Translate this page into:
Estimation of genetic variability, mutagenic effectiveness and efficiency in M2 flower mutant lines of Capsicum annuum L. treated with caffeine and their analysis through RAPD markers
⁎Corresponding author at: Cell Molecular Biology and Genetic Section, Aligarh Muslim University, Aligarh 202002, India. Tel.: +91 9456667758, +91 9412272845. raslam07@gmail.com (Rumana Aslam),
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
In the present investigation healthy and certified seeds of Capsicum annuum were treated with five concentrations of caffeine i.e. 0.10%, 0.25%, 0.50%, 0.75% and 1.0%. Germination percentage, plants survival and pollen fertility were decreased with the increase of caffeine concentrations. Similarly root length and shoot length were decreased as the concentrations increased in M1 generation. Different mutants were isolated in M1 generation. In M2 generation, various flower mutants with changes in number of sepals, petals, anther size colour i.e. Trimerous, tetramerous, pentamerous with fused petals, hexamerous etc were segregated. Heptamerous and anther change was not observed in lower concentration viz. 0.1%. All these mutants showed significant changes in morphological characters and good breeding values at lower and intermediate concentrations. Mutagenic effectiveness and efficiency was observed on the basis of M2 flower mutant frequency. It was generally decreased with the increase of mutagen concentrations. Cytological aberrations in mutants showed the decreasing trend at meiotic final stages. These mutants were further analysed through RAPD method and on the basis of appearance of polymorphic DNA bands, they distinguished these flower mutants genotypically. Among 93 bands 44 bands were polymorphic which showed great genetic variation produced by caffeine. As an outcome of that the above caffeine concentrations are good for the induction of genetic variability in Capsicum genotype.
Keywords
Capsicum
Caffeine
RAPD
Mutagenic effectiveness and efficiency
Genetic variability
1 Introduction
Capsicum (2n = 24, Family = Solanaceae) is an essential spice which is used in every Indian dish due to its pungency, flavour, aroma and colour. It also has various medicinal i.e. anti-inflammatory, analgesic, rubefacient, carminative and antioxidant (Sim and Sil, 2008), hypoglycaemic (Monsereenusorn, 1980), antifungal (De Lucca et al., 2006) and antimicrobial activities (Ribeiro et al., 2007). There are different morphological mutants, which have been isolated by various workers after the treatment of chemical mutagens in chilli (Raghuvanshi and Singh, 1982; Rostaino, 1983).
Mutation induction is an important and complementary method of plant breeding. At genic level mutation causes alterations in the structure and position of the gene on a chromosome then it is called as point mutation, which alter the phenotype of an organism. For any successful crop improvement programme, genetic variability plays an important role because it provides a spectrum of variants for effective and better selection which can be obtained using mutation, hybridization, recombination and selection processes (Dhumal and Bolbhat, 2012). Induced mutagenesis is an important and established method for plant improvement, where plant genes are altered by treated seeds and other plant parts with chemical mutagens (Sri Devi and Mullainathan, 2012). In any mutation breeding plan for the production of high frequency of desirable mutation, selection of an effective and efficient mutagen is indispensable (Roychowdhury and Tah, 2011). The application of molecular markers for the estimation of the variability of plant varieties and species is helpful in both detection of genetic relationships between them and making a system of plant genera, which involves the most important agricultural species (Sivolap et al., 2004). Caffeine (1,3,7-trimethylxanthine) is a purine alkaloid. It can interact with DNA and change the some of its physical properties (i.e. DNA denaturation temperature) and determine a higher rate of spontaneous mutations (Truta et al., 2007) and also inhibit DNA repair mechanism (Itoyama and Bicudo, 2000), causing DNA-DNA or DNA–protein linking (Amin, 2002). Due to its purine nature caffeine has mutagenic potential and it also has the ability to act synergistically in inducing chromosomal aberration in mammalian cells (Khursheed et al., 2009).
Capsicum has economic value so it is important to enlarge the genotypic and phenotypic variability, to observe some desired characters and to obtain new valuable genotypes through different methods, including chemical mutagenesis. For this reason, the present experiment was designed to report the genetic variability in M2 flower mutant lines in chilli which was isolated through the mutagenic effect of caffeine. For variability we used morphological characteristics, meiotic behaviour of mutants and their molecular analysis because it is a robust and reliable method to detect interspecific genetic variability.
2 Materials and methods
2.1 Experimental plan and procedure
Fresh, healthy and certified seeds of Capsicum annuum L. were collected from IARI-Pusa campus New Delhi. Seeds were pre-soaked in distilled water and 24 h after pre-soaking they were treated with five concentrations i.e. 0.10%, 0.25%, 0.50%, 0.75% and 1.0% of caffeine (issued from departmental chemical store) solution (pH4) prepared in phosphate buffer (6 h) along with one control set pre-soaked in distilled water in M1 generation. Now treated seeds were sown immediately to raise M1 generation. The desirable variants from M1 generation were selected on the basis of their phenotypical features and harvested separately for the calculation of frequency, meiotic aberrations, mutagen effectiveness and DNA damage in the next generation (M2). Flower variants were scored and classified.
2.2 Growth parameters
The experiments were done to determine the effect of caffeine on germination, survival, pollen fertility, root and shoot lengths in M1 generation. Each treatment was replicated five times and for each replication 50 seeds were sown and tested for all growth parameters. Effect on root and shoot was measured in terms of length of root and shoot respectively for 30 days and results recorded from randomly selected seedlings of each replicate. Some seedlings were transferred into pots and after maturity seeds with identified variants for M2 generation in all the concentrations were harvested.
2.3 Methodology of M2 flower mutant selection
M1 generation seeds were harvested and pre-soaked in distilled water (24 h), planted to raise the M2 generation in control and isolated mutants were also sown individually. In M2 generation, various flower mutants were identified with different morphological traits such as growth, plant height, number of fruits, No. of seed, number of sepals, petals and anthers colours in all the concentrations and they were harvested separately.
2.3.1 Mutation effectiveness and efficiency
Mutation frequency was calculated as M2 plant percentage while mutation effectiveness and efficiency were calculated on the basis of Konzak et al. (1965) formula.
Mp = Frequency expressed as percentage of flower mutation in M2 generation, estimated on M1 plant basis.
Conc. = Concentration of mutagen.
Duration = Duration of treatment (hrs).
I = Percentage of inhibition in seed germination.
S = Percentage reduction of pollen fertility or sterility.
2.3.2 Meiotic studies
The young flower buds of selected flower mutant plants of each concentration and control plant were fixed in Carnoy’s fixative (absolute alcohol: chloroform: acetic acid, 6:3:1) for 24 h and preserved in 70% alcohol. For permanent slides, the anthers of these buds were crushed in 1% propinocarmine solution and followed the NBA + GAA series for pollen mother cells analysis (Bhaduri and Ghosh, 1954). Pollen fertility was determined by staining the pollen grain with acetocarmine and glycerine solution (1:1). Photographs were taken from temporary slides or permanent slides using X30 Olympus research photomicroscope.
2.3.3 Molecular analysis
For DNA isolation about 0.5 gm of young fresh leaves were taken from each mutant plant and ground in pre chilled pestle and mortar with liquid nitrogen. Now each sample powder was transferred into 15 ml polypropylene centrifuge tubes separately which contains 5 ml pre warmed extraction of buffer solution. DNA was extracted by following Saghai et al. (1984) CTAB method. This solution was gyred for 30–40 s for gentle mixing and emulsifed by adding 30 ml chloroform: iso-amyl alcohol (24:1). At room temperature, the solution was centrifuged (15,000 rpm) for 10 min. The aqueous phase of solution was removed and transferred into a tube, mixed by adding isopropanol (2/3 volume). Now DNA was spooled by a bent pasture pipette and relocated into another clean tube. If DNA appeared flocculent then centrifugation was done (5000 rpm, 2 min) by gently pouring off the supernatant. After that DNA pellet was washed by 70% ethanol and the dried pellet was dissolved in 500 μl TE buffer. Now DNA was purified through RNAse and treated with proteinase K. RAPD analysis was done separately with 10 randomly selected decamer primers. Denaturation was done (95 °C, 4 min), primer annealing (54 °C, 1 min) and primer extension (73 °C, 2 min).
2.3.3.1 Polymerase chain reaction
PCR amplification was conducted in Eppendrof thermalcycler (Eppendrof, Germany) with a temperature programme consisting of thermal cycling conditions with denaturation at 94 °C, 4 min: annealing (3 °C, 1 min) and extension (72 °C, 2 min). The amplification product along with 2 μl bromophenol blue was separated on 1.5% agarose gel using 0.5× Tris–borate-EDTA buffer (pH 8.0) containing ethidium bromide gel (0.5 μg/ml). This gel was examined under ultraviolent transilluminator and photographs were taken through gel documentation.
2.4 Statistical analysis
For each treatment a total of 5 replicates were organised. Statistical analysis of data was done with SPSS 17.0 for Windows (SPSS, Chicago, IL, USA). One way ANOVA was done through DMRT test to determine the least significant difference (LSD).
3 Results
3.1 Germination, survival, pollen fertility
Noticeable variations were recorded in germination percentage, plant survival and pollen fertility after the treatment of caffeine in chilli. Fig. 1 shows that all these characters were significantly (p < 0.05 and p < 0.01) decreased as the mutagen concentration increased. At 1.0% caffeine concentration germination, survival and pollen fertility at maturity was found 66.20%, 75.72% and 74.53% respectively.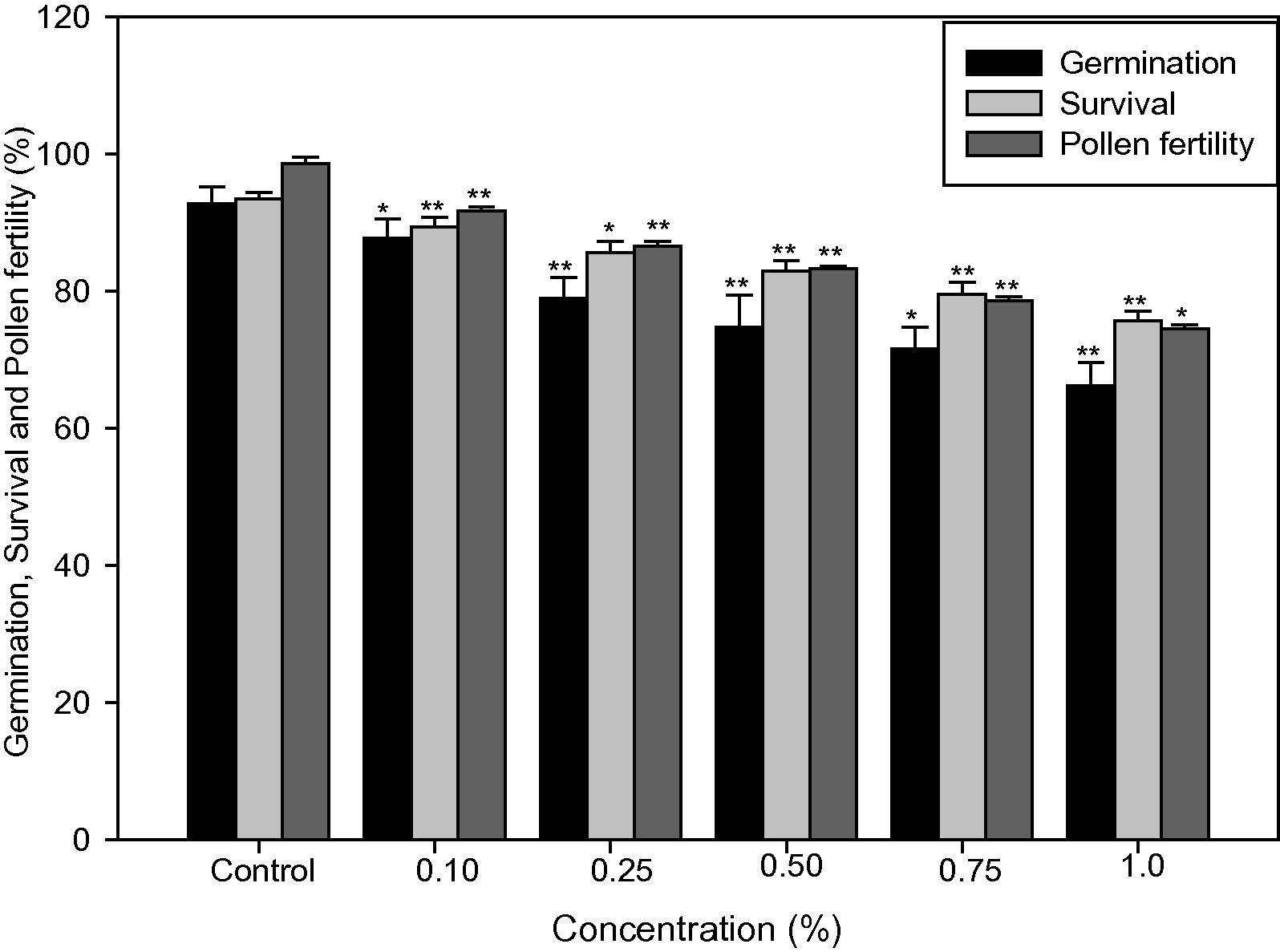
Effect of caffeine on germination, survival, and pollen fertility in Capsicum annuum L. (M1 generation). ANOVA using DMRT *p < 0.05 and **p < 0.01.
3.2 Effect on root and shoot length (cm)
Fig. 2 shows that the root and shoot length of control plant were 16.57 ± 1.02 and 46.57 ± 0.29 respectively, while it significantly (p < 0.01 and p < 0.05) decreased from 14.65 ± 0.66 to 7.65 ± 0.78 and 42.81 ± 0.84 to 30.35 ± 0.86 respectively in 0.10–1.0% concentrations. The results shows that the root and shoot length decreased with an increase in caffeine concentrations in a linear way.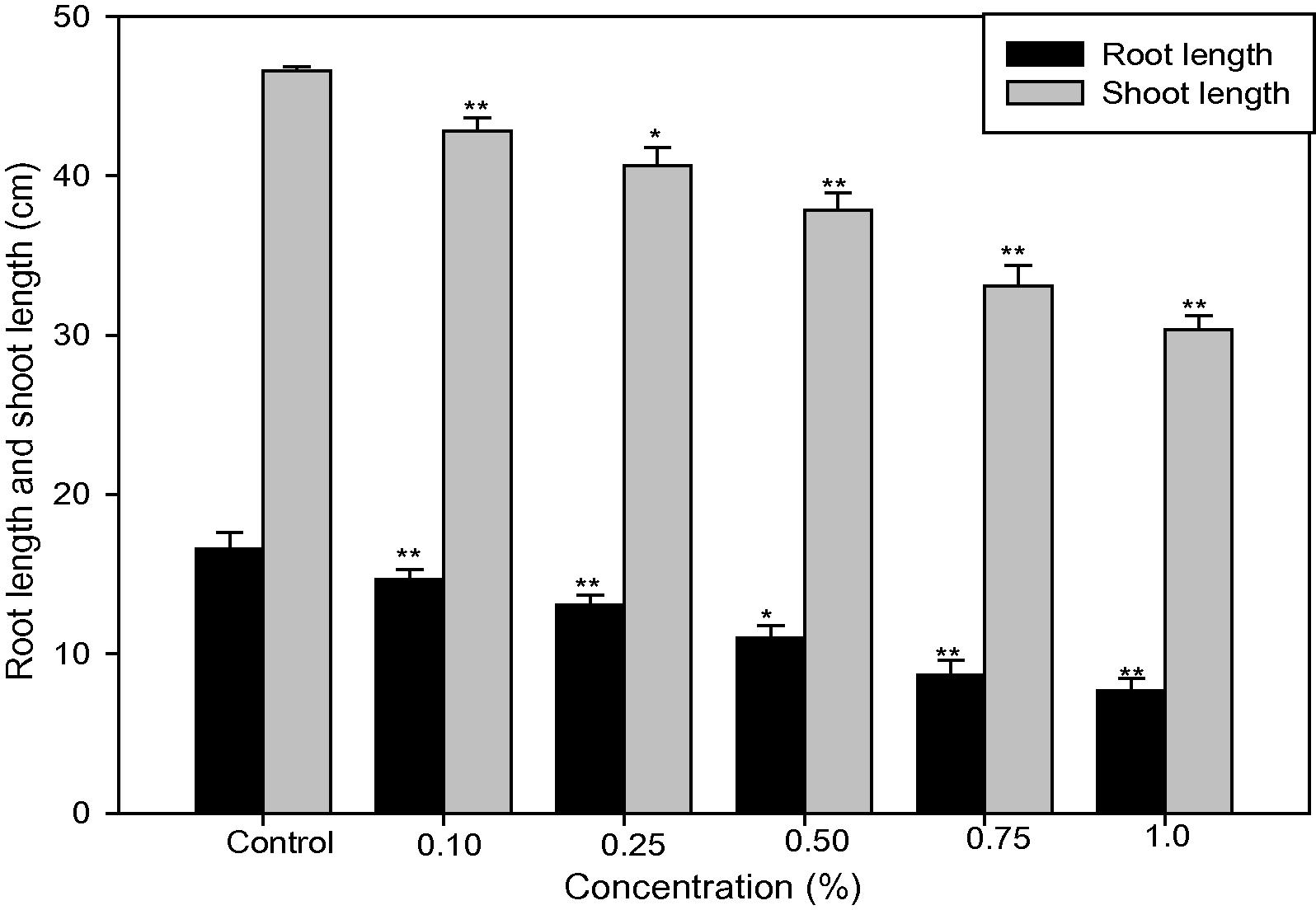
Effect of caffeine on root length (cm) and shoot length (cm) of Capsicum annuum L. Data mean of five replicates ± SD (ANOVA using DMRT *p < 0.05 and **p < 0.01).
3.3 Flower mutants
Different types of flower mutants were observed in M2 progeny. Present experiment is dedicated to induce the broad spectrum of mutation frequencies of flower morphology with their effective concentrations and drastic flower mutants (Table 1). In the present investigation various flower abnormalities i.e. tetramerous, fused petals, hexamerous, heptamerous, flower with different colour anthers mutants showed the presence and absence of sepals, petals, anthers etc. (Fig. 3A–I). Among the concentrations of caffeine, 0.10% produced highest frequency of tetramerous and pentamerous with fused petals flower mutants, while lowest frequency observed in 0.50% concentration (Table 1). Flower mutants with hexamerous and heptamerous characters showed highest frequency at 0.10% and 0.50% concentrations respectively. Flowers with changed colour anthers showed 11.76 higher frequency in 0.50% concentration while 0.25% shows minimum frequency.
Conc. caffeine
Mutant seedlings
Frequency of flower mutants
Tetramerous
Pentamerous (fused petals)
Hexamerous
Heptamerous
Anther colour change
Control
–
–
–
–
–
–
0.10%
10
30.00
30.00
40.00
–
–
0.25%
14
28.57
21.42
28.57
14.28
7.14
0.50%
17
17.65
17.64
23.52
29.41
11.76
0.75%
20
25.00
25.00
20.00
20.00
10.00
1.0%
24
25.00
25.00
20.83
20.83
8.33

Different flower mutants induced by Caffeine (A) Control (B) fused 5 petals (C) tetramerous (D) hexamerous with change aestivation (E) heptamerous (F) hexamerous with yellow and purple anthers (G) 5 fused petals with small anthers (H) deshaped hexamerous with 5 long blue anthers (I) off white heptamerous flower.
3.4 Meiotic studies of mutant progeny
Meiotic studies of flower mutants which were isolated in all concentrations were carried out in M2 generation to determine that they occurred due to point mutations or chromosomal changes. Control plant was showing normal pollen mother cells with 12 bivalents at meiotic division, while the isolated flower mutant exhibited various PMCs with chromosomal anomalies. The most common type of chromosomal anomalies observed in flower mutants were univalent, multivalent, precocious separation of chromosomes, stray bivalent, stickiness, laggards, bridges, disturb polarity etc. (Fig. 4a–i). The frequency of various meiotic aberrations in mutant seedlings with total abnormal pollen mother cells percentage in each concentration has been recorded in (Table 2). The maximum aberration frequency was observed at highest concentration (1.0%) of the mutagen or meiotic anomalies were dose dependent as well as the concentration increase abnormalities also increased (Table 2). The total percentage of abnormal PMCs varies from 1.95% to 18.70%. PMCs = pollen mother cells, Chrom. Abn. = chromosomal abnormality, Uni. = univalents, Multi = multivalents, Prec. Sep. = precocious separation, Str. Biv. = stray bivalents, Sti. = stickiness, Lagg. = laggards, Bri. = bridge, Uneq. Sep. = unequal separation, Dis. Pol. = disturb polarity.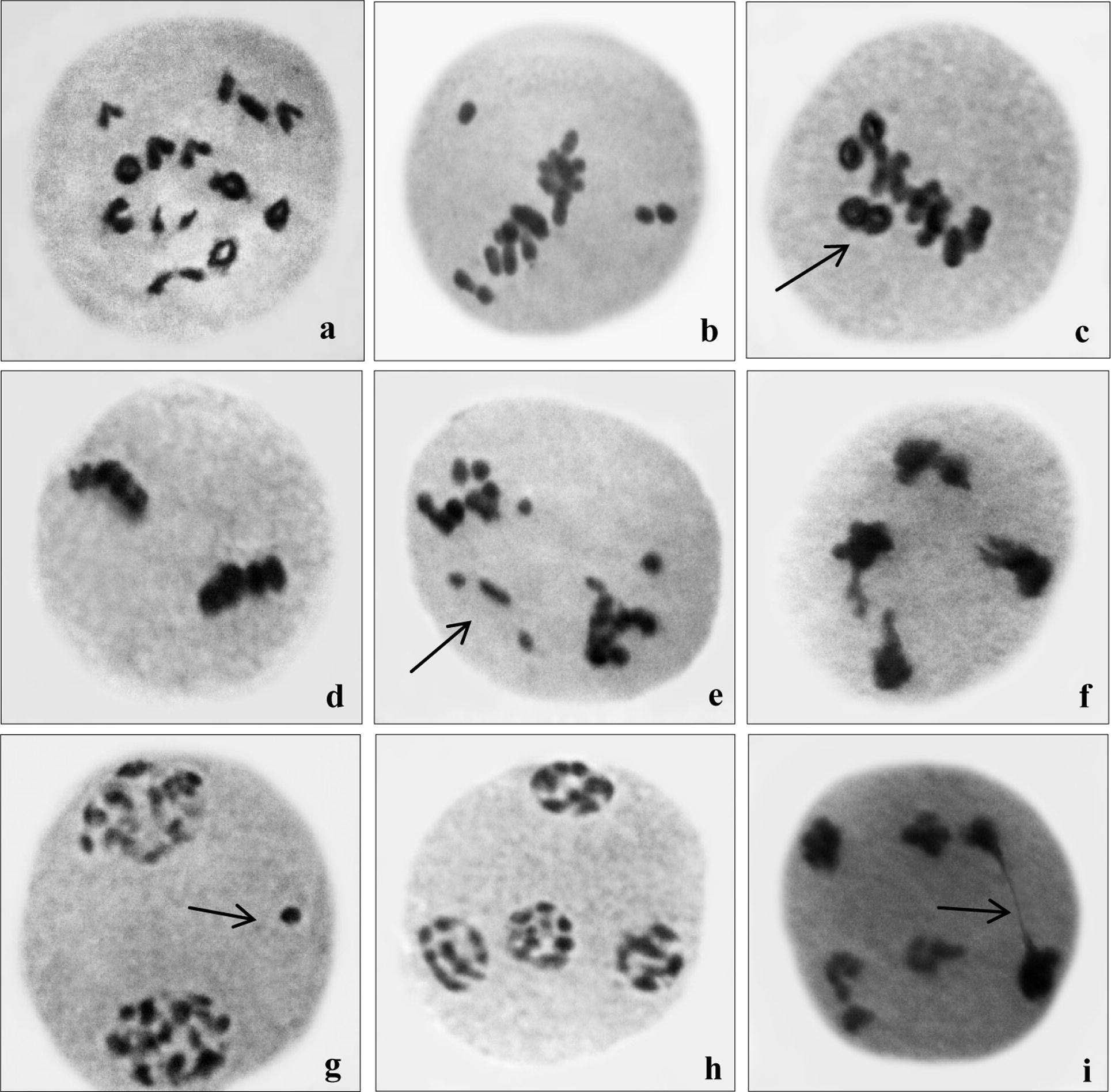
Chromosomal aberrations in mutant plant (a) diakinesis (b) precocious separation at meta I (c) two ring stray bivalent at meta I (d) sticky meta II (e) laggards at ana. I (f) sticky broken bridge at ana II (g) laggard at telo I (h) disturb polarity at telo. II (i) bridge and 6 nucleate condition at telo II. (magnification 100×, cropped).
Conc. caffeine
Mutant seedlings
Total No. of PMCs observed
Prophas.
Metaphase I/II
Anaphase I/II
Telophase I/II
Total % of Chrom. Abn. PMCs
Uni.
Multi.
Multi.
Prec. Sep.
Str. Biv.
Sti.
Lagg.
Bri.
Uneq. Sep.
Lag.
Bri.
Dis. Pol.
Control
–
261
–
–
–
–
–
–
–
–
–
–
–
–
–
0.10%
10
256
–
–
–
0.39
–
0.39
0.39
0.39
–
0.39
–
–
1.95
0.25%
14
259
0.38
0.38
0.38
0.77
0.38
0.77
0.38
–
0.38
0.38
0.38
–
4.63
0.50%
17
264
0.75
0.37
1.13
1.13
1.13
1.51
1.13
0.37
0.37
0.75
0.75
0.37
9.84
0.75%
20
263
0.76
1.14
1.14
1.52
1.14
1.52
1.14
1.52
1.14
1.14
0.76
0.76
13.68
1.0%
24
262
1.14
1.14
1.52
1.90
1.52
2.29
1.90
1.90
1.52
1.52
1.14
1.14
18.70
3.5 Effect on qualitative and quantitative traits
Table 3 shows the mean values of quantitative and qualitative parameters in flower mutant seedlings. The maximum significant increase in plant height (64.33 ± 0.12, at p < 0.01) was observed in 0.10% concentration, while it is significantly minimally decreased (53.98 ± 0.05, at p < 0.01) in 1.0% concentrations compared to the control plant (59.13 ± 0.07). Significant increase in branches per plant was observed of 14.26 ± 0.06 and decreased by 11.16 ± 0.14 (at p < 0.01) in 0.10% and 1.0% concentration respectively. Maximum increase in values of fruits/plant and fruits length were 7.64 ± 0.05–6.95 ± 0.04 and 25.97 ± 0.03 to 24.53 ± 0.13 in 0.10–1.0% concentration respectively and significant (at p < 0.01) while minimum was found in 1.0% and 0.75% compared to control. 0.10% concentration shows maximum mean values in both seeds per fruit and seed weight and minimum in 1.0% concentration and these are significant (at p < 0.01). Maximum 1000-seed weight (g) was observed in 0.10% (4.16 ± 0.03, at p < 0.05), while fruit yield per plant (g) observed at both 0.10% and 0.25% concentrations (at p < 0.01) while both were minimum at 1.0% as compared to that of the control plant (Table 3). Each datum represents mean ± SD (5 for each concentration) ANOVA using DMRT. * = p < 0.05, ** = p < 0.01 and ns = p > 0.05, ns = non-significant.
S. No.
Morphological traits
Control Mean ± SD
Flowers mutant lines
0.10% Mean ± SD
0.25% Mean ± SD
0.50% Mean ± SD
0.75% Mean ± SD
1.0% Mean ± SD
1.
Plant Height (cm)
59.13 ± 0.07
64.33∗∗ ± 0.12
62.27∗∗ ± 0.07
58.78∗∗ ± 0.05
56.60∗∗ ± 0.06
53.98∗∗ ± 0.05
2.
Branches/plant
13.48 ± 0.07
14.26∗∗ ± 0.06
13.65ns ± 0.04
13.08∗∗ ± 0.03
12.66∗∗ ± 0.07
11.16∗∗ ± 0.14
3.
Fruits/plant
6.47 ± 0.05
7.64∗∗ ± 0.05
6.95∗∗ ± 0.04
6.28∗∗ ± 0.06
6.37ns ± 0.05
6.05∗∗ ± 0.06
4.
Fruits length (cm)
23.06 ± 0.05
25.97∗∗ ± 0.03
24.53∗∗ ± 0.13
21.87∗∗ ± 0.07
19.17∗∗ ± 0.19
19.79∗∗ ± 0.06
5.
Seeds/fruit
26.72 ± 0.06
32.59∗∗ ± 0.06
30.41∗∗ ± 0.13
26.69ns ± 0.08
24.96∗∗ ± 0.04
24.11∗∗ ± 0.10
6.
Seeds weight/plant g)
1.97 ± 0.01
2.12∗∗ ± 0.02
1.99ns ± 0.01
1.93∗ ± 0.005
1.82∗∗ ± 0.01
1.77∗∗ ± 0.01
7.
1000-seed weight (g)
4.08 ± 0.03
4.16∗ ± 0.03
3.83∗∗ ± 0.03
3.71∗∗ ± 0.02
4.00∗ ± 0.03
3.81∗∗ ± 0.04
8.
Fruits yield/plant (g)
11.66 ± 0.08
12.79∗∗ ± 0.06
12.09∗∗ ± 0.03
11.54∗ ± 0.07
11.45∗∗ ± 0.05
11.39∗∗ ± 0.05
3.6 Mutagenic effectiveness and efficiency
Mutagenic effectiveness and efficiency varies with concentration in the mutant progeny (Table 4). It was observed that mutagenic effectiveness was linearly decreased from 4.16 to 1.00 in lowest to higher concentrations of caffeine. It was also recorded that maximum mutagenic efficiency was found to be 1.85 at the lowest concentration i.e. 0.10% and minimum at higher concentration (Table 4). Moreover in the lower concentrations of caffeine the mutagenic effectiveness and efficiency were generally higher. Mp = frequency of mutant progeny, I = germination inhibition percentage.
Conc.
Mutagenic effectiveness
Mutagenic efficiency
Mp/I
Control
–
–
Caffeine
0.10%
4.16
1.85
0.25%
2.33
0.93
0.50%
1.41
0.87
0.75%
1.11
0.85
1.0%
1.00
0.83
3.7 RAPD analysis
Caffeine interacts with DNA and it produced different banding patterns as compared to control. In RAPD analysis ten primers were selected with their G + C content percentage. Fig. 5 showing the altered pattern of monomorphic and polymorphic bands with variation in their intensity and all these bands has been summarised in Table 5. A total number of 93 bands were recorded in 5 different flower mutants and control plant. Out of 93 bands, 44 polymorphic bands and 49 monomorphic bands were observed. These polymorphic bands were varied from 3 to 7 numbers in all the primers. Highest number of polymorphic bands was 7 as found in OPB-03 and OPD-02 and lowest was 3 in OPA-05 followed by OPB-04, OPK-10 and OPL-14 with an average of 9.3 bands per primer. The polymorphism percentage was highest (63.63%) in primer OPB-03 followed by primer OPD-02 having 58.33% polymorphism and the lowest polymorphism percentage was produced by primer OPB-04 which showed 37.50% polymorphism. The average of monomorphic bands per primer was 4.9 while average polymorphic band per primer was 4.4 (Table 5).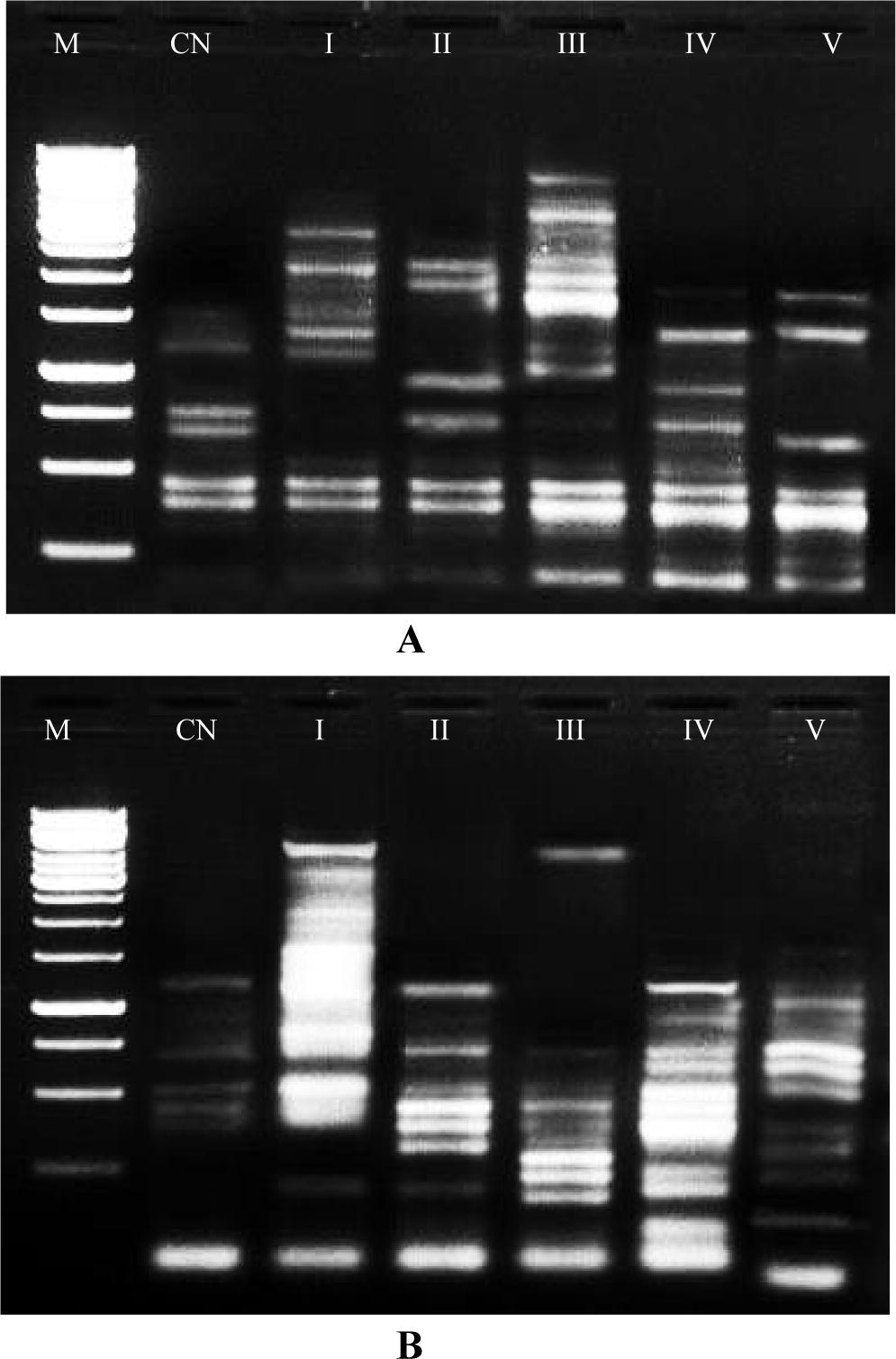
DNA Band Profile of five different flower mutants in Capsicum annuum L. using RAPD with primer OPB-03, with primer OPD-02. *(M-marker, CN-control, I-tetramerous, II-fused pentamerous, III-hexamerous, IV-heptamerous, V-anther colour change).
S. No.
Primer names
Nucleotide sequences (5′–3′)
No. of polymorphic Bands
No. of monomorphic Bands
Total No. of bands
G + C content %
Polymorphism %
1.
OPA-02
TGCCGAGCTG
4
5
9
70
44.44
2.
OPA-05
AGGGGTCTTG
3
4
7
60
42.85
3.
OPA-07
GAAACGGGTG
5
5
10
60
50.00
4.
OPA-09
GGGTAACGCC
4
5
9
70
44.44
5.
OPB-03
CATCCCCCTG
7
4
11
70
63.63
6.
OPB-04
GGACTGGAGT
3
5
8
60
37.50
7.
OPC-05
GATGACCGCC
5
6
11
70
45.45
8.
OPD-02
GGACCCAACC
7
5
12
70
58.33
9.
OPK-10
GTGCAACGTG
3
7
10
60
41.66
10.
OPL-14
GTGACAGGCT
3
3
6
60
50.00
Total
44
49
93
–
478.30
Avg./primer
4.4
4.9
9.3
–
47.83
4 Discussion
Germination is a process by which a seed initiates growth after a period of quiescence. It is a process of resumption of active metabolism manifested in visible growth, an important parameter used to measure the response of plant to mutagenic treatments (Shah et al., 2008). The delayed germination in treated populations might be due to chromosomal aberrations/delay in DNA synthesis/delayed metabolic process. Similar results have also been reported by Kumar and Yadav (2010), Gnanamurthy et al. (2012), Goyal and Khan (2010) and Gandhi and Sri Devi (2014), etc. Reduction in plant survival in treated population may occur due to various factors such as cytogenetic damage and physiological disturbances (Sato and Gaul, 1967) and disturbances in balance between inhibitors of growth regulators and promoters (Meherchandani, 1975). Kumar and Rai (2007) recorded that the accumulation of more and more chromosomal abnormalities greatly affected microsporogenesis leading to the formation of non-viable gametes, which considerably reduced the plant fertility.
It was also observed that root and shoot lengths decreased in a linear mode from lower to higher concentrations of caffeine. Similar results have also been reported by Talebi and Talebi (2012) and Ambavane et al. (2015). According to Blixt (1970) when gene controlled biochemical processes or chromosomal anomalies caused major lesion at cellular level then it inhibits the seedling growth.
In the present study some flower mutants with different number of petals, anther number and coloured were observed. Various flower mutants were also observed by various workers such as change in sepals, petals number and ovules (De Haro and Del Rio, 1998), change in carpal number and morphology (Zeerak, 1998), change in flower colours (Mahamune and Kothekar, 2011). Sri Devi and Mullainathan (2012) observed that the potential of genotype-dependent mechanism of some flower mutant forms is very helpful in maintaining genetic purity of the variety of the next generation. They also identified that floral changes were due to genetic alterations which originate due to loss of function of the gene. Borkar and More (2010) depicted that flower mutation increased according to the dose or concentration of the mutagens.
Cytological investigation defines the specific responses of different genotypes to a specific mutagen and it is also provides significant evidences for the selection of desirable traits (Avijeet et al., 2011). In the present experiment various meiotic anomalies were recorded in all the selected flower mutants of all concentrations i.e. univalent, multivalent, precocious movement, bridge, laggard, stickiness, etc. Univalents may originate due to an early disjunction of some of the partially homologous chromosomes as a consequence of which they were unable to form normal bivalents (Srivastava and Srivastava, 2004). The formation of multivalents may also be attributed to the abnormal pairing and non-disjunction of bivalents (Siddiqui and Ansari, 2005). Jafri et al. (2011) suggested that precocious movement of chromosomes was probably caused by spindle dysfunction. According to Khan et al. (2009), precocious movement of univalent or bivalents toward one pole causes unequal distribution of chromosomes or loss of a complete set of chromosome (bivalent) called as stray chromosome. Minija et al. (1999) believed that laggard at anaphase I/II can be attributed to the delayed terminalization of chiasma or perhaps to stickiness of chromosomal ends. The defective functioning of target proteins may be due to gene mutation or direct action of mutagens on protein creates the disturbance during chromosome separation and it forms bridges (Kumar and Gupta, 2009; Basi et al., 2006). At anaphase and telophase stages disturbed polarity could occurr due to disturbances in spindle fibre and may result in multinucleate condition. In different plants similar results were also recorded by Aslam et al. (2012), Choudhary et al. (2012), Sharma et al. (2009), etc.
Plant height reduction can be attributed to chromosomal abnormalities after the treatment of chemical mutagens (Kumar and Tripathi, 2008). Yield is a very important parameter in mutation breeding, because ultimately the plant breeder wants to improve the yield along with other characters. These characters were significantly higher at lower concentration but at higher concentration it shows a reduction pattern. Anis and wani (1997) and Khursheed et al. (2009) also reported that lower caffeine doses exhibited stimulatory effects on morphological characters while higher concentrations showed inhibitory effects on the plant. Panigrahi et al. (2015) suggested that enhancement of yield at significant levels and variations in quantitative parameters may show stable gene mutations in the next generations, while Thilagavathi and Mullainathan (2011) concluded that the decrease in quantitative traits have been attributed to the physiological disturbance or chromosomal damage caused to the cells of the plant by the mutagen. The selection of effective and efficient mutagens is most important to recover the spectrum while high frequency of desirable mutations and efficiency of a mutagen indicates relatively less biological damage in relation to induced mutation (Solanki and Sharma, 1994). Mullainathan and Suthakar (2015) concluded that mutagenic effectiveness and efficiency were decreased as the mutagen concentration increased. Morsucci et al. (1991) observed that stomata opening in epidermis cell of Vicia faba plant was promoted by caffeine because it has stimulant or inhibiting effects. Caffeine application on a variety of crop plants showed contradictory results on plant growth ranging from stimulation of partial or complete growth inhibition. According to Montes et al. (2014) these variations may be mixed results of different caffeine concentrations, application periods, various type evaluations and different plant organs.
We can estimate the existence of mutation and structural changes in plant DNA after the impact of different caffeine concentrations. On the basis of altered DNA banding pattern the isolated mutants showed different polymorphism percentages. Williams et al. (1990) observed that due to nucleotide substitutions and insertion or deletions polymorphism occurred between individuals. Bhat et al. (2011) suggested that when the genetic base increased in mutant plants then it produced different types of suitable numbers of polymorphic bands in mutant progeny.
5 Conclusion
It is concluded that caffeine mutagen generates a variety of flower anomalies in C. annuum. It is noticed that these changes are differentially sensitive to caffeine and the appearance of new mutants would very helpful in maintaining the genetic purity of plant variety. Therefore these mutants should be isolated after chemical mutagenesis and desired characters also selected from it. The cytological analysis of these mutants showed that these changes were induced due to changes in chromosome number, structure, base substitution and deletion. Further DNA analysis using different primers should be carried out for the validation of induced mutations. It is also concluded that RAPD analysis provide considerable polymorphism in isolated Capsicum mutants. These mutants are used for the selection of diverse parents and monitoring of the genotypic variability of the Capsicum collection.
Conflict of interest statement
There is no conflict of interest.
Acknowledgements
The authors are thankful to Chairmen, Department of Botany, Aligarh Muslim University, Aligarh, India for providing the necessary facilities required for the completion of this experiment and University Grants Commission, New Delhi, India, for financial support.
References
- Studies on mutagenic effectiveness and efficiency of gamma rays and its effect on quantitative traits in finger millet (Eleusine coracana L. Gaertn) J. Radiat. Res. Appl. Sci.. 2015;8:120-125.
- [Google Scholar]
- Cytotoxicity testing of sewage water treatment using Allium cepa chromosome aberration assay. Pak. J. Biol. Sci.. 2002;5:184-188.
- [Google Scholar]
- Caffeine induced morpho-cytological variability in Fenugreek, Trigonella foenum-graecum L. Cytologia. 1997;62:343-349.
- [Google Scholar]
- Assessment of genetic variability induced by MMS and 6-AP in a medicinal herb Cichorium intybus L. Biosci. Int.. 2012;1(2):30-39.
- [Google Scholar]
- Impact of mutagenesis on cytological behavior in relation to specific alkaloids in Opium Poppy (Papaver somniferum L.) Caryologia. 2011;64(1):14-24.
- [Google Scholar]
- Cytogenetic effects of gamma rays on Indica Rice Radha-4. J. Inst. Agric. Anim. Sci.. 2006;27:25-36.
- [Google Scholar]
- Evaluation of variability in five linseed (Linum usitatissimum L.) genotypes using agromorphological characters and RAPD analysis. S. Asian J. Exp. Biol.. 2011;1:43-47.
- [Google Scholar]
- Studies of induced mutations in peas. XXV. Genetically Controlled Differences in Radiation Sensitivity. Agri. Hort. Genet.. 1970;28:55-116.
- [Google Scholar]
- Induced flower colour mutations in Phaseolus vulgaris Linn through physical and chemical mutagens. Adv. Biores.. 2010;1(1):22.
- [Google Scholar]
- Cytotoxic action of lead nitrate on cytomorphology of Trigonella foenum-graecum L. Turk. J. Biol.. 2012;36:267-273.
- [Google Scholar]
- Isolation of chemically-induced mutants in Borage (Borago officinalis L.) J. Am. Oil Chem. Soc.. 1998;75:281-283.
- [Google Scholar]
- Fungicidal properties of two saponins from Capsicum frutescens and the relationship of structure and fungicidal activity. Can. J. Microbiol.. 2006;52(4):336-342.
- [Google Scholar]
- Induction of genetic variability with gamma radiation and its applications in improvement of horsegram. In: Adrovic Feriz, ed. Gamma Radiation. Croatia: In Tech Publisher; 2012. p. :207-228.
- [Google Scholar]
- The effect of ethyl methane sulphonate and diethyl sulphate on chilli (Capsicum annuum L.) in M1 generation. Int. Lett. Nat. Sci.. 2014;5:18-23.
- [Google Scholar]
- Frequency in germination studies of chlorophyll mutants in effectiveness and efficiency using chemical mutagens. Int. J. Curr. Life Sci.. 2012;2(3):23-27.
- [Google Scholar]
- Induced mutagenesis in urdbean (Vigna mungo L. Hepper): a review. Int. J. Bot.. 2010;6:194-206.
- [Google Scholar]
- Effect of stannous chloride combined with caffeine on fecundity of Drosophila prosaltans. Genet. Mol. Biol.. 2000;23:105-107.
- [Google Scholar]
- Genotoxic effects of 5-bromouracil on cytomorphological characters of Cichorium intybus L. Afr. J. Biotechnol.. 2011;10(52):10595-10599.
- [Google Scholar]
- Methyl methane sulphonate induced chromosomal variations in a medicinal plant Cichorium intybus L. during microsporogenesis. Biol. Med.. 2009;1(2):66-69.
- [Google Scholar]
- Studies on the effect of caffeine on growth and yield parameters in Helianthus annuus L. variety Modern. Biol. Med.. 2009;1(2):56-60.
- [Google Scholar]
- Efficient chemical mutagenesis. The use of induced mutations in plant breeding (FAO/IAEA Meeting, Rome) Rad. Bot. (Suppl.). 1965;75:49-70.
- [Google Scholar]
- Induced karyomorphological variations in three phenodeviants of Capsicum annuum L. Turk. J. Biol.. 2009;33:123-128.
- [Google Scholar]
- Comparative genotoxic potential of mercury and cadmium in soybean. Turk. J. Biol.. 2007;31:13-18.
- [Google Scholar]
- Lead-induced cytotoxicity and mutagenicity in grass pea. Turk. J. Biol.. 2008;32:73-78.
- [Google Scholar]
- EMS induced genomic disorders in sesame (Sesamum indicum L.) Rom. J. Biol. – Plant Biol.. 2010;55(2):97-104.
- [Google Scholar]
- Gamma ray induced flower colour and seed mutants in French bean (Phaseolus vulgaris l.) Recent Res. Sci. Technol.. 2011;3(5):33-35.
- [Google Scholar]
- Effect of gamma radiation on dormant seeds of Avena sativa L. Rad. Bot.. 1975;15:439-445.
- [Google Scholar]
- Mitoclastic properties of Mentha rotundifolia L. J. Cytol. Genet.. 1999;34:169-171.
- [Google Scholar]
- Doses of caffeine on the development and performance of pepper crops under greenhouse. Hortic. Bras.. 2014;32:398-403.
- [Google Scholar]
- Involvement of cytokinin and adenosine 3,5 cyclic monophosphate in stomatal movement in Vicia faba. Plant Physiol. Biochem.. 1991;29:537-547.
- [Google Scholar]
- Effectiveness and efficiency of Chemical and Physical Mutagens in Sorghum (Sorghum bicolor (L.) Moench) in M2 generation. Int. J. Curr. Res.. 2015;3(1):1-4.
- [Google Scholar]
- Mutagenic efficiency and effectiveness of gamma rays, ethyl methane sulphonate (ems), nitrosoguanidine (ng) and their synergistic effect for different polygenic traits in black gram (Vigna mungo (l.) hepper) through induced mutagenesis. Int. J. Plant Anim. Environ. Sci.. 2015;5(1):292-299.
- [Google Scholar]
- Induced mutagenesis in Capsicum. II. Effects of single and combined mutagenic treatments on habit and fruit. Curr. Sci.. 1982;51:235-237.
- [Google Scholar]
- Isolation and characterization of novel peptides from chilli pepper seeds, antimicrobial activities against pathogenic yeasts. Toxicology. 2007;50(5):600-611.
- [Google Scholar]
- Pepper brachytic forms (Capsicum annuum L.) obtained through induced mutagenesis. Genet. Agrar.. 1983;37:204-205.
- [Google Scholar]
- Germination behaviors in M2 generation Dianthus after chemical mutagenesis. Int. J. Adv. Sci. Technol. Res.. 2011;1(2):448-454.
- [Google Scholar]
- Mutagenic efficiency and effectiveness of gamma rays, ethyl methane sulphonate (ems), nitrosoguanidine (ng) and their synergistic effect for different polygenic traits in black gram (Vigna mungo (l.) hepper) through induced mutagenesis. Int. J. Plant. Anim. Environ. Sci.. 2015;5(1):292-299.
- [Google Scholar]
- Radio sensitivity of various chickpea genotypes in M1 generation 1-laboratory studies. Pak. J. Bot.. 2008;40:649-665.
- [Google Scholar]
- Assessment of mutagenicity of individual and combination treatments of gamma rays and MMS in Broad bean (Vicia faba L.) Cytologia. 2009;74:235-241.
- [Google Scholar]
- Studies on the genotoxic effect of pollution on brinjal (Solanum melongena L.) growing around Harduaganj thermal power plant. Nat. Environ. Pollut. Technol.. 2005;4(1):13-17.
- [Google Scholar]
- Antioxidant activities of red pepper pericarp and seed extracts. Int. J. Food Sci. Technol.. 2008;43:1813-1823.
- [Google Scholar]
- Sivolap, Yu.M., Volkodav, V.V., Bal’vins’ka, M.S., Kozhukhova, N.E., Solodenko, A.E., Chebotar, S.V., 2004. Identification and Registration of Genotypes of Common Wheat (Triticum aestivum L.), Barley (Hordeum vulgare L.), maize (Zea mays L.), and Sunflower (Helianthus annuus L.) suing Microsatellite Locus Analysis: Guide lines Manual, Odessa, p. 14.
- Mutagenic effectiveness and efficiency of gamma rays, ethylene imine and N-nitroso-N-ethyl urea in macrosperma lentil (Lens culinaris Medik.). Indian. J. Genet.. 1994;54(1):72-76.
- [Google Scholar]
- The use of ethyl methanesulfonate to study the flower development in Capsicum annuum L. mutants. Bot. Res. Int.. 2012;5:4-9.
- [Google Scholar]
- Meiotic abnormalities and tetraploidy (by Lead Nitrate) induced by heavy metals (Pb, Cu, Hg, Zn) on Helianthus annuus L. Cytologia. 2004;69(2):119-124.
- [Google Scholar]
- Radiosensitivity study for identifying the lethal dose in MR219 (Oryza sativa L. spp. Indica Cv. MR219) Int. J Agric. Sci. Res. Technol.. 2012;2(2):64-67.
- [Google Scholar]
- Influence of physical and chemical mutagens on quantitative characters of Vigna mungo (L. Hepper) Int. Multidiscip. Res. J.. 2011;1(1):6-8.
- [Google Scholar]
- Effects induced by diethyl sulphate on some cytogenetical parameters and length growth of hemp plantlets. Secţiunea Genetică şi Biologie Moleculară. 2007;TOM VIII:243-247.
- [Google Scholar]
- DNA polymorphism amplification by arbitrary primers are useful as genetic markers. Nucleic Acid Res.. 1990;18:6531-6535.
- [Google Scholar]
- Some induced mutations of phylogenetic interest in Solanum melongena. J. Indian Bot. Soc.. 1998;77:253-254.
- [Google Scholar]







