M’barek, which constitutes one of the most important breeding areas in the Eastern High Plateaux, northeast Algeria. The breeding biology of the Kentish plover in this natural ecosystem appears to be different from other populations. It is mainly characterised by a late start of egg laying, a short breeding period and a high hatching success. We assume that the late onset of egg laying and the short-laying period is a different strategy employed by this population as an adaptation to local conditions (mainly the high altitude). We hypothesise that the breeding period is largely influenced by the disturbance of livestock late in the season although we cannot exclude alternative explanations. It appears that the disturbance by livestock after a decrease in the water level was the major contributor of nesting failure, so we believe that maintaining stable water levels during the breeding season will greatly increase hatching success. This may also extend the breeding period for the Kentish plover and likewise benefit other breeding species in this wetland such as the Pied Avocet and the Black-winged Stilt (Saheb, 2009; Belhassini, unpublished data). We recommended establishing new protected area to prevent habitat loss and improve the habitat quality for the breeding of most waterfowls, which experience great natural and anthropogenic disturbances. We suggest that, to enhance the nest success of birds, mammalian predators and livestock should be prohibited by fencing the new protected breeding area to protect the Kentish plover from predators and anthropogenic pressure which may provide safe and adequate nesting sites (Mabee and Estelle, 2000; Neuman et al., 2004; Hardy and Colwell, 2008; Székely et al., 2008; Page et al., 2009).
The Kentish plover population in Sebkhet Ouled M’barek offers an excellent opportunity to investigate the life-history traits of this species and it is ideal for long-term monitoring. Further research should focus on the following topics: (1) investigate the start and the length of breeding season over a longer time-scale with covering a large area of this saline marsh to verify our present findings; (2) evaluate the link between climatic conditions (temperature and precipitation) and the start of egg laying; (3) evaluate the link between water level, events of trampling and nest mortality; (4) estimate the daily survival rate, nesting success and identify avian and mammalian predators.
Acknowledgments
We are grateful to Menouar Saheb for providing the map of the study area. We would like to thank Saâd Hanane, Patrick Triplet and three anonymous reviewers for commenting on an early version of our manuscript.
References
- Breeding ecology of the Kentish plover, Charadrius alexandrinus, in the Farasan Islands, Saudi Arabia. Zool. Middle East. 2011;53:15-24.
- [Google Scholar]
- The influence of a hot environment on parental cooperation of a ground-nesting shorebird, the Kentish plover (Charadrius alexandrinus) Fron. Zool.. 2010;7:1.
- [Google Scholar]
- Predation risk on incubating adults constrains the choice of thermally favourable nest sites in a plover. Anim. Behav.. 2004;67:293-300.
- [Google Scholar]
- Brood desertion and polygamous breeding in the Kentish plover Charadrius alexandrinus. Ibis. 1999;141:596-607.
- [Google Scholar]
- Variations in body condition and egg characteristics of female Kentish plovers Charadruis alexanderinus. Ardea. 2001;89:293-299.
- [Google Scholar]
- Intraclutch egg-size variation and offspring survival in the Kentish plover Charadrius alexandrinus. Ibis. 2001;143:17-23.
- [Google Scholar]
- Brood desertion and polygamous breeding in the Kentish plover (Charadrius alexandrinus) Ibis. 2008;141:596-607.
- [Google Scholar]
- Avian life history variation along altitudinal gradients: an example with cardueline finches. Oecologia. 1997;111:365-374.
- [Google Scholar]
- The breeding biology of hooded plovers, Thinornis rubricollis, on Phillip Island, Victoria. Emu. 2003;103:323-328.
- [Google Scholar]
- Breeding in high-elevation habitat results in shift to slower life-history strategy within a single species. J. Anim. Ecol.. 2009;78:365-375.
- [Google Scholar]
- Contribution à l’étude écologique des passereaux nicheurs dans le Nord-Est d’Algérie. Annaba: Badji Mokhtar University; 2015. (Ph.D. thesis)
- Breeding biology of the European Greenfinch Chloris chloris in the loquat orchards of Algeria (North Africa) Zool. Ecol.. 2014;24:199-207.
- [Google Scholar]
- BirdLife International (2015) Species Factsheet: Charadrius Alexandrinus. Downloaded from http://www.birdlife.org on 23/11/2015.
- Patterns and drivers of intraspecific variation in avian life history along elevational gradients: a meta-analysis. Biol. Rev. 2015
- [CrossRef] [Google Scholar]
- Snowy plover reproductive success in beach and river habitats. J. Field Ornithol.. 2005;76:373-382.
- [Google Scholar]
- Shorebird breeding biology in wetlands of the Playa Lakes, Texas, USA. Waterbirds. 2005;28:129-138.
- [Google Scholar]
- The Birds of the Western Palearctic. Vol vol. 3. Oxford, UK: Oxford University Press; 1983.
- Hatching success in avocet Recurvirostra avosetta and black-winged stilt Himantopus himantopus. Bird Study. 2005;52:166-172.
- [Google Scholar]
- Hoatzin to Auks. Vol vol. 3. Barcelona, Spain: Lynx Edicions; 1996.
- An Atlas of Wader Populations in Africa and Western Eurasia. Wetlands International: Wageningen; 2009.
- Volume determination in reptilian and avian eggs with practical applications. S. Afr. J. Wildl. Res.. 1990;20:111-117.
- [Google Scholar]
- Comparison of the breeding habitats of Little Ringed plover Charadrius dubius and Kentish plover Charadrius alexandrinus on the shingle bed. Bird Study. 2000;47:8-12.
- [Google Scholar]
- Breeding biology of a Kentish plover (Charadrius alexandrinus) population in an inland saline lake. Ardeola. 1996;43:69-85.
- [Google Scholar]
- A method to quantify the effects of human disturbance on animal populations. J. Appl. Ecol.. 1996;33:786-792.
- [Google Scholar]
- Breeding ecology of Kentish plovers Charadrius alexandrinus in rocky and sandy habitats of north-west Morocco (North Africa) Ostrich. 2011;82:217-223.
- [Google Scholar]
- Breeding ecology of Collared Pratincoles Glareola pratincola in two coastal habitats in northwest Morocco. Bird Study. 2010;57:236-243.
- [Google Scholar]
- The impact of predator exclosures on Snowy plover nesting success: a seven-year study. Wader Study Group Bull.. 2008;115:161-166.
- [Google Scholar]
- Les Oiseaux du Nord-Ouest de l’Afrique: distribution géographique, écologie, migration, reproduction. Paris: Le chevalier; 1962.
- Predation on common tern eggs in relation to sub-colony size, nest aggregation and breeding synchrony. Waterbirds. 2003;26:280-289.
- [Google Scholar]
- Elevational trends in life histories: revising the pace-of-life framework. Biol. Rev.. 2014;90:204-213.
- [Google Scholar]
- Oiseaux D’Algérie. Paris: SEOF; 2000.
- Using a transponder system to monitor incubation routines of snowy plovers. J. Field Ornithol.. 2002;73:199-205.
- [Google Scholar]
- Ecological constraints on breeding system evolution: the influence of habitat on brood desertion in Kentish plover. J. Anim. Ecol.. 2006;75:257-265.
- [Google Scholar]
- The function of habitat change during brood-rearing in the precocial Kentish plover Charadrius alexandrinus. Acta Ethol.. 2007;10:73-79.
- [Google Scholar]
- Breeding ecology of Kentish plover Charadrius alexandrinus in an extremely hot environment. Bird Study. 2009;56:244-252.
- [Google Scholar]
- Le Gravelot à collier interrompu (Charadrius alexandrinus): Structure de population, phénologie de reproduction et régime alimentaire dans le Chott Ain El Beida, Ouargla, Algérie. France: Presses académiques francophones; 2013.
- Kentish versus Snowy plover: phenotypic and genetic analysis of Charadrius alexandrinus reveal divergence of Eurasian and American subspecies. Auk. 2009;126:839-852.
- [Google Scholar]
- Restoration of breeding by Snowy plovers following protection from disturbance. Biodivers. Conserv.. 2006;15:2217-2230.
- [Google Scholar]
- Spatial differences in breeding success in the Pied Avocet Recurvirostra avosetta: effects of habitat on hatching success and chick survival. J. Avian Biol.. 2006;37:381-395.
- [Google Scholar]
- The mating system of Kentish plovers Charadrius alexandrinus. Ibis. 1984;126:474-483.
- [Google Scholar]
- Conservation status and reproductive ecology of Giant babax babax waddelli (Aves, Timaliinae), endemic to the Tibet plateau. Oryx. 2004;38:418-425.
- [Google Scholar]
- Reproductive ecology of blackbirds (Turdus merula maximus) in a high-altitude location. Tibet. J. Ornithol. 2005;146:72-78.
- [Google Scholar]
- Reproductive ecology of brown-cheeked laughing thrushes (Garrulus henrici) in Tibet. J. Field Ornithol.. 2008;79:152-158.
- [Google Scholar]
- Comparative breeding ecology of two White-bellied Redstart populations at different altitudes. J. Field Ornithol.. 2010;81:167-175.
- [Google Scholar]
- Nest fate and vegetation characteristics for Snowy plover and Killdeer in Colorado, USA. Wader Study Group Bull.. 2000;93:67-72.
- [Google Scholar]
- Breeding biology and nesting site selection by the spur-winged plover Hoplopterus spinosus in the Evros Delta, NE Greece. J. Nat. Hist.. 2008;42:333-344.
- [Google Scholar]
- Demography of an alpine population of Savannah Sparrows. J. Field Ornithol.. 2009;80:253-264.
- [Google Scholar]
- The distribution and conservation of the Kentish plover Charadrius alexandrinus in Catalonia. Revista Catalana d’Ornitologia. 2006;22:1-8.
- [Google Scholar]
- Effect of mammalian predator management on Snowy plover breeding success. Waterbirds. 2004;27:257-263.
- [Google Scholar]
- Nest success and habitat selection of the Semipalmated plover on Akimiski Island, Nunavut. Wilson Bulletin. 2003;115:285-291.
- [Google Scholar]
- Influence of arctic Terns on survival of artificial and natural Semipalmated plover nests. Waterbirds. 2006;29:100-104.
- [Google Scholar]
- Nest-site selection and breeding biology of Kentish plover Charadrius alexandrinus on sandy beaches of the Portuguese west coast. Ardeola. 2004;51:255-268.
- [Google Scholar]
- Snowy plover Charadrius alexandrinus. In: Poole A., Gill F., eds. The Birds of North America. Vol 154. Philadelphia/Washington, DC: Academy of Natural Sciences/American Ornithologists’ Union; 1995.
- [Google Scholar]
- Snowy plover (Charadrius nivosus) In: Poole A., ed. The birds of North America Online. Ithaca: Cornell Lab of Ornithology; 2009. Retrieved from the Birds of North America Online: http://bna.birds.cornell.edu/bna/species/154. doi:10.2173/bna.154 Accessed 22 Nov 2015
- [Google Scholar]
- Testing the effects of recreational disturbance on two upland breeding waders. Ibis. 2007;149(Suppl. 1):45-55.
- [Google Scholar]
- Avian Ecology. Glasgow: Blackie; 1983.
- Habitat characteristics and nest success of Snowy plovers associated with California Least Tern colonies. Condor. 2001;103:785-792.
- [Google Scholar]
- Habitat use and reproductive success of Western Snowy plovers at new nesting areas created for California Least Terns. J. Wildl. Manag.. 2000;64:24-33.
- [Google Scholar]
- Status of breeding and wintering Snowy plovers in San Diego County, California, 1994–1999. J. Field Ornithol.. 2002;73:156-165.
- [Google Scholar]
- Effects of a changing environment on nesting Snowy plovers at Owens Lake, California. Western Birds. 2006;37:126-138.
- [Google Scholar]
- Ecologie de la reproduction de l’Echasse blanche Himantopus himantopus et de l’Avocette élégante Recurvirostra avosetta dans les hautes plaines de l’Est-algérien. Annaba: Badji Mokhtar University; 2009. (Ph.D. thesis)
- An appraisal of the status and distribution of waterbirds of Algeria: indicators of global changes? Ardeola. 2011;58:137-163.
- [Google Scholar]
- Use of dredge islands by a declining European shorebird, the Kentish plover Charadrius alexandrines. Wetlands Ecol. Manage.. 2013;21:15-27.
- [Google Scholar]
- Song bird populations in fluctuating environments: predator responses to pulsed resources. Ecology. 2003;84:406-415.
- [Google Scholar]
- Population status of the Kentish plover Charadrius alexandrinus in Eastern Romania. Wader Study Group Bull.. 2001;95:51-54.
- [Google Scholar]
- Status and breeding biology of Kentish plover (Charadrius alexandrinus) in Hungary – a progress report. Wader Study Group Bull.. 1990;62:17-23.
- [Google Scholar]
- Brood desertion in Kentish plover Charadrius alexandrinus: An experimental test of parental quality and remating opportunities. Ibis. 1996;138:749-755.
- [Google Scholar]
- Brood desertion in Kentish plover: the value of parental care. Behav. Ecol.. 1999;10:191-197.
- [Google Scholar]
- Costs and benefits of brood desertion in female Kentish plovers, Charadrius alexandrinus. Behav. Ecol. Sociobiol.. 1995;37:155-161.
- [Google Scholar]
- Determination of clutch size in the Kentish plover Charadrius alexandrinus. Ibis. 1994;136:341-348.
- [Google Scholar]
- Brood desertion in Kentish plover: sex differences in remating opportunities. Behav. Ecol.. 1999;10:185-190.
- [Google Scholar]
- Sexual conflict, ecology, and breeding systems in shorebirds. Bioscience. 2006;56:801-808.
- [Google Scholar]
- Székely, T., Kosztolányi, A., Küpper, C., 2008. Practical guide for investigating breeding ecology of Kentish plover Charadrius alexandrinus, Version 3. Unpublished Report, University of Bath. (available at http://www.bath.ac.uk/bio-sci/biodiversity-lab/pdfs/KP_Field_Guide_v3.pdf).
- Nest success of Black-winged Stilt Himantopus himantopus and Kentish plover Charadrius alexandrinus in rice fields, southwest Spain. Ardea. 2012;100:29-36.
- [Google Scholar]
- Habitat selection and nesting association in four species of Charadriiformes in the Po delta (Italie) Ardeola. 1999;46:1-12.
- [Google Scholar]
- Reproductive tactics of the ringed plover Charadrius hiaticula. J. Avian Biol.. 2003;34:259-266.
- [Google Scholar]
- Mating system and reproductive success of a small population of polygamous Snowy plovers. Wilson Bull.. 1986;98:15-37.
- [Google Scholar]
- Human disturbance and stage-specific habitat requirements influence snowy plover site occupancy during the breeding season. Ecol. Evol.. 2013;3:853-863.
- [Google Scholar]
- Constraints on, and determinants of, the annual number of breeding attempts in the multibrooded Black Redstart Phoenicurus ochruros. Ibis. 2006;148:273-284.
- [Google Scholar]
- Breeding ecology and management of Snowy plovers in north-central Oklahoma. J. Field Ornithol.. 2000;71:573-584.
- [Google Scholar]
- Breeding distribution and ecology of the endangered Madagascar plover Charadrius thoracicus. Ostrich. 2008;79:43-51.
- [Google Scholar]







