Translate this page into:
Eco-friendly and effective dyeing of wool with anthraquinone colorants extracted from Rubia cordifolia roots: Optimization, colorimetric and fastness assay
⁎Corresponding author. yusuf1020@gmail.com (Mohd Yusuf),
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
The present study is aimed to explore the dyeing ability of anthraquinone colorants extracted from Rubia cordifolia Lin. roots. Sustainable approach for textile dyeing with a systematic protocol is followed with the effect of AlCl3 and CaCl2 metallic mordants. The optimization of extraction and dyeing conditions was assessed by Reflectance Spectrophotometry. The optimum extraction and dyeing conditions were found to be pH = 2, time = 45 min and temperature 90 °C and pH = 4, time = 90 min and temperature 90 °C respectively. At optimized conditions, dyeing abilities of pre-mordanted with metallic salts as anchoring agent (AlCl3 and CaCl2) were compared. Wool samples dyed with Madder root extract impart radiant red shades with or without mordants having different hue and tones with commercially acceptable colorimetric and fastness properties without sacrificing much of the resources. Effective improvement in color as well as fastness properties was observed using anchoring agents.
Keywords
Extraction
Dyeing
Fastness
Color strength
Rubia cordifolia
1 Introduction
In recent times, use of synthetic coloring and finishing agents is under severe censuring for their high pollution risks at the stage of manufacturing as well as during applications (Shahid et al., 2013; Javadian et al., 2014). Synthetic dyes and finishing agents are commonly used for their advantageous nature such as high substantivity, better durability and broad range of applications, except the environmental risks. Alternatively, dyes obtained from plants, insects/animals and minerals are proven to have better biodegradability, sustainability, eco-friendliness and generally higher compatibility with the environment. As a result, green progress has created awareness in sustainable practices in the apparel and textile industries (Shahid et al., 2013; Ali et al., 2009; Shahid et al., 2012; Shabbir et al., 2016a,b). So, dyes and pigments that originated from nature are healthier and eco-friendly substitutes to synthetic dyes and their synthetic counterparts.
Natural dyes are considered healthier over synthetic ones, but due to lower substantivity, durability and narrow shade range on textiles of them need some advancement in the use of natural dyes for coloration and finishing of textile materials. These drawbacks of natural dyes can be overcome with the use of appropriate mordants which are permissible up to some levels for textile dyeing (Shabbir et al., 2016a,b). Aluminum, copper, iron, chromium and tin salts were used for textile dyeing with natural dyes. In recent times some modern techniques have been employed for textile dyeing and functionalization simultaneously by researchers. For better color strength and dyeability radiation treatments of textile substrates are also trending such as UV (Adeel et al., 2014) and gamma radiations (Gulzar et al., 2015; Khan et al., 2014; Ajmal et al., 2014). Textile dyeing that has been overloading the effluents into the water bodies may be a major concern and so, waste water treatment or dye removal is needed (Tahir et al., 2016). To avoid the extra burden of water treatment or dye removal, some systematic approaches can be utilized to make dyeing sustainable up to a conventional level. Application of natural resources as dye, with systematic protocols can be a better substitute to get maximum results with minimum resources utilized keeping ecosystem safe up to a great extent. When sustainability and environment safety are concerned optimization process can be a primary step for dyeing to get maximum output with minimal waste, as far as it is considered also in literature in recent past whether it may be response surface methodology or other UV characterizations (Sinha et al.,2016; Mansour et al., 2016; Swamy et al., 2016).
Rubia cordifolia Lin., belongs to the family Rubiaceae, commonly known as Majith and Indian Madder, and is one of the most important multipurpose species of genus Rubia in the Indian subcontinent. It is an old and famous plant that is used in the Indian Traditional System of Medicine (ITSM). It is a variable, prickly creeper, perennial, herbaceous climbing plant, up to 10 m long, with very long roots which are cylindrical, flexuous, and with a thin red bark. Its stems often have a long, rough, grooved, woody base. The valuable portion of this plant is entirely its root, which is usually of considerable length but does not exceed ordinary slate pencil in thickness (Fig. 1). Outer covering of Madder roots are enriched with several anthraquinone derivatives. Roots contain approximately 1.9% dye present in the free or bound as the glucosides which are anthraquinone derivatives; mainly purpurin (CI-75410) and munjistin (CI-75370). The roots (Fig. 1) also contain small amount of xanthopurpurin (CI-75340), pseudopurpurin (CI-75420), nordamncanthal and rubiadin (Color Index, 1971; Mayer and Cook, 1943; Perkin and Everest, 1918; Anonymous, 1972), responsible for color fabrication onto textile substrates imparting a range of red shades (Zarkogianni et al., 2010; Parvinzadeh, 2007). A lot of research work has been conducted on dyeing behavior of R. cordifolia on textile substrates (Zarkogianni et al., 2010; Parvinzadeh, 2007; Gupta et al., 2001a,b; Sarkar, 2004; Uslu and Bamu, 2016; Khan, 2012; Gupta et al., 2004), still due to its promising dyeing abilities and beautiful red color palette obtained, extensions in the studies on R. cordifolia make sense.
Rubia cordifolia: (a) plant, (b) roots and (c) powdered dye.
In this study, a systemic approach to extend the knowledge for sustainability of dyeing for coloration of textile substrate has been evaluated. Therefore, conditions for extraction in aqueous medium and dyeing of R. cordifolia extract using Reflectance Spectrophotometry, with the effect of AlCl3 and CaCl2 as metallic mordants was evaluated. To get the highest extent of reproducibility, each step for optimization of extraction and dyeing were accessed after complete dyeing process by Reflectance Spectroscopy. Dyed wool yarns were also assessed for color in terms of lightness (L*), redness-yellowness (a*), blueness-greenness (b*), and fastness in terms of washing, light exposure and rubbing.
2 Materials and methods
2.1 Wool and dye
100% pure NZ semi-worsted wool yarns were purchased from MAMB Ltd., Bhadohi, India. Commercially powdered Madder roots were purchased from SAM Vegetables, Moradabad, India. All other chemicals used were of Laboratory grade and used without further purification.
2.2 Pre-treatment of wool and mordanting
Wool skeins were immersed in aqueous solution of non-ionic detergent (5 mL/L) for 30 min to remove dirt and swelling of wool. Mordanting performed by pre-mordanting method to analyze the influence of metal mordants on the shades produced with different concentrations of Madder on wool yarns was executed through comparison with un-mordanted samples. 5% o.w.f (optimized concentration) of each mordant (AlCl3·6H2O and CaCl2·2H2O) as anchoring agent was added to water in separate baths and the temperatures of the mordant solutions were raised to 30 °C and then water soaked wool yarns were added to the baths. The temperatures of the mordanting baths were brought to 90 °C for 1 h with continuous stirring. Mordant baths were cooled and mordanted samples were taken out of the mordanting baths and rinsed with tap water.
2.3 Optimization of mordant concentration
Mordants used in this study were optimized (0.5–10.0% o.w.f.) with respect to percent dye exhaustion for their better performance on wool in terms of achieving higher color strength and improved fastness properties.
2.4 Optimization for Extraction of R. cordifolia extract
1:20 M:L ratio (material to liquor ratio) was taken for the study in an exhaust bath. In order to find optimum extraction conditions, a series of trials were performed in accordance with parameters such as pH, time and temperature. Hydrochloric acid (HCl) and sodium carbonate (Na2CO3) were used to maintain the pH. Trials for optimization are summarized in Table 1.
2.5 Optimization for dyeing with R. cordifolia extract
1:40 M:L ratio was taken for the study in an exhaust bath. In order to find optimum dyeing conditions, progressions of trials were performed in accordance with parameters pH, time and temperature. Trials for optimization are summarized in Table 2.
Dyed wool yarn samples were washed with non-ionic detergent (5 mL/L), and thereafter, rinsed with tap water and dried in shade at room temperature. The dyed samples so obtained were subjected to assess for color characteristics and fastness properties.
50% o.w.f. powdered dye was taken throughout the study.
2.6 Color measurement
Gretag Macbeth Color-Eye 7000 A Reflectance Spectrophotometer integrated with a computer is used for measurement of color characteristics in terms of CIELab color coordinates (L*, a*, b*, c*, h°) and color strength values (K/S). The color strength value (K/S) in the visible region of the spectrum (400–700 nm) was calculated based on the Kubelka–Munk equation:
2.7 Fastness tests
2.7.1 Light fastness
The light fastness of the dyed wool yarn samples was conducted on Digi light Nx™, having water cooled Mercury Blended Tungsten lamp as per Test method AATCC 16e-2004 similar to ISO 105-B02:1994 (Amd.2:2000), which has the nearest approach to that of sunlight. Light fastness ratings have been done on 1–8 scale as per ISO 105-B02:1994 (Amd.2:2000).
2.7.2 Wash fastness
The wash fastness of the dyed wool yarn samples was measured in Digi wash SS™ (Launder-o-meter) as per the ISO 105-C06:1994 (2010) specifications. The changes in color of the dyed specimens were assessed, both in terms of alteration of shades and the degree of staining on white adjacent fabrics (cotton and wool) against the standard five-point gray scale. A grade of 5 is the best and the grade of 1 is the poor. Color fastness rating of less than grade 3 indicates considerable alteration in color after washing.
2.7.3 Rub fastness
The samples were assessed for staining on white adjacent cotton fabric. Dry and wet rub fastness of the dyed wool yarn samples was tested using a Digi crock™ (Crockmeter) as per Indian standard IS 766:1988 (Reaffirmed 2004) based on ISO 105-X12:2001 by mounting the fabric on panel and giving ten strokes for both dry and wet rub fastness tests.
3 Results and discussion
3.1 Optimization for extraction
Table 1 summarizes the optimum results for extraction of R. cordifolia.
Parameters
Trials
Control conditions
Optimized condition
pH
1, 2, 3, 4, 5, 6, 7, 8, 9, 10
t = 60 min, T = 90 °C
2
Time (min)
15, 30, 45, 60, 75
pH = 2, T = 90 °C
45 min
Temperature (°C)
40, 50, 60, 70, 80, 90, 100
pH = 2, 60 min
90 °C
3.1.1 Effect of pH
Fig. 2a represents the effect of pH on color strength (K/S) values of dyed wool with 50% R. cordifolia extract with respect to controlled conditions (Table 1). From the Fig. 2a, it can be depicted that maximum dye contents are extracted in acidic medium at pH 2. Phenols are weak acids as these are having –OH groups in their structure. At higher pH values these phenolic contents of extract transforms into their anionic forms and equilibrium yield lowers with an increase in pH value (Uslu and Bamu, 2016).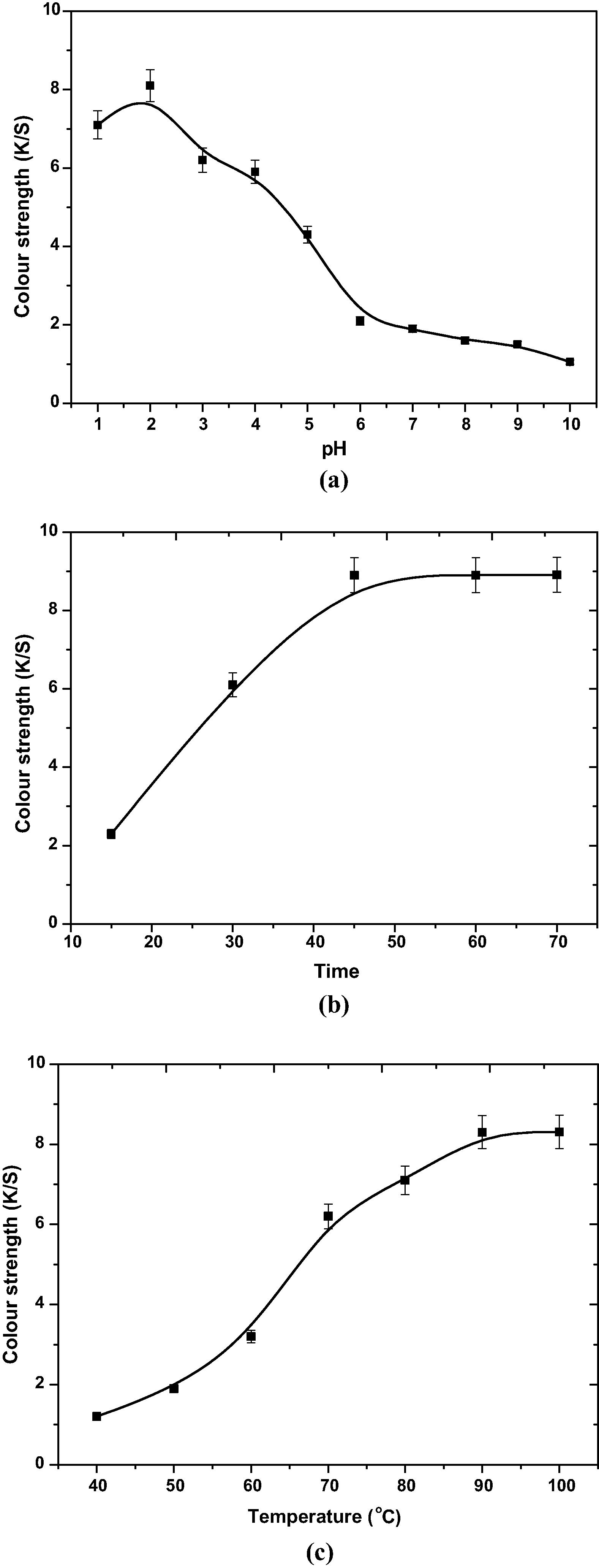
Effect of (a) pH, (b) time and (c) temperature on extraction.
3.1.2 Effect of extraction time
Fig. 2b, illustrates the optimum time for extraction of color components from powdered Madder roots at control conditions (Table 1) and is 45 min and after 45 min, extraction was almost completed. Optimization of extraction time can be an important step in sustainable textile dyeing with respect to the energy savings.
3.1.3 Effect of extraction temperature
Fig. 2c represents the effect of temperature on control conditions (Table 1) of extraction of dye. The optimum extraction temperature is 90 °C.
From Table 1 and Fig. 2a –c, the optimized conditions for extraction of coloring matter of R. cordifolia are as follows:
Dye extraction medium pH = 2, extraction time = 45 min and temperature = 90 °C.
3.2 Optimization for dyeing
Dyeing of textile or textile substrate is the main step in the process of textile coloration with respect to resources and energy also. Generally dyeing parameters such as pH, time and temperature of dyeing, play an important role in controlling dye adsorption on textile materials. Table 2 summarizes the results for optimization to dyeing of R. cordifolia.
Parameters
Trials
Control conditions
Optimized condition
pH
1, 2, 3, 4, 5, 6, 7, 8, 9, 10
t = 60 min, T = 90 °C
4
Time (min)
15, 30, 45, 60, 75, 90, 105, 120
pH = 4, T = 90 °C
90 min
Temperature (°C)
40, 50, 60, 70, 80, 90, 100
pH = 4, 60 min
90 °C
3.2.1 Effect of pH
Fig. 3a represents the effect of pH on color strength values of dyed wool with 50% R. cordifolia extract with respect to control conditions (Table 2). From Fig. 3a, it can be revealed that the optimum pH for dyeing is 4. At isoelectric point wool is neutral and main reactive sides of wool are COO− and NH3+. Therefore pH of the medium decides which side will react with the dye molecule. In acidic medium COO− becomes COOH and NH3+ remains positively charged, consequently, making protein fibers positively charged. However, wool is being dyed mostly in an acidic medium (pH ⩽ pH of isoelectric point) because there are more acidic groups than basic groups in these protein fibers as per the results (Khan, 2012).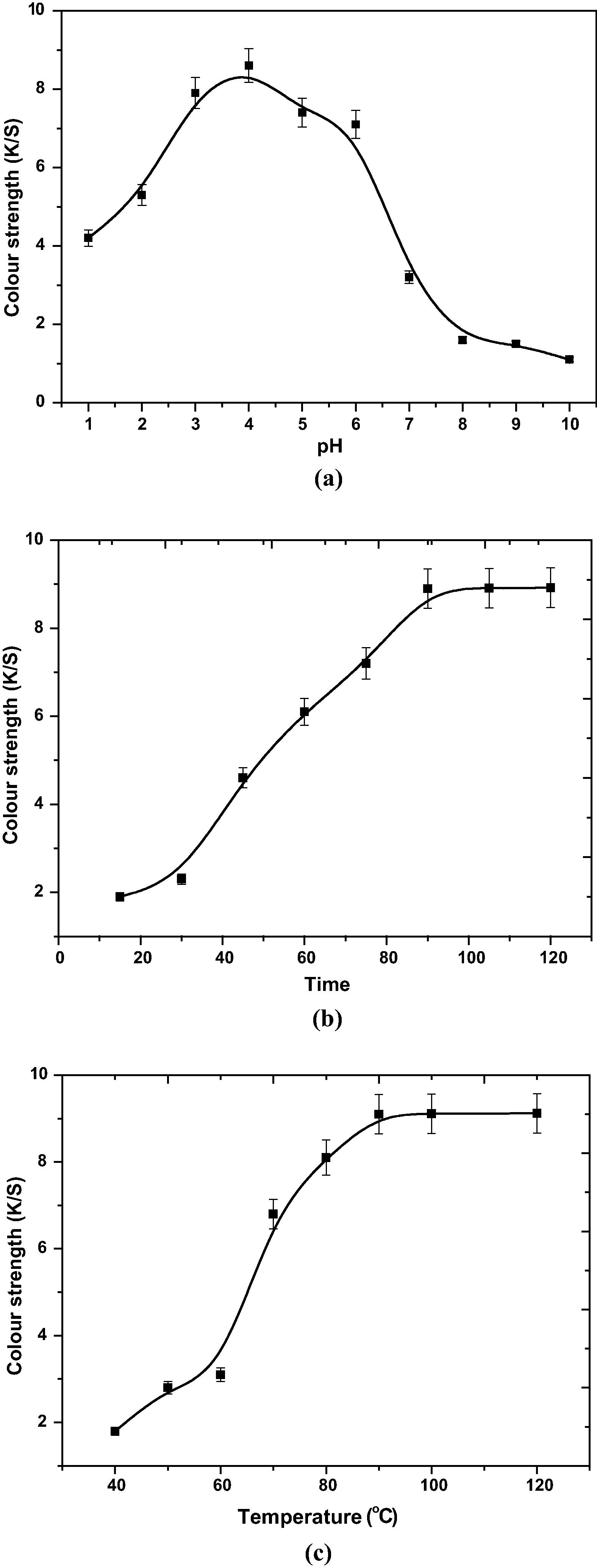
Effect of (a) pH, (b) time and (c) temperature on dyeing.
3.2.2 Effect of dyeing time
From the Fig. 3b, it can be shown that on control conditions (Table 2) the optimum time for dyeing is 90 min.
3.2.3 Effect of temperature
Temperature controls the dyeing mechanism by increasing the swelling extent of wool and makes the interaction of wool surface to dye molecules more feasible. Fig. 3c represents the effect of temperature on control conditions (Table 2). The optimum dyeing temperature is 90 °C. Dyeing of textiles occurs mainly by adsorption of dye molecules over the surface and temperature highly affects the adsorption by adsorption–desorption equilibrium. Temperatures above 90 °C shift the adsorption–desorption equilibrium toward right and so the dye exhaustion decreases with the increase in dyeing temperature further (Shabbir et al., 2016a,b).
From Table 2 and Fig. 3a –c, the optimized conditions for dyeing are as follows:
Dye bath medium pH = 4, dyeing time = 90 min and temperature = 90 °C.
3.3 Optimization of mordant concentration
Metal salt mordants have different linking interactions with wool and thereby may darken, brighten or alter the overall color of the dyed wool samples (Uslu and Bamu, 2016; Khan, 2012; Gupta et al., 2004). In this study, wool yarns were pre-treated with different types of metal salts (aluminum chloride and calcium chloride).
Fig. 4a and b show the effect of mordant concentrations on the percentage exhaustion of R. cordifolia on dyed wool yarns in terms of color strength of dyed (a fixed % age of dye) samples. In general, increasing the concentration of mordants enhances the % dye exhaustion. From the Fig. 4a and b, it can be seen that dye exhaustion gradually increases up to 5% and thereafter increment goes to saturation. Thus, 5% mordant concentration is believed to be sufficient for mordanting of wool.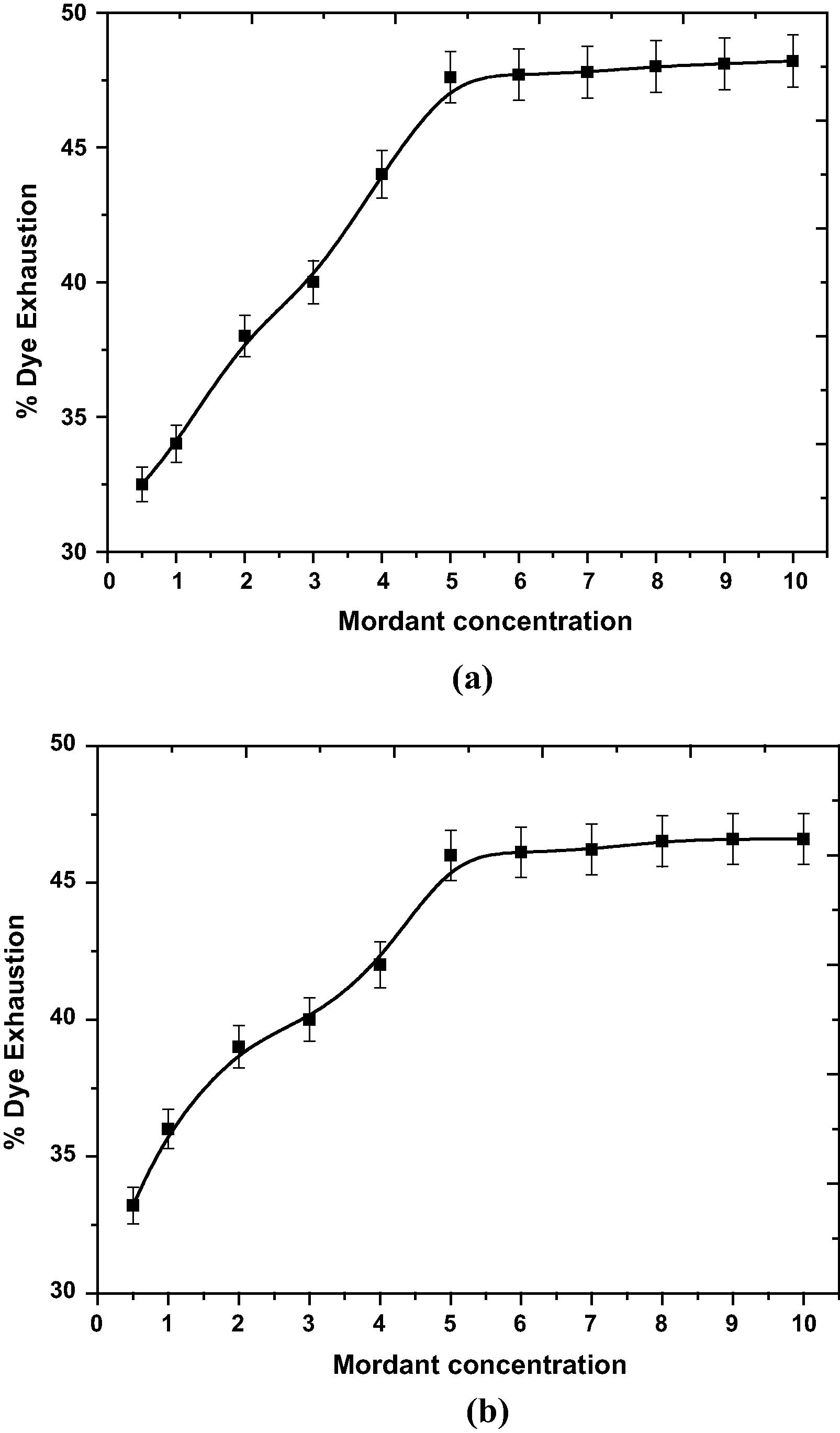
Optimization of (a) AlCl3 and (b) CaCl2 mordant.
3.4 Effect of dye concentration on color strength
In order to assess the effect of concentration of dye on color strength of dyed woolen yarn different concentrations of dye were used (5–50.0% o.w.f.). With the increase in dye concentration, an increase in color strength (K/S) is observed resulting in deeper shades having maximum color depth with an increase in the dye adsorption (Fig. 5).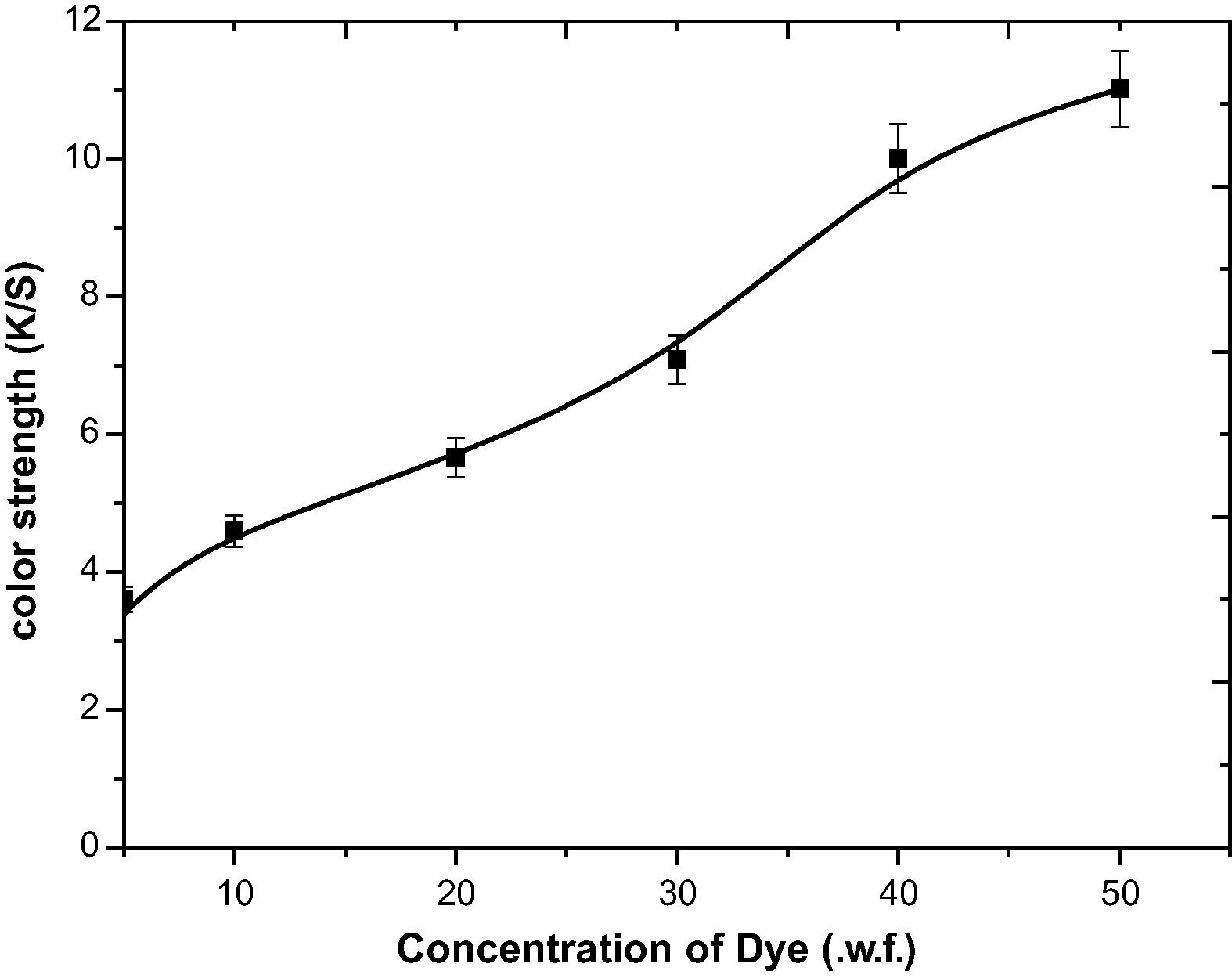
Effect of dye concentration on color strength.
3.5 Effect of mordants on colorimetric properties
Mordants are metal salts that enhance the dyeability and durability of textile substrates by forming an insoluble complex with dye molecules. The results of colorimetric properties of shades obtained are presented in Table 3 in terms of CIE L*a*b* color coordinates (L*, a*, b*) and color strengths (K/S). In the view of several published reports (Vankar et al., 2008; Yusuf et al., 2013) mordants generally have a propensity to combine with dye and fiber that possibly contribute imparting stable color, attributed to a chemical bridging/bonding between dye and fiber molecules. Mordanting has little effect on colorimetric properties as subsidiary changes were observed in L*, a*, b* values for mordanted samples in comparison to un-mordanted ones. From Table 3, it can be shown that the darkest shades were perceived with AlCl3 as an anchoring agent. From a*–b* plot (Fig. 6), it can be seen that mordanted samples were found to have a little change toward yellow co-ordinate in red-yellow zone of CIE L*a*b* color space. Shift toward yellow co-ordinate was higher in case of CaCl2 mordanted samples. Fig. 7 is a representation of color strength in terms of K/S values for dyed wool yarn samples at optimized conditions. When AlCl3 and CaCl2 mordants were used, color of dyed samples became more intense resulting in higher K/S values. AlCl3 mordant has more prominent effect on color strength than CaCl2 mordant. Probable schematic representation of dyeing mechanism of R. cordifolia dye extract on wool is shown in Fig. 8 also described in literature for various dyeing components of extracts used for dyeing (Shabbir et al., 2016a,b; Khan et al., 2015).
Mordant
L*
a*
b*
K/S
Nil
48.12
40.06
36.21
11.02
5.0% AlCl3
44.33
39.59
37.13
17.79
5.0% CaCl2
44.91
38.67
37.11
14.14
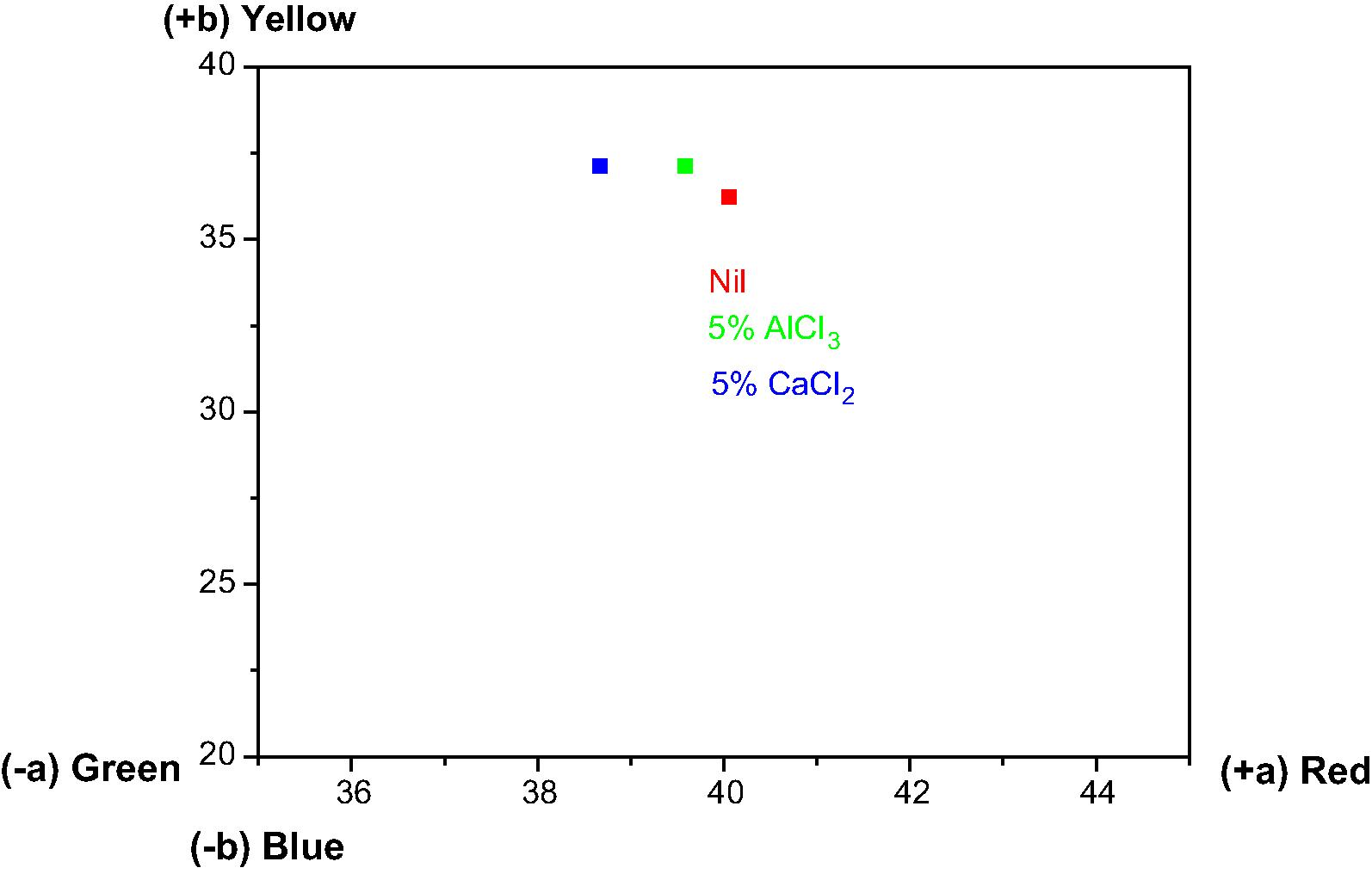
a*–b* plot of Rubia cordifolia dyed wool: effect of mordants.
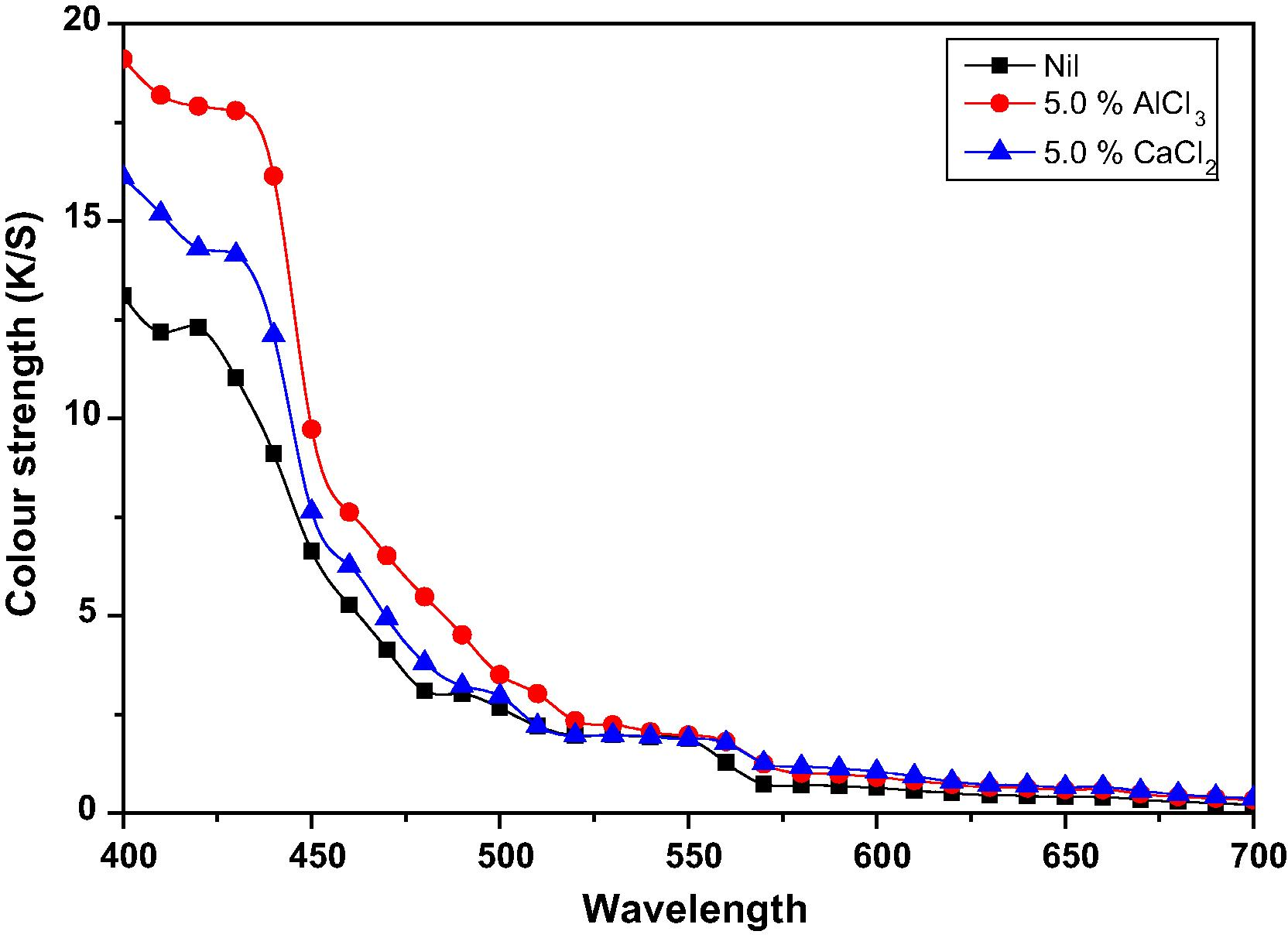
Effect of mordants on color strength (λmax = 430 nm).
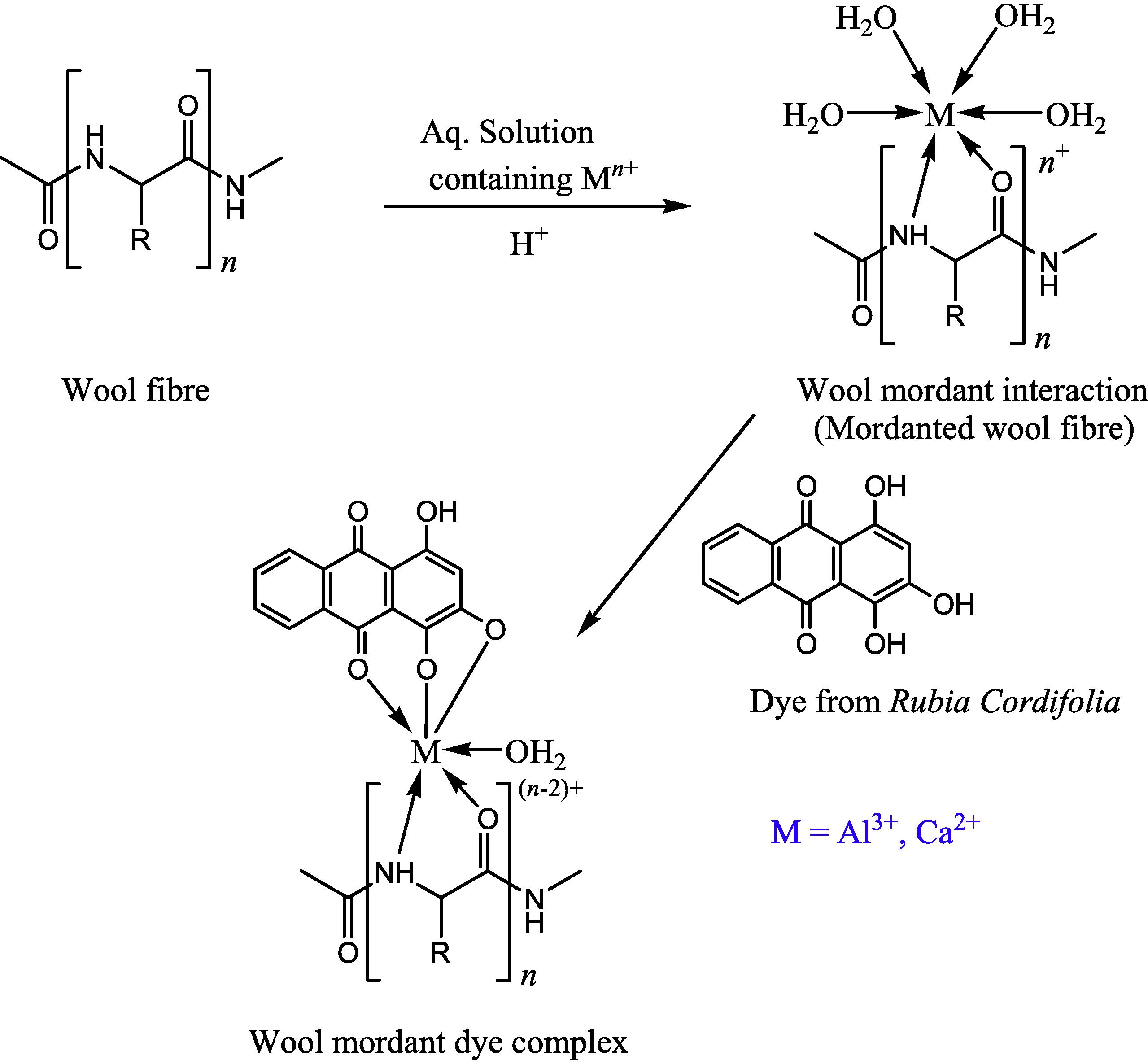
Probable complex of wool functional groups, mordant and purpurin dye molecule.
3.6 Effect of mordants on color fastness
Plant extracts are enriched with phytochemical constituents attributed to their specific role such as colorants, cosmetics, antimicrobials, anticancer, anti-diabetic, antioxidants, etc. (Yusuf et al., 2012; Khan et al., 2012; Modolo et al., 2015; Ahmed et al., 2016). Table 4 represents the color fastness properties of wool samples dyed with 50% R. cordifolia. All samples have shown good light and wash fastness. Mordanting increase overall fastness properties compared to un-mordanted samples. AlCl3 mordant was more efficient than CaCl2 mordant for fastness properties. Dry rub fastness was found to be relatively better than wet rub fastness as far as to be expected. Fastness data from Table 4 indicate that mordanting increased resistance of transfer of color to adjacent fabrics (cotton and wool). c.c., color change; c.s., color staining on cotton; c.w., color staining on wool.
Mordant concentrations
Light fastness
Wash fastness
Rub fastness
c.c.
c.s.
c.w.
Dry
Wet
Nil
4/5
3/4
4/5
4/5
4
3
5.0% AlCl3
5
4/5
5
5
5
4/5
5.0% CaCl2
5
4
5
5
4/5
3/4
4 Conclusion
The feasibility of R. cordifolia root’s extract as a natural dye for dyeing woolen yarn via color measurement and color fastness testing is investigated in this study. The order of mordanting on the color strength and fastness properties was studied in the course of the investigation. And we also investigated the optimal conditions for extraction and dyeing of R. cordifolia. A systematic protocol is successfully evaluated for textile dyeing with anthraquinone dye.
Following conclusions were obtained on the basis of above study:
-
The optimum extraction condition is found to be pH = 2, time = 45 min and temperature 90 °C.
-
The optimum dyeing condition is found to be pH = 4, time = 90 min and temperature 90 °C.
-
R. cordifolia can be applied on wool as substrate that imparts radiant red shades with or without mordants having different hue and tones with commercially acceptable colorimetric properties.
-
Mordanting with anchoring agents improves overall fastness properties against light, washing and rubbing.
Thus R. cordifolia, a natural dye containing anthraquinone colorants may serve as an eco-friendly alternative to their synthetic counterparts to make a greener life. It is hoped, that natural colorants can be tailored to approach the stringent functional parameters of synthetic dyes and can be a viable commercial alternative for the textile and apparel industries with systematic research.
References
- Ecofriendly dyeing of UV-irradiated cotton using extracts of Acacia nilotica Bark (Kikar) as source of Quercetin. Asian J. Chem.. 2014;26:830-834.
- [Google Scholar]
- A review on plants extract mediated synthesis of silver nanoparticles for antimicrobial applications: a green expertise. J. Adv. Res.. 2016;7:17-28.
- [Google Scholar]
- Modulation of pomegranate peel colourant characteristics for textile dyeing using high energy radiations. Ind. Crops Prod.. 2014;58:188-193.
- [Google Scholar]
- Optimization of alkaline extraction of natural dye from Henna leaves and its dyeing on cotton by exhaust method. J. Cleaner Prod.. 2009;17:61-66.
- [Google Scholar]
- The Wealth of India Raw Materials, National Institute of Science Communication and Information Resources. Vol 9. New Delhi: CSIR; 1972. p. :82-83. Rh-So
- Society of Dyers and Colorists Vol 4. (third ed.). 1971. p. :4630-4631. (Bradford, UK)
- Eco-friendly dyeing of gamma ray induced cotton using natural quercetin extracted from Acacia Bark (A. nilotica) J. Nat. Fibers. 2015;12:494-504.
- [Google Scholar]
- Dyeing studies with hydroxyanthraquinones extracted from Indian Madder. Part 1: dyeing of nylon with purpurin. Color. Technol.. 2001;117:328-332.
- [Google Scholar]
- Dyeing studies with hydroxyanthraquinones extracted from Indian Madder. Part 2: dyeing of nylon and polyester with nordamncanthal. Color. Technol.. 2001;117:333-336.
- [Google Scholar]
- Light fastness of naturally occurring anthraquinone dyes on nylon. Color. Technol.. 2004;120:205-212.
- [Google Scholar]
- Synthesis and characterization of polyaniline/g-alumina nanocomposite: a comprehensive study for the adsorption of three different anionic dyes. J. Ind. Eng. Chem.. 2014;20:3890-3900.
- [Google Scholar]
- Extraction of natural dye from red calico leaves: gamma ray assisted improvements in colour strength and fastness properties. Dyes Pigm.. 2014;103:50-54.
- [Google Scholar]
- Khan, M.A., 2012. Dyeing of wool and silk fibres with a conductive polyelectrolyte and comparing their conductance. <http://bada.hb.se/handle/2320/10426> (retrieved on 18.05.2016).
- Antimicrobial activity of wool yarn dyed with Rheum emodi (Indian Rhubarb) Dyes Pigm.. 2012;95(2) 206-204
- [Google Scholar]
- Mixed Metal mordant dyeing of wool using root extract of Rheum emodi (Indian Rhubarb/Dolu) J. Nat. Fibers. 2015;12:243-255.
- [Google Scholar]
- The use of response surface method to optimize the extraction of natural dye from winery waste in textile dyeing. J. Text. Inst.. 2016;2016:1-12.
- [Google Scholar]
- The Chemistry of Natural Coloring Matters. Reinhold Publishing Corporation, USA. 1943;120:124.
- [Google Scholar]
- An overview on the potential of natural products as ureases inhibitors: a review. J. Adv. Res.. 2015;6(1):35-44.
- [Google Scholar]
- Effect of proteolytic enzyme on dyeing of wool with Madder. Enzyme Microb. Technol.. 2007;40:1719-1722.
- [Google Scholar]
- Natural Organic Coloring Matters. London: Longmans Green and Co.; 1918. p. :41-42.
- An evaluation of UV protection imparted by cotton fabrics dyed with natural colorants. BMC Dermatol.. 2004;4:15.
- [Google Scholar]
- An eco-friendly dyeing of woolen yarn by Terminalia chebula extract with evaluations of kinetic and adsorption characteristics. J. Adv. Res.. 2016;7:473-482.
- [Google Scholar]
- Application of Terminalia chebula natural dye on wool fiber-evaluation of color and fastness properties. Text Clothing Sustainable. 2016;2016(2):1-8.
- [Google Scholar]
- Dyeing, fastness and antimicrobial properties of woolen yarns dyed with gallnut (Quercus infectoria Olivier) extract. Dyes Pigm.. 2012;95:53-61.
- [Google Scholar]
- Recent advancements in natural dye applications: a review. J. Cleaner Prod.. 2013;53:310-331.
- [Google Scholar]
- Dyeing of modified cotton fiber with natural Terminalia arjuna dye: optimization of dyeing parameters using response surface methodology. Environ. Prog. Sustainable Energy. 2016;35:719-728.
- [Google Scholar]
- Extraction and dyeing conditions of natural dye from flowers of Plumeria rubra L. on textiles and fastness properties. Indian J. Traditional Knowledge. 2016;15:278-284.
- [Google Scholar]
- Phytoremediation: potential flora for synthetic dyestuff metabolism. J. King Saud Univ. Sci.. 2016;28:119-130.
- [Google Scholar]
- Effect of solvent and pH on the extraction of carbolic acid from aqueous solution by TOMAC. J. Chem. Eng. Data. 2016;61:1676-1680.
- [Google Scholar]
- Ecofriendly sonicator dyeing of cotton with Rubia cordifolia Linn. using biomordant. Dyes Pigm.. 2008;76:207-212.
- [Google Scholar]
- Assessment of colorimetric, antibacterial and antifungal properties of woollen yarn dyed with the extract of the leaves of henna (Lawsonia inermis) J. Cleaner Prod.. 2012;27:42-50.
- [Google Scholar]
- Eco-dyeing of wool using aqueous extract of the roots of Indian Madder (Rubia cordifolia) as natural dye. J. Nat. Fibers. 2013;10:14-28.
- [Google Scholar]
- Colour and fastness of natural dyes: revival of traditional dyeing techniques. Color. Technol.. 2010;127:18-27.
- [Google Scholar]







