Translate this page into:
Iron oxide nanoparticles to an Indian major carp, Labeo rohita: Impacts on hematology, iono regulation and gill Na+/K+ ATPase activity
*Corresponding author. Tel.: +91 422 2428493; fax: +91 422 2422387 mathanramesh@yahoo.com (Mathan Ramesh)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Available online 23 November 2014
Peer review under responsibility of King Saud University.
Abstract
In this study, the chronic toxicity effects of iron oxide (Fe2O3) nanoparticles (NPs) (500 mg l−l) on certain hematological, ionoregulatory and gill Na+/K+ ATPase activity of an Indian major carp, Labeo rohita were estimated for a period of 25 days under static bioassay. A significant increase in hemoglobin (Hb) content, red blood cell (RBC) count and hematocrit (Ht) value was noticed throughout the study period when compared to control groups. In contrast, mean cellular volume (MCV), mean cellular hemoglobin (MCH) (except on 5th day) and mean cellular hemoglobin concentration (MCHC) levels and white blood cell (WBC) counts were found to be decreased during the above study period. Fe2O3 NPs also caused alterations in iono regulation resulting in hyponatremia (Na+), hypochloremia (Cl−) (except on 5th day) and hypokalemia (K+) (except up to 15th day). A biphasic trend in gill Na+/K+-ATPase activity was noticed during the above treatment period. Our results demonstrate that high Fe2O3 NP concentrations in the aquatic environment may have adverse physiological effects on fish. These data may be useful to assess the environmental risk posed by NPs. However the toxicity of various sizes of the nanoparticle could be evaluated using different aquatic organisms.
Keywords
Iron oxide
Nanoparticle toxicity
L. rohita
Hematology
Iono regulation
Gill Na+/K+-ATPase
1 Introduction
The production of nanomaterials is growing exponentially (millions of tonnes of NPs yearly) throughout the world (Simonet and Valcarcel, 2009; Klaine et al., 2012) due to their unique physicochemical properties such as high surface area and an enhanced reactivity (Farkas et al., 2011; Petersen and Henry, 2012). The increasing number and quantity of manufactured NPs in various fields like chemical industry, electronics, biomedicine, food additives, and semiconductors and others may pave a way for the discharge of these particles in the various segments of the environment (Zhang and Elliott, 2006; Blaise et al., 2008; Klaine et al., 2012). The properties of NPs differ remarkably from small molecules which may cause a lot of hazardous effects on environment and also human health (Colvin, 2003; Moore, 2006; Gaiser et al., 2012). The aquatic environment may act as a sink for the entry of these NPs (Farre et al., 2009; Scown et al., 2010; Gaiser et al., 2012) that are easily taken by aquatic organisms such as mollusks, crustaceans and fish (Ward and Kach, 2009; Johnston et al., 2010; George et al., 2014). The uptake and effects of NPs in the aquatic biota may be a major concern (Moore, 2006) which leads to extensive toxicological studies (Griffitt et al., 2008).
Metallic nanoparticles (NPs) which include particles made from Au, Ag, Pt, Fe, and Cu and metal oxides such as ZnO and TiO2 particles (Glenn et al., 2012) are widely used due to their unique properties such as diverse surface chemistries and can be prepared into a variety of shapes and sizes (Murphy et al., 2005). Among the metal oxide nanoparticles, Iron oxide NPs (particularly magnetite (Fe2O3) and hematite forms (α-Fe2O3 and ß-Fe2O3) are widely used in biomedical, bioengineering and clinical applications due to their unique magnetic properties and high catalytic abilities (Weissleder et al., 1990; Huber, 2005; Smith et al., 2007; Kadar et al., 2011; Naqvi et al., 2010; Singh et al., 2010; Chen et al., 2012). Furthermore, iron-based nanoparticles (NPs) are also used for soil and groundwater remediation and water treatment processes (Yavuz et al., 2006; Chen et al., 2012). Iron NPs may enter the surface of water via effluent discharge or waterborne transportation, underground and surface aquifers and may pose a risk to aquatic organisms and humans (Chen et al., 2012; Shen et al., 2012; Zhu et al., 2012). Hence studies on the fate and ecotoxicological risk due to iron NPs in aquatic environment are urgently needed (Chen et al., 2012).
Fish species have been extensively used for assessing the effects of NPs on aquatic ecosystem. For example, fluorescent silica nanoparticles (10 and 50 mg l−l) affect the early development of Oryzias latipes embryos (Lee et al., 2011). Rainbow trout (Oncorhynchus mykiss) exposed to dietary titanium dioxide nanoparticles (10 or 100 mg kg−1) shows biochemical disturbances in the brain (Ramsden et al., 2009). Smith et al. (2007) observed a dose dependant rise in ventilation rate, gill pathologies (edema, altered mucocytes, hyperplasia) and mucus secretion in juvenile rainbow trout exposed to single walled carbon nanotubes (SWCNT). Wise et al. (2010) reported that silver nanoparticles are cytotoxic and genotoxic to fish cells. Likewise nano zinc oxide (nZnO) was found to be toxic to crustaceans and fish (Wong et al., 2010). Previous literature indicates that nanometals can be lethal to fish in mg to μg l−1 range, depending on the type of material (Shaw and Handy, 2011).
However, the information on the harmful effects of metal oxide NPs particularly iron oxide nanoparticles on aquatic animals is very limited (Bystrzejewska-Piotrowska et al., 2009; Zhu et al., 2012; Castro-Bugallo et al., 2014). Moore (2006) suggested that uptake, bioavailability and harmful effects of nanoscale materials in the aquatic environment can be evaluated by suitable biomarkers. Biological end points or biomarkers such as hormonal, hematological, iono regulation, biochemical parameters and histopathology which are used in the field of aquatic toxicology can be also used to evaluate the impact of engineered nanoparticles on aquatic organisms (Klaine et al., 2008; Handy et al., 2012). Recently, Shaw and Handy (2011) suggested that the risk of nanomaterials on fish hematology, plasma biochemistry and biochemical alterations in internal organs needs to be investigated. This paper critically evaluated the toxic effect of iron oxide nanoparticles at higher concentration on the physiology of an Indian major carp Labeo rohita using certain hematological, ionoregulatory and gill Na+/K+-ATPase biomarkers. The alterations of these parameters may help to generate data on the possible mechanism of Fe2O3 NPs toxicity at an unrealistic dose in the aquatic environment.
2 Materials and methods
The Department of Zoology, School of Life Sciences, Bharathiar University, Coimbatore 46, Tamil Nadu, India, has been registered with the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Government of India. The experiments and the handling of the organisms were carried out as per the guidelines of CPCSEA.
2.1 Fish
Healthy specimens of L. rohita fingerlings were procured from Tamil Nadu Fisheries Development Corporation Limited, Aliyar Fish Farm, Aliyar, Tamil Nadu, India and acclimatized to laboratory conditions for about 20 days before the commencement of the experiment. During acclimatization, fish were fed with rice bran and ground nut oil cake (ad libitum) once a day. Feeding was given at least one hour prior to replacement of water. Water (one-third) was changed frequently to remove the excretory wastes. Feeding was withheld for 24 h before the commencement of the experiment to keep the experimental animals more or less in the same metabolic state. During acclimatization, the fish stock was maintained at natural photoperiod and ambient temperature. This ensures sufficient oxygen for the fish and the environment is devoid of any accumulated metabolic wastes. In the present study, tap water free from chlorine was used with the following physicochemical characteristics (APHA, 1998); temperature: 27.0 ± 2.0 °C; pH: 7.0 ± 1, dissolved oxygen: 6.8 ± 0.02 mg l−l; total alkalinity: 32.0 ± 5 mg l−l; salinity: 1.3 ± 0.1 ppt; total hardness: 18.0 ± 0.2 mg l−l; calcium: 3.4 ± 0.4 mg l−l and magnesium: 2.05 ± 0.2 mg l−l.
2.2 Preparation of Fe2O3 NPs
Iron oxide nanoparticles (Fe2O3, spindle shaped) were prepared in the Thin Films and Nanomaterials Laboratory, School of Physical Sciences, Bharathiar University. The particles were prepared by using forced hydrolysis or the reflux condensation method. The structure and morphological analysis are shown in Fig. 1. The prepared sample was characterized using X-ray diffraction (XRD), scanning electron microscope (SEM) and Fourier transforms infra red spectroscopy. The structural analysis of the prepared sample made using XRD shows the pure hematite phase of iron oxide. The average size of the spindle shaped particle is about 200 nm in length and 100 nm in diameter.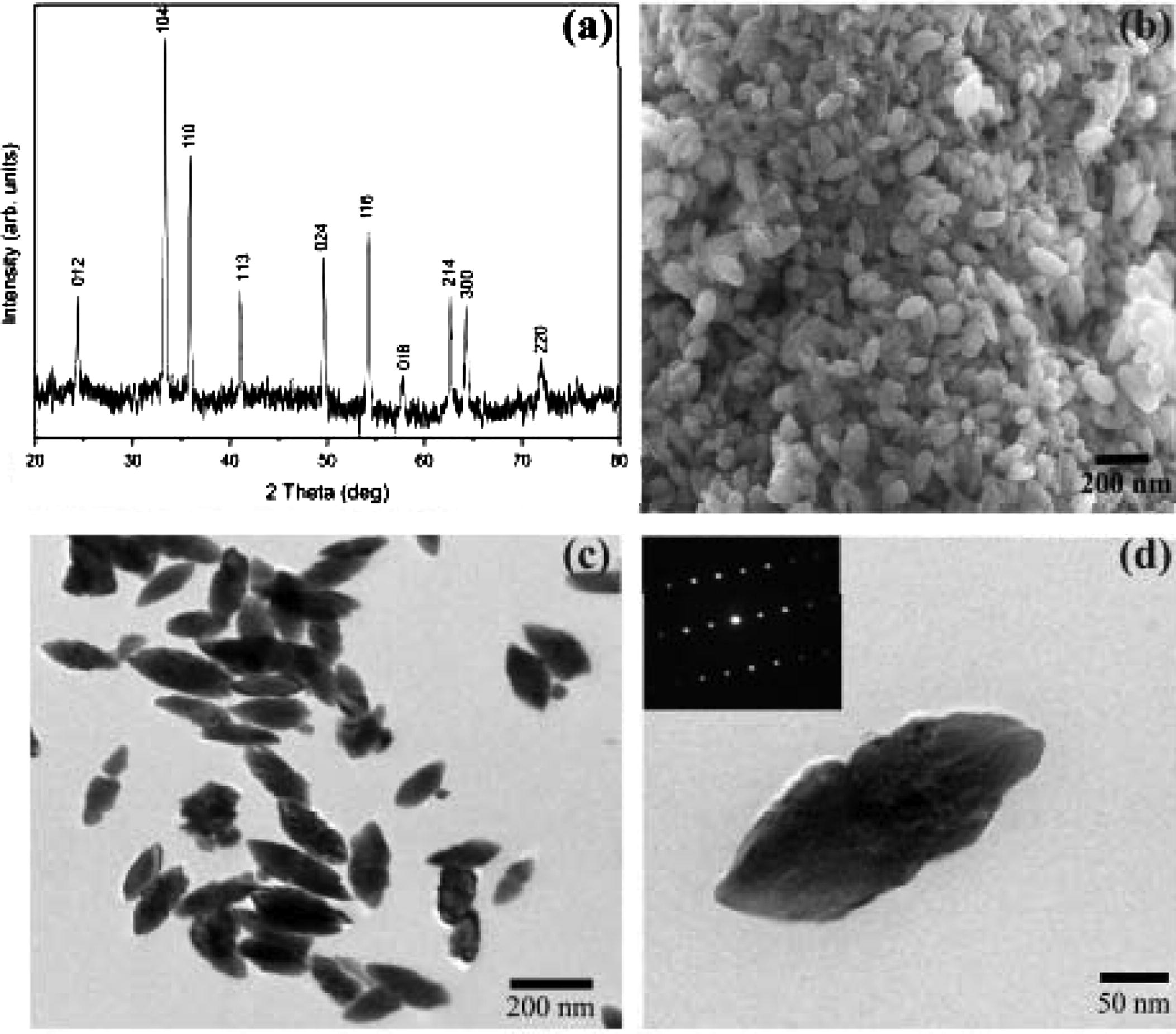
Structure and morphological analysis of Fe2O3 NPs (a) XRD pattern of spindle like hematite nanostructure (b) FESEM image of spindle-like nanostructure (c) TEM Image of spindle-like nanostructure (d) TEM Image of a single spindle-like nanostructure (inset shows the corresponding SAED pattern).
2.3 Preparation of stock solution
Powder form of ultra fine Fe2O3 NPs was disposed in distilled water. A stock solution of 15 g l−l Fe2O3 NPs was prepared by dispersing nanoparticle in distilled water with sonication 6 h bath type sonicator (40 kHz frequency Vibronics-250 wts) and subsequently for a further 30 min sonication immediately prior to dosing each day. NPs were kept in suspension in the water using aeration or a peristaltic pump for minimizing the settling of NPs. The dispersion was very good at final working concentration. In addition, and despite extensive sonication, a few aggregates of NPs were also observed in stock solution.
2.4 Iron oxide (Fe2O3) NP exposure
For the assessment of Fe2O3 NP toxicity, aquarium with 10 L of water was taken. Then to each aquarium, different concentrations of the Fe2O3 NPs (i.e. 2, 10, 100, 250, 500, 750, 1000 ppm) were added. A control aquarium with 10 L of water was also taken. Ten healthy fish, with an average length of 7.0 cm and average weight of 5.0 g were selected and introduced into each aquarium. The manifestation and survival time of fish was observed in each concentration after 24. There was no mortality of fish exposed to 2, 10, 100 and 250 ppm of Fe2O3 NPs. However, mortality was observed at 500, 750, 1000 ppm. Based on the above observation, 500 ppm was selected for the present study. For the chronic assay 300 fingerlings were selected from the stock and divided into three groups (one control and two experiments) with 100 fish in aquarium filled with water. Simultaneously, three replicates were also maintained. Then 500 ppm of Fe2O3 NPs was added into two experimental aquariums after removal of the same volume of water. Experiments were conducted for a period of 25 days with 5 day sampling frequency. Upon completion of the stipulated exposure period of 5, 10, 15, 20 and 25 days, 20 fish were taken out and sacrificed without anesthetizing for further analysis. After removal of fish at various intervals of time, the volume of the experimental and control media was adjusted to maintain a constant density of fish per unit volume of water.
2.5 Hematological studies
Fish from control and iron oxide treated aquarium were sacrificed and blood was drawn by cardiac puncture using a plastic disposable syringe. The collected blood sample was transferred into small vials, which were previously rinsed with heparin. The whole blood sample was used for the estimation of hematological parameters like Hb, RBC and WBC counts. The remainder of the blood sample was centrifuged in a cooling centrifuge at 10,000 rpm for 20 min to separate the plasma, which was used for the estimation of electrolytes (sodium, potassium and chloride). Hb was estimated by the Cyanmethemoglobin method. Hct was estimated by the microhematocrit (capillary) method (Nelson and Morris, 1989). RBC and WBC were counted by the method of Rusia and Sood (1992) using hemocytometer. Erythrocyte indices of fish viz., MCV (Mean cell volume), MCH (Mean cell hemoglobin) and MCHC (Mean cell hemoglobin concentration) were also calculated according to standard formulas.
2.6 Ionic regulation
Sodium and potassium were estimated by the method of Maruna (1958) and chloride was estimated by the modified method of Tietz (1990), and Young et al. (1975).
2.7 Gill Na+/K+-ATPase assay
After drawing the blood fish were washed with double distilled water and blotted dry with absorbent paper. Then the gills were separated from the control and iron oxide treated fish and 100 mg tissue from each was weighed and homogenized with 1.0 ml of 0.1 M Tris–HCl buffer (pH 7.5) using a Teflon homogenizer. Then the contents were centrifuged at 1000 rpm at 4 °C for 15 min and the clear supernatant was used for the sodium potassium ATPase activity. The specific activities of gill Na+/K+-ATPase were estimated following the method of Shiosaka et al. (1971).
0.3 ml of Tri-HCl buffer (pH7.5), 0.1 ml of 0.02 M ATP, 0.1 ml of 100 mM NaCl and 0.1 ml of KCl were taken in test tubes; 0.1 ml of distilled water was added to ‘Blank’ and 0.1 ml of tissue extract (gill) of control and Fe2O3 NP treated fish was added to respective tubes. The reaction mixture was mixed and incubated in water bath at 37 °C for 15 min and the reaction was terminated with 2.00 ml of 5% TCA. Tubes were kept at 4° for 30 min and centrifuged for 5 min at 500 rpm. To the supernatant, 1 ml of ammonium molybdate and 0.4 ml of ANSA reagent were added and allowed to stand for 10 min at room temperature and the intensity of the blue color developed was read at 680 nm against reagent. Suitable standards were also run through each batch of assays. The enzyme activity was expressed in terms of micrograms of inorganic phosphorous formed per gram of tissue.
2.8 Statistical analysis
The significance (p < 0.05) between control and Fe2O3 NP treated fish was analyzed by using Student’s t test.
3 Results
3.1 Hematological responses
Hematological parameters of L. rohita exposed to Fe2O3 NPs for a period of 25 days are presented in Table 1. During the above treatment period, both Hb and Hct contents were increased up to 10th day in Fe2O3 NP treated fish and then declined from that of the respective control groups (Table 1). RBC count was increased in Fe2O3 NP treated fish throughout the exposure period registering a direct relationship with the exposure period (10–237%) (Table 1). WBC count was decreased significantly (P < 0.05) in Fe2O3 NP treated fish when compared with their respective controls (Table 1). Hematological indices such as MCV and MCH were decreased (except at the end of 5th day) significantly (p < 0.05) when compared to their respective control groups (Table 1). However, a non significant decrease in MCHC value was observed in Fe2O3 NP treated fish throughout the study period (except at the end of 15 and 20th day). Values are means ± S.E. of five individual observations, (+) denotes percent increase over control, (−) denotes percent decrease over control.
Hematological parameter
Exposure time (d)
Control
Experiment
Percent change
Hb (g/dl)
5
5.550 ± 0.158
7.014 ± 0.026**
(+26.37)
10
4.362 ± 0.389
6.057 ± 0.005*
(+38.86)
15
5.710 ± 0.013
3.710 ± 0.110**
(−35.02)
20
5.900 ± 0.027
4.269 ± 0.182*
(−27.63)
25
5.360 ± 0.017
4.104 ± 0.048**
(−23.40)
Hct (%)
5
12.80 ± 1.173
17.91 ± 0.003*
(+39.92)
10
16.60 ± 0.510
20.68 ± 0.052*
(+24.58)
15
16.39 ± 0.295
11.38 ± 0.337*
(−30.57)
20
15.03 ± 0.005
12.13 ± 0.045*
(−19.32)
25
17.53 ± 0.094
12.84 ± 0.580*
(−26.76)
RBC (million/cu mm)
5
0.22 ± 0.007
0.24 ± 0.011**
(+10.00)
10
0.25 ± 0.005
0.42 ± 0.032*
(+67.46)
15
0.22 ± 0.022
0.51 ± 0.165**
(+131.91)
20
0.24 ± 0.016
0.72 ± 0.048**
(+198.33)
25
0.23 ± 0.009
0.78 ± 0.027*
(+237.39)
WBC (1000/cu mm)
5
83.51 ± 0.757
40.86 ± 0.991*
(−51.07)
10
84.22 ± 0.213
48.00 ± 1.122*
(−43.01)
15
84.15 ± 0.375
50.25 ± 1.117*
(−40.28)
20
84.33 ± 0.373
57.85 ± 0.859*
(−36.83)
25
83.95 ± 0.678
53.27 ± 0.815*
(−31.08)
MCV (fl)
5
568.00 ± 47.67
746.13 ±.32.70*
(+31.38)
10
659.72 ± 24.16
502.17 ± 40.67*
(−23.88)
15
767.01 ± 26.24
147.08 ±.17.04*
(−80.71)
20
666.35 ± 54.18
172.91 ± 13.39*
(−74.05)
25
780.91 ± 34.35
165.48 ± 4.71*
(−78.81)
MCH (picograms)
5
192.93 ± 15.96
252.31 ± 11.11*
(+30.77)
10
220.75 ± 11.56
170.35 ± 13.89*
(−22.83)
15
259.18 ± 16.58
48.04 ± 5.10*
(−81.46)
20
243.96 ± 11.37
58.44 ± 4.20*
(−76.05)
25
263.08 ± 11.10
55.05 ± 1.820*
(−79.08)
MCHC (g/dl)
5
33.92 ± 0.159
33.82 ± 0.028**
(−0.29)
10
33.46 ± 0.195
33.01 ± 0.069*
(−1.34)
15
33.59 ± 0.477
32.61 ± 0.056**
(−2.91)
20
35.64 ± 0.113
33.83 ± 0.284*
(−5.07)
25
33.69 ± 0.081
33.25 ± 0.335**
(−1.30)
3.2 Ionoregulation
Fish exposed to Fe2O3 NPs showed a significant increase (p < 0.05) in plasma sodium level (except at the end of 5th day) throughout the study period when compared with the control groups (Fig. 2). Plasma potassium level in Fe2O3 NP treated fish was found to be increased up to 15th day and underwent a significant (p < 0.05) decrease during subsequent exposure period (Fig. 3). However, the decrease in plasma chloride level was not significant on 5th day when compared to control groups (Fig. 4).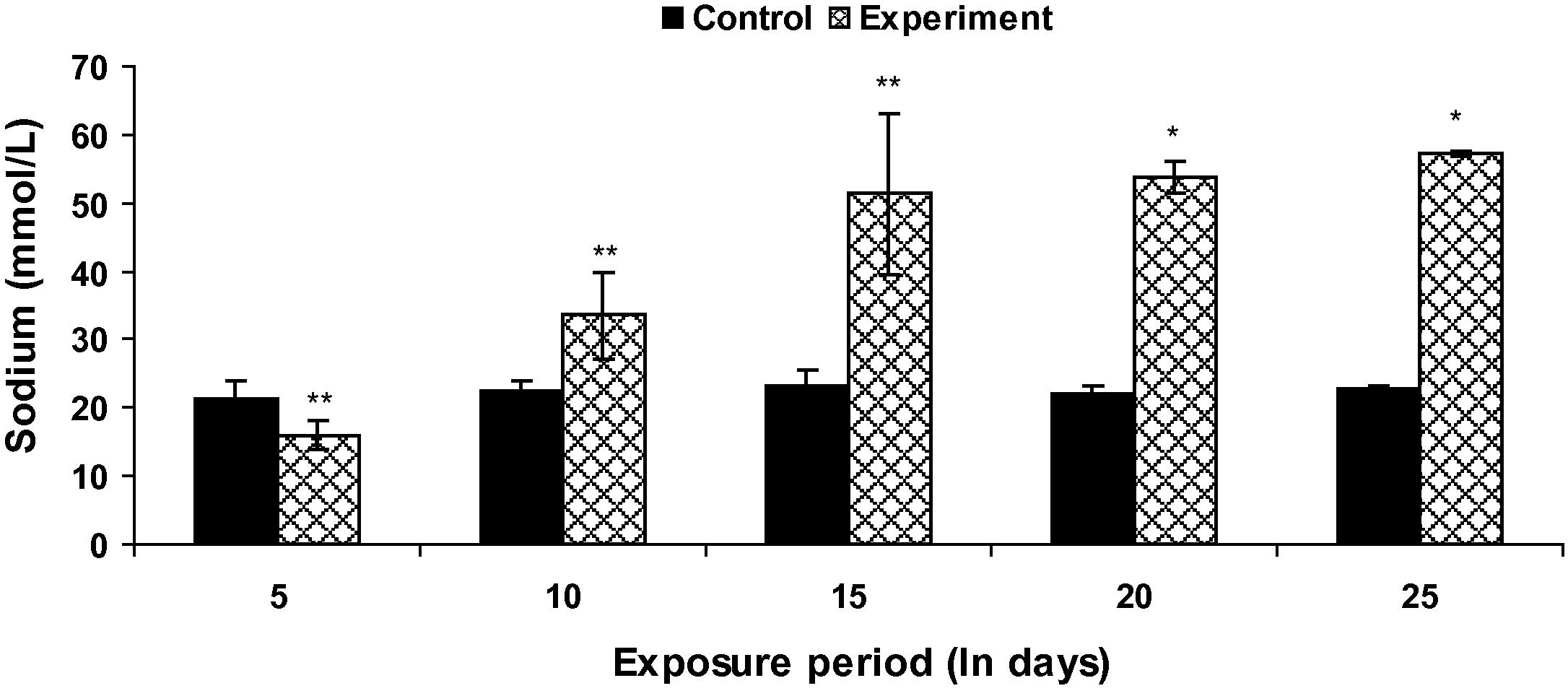
Plasma Na+ level in control and Fe2O3 NPs treated L. rohita (500 mg l−l; 25 days). Bars represent means of the SE of five individual observations with (∗) significant and ∗∗ not significant at p < 0.05 (based on t test).
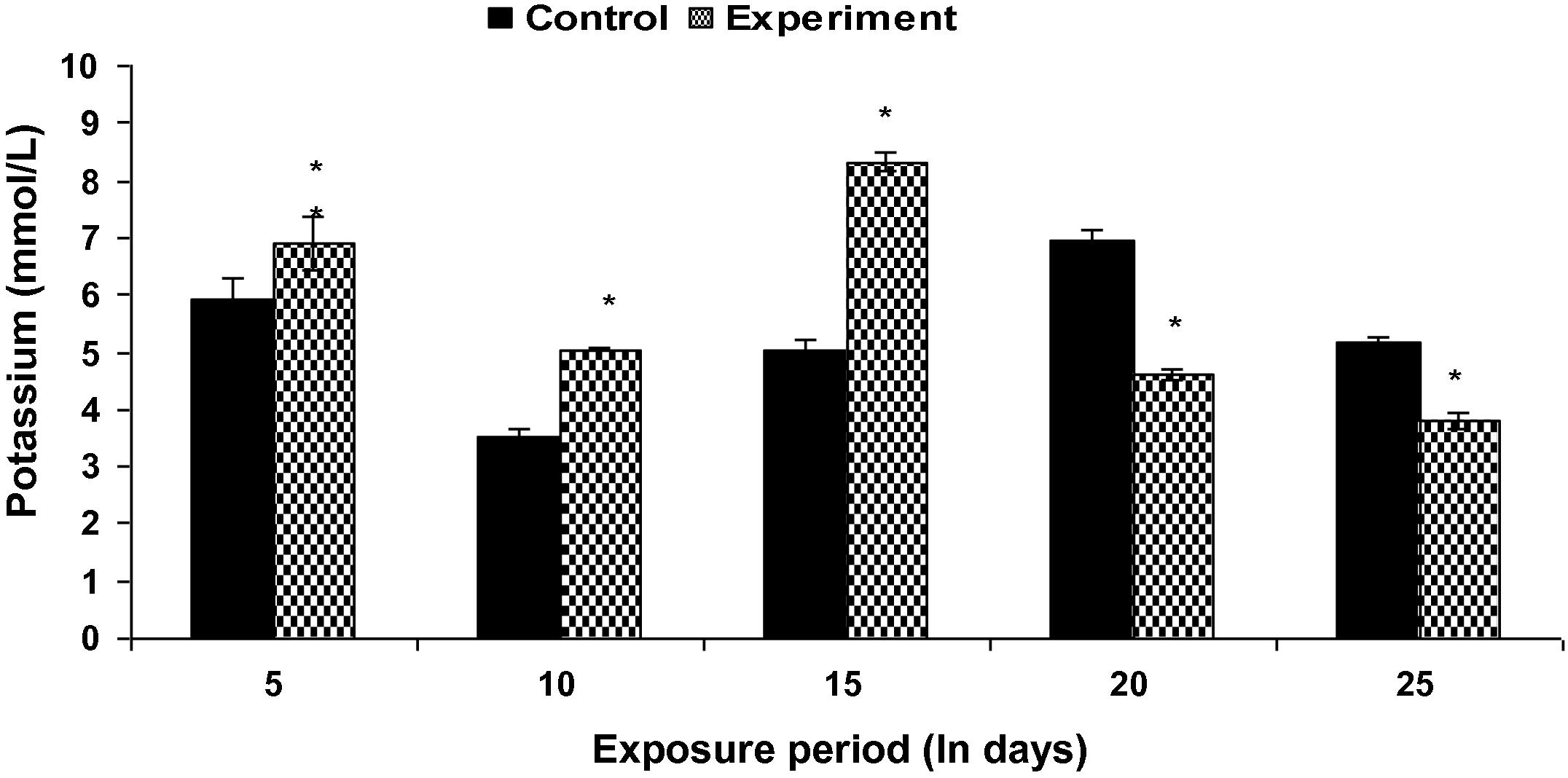
Plasma K+ level in control and Fe2O3 NP treated L. rohita (500 mg l−l; 25 days). Bars represent means of the SE of five individual observations with (∗) significant and ∗∗ not significant at p < 0.05 (based on t test).
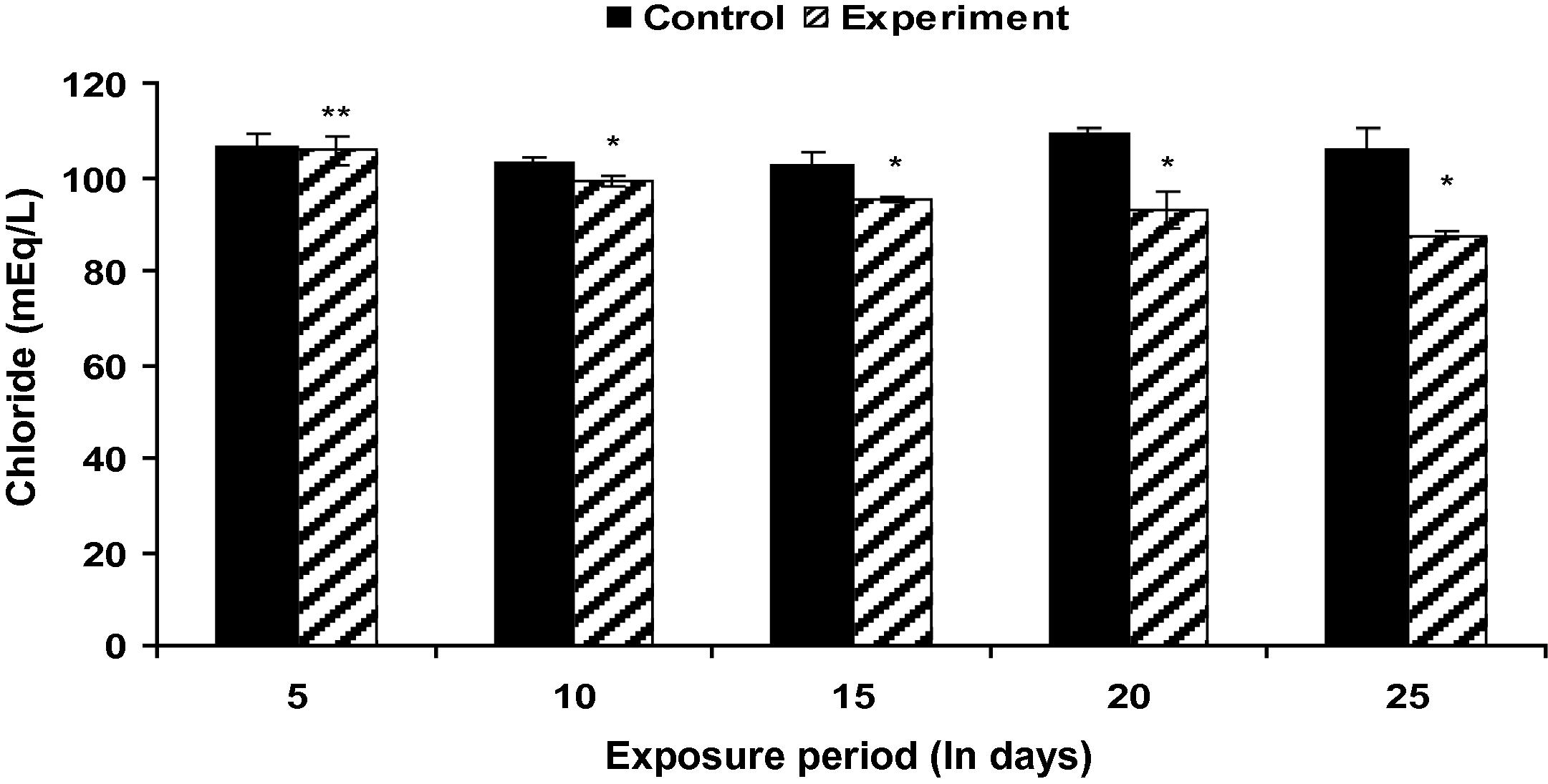
Plasma Cl− level in control and Fe2O3 NP treated L. rohita (500 mg l−l; 25 days). Bars represent means of the SE of five individual observations with (∗) significant and ∗∗ not significant at p < 0.05 (based on t test).
3.3 Gill Na+/K+-ATPase activity
Gill Na+/K+-ATPase in Fe2O3 NP treated fish was significantly (p < 0.05) increased up to 20th day and then slightly decreased at the end of the study period when compared to that in the control groups (Fig. 5).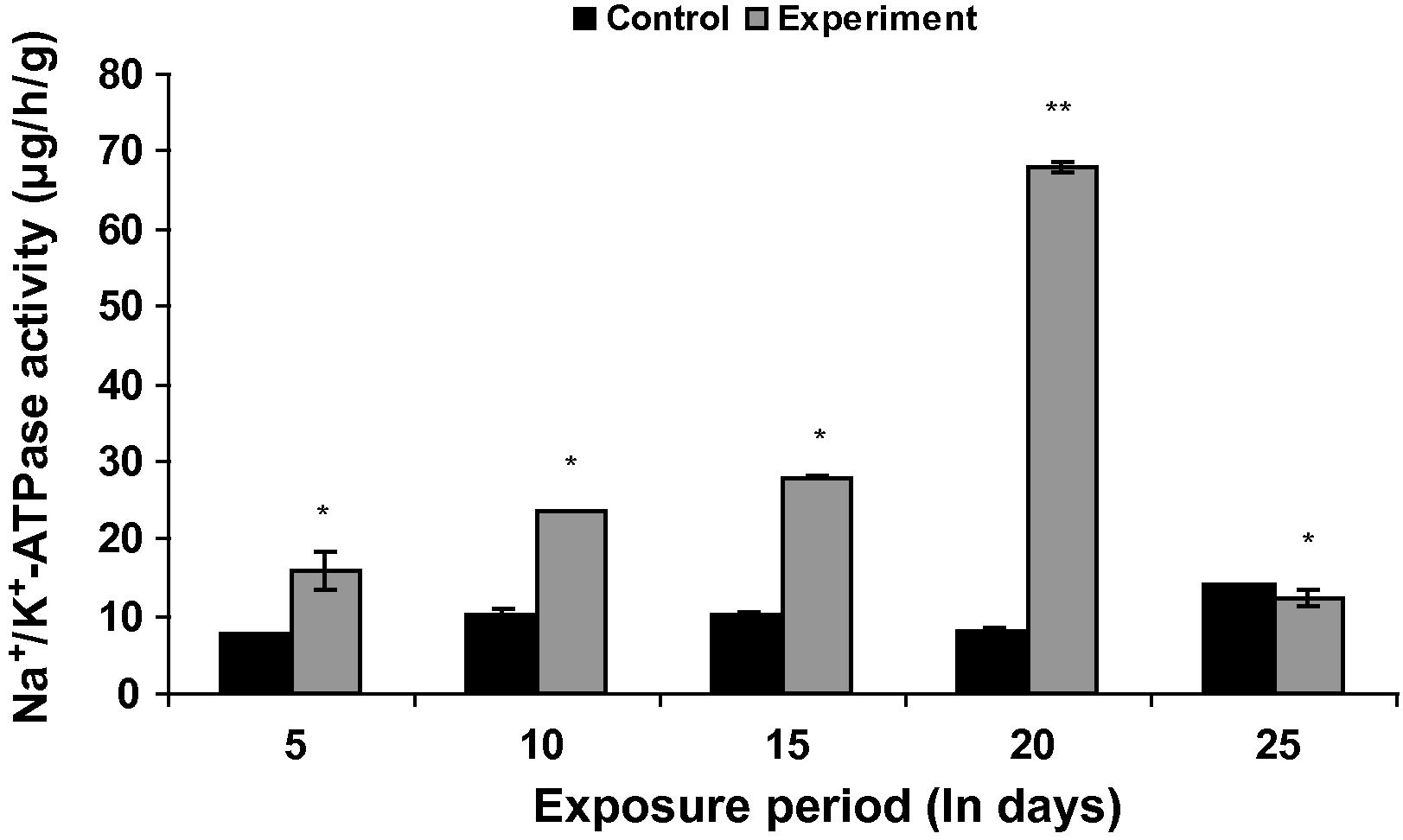
Gill Na+/K+-ATPase activities in control and Fe2O3 NP treated L. rohita (500 mg l−l; 25 days). Bars represent means of the SE of five individual observations with (∗) significant and ∗∗ not significant at p < 0.05 (based on t test).
4 Discussion
Scown et al. (2009) reported that aquatic environment is particularly vulnerable to contamination from ENMs (engineered nanomaterials) and the knowledge on the behavior; entry and toxicity of these ENMs are very limited. However, nanoparticles may produce reactive oxygen species (ROS) upon their interaction with organisms or other agents present in the environment (Castro-Bugallo et al., 2014). Manufactured NPs upon their release in the aquatic environment may conjugate with biological molecules and gain soluble properties which may affect the aquatic organisms through oxidative stress resulting damages in lipids, carbohydrates, proteins and DNA (Kohen and Nyska, 2002; Niazi and Gu, 2009). In this line, Li et al. (2009) and Chen et al. (2012) reported that attachment of nano-iron particles with the gill region may lead to damage in the epithelial cell resulting in the entry of these particles into the fish body and alter the antioxidants and antioxidant enzymatic activity. Furthermore incorporation or adsorption of dissolved cations may be the mechanism of NP toxicity in aquatic organisms (Franklin et al., 2007; Aruoja et al., 2009). Previous studies reported direct adherence/adsorption of nFe2O3 aggregates on the surface of the exposed organisms and their effects such as delay of hatching, damage in cell wall and outer membranes, depletion of oxygen exchange and hypoxia (Cheng et al., 2007; He et al., 2011).
Furthermore, direct adherence/adsorption of nFe2O3 aggregates on the surface of the exposed organisms leads to a high level of free ions resulting in the accumulation of nFe2O3 (Zhu et al., 2012). In the present study the observed mortality of fish in higher concentrations of Fe2O3 NP treated groups might have resulted from the excessive accumulation of Fe2O3 NPs in the body of fish. Imbalance in homeostasis and aberrant cellular responses, DNA damage, oxidative stress and inflammatory processes has been reported at cellular level (Singh et al., 2010). Iron nanoparticles may penetrate the body of animals, accumulate and induce toxic effects (Chen et al., 2011). Excessive accumulation of free ion can cause toxicity via generation of reactive oxygen species (ROS) (Dixon and Stockwell, 2014). A similar mechanism may be operated in the present study also. Furthermore release of free ion can potentially cross the nuclear or mitochondrial membrane and cause cytotoxic effect due to its catalytic function in the production of ROS in the Fenton reaction (H2O2 is converted into highly reactive hydroxyl or superoxide radicals catalyzed by Fe2+/Fe3+ ions) (Muller et al., 2007; Singh et al., 2010; Huang et al., 2013).
Hematological parameters such as Hb, Hct, RBCs and WBCs are frequently used as indicators of metal pollution in the aquatic environment and also to determine the sublethal toxicity of pollutants (Nussey et al., 1995). In the present study exposure of fish L. rohita to Fe2O3 NPs showed significant alterations in the hematological parameters. Similar to our findings, Smith et al. (2007) reported a significant decrease in the hematocrit and blood hemoglobin in rainbow trout exposed to SWCNT. In contrast to above findings, TiO2 NPs did not cause any major disturbances in hematology of rainbow trout (Federici et al., 2007; Handy and Shaw, 2007). The observed reduction in Hb and Hct contents might have resulted from structural changes in gill structure due to Fe2O3 NP accumulation and toxicity. The observed increase in Hb and Hct level may reflect the increased demand for oxygen under Fe2O3 NP toxicity.
It has been already reported that nanoparticles may accumulate in gills and damage the organ resulting in respiratory disturbances (Griffitt et al., 2007; Handy et al., 2008; Li et al., 2009; Ates et al., 2013). Further, excess amount of iron can result in iron flocs on the gills which may also lead to respiratory disturbances (Dalzell and MacFarlane, 1999). In the present study the observed increase in RBC count might have resulted from oxygen deficiency due to gill damage caused by Fe2O3 NP toxicity. A significant decrease in WBC count during Fe2O3 NP exposure may indicate a decrease in nonspecific immunity of the fish due to Fe2O3 NP stress. Moreover, attachment of nanoparticles to the membranes or storage of these particles inside the cells may impair cellular functions (Bystrzejewska-Piotrowska et al., 2009). In contrast to our findings no significant changes in WBC count were observed in fish exposed to SWCNT (Smith et al., 2007). The observed alterations in erythrocyte indices may be a response to the compensation for the impaired oxygen uptake caused by the toxicant (Fe2O3 NPs). A significant decrease in MCH was noted in fish exposed to SWCNT at high concentration (Smith et al., 2007). Furthermore Fe2O3 NPs may affect the immune system of fish resulting in alterations in the hematological parameters.
Plasma electrolytes such as sodium (Na+), potassium (K+), and chloride (Cl−) are highly sensitive to environmental changes and their measurement can be used as potential biomarkers of chemical exposure in aquatic organisms (Mayer et al., 1992). The gills of fish due to their large surface area and intimate contact with water are likely to be the important target organ for aquatic pollutants such as metals, pesticides and nanoparticles (Griffitt et al., 2007; Farkas et al., 2011). Similar to our study, depletion of plasma Na+ and Cl− and Na+/K+−ATPase has been reported in fish exposed to silver nanoparticles (Farmen et al., 2012). A decrease in plasma electrolytes has been reported in zebra fish (Danio rerio) exposed to silver nanoparticles indicating that AgNPs might have inhibited the Na+/K+−ATPase activity (Katuli et al., 2014) because in teleost fish gill Na+/K+−ATPase plays an important role in the maintenance of electrolytes between extra and intra cellular milieus (McCormick, 1993). Likewise significant alterations in plasma Na+ and K+ were noted in fish exposed to SWCNT (Smith et al., 2007). In the present study the decrease in plasma electrolytes during Fe2O3 NP exposure indicates that the Fe2O3 NPs may act as a stressor and inhibit the Na+/K+−ATPase activity resulting in alterations in ionoregulation of fish. Furthermore alteration of electrolytes may be due to accumulation and toxic effect of Fe2O3 NPs in the gill surface. A histological alteration in gill such as edema was noted in rainbow trout exposed to TiO2 NPs (Federici et al., 2007). Similarly, histological alterations such as cell swelling and hyperplasia were also noticed in gill of fish exposed to Fe-NPs (Li et al., 2009). Osmoregulatory failure may be another possible reason for the observed decreased levels of major plasma ions. In this line impaired osmoregulation has been reported in fish exposed to nanoparticles (Farmen et al., 2012).
In freshwater fish the enzyme Na+/K+-ATPase plays an important role in active transport mechanisms for ions. Inhibition of Na+/K+-ATPase activity in gills of Fe2O3 NPs treated fish L. rohita may be due to a change in the physical properties of the membrane or alterations in the lipid content of the membrane due to the accumulation of iron oxide nanoparticles. Furthermore release of free ions from Fe2O3 NPs might have affected the gill structure. Inhibition of Na+/K+−ATPase activity was also reported in fish exposed to various nanoparticles (Griffitt et al., 2007; Ramsden et al., 2009; Shaw and Handy, 2011; Farmen et al., 2012). Farmen et al. (2012) reported that silver nanoparticles may impair the osmoregulatory capacity by inhibiting the Na+/K+−ATPase activity. In the present study the accumulation of dissolved iron particles in the gill region may impair the osmoregulation. However at the end of the study period gill Na+/K+−ATPase activity was found to be increased. Similar to our findings a significant increase in Na+/K+−ATPase activity was noted in fish exposed to carbon nanotubes (Smith et al., 2007; Fent et al., 2010). Towle (1981) suggested that high Na+/K+-ATPase activity in gills of the teleosts appeared to provide an adaptive mechanism to support the increased Na+ uptake, required in the dilute freshwater environments.
In the present study the alterations in hematological, ionoregulatory and enzymological parameters might have resulted from the release of metals into solution (Keenan et al., 2009; Phenrat et al., 2009; Chen et al., 2012) or the specific physicochemical properties of the iron NPs (Chen et al., 2012). They also reported that nFe3O4 aggregates may be loosely formed and can easily penetrate the cell membrane. Lee et al. (2007) reported that AgNPs upon exposure cross the chorion of zebrafish embryos by Brownian diffusion indicating that early life stages of fish are sensitive to nanometals. Moreover, the toxicity of nanoparticles also depends upon their surface properties, size, ionic strength, redox state, organic matter (Illés and Tombácz, 2005; Zhang et al., 2007; Klaine et al., 2008; Farkas et al., 2011; McShane et al., 2012).
5 Conclusion
The concentration used in this study has a profound influence on the hematological, ionoregulatory and gill Na+/K+−ATPase activity of an Indian major carp, L. rohita. These parameters could be effectively used as potential biomarkers or biological end points in assessing the toxicity of engineered nanoparticles on aquatic organisms. The results of the present study highlight the need for safe disposal and protocols for these metal oxides. However, further research is needed on the direct effect of metal ion and/or release of metal ions from nanoparticles.
Acknowledgement
Authors are very thankful to Dr. Mangalraj, Professor and Head, Department of Nanoscience and Technology, Bharathiar University, Coimbatore, India for his valuable suggestions.
References
- Standard methods for the examination of water and wastewater (20th ed.). Washington DC: American Public Health Association; 1998.
- Toxicity of nanoparticles of CuO, ZnO and TiO2 to microalgae Pseudokirchneriella subcapitata. Sci. Total Environ.. 2009;407:1461-1468.
- [CrossRef] [Google Scholar]
- Bioaccumulation, subacute toxicity, and tissue distribution of engineered titanium dioxide nanoparticles in goldfish (Carassius auratus) J. Nanomater. 2013:1-6.
- [CrossRef] [Google Scholar]
- Ecotoxicity of selected nanomaterials to aquatic organisms. Environ. Toxicol.. 2008;223:591-598.
- [CrossRef] [Google Scholar]
- Nanoparticles: their potential toxicity, waste and environmental management. Waste Manage.. 2009;29(9):2587-2595.
- [CrossRef] [Google Scholar]
- Comparative responses to metal oxide nanoparticles in marine phytoplankton. Arch. Environ. Contam. Toxicol.. 2014;67:483-493.
- [CrossRef] [Google Scholar]
- Effects of titanium dioxide nano-particles on growth and some histological parameters of zebra fish (Danio rerio) after a long-term exposure. Aquat. Toxicol.. 2011;101:493-499.
- [CrossRef] [Google Scholar]
- Stabilization or oxidation of nanoscale zerovalent iron at environmentally relevant exposure changes bioavailability and toxicity in medaka fish. Environ. Sci. Technol.. 2012;46:8431-8439.
- [Google Scholar]
- Effect of carbon nanotubes on developing zebrafish (Danio rerio) embryos. Environ. Toxicol. Chem.. 2007;26:708-716.
- [CrossRef] [Google Scholar]
- The potential environment impact of engineered nanomaterials. Nat. Biotechnol.. 2003;21:1166-1170.
- [CrossRef] [Google Scholar]
- The toxicity of iron to brown trout and effects on the gills: a comparison of two grades of iron sulphate. J. Fish Biol.. 1999;55:301-315.
- [CrossRef] [Google Scholar]
- The role of iron and reactive oxygen species in cell death. Nat. Chem. Biol.. 2014;10:9-17.
- [Google Scholar]
- Characterization of the effluent from a nanosilver producing washing machine. Environ. Int.. 2011;37:1057-1062.
- [Google Scholar]
- Acute and sub-lethal effects in juvenile Atlantic salmon exposed to low μg/L concentrations of Ag nanoparticles. Aquat. Toxicol.. 2012;108:78-84.
- [CrossRef] [Google Scholar]
- Ecotoxicity and analysis of nanomaterials in the aquatic environment. Anal. Bioanal. Chem.. 2009;393:81-95.
- [CrossRef] [Google Scholar]
- Toxicity of titanium dioxide nanoparticles to rainbow trout, (Oncorhynchus mykiss): Gill injury, oxidative stress, and other physiological effects. Aquat. Toxicol.. 2007;18:175-197.
- [CrossRef] [Google Scholar]
- Assessment of uptake and toxicity of fluorescent silica nanoparticles in zebrafish (Danio rerio) early life stages. Aquat. Toxicol.. 2010;100:218-228.
- [CrossRef] [Google Scholar]
- Comparative toxicity of nanoparticulate ZnO, bulk ZnO and ZnCl2 to a freshwater algae (Pseudokirchneriella subcapitata): the importance of particle solubility. Environ. Sci. Technol.. 2007;41:8484-8490.
- [CrossRef] [Google Scholar]
- Stone, Interspecies comparisons on the uptake and toxicity of silver and cerium dioxide nanoparticles. Environ. Toxicol. Chem.. 2012;31(1):144-154.
- [CrossRef] [Google Scholar]
- Differential effect of solar light in increasing the toxicity of silver and titanium dioxide nanoparticles to a fish cell line and zebrafish embryos. Environ. Sci. Technol.. 2014;48:6374-6382.
- [Google Scholar]
- Interactions of gold nanoparticles with freshwater aquatic macrophytes are size and species dependent. Environ. Toxicol. Chem.. 2012;31(1):194-201.
- [CrossRef] [Google Scholar]
- Exposure to copper nanoparticles causes gill injury and acute lethality in zebrafish (Danio rerio) Environ. Sci. Technol.. 2007;41(23):8178-8186.
- [CrossRef] [Google Scholar]
- Effects of particle composition and species on toxicity of metallic nanomaterials in aquatic organisms. Environ. Toxicol. Chem.. 2008;27:1972-1978.
- [CrossRef] [Google Scholar]
- Toxic effects of nanoparticles and nanomaterials: implications for public health, risk assessment and the public perception of nanotechnology. Health Risk Soc.. 2007;9:125-144.
- [CrossRef] [Google Scholar]
- The ecotoxicology and chemistry of manufactured nanoparticles. Ecotoxicology. 2008;17:287-314.
- [CrossRef] [Google Scholar]
- Ecotoxicity test methods for engineered nanomaterials: practical experiences and recommendations from the bench. Environ. Toxicol. Chem.. 2012;31:15-31.
- [CrossRef] [Google Scholar]
- The effect of γ-Fe2O3 nanoparticles on Escherichia coli genome. Environ. Pollut.. 2011;159:3468-3473.
- [CrossRef] [Google Scholar]
- Superparamagnetic iron oxide nanoparticles: amplifying ROS stress to improve anticancer drug efficacy. Theranostics. 2013;3(2):116-126.
- [Google Scholar]
- Synthesis, properties, and applications of iron nanoparticles. Small. 2005;1:482-501.
- [Google Scholar]
- The effect of humic acid adsorption on pH-dependent surface charging and aggregation of magnetite nanoparticles. J. Colloid Interface Sci.. 2005;295:115-123.
- [Google Scholar]
- A critical review of the biological mechanisms underlying the in vivo and in vitro toxicity of carbon nanotubes; the contribution of physicochemical characteristics. Nanotoxicology. 2010;4(2):207-246.
- [Google Scholar]
- Stabilization of engineered zero-valent nanoiron with Na-acrylic copolymer enhances spermiotoxicity. Environ. Sci. Technol.. 2011;45(8):3245-3251.
- [Google Scholar]
- Silver nanoparticles inhibit the gill Na+/K+-ATPase and erythrocyte AChE activities and induce the stress response in adult zebrafish (Danio rerio) Ecotoxicol. Environ. Saf.. 2014;106:173-180.
- [Google Scholar]
- Oxidative stress induced by zerovalent iron nanoparticles and Fe(II) in Human Bronchial Epithelial Cells. Environ. Sci. Technol.. 2009;43:4555-4560.
- [Google Scholar]
- Nanomaterials in the environment: behavior, fate, bioavailability, and effects. Environ. Toxicol. Chem.. 2008;27:1825-1851.
- [Google Scholar]
- Paradigms to assess the environmental impact of manufactured nanomaterials. Environ. Toxicol. Chem.. 2012;31(1):3-14.
- [Google Scholar]
- Oxidation of biological systems: oxidative stress phenomena, antioxidants, redox reactions, and methods for their quantification. Toxicol. Pathol.. 2002;30:620-650.
- [Google Scholar]
- In vivo imaging of transport and biocompatibility of single silver nanoparticles in early development of zebrafish embryos. ACS Nano. 2007;1(2):133-143.
- [Google Scholar]
- Effect of fluorescent silica nanoparticles in embryo and larva of Oryzias latipes: Sonic effect in nanoparticle dispersion. Chemosphere. 2011;82(3):451-459.
- [Google Scholar]
- Effects of waterborne nano-iron on medaka (Oryzias latipes): antioxidant enzymatic activity, lipid peroxidation and histopathology. Ecotoxicol. Environ. Safe.. 2009;72:684-692.
- [Google Scholar]
- Quantitative estimation of sodium (Na+) and potassium (K+) in human serum by colorimetric method. Clin. Chim. Acta. 1958;2:581-585.
- [Google Scholar]
- Mayer, F.L., Versteeg, D.J., McKee, M.J., Folmar, L.C., Graney, R.L., McCume, D.C., Rattne, B.A., 1992. Physiological and nonspecific biomarkers. In: Huggett, R.J., Kimerle, R.A., Mehrle, Jr P.M., Bergman H.L., (eds), Biomarkers, biochemical, physiological, and histological markers of anthropogenic stress, Proceedings of the Eighth Pellston Workshop, Keystone, Colorado, July 23–28, 1989. Lewis Publishers, Boca Raton, USA, pp. 5–85.
- Methods for nonlethal gill biopsy and measurement of Na+, K+−ATPase activity. Can. J. Fish. Aquat. Sci.. 1993;50:656-658.
- [Google Scholar]
- Reproductive and behavioral responses of earthworms exposed to nano-sized titanium dioxide in soil. Environ. Toxicol. Chem.. 2012;31(1):184-193.
- [Google Scholar]
- Do nanoparticles present ecotoxicological risks for the health of the aquatic environment? Environ. Int.. 2006;32:967-976.
- [Google Scholar]
- Effect of ultra small superparamagnetic iron oxide nanoparticles (Ferumoxtran-10) on human monocyte-macrophages in vitro. Biomaterials. 2007;28:1629-1642.
- [Google Scholar]
- Anisotropic metal nanoparticles: synthesis, assembly, and optical applications. J. Phys. Chem.. 2005;109B:13857-13870.
- [Google Scholar]
- Concentration-dependent toxicity of iron oxide nanoparticles mediated by increased oxidative stress. Int. J. Nanomed.. 2010;5:983-989.
- [Google Scholar]
- Basic methodology. Hematology and coagulation, part IV. In: Nelson D.A., Henry J.B., eds. Clinical Diagnosis and Management by Laboratory Methods (17th ed.). Philadelphia, USA: Saunder Company; 1989. p. :578-724.
- [Google Scholar]
- Toxicity of metallic nanoparticles in microorganisms- a Review. In: Kim Y.J., Platt U., Gu M.B., Iwahashi H., eds. Atmospheric and biological environmental monitoring. Springer Science+Business Media B.V.; 2009.
- [CrossRef] [Google Scholar]
- Effect of copper on blood coagulation of Oreochromis mossambicus (Cichlidae) Comp. Biochem. Physiol. C: Toxicol. Pharmacol.. 1995;111:359-367.
- [Google Scholar]
- Methodological considerations for testing the ecotoxicity of carbon nanotubes and fullerenes: review. Environ. Toxicol. Chem.. 2012;31(1):60-72.
- [Google Scholar]
- Partial oxidation (“aging”) and surface modification decrease the toxicity of nanosized zerovalent iron. Environ. Sci. Technol.. 2009;43:195-200.
- [Google Scholar]
- Dietary exposure to titanium dioxide nanoparticles in rainbow trout, (Oncorhynchus mykiss): no effect on growth, but subtle biochemical disturbances in the brain. Ecotoxicology. 2009;18:939-951.
- [Google Scholar]
- Routine hematological tests. In: Mukerjee K.L., ed. Medical laboratory technology. Vol vol. I. New Delhi: Tata McGraw Hill Publishing Company Limited; 1992. p. :252-258. Fifth reprint
- [Google Scholar]
- High doses of intravenously administered titanium dioxide nanoparticles accumulate in the kidneys of rainbow trout but with no observable impairment of renal function. J. Toxicol. Sci.. 2009;109:372-380.
- [Google Scholar]
- Review: Do engineered nanoparticles pose a significant threat to the aquatic environment? Crit. Rev. Toxicol.. 2010;40(7):653-670.
- [Google Scholar]
- Physiological effects of nanoparticles on fish: A comparison of nanometals versus metal ions. Environ. Int.. 2011;37(6):1083-1097.
- [Google Scholar]
- Iron oxide nanoparticles suppressed T helper 1 cell-mediated immunity in a murine model of delayed-type hypersensitivity. Int. J. Nanomed.. 2012;7:2729-2737.
- [Google Scholar]
- Mechanisms of phosphorylation of thymidine by the culture filtrate of Clostridium perfringens and rat liver extract. Biochem. Biophys. Acta. 1971;246:171-183.
- [Google Scholar]
- Potential toxicity of superparamagnetic iron oxide nanoparticles (SPION) Nano Rev.. 2010;1:5358.
- [Google Scholar]
- Toxicity of single walled carbon nanotubes on rainbow trout, (Oncorhynchus mykiss): respiratory toxicity, organ pathologies, and other physiological effects. Aquat. Toxicol.. 2007;82:94-109.
- [Google Scholar]
- Clinical Guide to Laboratory Test (2nd ed.). Philadelphia: WB Saunders Co.; 1990. p. :118.
- Role of Na+/K+-ATPase in ionic regulation by marine and estuarine animals. Marine Biol. Lett.. 1981;2:107-122.
- [Google Scholar]
- Marine aggregates facilitate ingestion of nanoparticles by suspension feeding bivalves. Mar. Environ. Res.. 2009;68:137-142.
- [Google Scholar]
- Ultra small superparamagnetic iron oxide: characterization of a new class of contrast agents for MR imaging. Radiology. 1990;175(2):489-493.
- [Google Scholar]
- Silver nanospheres are cytotoxic and genotoxic to fish cells. Aquat. Toxicol.. 2010;97:34-41.
- [Google Scholar]
- Toxicities of nano zinc oxide to five marine organisms: influences of aggregate size and ion solubility. Anal. Bioanal. Chem.. 2010;396:609-618.
- [Google Scholar]
- Low-field magnetic separation of monodisperse Fe3O4 nanocrystals. Science. 2006;314(5801):964-967.
- [Google Scholar]
- Applications of iron nanoparticles for groundwater remediation. Remediation. 2006;16(2):7-21.
- [Google Scholar]
- Enhanced bioaccumulation of cadmium in carp in the presence of titanium dioxide nanoparticles. Chemosphere. 2007;67:160-166.
- [Google Scholar]
- Toxicity assessment of iron oxide nanoparticles in zebrafish (Danio rerio) early life stages. PLoS ONE. 2012;7(9):46286.
- [Google Scholar]







