Translate this page into:
Impact of habitat variability and altitude on growth dynamics and reproductive allocation in Ferula jaeschkeana Vatke
⁎Corresponding author. Cell: +91 9796186479. ubaidyaqoob@yahoo.in (Ubaid Yaqoob)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Ferula jaeschkeana Vatke is an important threatened medicinal plant of the Himalayan region. The present study was carried out to determine the impact of the habitat variability and altitudinal gradient on the morphological and reproductive features of the species under study. The species exhibited great variability in its morphological traits under different environmental conditions. The plants were more vigorous and taller at a low altitude site, Kashmir University Botanical Garden (KUBG) while the plants of a high altitude site, Gulmarg were shorter. With increased altitude, a significant reduction in the number of umbels per flowering stem, umbellules per umbel and flowers per umbellule occurred. An increase in the number of stigma and anthers was also observed in some plants at higher altitudes. Principal component analysis (PCA) revealed that the habitat of KUBG and Dachigam proved relatively better for the growth of F. jaeschkeana. Maximum resources were allocated to the growth and development of the stem followed by root tubers, leaves and inflorescence. Reproductive success of the plant species varied along the altitudinal gradient and ranged from 64% to 72%. Increasing altitude resulted in a decrease in the allocation of biomass to reproductive structures in the form of decreasing dry weight. The total resource budget per plant was maximum in low altitude Drang (572.6 ± 158.36 g) and Dachigam (568.4 ± 133.42 g) populations and was least in the Gulmarg population (333.4 ± 82.89 g). The reproductive effort was higher (50.83%) for the high altitude Gulmarg population. The regression analysis revealed a positive correlation and predicts that plant height has a direct impact on the umbel diameter and leaf length. Our results present a detailed account on the variation of growth characteristics, reproductive success and changes in allocation patterns in relation to the environmental conditions of this valuable medicinal plant species. This information is very useful to introduce the species into cultivation and developing strategies for conservation and sustainable use of the wild populations.
Keywords
Apiaceae
Ferula jaeschkeana
Habitat variability
Kashmir valley
1 Introduction
In comparison with often broad and poorly defined latitudinal distributions in the lowlands, the distributions of species in mountain regions are typically restricted to relatively narrow altitudinal bands (Jump et al., 2009). The variation of size and placement of resource acquiring parts such as leaves are critical to a plant’s adjustments to resource availability (Sattarian et al., 2011). In order to study fundamental issues in plant ecology like plant response to fluctuating environmental conditions, abiotic gradients represent unique natural laboratories (Gimenez-Benavides et al., 2007; Gonzalo-Turpin and Hazard, 2009; Montesinos-Navarro et al., 2011).
To survive in harsh conditions, plants can either adapt to the local conditions genetically or respond to the environmental variability by phenotypic plasticity (Becker et al., 2006). Phenotypic plasticity is chiefly important for plants since they cannot move and have to deal with changing ambient conditions. It further allows for faster adjustments to a changing environment (Sultan, 2000; Hoffmann and Sgro, 2011). For alpine plants, phenotypic plasticity is also expected to occur more frequently at higher elevations as it enables fast adaptation to a temporally highly variable environment (Stocklin et al., 2009; Pluess et al., 2011). However, plasticity is not always advantageous, but can also be non-adaptive, with neutral or negative effects to fitness (Ghalambor et al., 2007).
In the case of altitudinal gradients, with increasing altitude plants must cope with fluctuating environmental conditions (Korner, 2007). In response to the climatic shifts, plants adjust key physiological processes such as gas exchange and photosynthetic rates (Korner, 2007) and morphological traits (Ma et al., 2010; Phillips et al., 2011). The most conspicuous structural alteration in plants is the reduction in overall plant size observed along elevational gradients (Korner et al., 1989).
Reproduction is the currency of natural selection, but plants must grow to build the machinery in order to reproduce (Bazzaz and Reekie, 1985). The biomass distribution and the proportion of plant allocated resource to reproductive structures is an important embodiment of plant fitness (Vega et al., 2000). Resource allocation to vegetative and reproductive parts has for long been known as an important determinant of reproductive success (Elle, 1999). The patterns of allocation reveal evolved strategies that are the results of different selection pressures and constraints (Weiner, 2004). In some alpine and subalpine species, phenotypic plasticity allows the high altitude populations to compensate the short growing season by reproducing more rapidly (Stinson, 2004).
Ferula L. is the third largest genus of family Apiaceae (Yaqoob and Nawchoo, 2015) and consists of 180–185 species (Pimenov and Leonov, 2004). Ferula jaeschkeana Vatke locally known as “Krandel” is a monocarpic herbaceous perennial plant. The species grows from plains to sub-alpine regions of the Kashmir valley along an altitudinal gradient of 1800–2800 m asl. The resin of F. jaeschkeana (collected from mature plants) is used in folk medicine in the treatment of tumors, chronic wounds and ulcers (Khalilova and Saidkhodzhaev, 1998). The powder prepared from the dried root tubers is used to cure chest pain (Yaqoob et al., 2015) and joint pain. The seeds are also used to cure chest pain. The leaf paste is applied on wounds for healing purposes. The whole plant is destructively collected and is used as fodder and firewood. Being a monocarpic perennial, most of the annual and biennial plants are wiped off before seed set leading to a decline in its population (personal observation of authors). The species has immense medicinal and traditional importance and is facing threat due to local usage, susceptibility to cattle grazing and other anthropogenic activities (Table 1). The present study was devised for the first time to understand the variation in growth characteristics, reproductive success and changes in allocation patterns in relation to the environmental conditions along the altitudinal gradient. This study aimed at developing strategies for cultivation, conservation and sustainable use of wild populations and to find the environments that are most favorable and productive for the growth of F. jaeschkeana. The sites are ordered by increasing elevation.
Location
Altitude (m asl)
Latitude and longitude
Climatic zone
Habitat
Threat factor
KUBG#
1595
34°7′57.17″N
74°50′15.19″EPlain
Open shady field
Nil
Dachigam
1900
34°8′9.99″N
75°29′52.40″EPlain
Sunny slope with partial shade
Local use, overgrazing
Drang
2235
34°2′8.70″N
74°25′4.57″ESub alpine
Sunny open slope
Local use, overgrazing, landslides, habitat destruction and construction of roads
Betab valley – Pahalgam
2405
34°3′15.17″N
75°21′49.83″ESub alpine
Sunny open slope
Local use, overgrazing, landslides, habitat destruction and construction of roads
Gulmarg
2590
34°3′40.2″N
74°23′15.95″ESub alpine
Moist open slope
Local use, overgrazing, landslides
2 Materials and methods
2.1 Study sites
F. jaeschkeana was found growing in Daara, Dachigam, Betab valley – Pahalgam, Chandanwari, Gulmarg, Aharbal, Jawahar tunnel, Ferozpora, Drang, Narayanag, Daksum, Kokernag and Drass. Four natural populations (Dachigam, Drang, Betab valley – Pahalgam and Gulmarg) and one transplanted control population grown at KUBG (Kashmir University Botanical Garden) were selected for the present study (Fig. 1). The origin of plants growing at KUBG is not known and has been collected earlier from natural habitats. The salient features of the selected sites are depicted in Table 1.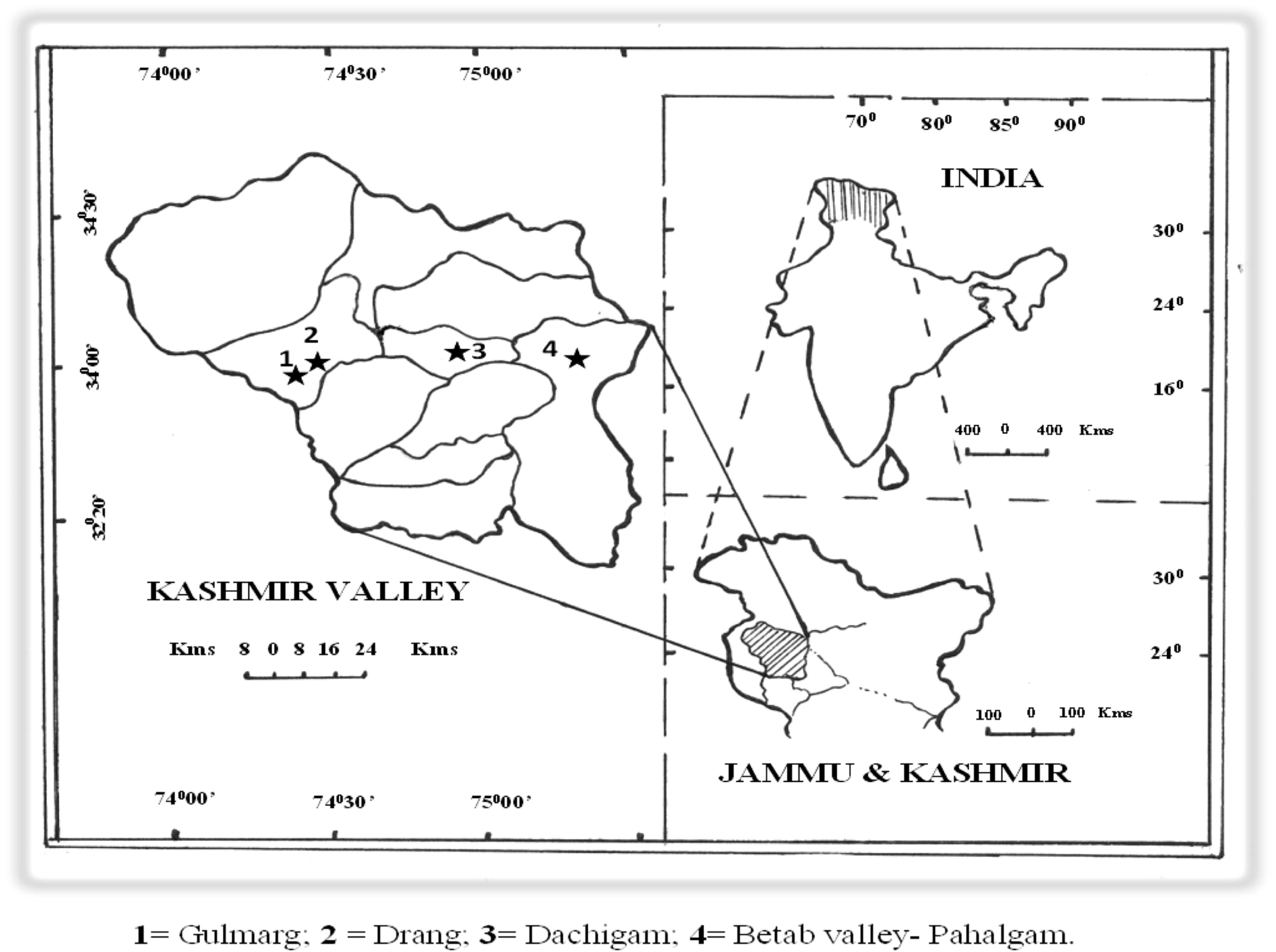
Map showing the location of study sites of Ferula jaeschkeana in Kashmir Valley.
2.2 Morphological characterization
The studies were carried out in both ex situ as well as in natural habitats and photographs were taken using Canon A810 camera. Ten mature flowering individuals were selected randomly and tagged from each population in order to observe the various morphological parameters of the species and to record the variability in floral and vegetative traits. The populations were analyzed for morphological traits like number of shoots per plant, root tuber dimensions, plant height, basal leaf length, pinnae number, pinnae length, pinnule length, number of flowering stems per plant, flowering stem length, sheath number per plant, sheath length, number of umbels, umbel diameter, umbels per flowering stem, umbellule’s per umbel, number of flowers, fruit morphology and fruit number. The plants were measured in situ and most of these parameters were recorded at flowering stage in all the populations. We used linear regression analysis to determine the correlation between various morphological parameters across the altitudinal gradient so as to find the sites of better growth performance of this species. Principal component analysis (PCA) was carried out to analyze the morphological traits in relation to habitat dynamics. In addition to this, it was also used to infer the coherence between different vegetative and reproductive parameters.
2.3 Reproductive success
The umbels were dissected under the stereo zoom microscope (Zeiss Discovery. V8) and the number of flowers per umbellule were counted to examine the proportion of variation within and among plants as well as among populations. The mature umbels containing mericarps were collected from all the selected populations. We determined the percentage seed set of each plant expressed as the ratio of developed fruits to the total number of flowers.
2.4 Reproductive allocation
Fifteen mature and healthy flowering plants from all the natural populations were harvested for the study of resource partitioning in different parts of a plant. The plants were fragmented into individual parts such as root tubers, leaves, stem and inflorescence. Using an electronic balance, fresh weight (weighing as fresh) and dry weight (after oven-drying for 48 h at 80 °C) of the plants were determined following Kawano and Masuda (1980). Reproductive effort (RE) was calculated from the estimates of dry weight or biomass allocated to reproductive and vegetative structures (Abrahamson and Gadgil, 1973):
2.5 Data analysis
ANOVA was used to test for differences between populations for all the morphological characters measured using the SPSS 16.0 software. Tukey’s multiple comparison of means was used to compare all populations and the differences between individual means were deemed to be significant at p ⩽ 0.05.
3 Results
3.1 Growth characteristics
Present investigation revealed a wide range of suitable habitats for the growth of F. jaeschkeana (Fig. 2). The species prefers to grow in the shady plains, rocky open slopes and open slopes with loose textured soils (Table 1). F. jaeschkeana is a herbaceous perennial plant with 1–2.5 m tall solitary, thick, reddish brown stem and a huge woody taproot, that grows 19–22 cm deep below the soil surface. The stem base is clothed in fibrous remnant sheaths. Upper leaves are reduced to large sheaths often with umbels in their ascils.
Different types of habitats of Ferula jaeschkeana in Kashmir Valley. (A) Open plains; (B) shady slope; (C) mountainous slope; (D) open slopes with loose textured soils.
At both inter and intra-population levels, the species exhibits a considerable variability in its morphological traits under different environmental conditions (Table 2). Highly significant differences among populations were observed in plant height, leaf dimensions, umbel size and umbellule number. The main difference between the populations was obvious in the plant height which was almost double in plants growing at low altitude sites KUBG and Dachigam (210.05 ± 17.42 cm and 197.61 ± 13.15) than in Gulmarg (106.17 ± 27.17). The flowering stem length and basal leaf length were also highest (35.05 ± 6.52 and 108.45 ± 18.56 cm, respectively) at low altitude KUBG and were the least (26.16 ± 4.77 and 74.93 ± 10.03 cm, respectively) at Gulmarg. Besides, the pinnae number per plant, pinnae length and pinnule length were also maximum (5.2 ± 1.47, 58.92 ± 10.54 cm and 17.52 ± 3.47 cm, respectively) in the plants of low altitude site, KUBG and were minimum (2.2 ± 0.5, 33.52 ± 7.87 and 7.36 ± 2.13) in the plants of high altitude site, Gulmarg. The root tuber length and root tuber breadth followed the same trend and ranged from 19.3 to 22.86 cm and 10.66 to 15.49 cm, respectively.
Plant characteristics
KUBG
Dachigam
Drang
Betab valley – Pahalgam
Gulmarg
F value
LSD
Plant height (cm)
210.0 ± 17.42a⁎
197.61 ± 13.15a
154.6 ± 21.38b
137.6 ± 18.56b
106.17 ± 27.17c
45.40
15.13
Basal leaf length (cm)
108.4 ± 18.56a
103.37 ± 15.62a
90.67 ± 15.97abc
77.47 ± 6.78bc
74.93 ± 10.03c
11.05
10.73
Pinnae number
5.2 ± 1.47a
3.0 ± 0.94b
3.0 ± 0.64b
2.5 ± 0.68bc
2.2 ± 0.5b
15.02
0.724
Pinnae length (cm)
58.92 ± 10.54a
42.16 ± 7.56b
40.13 ± 8.22b
36.83 ± 6.09bc
33.52 ± 7.87b
14.40
6.18
Pinnule length (cm)
17.52 ± 3.47a
9.39 ± 2.08b
8.63 ± 1.8b
7.87 ± 2.79bc
7.36 ± 2.13b
28.81
1.85
Flowering stems per plant
18.2 ± 1.98a
26.6 ± 4.08b
15.9 ± 1.52ac
14.7 ± 2.58ac
13.3 ± 3.49c
33.13
2.18
Flowering stem length (cm)
35.05 ± 6.52a
32 ± 3.98a
31.24 ± 6.55a
29.21 ± 5.23a
26.16 ± 4.77b
3.58
4.15
Sheath number per plant
18.9 ± 3.28a
19.7 ± 2.83a
17.6 ± 2.54ab
17.1 ± 2.84ab
14.2 ± 3.64b
4.78
2.30
Sheath length (cm)
32.13 ± 6.6a
34.29 ± 7.95a
30.22 ± 5.61a
28.19 ± 6.14a
27.68 ± 7.49a
1.62
5.13
Umbel diameter (cm)
38.35 ± 6.37a
32 ± 5.63ab
28.7 ± 4.77b
27.94 ± 4.77bc
27.68 ± 4.85b
7.55
3.89
Umbels per flowering stem
7.1 ± 2.13a
7.3 ± 2.21a
5.7 ± 1.76a
5.4 ± 1.07a
5.3 ± 1.49a
2.59
1.38
Umbellule’s per umbel
14.8 ± 3.82a
13.4 ± 1.42ab
10.7 ± 2.11bc
9.3 ± 1.33c
8.5 ± 2.06c
14.44
1.68
Flowers per umbellule
21.2 ± 3.22ab
22.7 ± 3.46a
19.6 ± 1.95ab
18.5 ± 2.46bc
15.9 ± 1.66c
9.65
1.99
Number of ramets
3.4 ± 1.34a
2.3 ± 0.94ab
2 ± 0.66b
1.7 ± 0.67b
1.4 ± 0.51b
7.62
0.66
Root tuber length (cm)
22.86 ± 3.58a
21.59 ± 5.63a
20.57 ± 4.69a
19.81 ± 3.73a
19.3 ± 3.81a
1.06
3.29
Root tuber breadth (cm)
15.49 ± 5.25a
15.24 ± 3.78a
13.46 ± 2.92a
11.68 ± 3.81a
10.66 ± 3.32a
2.95
2.94
Plants flowered for a period of 7–9 weeks and the highest percentage of flowering plants was recorded between May and early-June. Transplanted population at KUBG showed the highest values of almost all the morphological traits as compared to the natural populations. An increase in the number of stigma and anthers was also observed in some plants (Fig. 3) growing at the Betab valley – Pahalgam and Gulmarg. A positive correlation between root tuber length and plant height (r2 = 0.1459), plant height and basal leaf length (r2 = 0.3497), plant height and umbel diameter (r2 = 0.2843), umbellules per umbel and flowers per umbellule (r2 = 0.396), number of ramets and umbellules per umbel (r2 = 0.1287) and number of ramets and flowering stems per plant (r2 = 0.0734) was observed (Fig. 4).
Increase in the number of stigma and anthers of flowers. (A) Gynoecium with three stigmas and styles; (B) gynoecium with four stigmas and styles; (C) flower with six anthers; (D) flower with seven anthers.
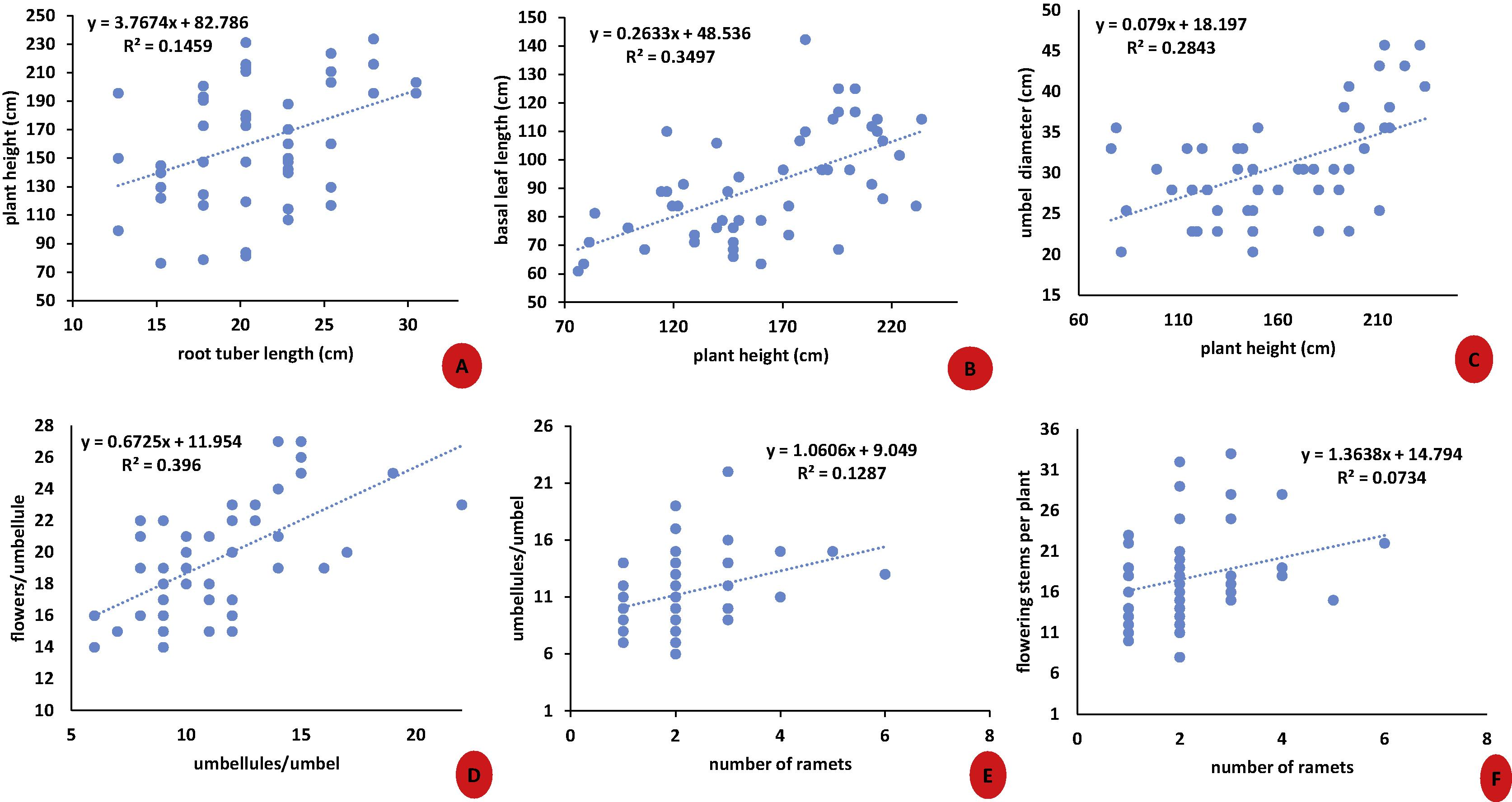
(A–F) Regression analysis between several morphological characters of F. jaeschkeana.
Principal component analysis (PCA, Fig. 5) of all morphological characters across the study sites reveals that the major differences between populations were due to size characteristics (axis 1; 85% of total variance) and separated KUBG and Dachigam (with high character values) from the other populations. PCA reveal that the high altitude populations are non-favorable to most of the vegetative and reproductive traits indicating better growth conditions at lower altitudes. Flowering stem length, root tuber length (cm), number of ramets, umbel diameter, pinnae number, pinnae length, and pinnule length were found to be favoring KUBG population. Basal leaf length, number of flowering stems per plant, sheath length, umbels per flowering stem, umbellules per umbel, plant height, root tuber breadth (cm), flowers per umbellule, and sheath number per plant were found to be favoring the Dachigam population. Thus, the habitat of KUBG and Dachigam proved relatively better for the growth of F. jaeschkeana.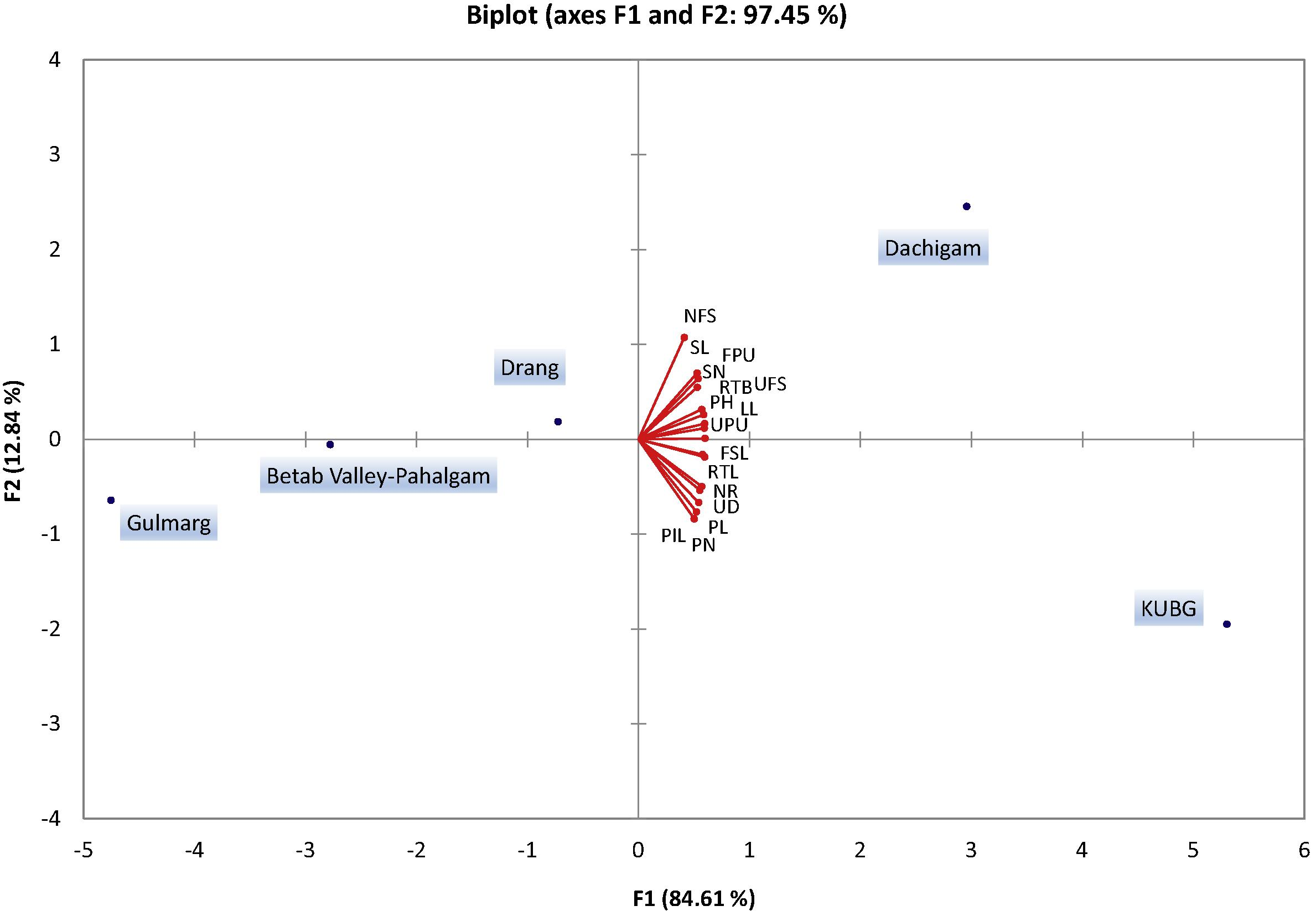
Principal component analysis (PCA) of morphological characteristics of Ferula jaeschkeana across the different study sites. LL – basal leaf length, NFS – number of flowering stems per plant, SL – sheath length, UFS – umbels per flowering stem, UPU – umbellules per umbel, PH – plant height, RTB – root tuber breadth (cm), FPU – flowers per umbellule, SN – sheath number per plant, PN – pinnae number, PL – pinnae length, NR – number of ramets, FSL – flowering stem length, UD – umbel diameter, PIL – pinnule length, RTL – root tuber length (cm).
3.2 Reproductive success
With increased altitude, a significant reduction in the number of umbels per flowering stem, umbellules per umbel and flowers per umbellule occurred (Table 2). Percentage seed set of the plant species also varied along the altitudinal gradient and ranged from 64% to 72%. The number of umbellule per umbel varied with overall plant height from 5 to 7 across populations. Moreover, the number of ramets per root tuber varied from 1 to 4 which represents a copious variation in numbers of umbellules per umbel, umbels per flowering stem, number of umbels and thus the reproductive success.
3.3 Reproductive allocation at flowering
It is evident from the data (Table 3) that partitioning of resources is not uniform among different parts of a plant. A striking difference was observed in total above ground dry weight and dry weight of different vegetative structures among the plants of studied populations, growing at different altitudes. The total resource budget per plant and the above ground dry weight of low altitude populations (Drang and Dachigam) and high altitude population (Gulmarg) varied to a greater extent. The values were maximum in low altitude Drang (572.6 ± 158.36 g and 407.2 ± 123.5 g, respectively) and Dachigam (568.4 ± 133.42 g and 359.6 ± 104.97 g, respectively) populations and were least in the Gulmarg population (333.4 ± 82.89 g and 236.8 ± 56.18 g, respectively). Also the percentage allocation to reproductive parts, i.e., reproductive effort was more (50.83) for the high altitude Gulmarg population. Maximum resources were allocated toward the growth and development of stem (156.9 ± 62.24 g to 275.3 ± 85.18 g) followed by root tuber (96.6 ± 26.71 g to 208.8 ± 28.45 g) and leaves (67.3 ± 10.19 g to 112.5 ± 35.69 g) while the least resources were allocated toward inflorescence (12 ± 3.12 g to 19.4 ± 2.63 g).
Traits
Dachigam
Drang
Betab valley – Pahalgam
Gulmarg
F value
LSD
Root tubers
208.8 ± 28.45a⁎
165.4 ± 34.86b
102.4 ± 26.12c
96.6 ± 26.71c
33.64
22.07
Stem
253.3 ± 78.22a
275.3 ± 85.18a
156.9 ± 62.24b
157.5 ± 42.87b
8.18
52.14
Leaves
91.4 ± 22.71ab
112.5 ± 35.69b
79.6 ± 24.65a
67.3 ± 10.19a
5.91
18.87
Inflorescence
14.9 ± 4.04a
19.4 ± 2.6b
14.7 ± 4.11a
12 ± 3.12a
7.53
2.66
Total resource budget per plant (g)
568.4 ± 133.42a
572.6 ± 158.36a
353.6 ± 117.12b
333.4 ± 82.89b
10.89
95.0887
Reproductive effort per plant
47.18 ± 1.19a
51.46 ± 1.5b
48.52 ± 1.58a
50.83 ± 1.39b
19.70
1.079
4 Discussion
Present investigation revealed that F. jaeschkeana grows in plains, rocky open slopes and open slopes with loose textured soils with low moisture retention. Hamzeloomoghadam et al. (2013) reported that the genus Ferula has a wide distribution and the species of the genus mostly grows in mountainous regions and some are distributed in desert areas. Dhancholia (2013) also reported the wide altitudinal range to be suitable for the growth of F. jaeschkeana. During the present study on plant morphology at inter- and intra-population levels, the morphological characters varied significantly across the selected populations. The present study revealed that the plants growing at shady habitats (KUBG and Dachigam) are more vigorous, taller and bear larger number of leaves and inflorescences in contrast to the plants growing in direct exposure to sunlight. This finding is in conformity with the observations of Siddique et al. (1997). It is known that the shady environments provide conditions where the plants can grow taller and can compete for light.
Our observations also reveal a decrease in the plant size with an increase in altitude. This is in accordance with the findings of Korner (2003), Baret et al. (2004) and Willis and Hulme (2004) who also reported a decrease in the plant size as an adaptation to increasing altitude. Decrease in plant height with increasing altitude results from slower growth rate that allows plants to use resources more efficiently in severe climatic environments (Bennington and McGraw, 1995) and may prove beneficial for the species as the stem shortening allows plants to avoid the detrimental effects of the strong winds (Korner and Cochrane, 1983). The leaf length was maximum in the plants growing at lower altitudes and was least in the plants growing at higher altitudes. Our results are in conformity with the observations of Bresson et al. (2011) who reported a general decrease in length, width and area of leaves with increasing altitude. An increase in the number of stigma and anthers in some plants suggest that the growth conditions are stressful at higher altitudes.
The pattern of biomass allocation of a plant to different structures is a fundamental aspect of its biology (Begon et al., 2006). Maximum amount of biomass is allocated toward the organs of support i.e., stem and root tubers. The reproductive effort of the plants growing at higher altitudes was more than the plants growing at lower elevations. These findings are in conformity with Fabbro and Korner (2004) and Molina-Montenegro et al. (2012) who reported the greater reproductive response with altitude. Furthermore, the relationship of increased allocation to reproduction (number of umbels) with plant size is in consistence with the observations of Aarssen and Taylor (1992) and Clauss and Aarssen (1994).
5 Conclusion
Our observation revealed a wide range of suitable habitats for the growth of F. jaeschkeana Vatke. The species was found to grow in the plains, rocky open slopes and open slopes with loose textured soils with great variability in its morphological traits under different environmental conditions. The plants were more vigorous and taller at low altitude site, KUBG while the plants of high altitude site, Gulmarg were shorter. With increased altitude, a significant reduction in the number of umbels per flowering stem, umbellules per umbel and flowers per umbellule occurred. PCA revealed that the habitat of KUBG and Dachigam proved relatively better for the growth of F. jaeschkeana. The total resource budget per plant and the above ground dry weight was higher for low altitude populations and was least for high altitude populations. Negative correlation of the morphological parameters to increasing altitude points toward the better growth performance of this species at lower elevations. This information is very useful when planning to introduce the species into cultivation and devoloping strategies for addressing conservation and sustainable use of wild populations.
Acknowledgement
The corresponding author is grateful to Council of Scientific and Industrial Research (CSIR) for providing financial assistance as JRF during this study.
References
- Growth form and reproductive effort in golden rods (Solidago, Compositae) Am. Nat.. 1973;107:651-661.
- [Google Scholar]
- Altitudinal variation in fertility and vegetative growth in the invasive plant Rubus alceifolius (Rosaceae), on Reunion Island. Plant Ecol.. 2004;172:265-273.
- [Google Scholar]
- The meaning and measurement of reproductive effort in plants. In: White J., ed. Studies on Plant Demography. London: Academic Press; 1985. p. :373-387.
- [Google Scholar]
- Local adaptation in the monocarpic perennial Carlina vulgaris at different spatial scales across Europe. Oecologia. 2006;150:506-518.
- [Google Scholar]
- Ecology: Individuals, Populations and Communities (fourth ed.). Blackwell: Oxford; 2006.
- Natural-selection and ecotypic differentiation in Impatiens pallida. Ecol. Monogr.. 1995;65:303-323.
- [Google Scholar]
- To what extent is altitudinal variation of functional traits driven by genetic adaptation in European oak and beech? Tree Physiol.. 2011;31:1164-1174.
- [Google Scholar]
- Phenotypic plasticity of size-fecundity relationships in Arabidopsis thaliana. J. Ecol.. 1994;82:447-455.
- [Google Scholar]
- Pleurotus himalayaensis Dhancholia sp. nov. A highly delicious edible mushroom from dry temperate cold desert zone of lahoul valley in Himachal Pradesh (India) American-Eurasian J. Agric. Environ. Sci.. 2013;13(1):44-49.
- [Google Scholar]
- Sex allocation and reproductive success in the andromonoecious perennial Solanum carolinensis (Solanaceae). I. Female success. Am. J. Bot.. 1999;86:278-286.
- [Google Scholar]
- Altitudinal differences in flower traits and reproductive allocation. Flora. 2004;199:70-81.
- [Google Scholar]
- Adaptive versus non-adaptive phenotypic plasticity and the potential for contemporary adaptation in new environments. Funct. Ecol.. 2007;21:394-407.
- [Google Scholar]
- Local adaptation enhances seedling recruitment along an altitudinal gradient in a high mountain Mediterranean plant. Ann. Bot.. 2007;99:723-734.
- [Google Scholar]
- Local adaptation occurs along altitudinal gradient despite the existence of gene flow in the alpine plant species Festuca eskia. J. Ecol.. 2009;97:742-751.
- [Google Scholar]
- In vitro evaluation for cytotoxic activity of three Ferula species. IJPSR. 2013;4(7):2673-2676.
- [Google Scholar]
- The altitude-for-latitude disparity in the range retractions of woody species. Trends Ecol. Evol.. 2009;24(12):694-701.
- [Google Scholar]
- Resource allocation and reproductive capacity in wild populations of Heloniopsis orientalis (Thunb). C. Tanaka (Liliaceae) Oecologia. 1980;45:307-317.
- [Google Scholar]
- Alpine Plant Life (second ed.). Heidelberg, Germany: Springer; 2003.
- Stomatal responses and water relations of Eucalyptus pauciflora in summer along an elevational gradient. Oecologia. 1983;66:443-455.
- [Google Scholar]
- Seasonal dynamics in alpine meadow seed banks along an altitudinal gradient on the Tibetan Plateau. Plant Soil. 2010;336:291-302.
- [Google Scholar]
- Higher plasticity in ecophysiological traits enhances the performance and invasion success of Taraxacum officinale (dandelion) in alpine environments. Biol. Invasions. 2012;14:21-33.
- [Google Scholar]
- Arabidopsis thaliana populations show clinal variation in a climatic gradient associated with altitude. New Phytol.. 2011;189:282-294.
- [Google Scholar]
- Demography, reproduction, and dormancy along altitudinal gradients in three intermountain Allium species with contrasting abundance and distribution. Flora. 2011;206:164-171.
- [Google Scholar]
- The Asian Umbelliferae biodiversity database (ASIUM) with particular reference to South-West Asian taxa. Turk. J. Bot.. 2004;28:139-145.
- [Google Scholar]
- Plant growth and fitness of Scabiosa columbaria under climate warming conditions. Plant Ecol. Divers.. 2011;4:379-389.
- [Google Scholar]
- Phenotypic variation and leaf fluctuating asymmetry in natural populations of Parrotia persica (Hamamelidaceae), an endemic species from the Hyrcanian forest (Iran) Acta Botanica Mexicana. 2011;97:65-81.
- [Google Scholar]
- Siddique, M.A.A., Dar, N.A., Wafai, B.A., Beigh, Y.S., 1997. Reproductive biology of Podophyllum hexandrum Royle (Podophyllaceae) an important, rare and threatened Himalayan medicinal plant. Nat. Acad. Sci. India, Bhubaneshwar. pp. 10–11.
- Natural selection favours rapid reproductive phenology in Potentilla pulcherrima (Rosaceae) at opposite ends of a subalpine snowmelt gradient. Am. J. Bot.. 2004;91:531-539.
- [Google Scholar]
- Genetic diversity, phenotypic variation and local adaptation in the alpine landscape: case studies with alpine plant species. Botanica Helvetica. 2009;119:125-133.
- [Google Scholar]
- Phenotypic plasticity for plant development, function and life history. Trends Plant Sci.. 2000;5:537-542.
- [Google Scholar]
- Reproductive allometry in soybean, maize and sunflower. Ann. Bot.. 2000;85:461-468.
- [Google Scholar]
- Allocation, plasticity and allometry in plants. Perspect. Plant Ecol. Evol. Syst.. 2004;6:207-215.
- [Google Scholar]
- Environmental severity and variation in the reproductive traits of Impatiens glandulifera. Funct. Ecol.. 2004;18:887-898.
- [Google Scholar]
- Conservation and cultivation of Ferula jaeschkeana Vatke: a species with deep complex morphophysiological dormancy. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2015
- [CrossRef] [Google Scholar]
- Phytochemical screening of the root tuber extracts of Ferula jaeschkeana Vatke. J. Essent. Oil Bear. Plants 2015
- [CrossRef] [Google Scholar]







