Translate this page into:
Phenolic acids in the inflorescences of different varieties of buckwheat and their antioxidant activity
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Available online 4 August 2014
Peer review under responsibility of King Saud University.
Abstract
The comparative analysis of total phenolics and phenolic acid composition together with parameters of antioxidant activities was studied in the inflorescences of three varieties of buckwheat (F. esculentum, Fagopyrum tataricum rotundatum and Fagopyrum esculentum, forma green-flowers). Antioxidant activity of extracts of these buckwheat varieties has been found high and at the same time extracts of inflorescences of green flower buckwheat have been characterized by the highest total phenolic content. Eight phenolic acids (ferulic acid, vanillic acid, chlorogenic acid, p-coumaric acid, trans-ferulic acid, p-anisic acid, salicylic acid and methoxycinnamic acid) were found in the investigated buckwheat inflorescences with HPLC analysis. Inflorescences of F. esculentum, forma green-flowers have a high content of chlorogenic acid (16 mg 100 g−1 DW) and p-anisic acid (872 mg 100 g−1 DW). The highest content among the investigated buckwheat inflorescences of vanillic acid, trans-ferulic acid, chlorogenic acid and p-anisic acid was found in the F. tataricum, F. esculentum inflorescences have been characterized by the highest content of salicylic acid (115 mg 100 g−1 DW) and methoxycinnamic acid (74 mg 100 g−1 DW).
Keywords
Phenolic acids
Total phenolics
Buckwheat
Inflorescences
Antioxidant activity
1.Introduction
Buckwheat as a traditional pseudocereal crop (Polygonaceae) is widely used as food and as a medicinal plant. Particularly buckwheat has gained its fame due to the broad spectrum of flavonoids characterized by health benefits, i.e. cholesterol reduction (Kayashita et al., 1997), tumor inhibition (Chan, 2003), hypertension regulation (Ma et al., 2006), control of inflammation, carcinogenesis (Ishii et al., 2008), and diabetes (Kawa et al., 2003). Buckwheat-based products such as noodles, pancakes, buckwheat corn and muffins are consumed in many countries especially in India, China, Japan, Nepal, Canada, Russia, Slovenia and Ukraine. Unfortunately using of buckwheat plants is not existent any more in many EU countries. Tartary buckwheat (Fagopyrum tataricum Gaertn.) and common buckwheat (Fagopyrum esculentum Moench.) have been recognized as health foods because they contain antioxidant rutin and other secondary metabolites with antioxidative and anticancerogenic effects such as fagopyrin (Morishita et al., 2007).
Harvested buckwheat seeds go through a series of separation stages that give origin to fractions of diverse interest in the food industry. The hull (pericarp) constitutes 17–20% of total buckwheat production and is removed by impact milling. The resulting product is called groat (intact achene) and can be further separated into bran (10–24%) – usually a waste matter not commonly used in foods – and light flour (55–70%), which consists mainly of the starchy central endosperm and is commonly used in human nutrition (Bonafaccia et al., 2003).
Use of buckwheat to increase health promoting effects of different products has been the subject of intensive study in the last decades. In flour mixes, buckwheat has been shown to significantly increase AA when 15% of wheat flour in white bread preparations is replaced by buckwheat – without altering consumer perception (Lin et al., 2009). Additionally, it has been shown that consumption of boiled buckwheat groats or bread baked using 50% buckwheat flour significantly reduced post-prandial blood glucose and insulin responses compared to white wheat bread (Skrabanja et al., 2001). Positive effects of buckwheat consumption also extend to animal feeding. As an example, it has been recently shown that buckwheat bran can successfully replace 30% of corn soy based diet in laying hens, resulting in increased egg production without any changes in the egg’s quality (Benvenuti et al., 2012).
It is known that many nutraceutical compounds exist not just in buckwheat seeds but also in the vegetative mass. The vegetative mass of buckwheat plants which is usually not used in the agriculture industry can be an important source of fagopyrin, rutin, phenolic acids, vitamins and flavonoids (Ahmed et al., 2014; Sytar et al., 2013a).
Nowadays, health is considered as one of the key drivers in the food business, and both agricultural and pharmaceutical industries look for the natural flavonoid-rich material with high antioxidant capacity. Buckwheat herb as well as other plant extracts seems to be an attractive material for these requirements. Up till now, the antioxidant activity of tartary (F. tataricum (L.) Gaertn.) and common (F. esculentum Moench) buckwheat sprouts was reported (Liu et al., 2008), and only some reports are available on flavonoids in buckwheat seedlings focusing mostly on rutin (Fabjan et al., 2003; Kim et al., 2008; Bystrická et al., 2011).
The rank of antioxidant capacity provided for aerial parts of common and tartary buckwheat at early flowering stage was as follows: flowers > leaves > stems. The highest contribution of rutin to the antioxidant capacity of the aerial parts of common and tartary buckwheat was found for stems followed by leaves, flowers and unripe seeds. The results demonstrate that flowers from common and tartary buckwheat collected at early flowering as well as flowering and seed formation stages have the future potential to be a useful food ingredient. In China buckwheat tea (as organic product) originates from buckwheat grains and prevents hypertension, hyperglycemia and hyperlipidemia. To use inflorescences is a new step in developing organic tea production with buckwheat plants in European countries (Thwe et al., 2013).
Therefore the aim of this research work was to study phenolic acid composition, phenolics and antioxidant activity of inflorescences of different buckwheat varieties (F. esculentum, F. tataricum rotundatum and Fagopyrum esculentum, forma green flowers).
2 Materials and methods
The inflorescences of common buckwheat (F. esculentum Moench), tartary buckwheat (F. tataricum) and green-flower buckwheat (F. esculentum, forma green flowers) have been used for this experimental work.
A small plot field experiment was conducted in Kyiv Polissya region. The area of research plot was 2 m2. Soil of experimental areas was sod-podzol having humus content – 4.3%, nitrogen content – 0.06%, phosphorus content – 28.1 mg 100g−1, potassium content – 10.3 mg 100g−1, and general sulfur – 6.72 mg 100g−1. The agrochemical analyses of soil were conducted in accordance with standard methods of soil analysis (Zenova et al., 2001). The antioxidant activity and content of total phenols and phenolic acids have been evaluated in the inflorescences which were collected at the stage of flowering on 5 day (beginning of flowering). The experimental design was a split plot with four replications.
2.1 Determination of DPPH· Radical Scavenging Capacity
The 1,1-diphenyl-2-picrylhydrazyl (DPPH) assay (Lee et al., 2003) was utilized with some modifications. The stock reagent solution (1 * 10−3 M) was prepared by dissolving 22 mg of DPPH in 50 ml methanol and stored at 20 °C until use. The working solution (6 * 10−5 M) was obtained by mixing 6 ml of the stock solution with 100 ml methanol to obtain an absorbance value of 0.8 ± 0.02 at 515 nm, using a spectrophotometer (Jenway 6505 UV/Vis). The different extracts (0.1 ml of each) were allowed to react with 3.9 ml of the DPPH solution and vortex during 30 s and then the absorbance was measured at 515 nm, at reaction time 30 min. A control sample with no added extract was also analyzed and the scavenging percentage was calculated according to the following equation:
2.2 Determination of total phenolics
Total phenolics were determined by using Folin–Ciocalteu reagent (Singleton and Rossi, 1965). Twenty milligrams powdered samples (freeze-dried) was extracted for 10 min with 500 mL of 70% methanol at 70 °C. The mixtures were centrifuged at 3500g for 10 min and the supernatants were collected in separate tubes. The pellets were re-extracted under identical conditions. Supernatants were combined and used for total phenolics assay and for HPLC analysis. For total phenolics assay 20 mL of extract was dissolved into 2 mL of distilled water. Two hundred microliters of dissolved extract was mixed with 1 mL of Folin–Ciocalteu reagent (previously diluted tenfold with distilled water) and kept at 25 °C for 3–8 min; 0.8 mL of sodium bicarbonate (75 g L−1) solution was added to the mixture. After 60 min at 25 °C, absorbance was measured at 765 nm. The results were expressed as gallic acid equivalents.
2.3 Analysis of hydroxycinnamic acid derivatives
The leaves were harvested and frozen in liquid nitrogen for preventing phenolic compound volatilization and were lyophilized. Further, after finishing the freeze-drying process the material was ground by flint mill (20,000 g, 2 min). A total of 20 mg ground samples from leaf suspension were extracted for 15 min using 0.75 mL 70% methanol (v/v, pH 4.0, phosphoric acid) in an ultrasonic water bath on ice. Then samples were centrifuged for 5 min at 6000g. The supernatants were collected and the pellets were re-extracted twice more with 0.5 mL 70% methanol. Coumaric acid or cinnamic acid (40 mL of 3 mM solution) was added as internal standard to the first extraction. The combined supernatants from each sample were reduced to near dryness in a centrifugation evaporator (Speed Vac, SC 110) at 25 °C.
Samples were added up to 1 mL with 40% acetonitrile. The samples were filtrated using 0.22-mm filters, and then analyzed with HPLC. The chromatography was performed using a Dionex UltiMate 3000 HPLC System with a diode array detector (DAD-3000) with an WPS-3000 SL auto sampler, LPG-3400SD pump and a TCC-3000RS Column Compartment (Dionex Corp., Sunnyvale, CA, USA).
Extracts (1 mL) were analyzed at a flow rate of 0.4 mL min−1 and a column temperature of 35 °C. The column used is Narrow-Bore AcclaimPA C16-column (3 mm, 120A, 2.1 × 150 mm, Dionex). A 49-min gradient program was used with 0.1% v/v phosphoric acid in ultrapure water (eluent A) and 40% v/v acetonitrile in ultra-pure water (eluent B) as follows: 0–5 min: 0.5% B, 1–9 min: 0–40% B, 9–12 min: 40% B, 12–17 min: 40–80% B, 17–20 min: 80% B, 20–24 min: 80–99% B, 24–32 min: 99–100% B, 32–36 min: 100–40% B, 36–49 min: 40–1% B. The gradient program was followed by a 4-min period to return to 0.5% B and a 5-min equilibration period resulting in a total duration of 39 min. The eluent was monitored at 290, 330, and 254 nm. Phenolic acid quantity was calculated from HPLC peak areas at 290 nm against the internal standard.
2.4 Statistical analysis
The means and standard deviations were calculated using Microsoft Office Excel 2003. Significant differences of these data were calculated using analysis of variance (ANOVA-Duncan’s multiple test, SIGMASTAT 9.0). All results were expressed as mean ± standard deviations from replications n = 6.
3 Results
In early conducted studies, many experimental results presented antioxidant characteristics of common and tartary buckwheat but little information is available in the literature about antioxidant capacities and phenolic content of other buckwheat varieties, for example green-flower buckwheat. Therefore, efforts were made to evaluate the antioxidant activity and phenolic acid composition of inflorescence extracts from buckwheat and results have shown high antioxidant activity for all experimental extracts (F. tataricum, F. esculentum, and F. esculentum, forma green flowers) which were presented on the same level (Fig. 1).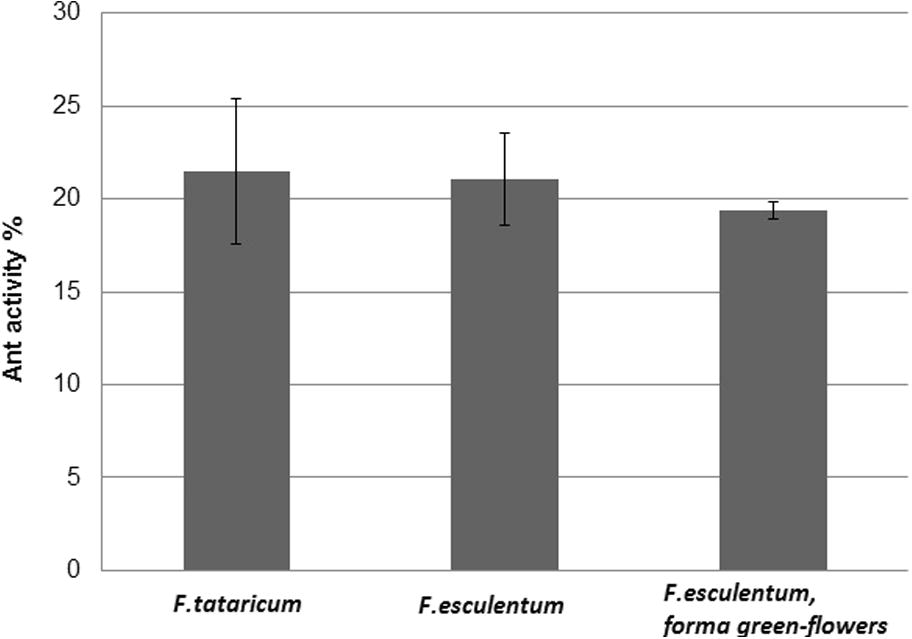
DPPH radical scavenging activity of methanolic extracts of experimental buckwheat inflorescences.
At the same time extract of F. esculentum, forma green flowers got the highest total phenolic content (73.1 mg g−1 DW) compared to the extracts of F. tataricum and F. esculentum (Fig. 2). The content of total phenolics 67.5 mg g−1 DW has been estimated in the inflorescences of common buckwheat (F. esculentum) whereas in tartary buckwheat (F. tataricum) was smallest (56.3 mg g−1 DW) (Fig. 2). The dependence between antioxidant activity and total phenolic content was not observed.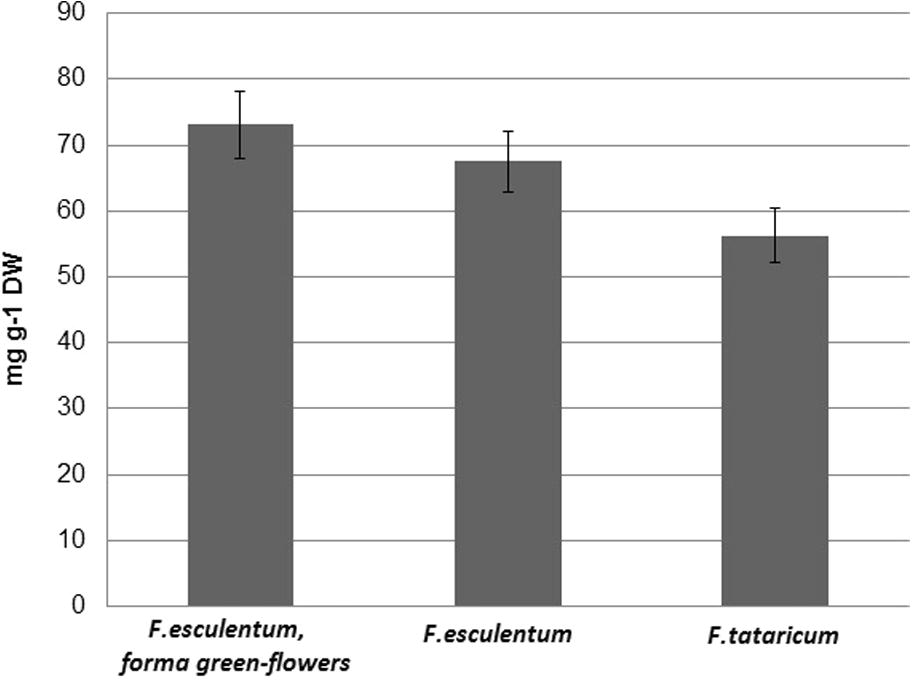
Total phenolic content of methanolic extracts of experimental buckwheat inflorescences.
We conclude that in inflorescences of different buckwheat varieties it is also important to study phenolic acid composition. In the buckwheat inflorescences of different varieties were detected phenolic acids: chlorogenic acid, vanillic acid, p-coumaric acid, ferulic acid, trans-ferulic acid, p-anisic acid, salicylic acid and methoxycinnamic acid (Table 1). In all investigated extracts of buckwheat inflorescences the highest content has been estimated for salicylic acid (72.2–114.7 mg 100 g−1 DW), vanillic acid (199.3–312.1 mg 100 g−1 DW) and p-anisic acid (745–1190 mg 100 g−1 DW) (Table 1).
Vanillic acid
Chlorogenic acid
p-Coumaric acid
Ferulic acid
Trans-ferulic acid
Salicylic acid
p-Anisic acid
Methoxy-cinnamic acid
F. esculentum, forma green-flowers
199.3 + 1.7
15.6 + 3.1
34.0 + 8.9
3.9 + 0.5
26.0 + 2.9
109.3 + 0.4
872.7 + 78.7
17.7 + 3.3
F. esculentum
221.1 + 42.0
3.1 + 1.1
28.3 + 2.6
5.6 + 1.1
31.7 + 1.4
114.7 + 0.2
744.5 + 100.1
74.7 + 24.0
F. tataricum rotundatum
311.7 + 40.4
16.6 + 4.1
28.9 + 3.7
7.3 + 1.3
65.7 + 12.6
72.2 + 0.3
1190.0 + 102.3
14.1 + 7.9
Among the investigated buckwheat inflorescence extracts the highest content of salicylic acid and also trans-ferulic acid has been found in the inflorescences of F. tataricum. Salicylic acid, an endogenous plant growth regulator has been found to generate a wide range of metabolic and physiological responses in plants thereby affecting their growth and development (Hayat et al., 2010; Kumar et al., 2013). The content of salicylic acid in the inflorescences of green-flower buckwheat and common buckwheat was on the same level but less by 20% compared to the content of salicylic acid in the inflorescences of F. tataricum. The content of trans-ferulic acid in the inflorescences of green-flower buckwheat and common buckwheat was on the same level but less twice compared to the content of trans-ferulic acid in the inflorescences of F. tataricum.
The highest vanillic acid content among experimental species of F. tataricum, F. esculentum, and F. esculentum, forma green flowers has been found in the inflorescence extract of F. tataricum where content of vanillic acid was 29% and 36% compared to the extracts of F. esculentum and F. esculentum, forma green flowers, respectively.
p-Anisic acid, also known as 4-methoxybenzoic acid or draconic acid, is one of the isomers of anisic acid. Anisic acid (p-methoxybenzoic acid) was characterized as a tyrosinase inhibitor (Kubo and Kinst-Hori, 1998). The highest content of p-anisic acid has been found in the inflorescences of F. tataricum. In the common buckwheat, forma green-flower and common buckwheat content of p-anisic acid was at the same level and lesser than in the inflorescences of F. tataricum.
The content of p-coumaric acid in all investigated inflorescences of buckwheat species was on the same level (near 30 mg 100 g−1 DW).
Ferulic acid has been identified at all experimental variants of buckwheat inflorescences. The highest content of ferulic acid was found in the inflorescences of F. tataricum. The smallest content of ferulic acid has been identified in the inflorescences of F. esculentum, forma green flowers (3.9 mg 100 g−1 DW).
The content of chlorogenic acid in the inflorescences of common buckwheat was 2 times less compared to the content of chlorogenic acid in the inflorescences of F. tataricum and F. esculentum, forma green flowers. The content of chlorogenic acid was near 0.16 mg g−1 DW in the inflorescences of F. tataricum and F. esculentum, forma green flowers. The inflorescences of F. tataricum had the highest content of vanillic acid, ferulic acids, trans-ferulic acid, salicylic acid and p-anisic acid. The content of vanillic acid in the inflorescences of F. esculentum was 28% less than that in the inflorescences of F. tataricum. The green-flower buckwheat content of vanillic acid was 36% compared to the content of vanillic acid in the extract of F. tataricum inflorescences.
The antihyperglycemic effect of p-methoxycinnamic acid (p-MCA), a cinnamic acid derivative was investigated. p-MCA exerts its antihyperglycemic effect by increasing insulin secretion and glycolysis, and by decreasing gluconeogenesis (Adisakwattana et al., 2005). The highest content of identified methoxycinnamic acid in the inflorescences of buckwheat varieties was found in the inflorescences of F. esculentum (75.0 mg 100 g−1 DW). It was higher more than 2 times compared to the content of methoxycinnamic acid in the inflorescence extracts of green-flower buckwheat and F. tataricum.
So the inflorescence extract of F. tataricum has been characterized by the highest content of phenolic acids: vanillic acid, trans-ferulic acid, chlorogenic acid and p-anisic acid. The inflorescence extracts of F. esculentum, forma green flowers have been estimated to have the highest content of chlorogenic acid and p-coumaric acid. The inflorescence extracts of F. esculentum have been characterized by the highest content of salicylic acid and methoxycinnamic acid.
4 Discussion
Results reported by Alvarez-Jubete et al. (2010) had shown that buckwheat plants have higher total phenolic content and antioxidant activity as compared to amaranth, wheat and quinoa and AOX is connected with total phenolic content. In the group of cereals and pseudocereals buckwheat is one of the best sources of polyphenols with a high antioxidant capacity (Vollmanova et al., 2013). Antioxidant activity of extracts of the investigated buckwheat inflorescences has been shown to have antioxidant activity which was at the same level in all experimental extracts. The rank of antioxidant capacity provided for aerial parts of common and tartary buckwheat at early flowering stage was as follows: flowers > leaves > stems. The results of Zielińska et al. (2012) demonstrate that flowers from common and tartary buckwheat collected at early flowering as well as flowering and seed formation stages have the future potential to be a useful food ingredient.
Kreft et al. (2006) had concluded that high antioxidant capacity of buckwheat seeds is connected with high polyphenol content, especially high content of rutin. The dependence between antioxidant activity and total phenolic content in the buckwheat inflorescences was not observed. The highest total phenolic content has been observed in the extract of F. esculentum, forma green flowers (73.1 mg g−1 DW) followed by the inflorescences of common buckwheat F. esculentum and tartary buckwheat. Vollmanova et al. (2013) had also confirmed a statistically significant influence of cultivar on total polyphenol and rutin contents as well as on total antioxidant capacity of pseudocereal seeds. At the same time Guo et al. (2011) had shown that growing conditions and the interaction between tartary buckwheat and environment may have more contribution than variety to individual phenolics and antioxidant properties. Environmental parameters such as higher altitudes may also have an increasing effect on rutin and phenolic acids. This study suggests that tartary buckwheat has potential health benefits because of its high phenolic content and antioxidant properties. These components could also be enhanced by optimizing the growing conditions of a selected variety (Guo et al., 2011). We suppose it can be same for all investigated buckwheat varieties but at this experimental work with three buckwheat varieties grown under the same growth conditions.
The tartary buckwheat samples (sprouts, microgreens and leafy greens) expressed higher total phenolic and flavonoid contents compared to the common buckwheat (Sharma et al., 2012). The high performance liquid chromatography results revealed that the tartary buckwheat leafy greens had higher rutin, sprouts higher quercetin content and in microgreens higher chlorogenic acid content was observed than those of common buckwheat. However, other phenolics like vitexin, isovitexin, orientin and isoorientin contents were more abundant in common buckwheat (Sharma et al., 2012). It is confirmed that both buckwheat species (common and tartary buckwheat) can be sources of different secondary metabolites with phenolic nature.
It is known that F. esculentum to be a rich source of bioactive natural products and a valuable potential ingredient of functional foods (Orcic et al., 2012). Dominant phenols for F. esculentum in the investigated 80% ethanolic extracts of rhizoma, stems, leaves, and flowers were quercetin and its glycosides – rutin, isoquercitrin, quercitrin and hyperoside, accounting for 16–18% of herb, flowers and leaves dry extract, thus making buckwheat a species extraordinarily rich in flavonols. Phenolic acids were less abundant, their total content not exceeding 4%, with quinic, 5-O-caffeoylquinic and (to a lesser extent) protocatechuic acid as the most significant. Both herb and rhizoma extracts exhibited high reduction capacity and radical-scavenging potential, in some cases higher than that of commercial antioxidants. In general, herb exhibited higher activity, which is in line with that found to have higher phenolic content.
Among phenolic compounds phenolic acids have a special place, which occurs in plants in free form as glycosides and can be integrated into larger molecules in an ester form. They are common as depsides — the intermolecular ester of two or more units composed of the same or different phenolic acids such as: caffeic, coumaric, ferulic, gallic and syringic. Depsides are, for example, ubiquitous chlorogenic as well as isochlorogenic, ellagic, lithospermic and rosmarinic acids. Due to the presence of a high number of hydroxyl groups and a carboxyl moiety, their antioxidant properties are more pronounced (Sroka, 2005). p-Hydroxybenzoic, ferulic and protocatechuic acids were the prominent phenolic acids in the seeds of different cultivars of tartary buckwheat and other phenolics, including p-coumaric, gallic, caffeic, vanillic and syringic acids were also detected (Guo et al., 2011). Among the eight phenolic acids, p-hydroxybenzoic, ferulic, protocatechuic, p-coumaric, gallic and vanillic acids were present in all six tartary buckwheat samples, whereas caffeic and syringic acids were detected in individual samples which differs from the phenolic acid composition of common buckwheat because protocatechuic acid was not detected in the latter (Hung and Morita, 2008; Inglett et al., 2011).
In the buckwheat inflorescences of different varieties were detected phenolic acids: chlorogenic acid, vanillic acid, p-coumaric acid, ferulic acid, trans-ferulic acid, p-anisic acid, salicylic acid and methoxycinnamic acid. In all experimental extracts of inflorescences (F. tataricum, F. esculentum, and F. esculentum, forma green flowers) same phenolic acids have been identified but their content was different. In the inflorescences of all experimental extracts p-hydroxybenzoic acid was not identified. At the same time in the leaves of buckwheat seedling p-hydroxybenzoic acid has been identified(Sytar et al., 2013b). The inflorescence extract of F. tataricum has been characterized by the highest content of phenolic acids: vanillic acid, trans-ferulic acid, chlorogenic acid and p-anisic acid. In the inflorescence extracts of F. esculentum, forma green flowers has been estimated highest content of chlorogenic acid and p-coumaric acid. The inflorescence extracts of F. esculentum have been characterized by the highest content of salicylic acid and methoxycinnamic acid.
Seeds of buckwheat (F. esculentum) contain small quantities of rutin, quercitrin, and chlorogenic acid. Chlorogenic acid and quercitrin are lesser known than rutin, but they are present in the buckwheat seeds and sprouts. In our experimental work we also identified chlorogenic acid in the inflorescences. As seeding days progressed, the contents of rutin (quercetin-3-O-rutinoside) and quercitrin (quercetin-3-O-rhamnoside) were gradually increased. The maximum contents of rutin and quercitrin were observed in 7 and 8 days after sowing of buckwheat sprouts, and their levels were 2236.7 and 2312 mg 100 g−1, respectively. Rutin (at 7 DAS) and quercitrin (at 8 DAS) contents were approximately 35 and 65 times higher than those of buckwheat seeds (rutin 63.3, quercitrin 35.4 mg 100 g−1). The interesting result is that chlorogenic acid showed a slight increment as germination days progressed (Kim et al., 2004). It was shown that content of chlorogenic acid has been increased in the leaves of buckwheat in the beginning of flowering phase more than twice compared to the content of chlorogenic acid in the leaves in the formation of buds phase (Sytar et al., 2014). So it is possible to suggest the presence of chlorogenic acid in the buckwheat inflorescences after transportation and changed plant metabolism during flowering period with an aim to concentrate on important secondary metabolites in the inflorescences. It is necessary to admit that screening of phenolic content and phenolic acid composition in the different buckwheat varieties and cultivars as potential sources of these antioxidants is better to estimate at the same period. In conclusion from the results of literature data and own experimental results it would be recommended to use the flowering period phase for such pre-screening.
5 Conclusion
The nutraceutical potential of buckwheat inflorescences has been confirmed and their further exploitation in food products will be very useful. The difference in contents of total phenol and phenolic acids among different buckwheat inflorescence varieties (F. esculentum, F. tataricum rotundatum and F. esculentum, forma green flowers) has been estimated. The highest total phenolic content among experimental buckwheat inflorescences has been observed in the inflorescences of green-flower buckwheat. Antioxidant activity of extracts of the experimental buckwheat varieties was on the same level and high in all variants. Inflorescences of green flower buckwheat got high content of chlorogenic acid and p-anisic acid. The highest content of ferulic acid, trans-ferulic acid, vanillic acid, p-anisic acid and salicylic acid among the investigated buckwheat inflorescences was found in the F. tataricum. More detailed biochemical characteristics of different vegetative organs of various buckwheat varieties and cultivars would be useful for creating a database on buckwheat sources of specific phenolic acids with high antioxidant capacity.
Acknowledgement
The authors thank the DAAD scholarship at the University of Applied Science Weihenstephan Triesdorf.
References
- Mechanisms of antihyperglycemic effect of p-methoxycinnamic acid in normal and streptozotocin-induced diabetic rats. Life Sci.. 2005;78(4):406-412.
- [Google Scholar]
- Phytochemicals and biofunctional properties of buckwheat: a review. J. Agric. Sci.. 2014;152(3):349-369.
- [Google Scholar]
- Polyphenol composition and in vitro antioxidant activity of amaranth, quinoa buckwheat and wheat as affected by sprouting and baking. Food Chem.. 2010;119:770-778.
- [Google Scholar]
- Buckwheat bran (Fagopyrum esculentum) as partial replacement of corn and soybean meal in the laying hen diet. Ital. J. Anim. Sci.. 2012;11(1):9-12.
- [Google Scholar]
- Composition and technological properties of the flour and bran from common and tartary buckwheat. Food Chem.. 2003;80:9-15.
- [Google Scholar]
- Bioactive compounds in different plant parts of various buckwheat (Fagopyrum esculentum Moench.) cultivars. Cereal Res. Commun.. 2011;39:436-444.
- [Google Scholar]
- Inhibition of tumor growth in vitro by the extract of Fagopyrum cymosum (fago-c) Life Sci.. 2003;72(16):1851-1858.
- [Google Scholar]
- Tartary buckwheat (Fagopyrum tataricum Gaertn.) as a source of dietary rutin and quercitrin. J. Agric. Food Chem.. 2003;51:6452-6455.
- [Google Scholar]
- Phenolics content and antioxidant activity of tartary buckwheat from different locations. Molecules. 2011;16(12):9850-9867.
- [Google Scholar]
- Effect of exogenous salicylic acid under changing environment: a review. Environ. Exp. Bot.. 2010;68(1):14-25.
- [Google Scholar]
- Distribution of phenolic compounds in the graded flours milled from whole buckwheat grains and their antioxidant capacities. Food Chem.. 2008;109(2):325-331.
- [Google Scholar]
- Antioxidant activity of commercial buckwheat flours and their free and bound phenolic compositions. Food Chem.. 2011;125(3):923-929.
- [Google Scholar]
- Anti-inflammatory effect of buckwheat sprouts in lipopolysaccharide-activated human colon cancer cells and mice. Biosci. Biotech. Biochem.. 2008;72:3148-3157.
- [Google Scholar]
- Buckwheat concentrate reduces serum glucose in streptozotocin diabetic rats. J. Agric. Food Chem.. 2003;51(25):7287-7291.
- [Google Scholar]
- Consumption of buckwheat protein lowers plasma cholesterol and raises fecal neutral sterols in cholesterol-fed rats because of its low digestibility. J. Nutr.. 1997;127:1395-1400.
- [Google Scholar]
- Introduction and nutritional evaluation of buckwheat sprouts as a new vegetable. Food Res. Int.. 2004;37(4):319-327.
- [Google Scholar]
- Comparison of phenolic compositions between common and tartary buckwheat (Fagopyrum) sprouts. Food Chem.. 2008;110(4):814-820.
- [Google Scholar]
- Rutin content in buckwheat (Fagopyrum esculentum Moench) food materials and products. Food Chem.. 2006;98(3):508-512.
- [Google Scholar]
- Enhancing seed yield and quality of Egyptian clover (Trifolium alexandrinum L.) with foliar application of bio-regulators. Field Crops Res.. 2013;146:25-30.
- [Google Scholar]
- Screening of medicinal plant extracts for antioxidant activity. Life Sci.. 2003;73(2):167-179.
- [Google Scholar]
- Quality and antioxidant property of buckwheat enhanced wheat bread. Food Chem.. 2009;112(4):987-991.
- [Google Scholar]
- Antioxidant activity of tartary (Fagopyrum tataricum (L.) Gaertn.) and common (Fagopyrum esculentum Moench) buckwheat sprouts. J. Agric. Food Chem.. 2008;56(1):173-178.
- [Google Scholar]
- Purification and identification of angiotensin I-converting enzyme inhibitory peptide from buckwheat (Fagopyrum esculentum Moench.) Food Chem.. 2006;96(1):36-42.
- [Google Scholar]
- The contribution of polyphenols to antioxidative activity in common buckwheat and tartary buckwheat grain. Plant Prod. Sci.. 2007;10(1):99-104.
- [Google Scholar]
- Phenolic profile and antioxidant activity of buckwheat (Fagopyrum esculentum) herb and root extracts. Planta Med. 2012 78–PL1
- [CrossRef] [Google Scholar]
- Phenolic contents, antioxidant and α-glucosidase inhibition properties of Nepalese strain buckwheat vegetables. Afr. J. Biotechnol.. 2012;11(1):184-190.
- [Google Scholar]
- Colorimetry of total phenolics with phosphomolybdic–phosphotungstic acid reagents. Am. J. Enol. Vitic.. 1965;16:144-158.
- [Google Scholar]
- Nutritional properties of starch in buckwheat products: studies in vitro and in vivo. J. Agric. Food Chem.. 2001;49(1):490-496.
- [Google Scholar]
- Antioxidative and antiradical properties of plant phenolics. Z. Naturforsch.. 2005;60C:833-843.
- [Google Scholar]
- Possible ways of fagopyrin biosynthesis and production in buckwheat plants. Fitoterapia. 2013;84:72-79.
- [Google Scholar]
- Foliar applied nickel on buckwheat (Fagopyrum esculentum) induced phenolic compounds as potential antioxidants. Clean Soil Air Water. 2013;41(11):1129-1137.
- [Google Scholar]
- Effect of chlorocholine chloride on phenolic acids accumulation and polyphenols formation of buckwheat plants. Biol. Res.. 2014;47:19.
- [CrossRef] [Google Scholar]
- Metabolomic analysis and phenylpropanoid biosynthesis in hairy root culture of tartary buckwheat cultivars. PLoS One. 2013;8(9):e65349.
- [CrossRef] [Google Scholar]
- Cultivar influence on total polyphenol and rutin contents and total antioxidant capacity in buckwheat, amaranth, and quinoa seeds. Czech J. Food Sci.. 2013;31(6):589-595.
- [Google Scholar]
- Comparative analysis of different fertilization systems and the duration of their effect on the complex of soil actinomycetes and properties of soddy-podzolic soil. Eur. Soil Sci.. 2001;34(6):639-644.
- [Google Scholar]
- Evaluation of flavonoid contents and antioxidant capacity of the aerial parts of common and tartary buckwheat plants. Molecules. 2012;17(8):9668-9682.
- [Google Scholar]







