Translate this page into:
Prenylated flavonoids from the stem wood of Commiphora opobalsamum (L.) Engl. (Burseraceae)
*Address: P. O. Box 9004, Faculty of Science, King Khalid University, Saudi Arabia. Tel.: +966 536344203 adamsuli67@hotmail.com (Mohamed Hammad Adam Suleiman)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Available online 24 April 2014
Peer review under responsibility of King Saud University.

Abstract
In this study, fractionation of acetone extract of the stem wood of Commiphora opobalsamum (L.) Engl. (Burseraceae) has been carried and two new prenylated flavonoids, 6-(3,3-Dimethylallyl)-2,3-dihydrokaempferol-3-β-O-glucoside 5 and 6-(3,3-Dimethylallyl) naringenin-7-O-β-glucoside 6, together with four known flavonoids 6-(3,3-Dimethylallyl)-2,3-diyhdrokaempferol 1, aromadendrin 3, kaempferol 2 and quercetin 4 were isolated. These compounds except quercetin are reported from this plant for the first time. Their structures were elucidated on the basis of spectroscopic analysis and comparison with published data for the known compounds.
Keywords
Burseraceae
Commiphora opobalsamum
Flavonoids
Flavanonols
Prenylated flavanone
1 Introduction
The genus Commiphora (Burseraceae), comprising more than 150 plant species, is distributed in the tropical and subtropical regions, especially occurring in north eastern Africa, southern Arabia and India (Langenheim, 2003). The plants of Commiphora species are characterized as small trees or shrubs with spinescent branches, pale-gray bark and reddish-brown resinous exudates.
Previous phytochemical investigations of this genus, afforded isolation and identification of more than 300 terpenoid molecules (Dekebo et al., 2002a,b; Meselhy, 2003; Abbas et al., 2007; Fraternale et al., 2011; Shen et al., 2007, 2012a; Xu et al., 2012) as the major constituents in this genus. Flavonoids are found in the flower, stem and bark (Fatope et al., 2003; Abbas et al., 2007), and lignans commonly occurred in the bark or stem (Francis et al., 2004). Steroids and polypodane triterpenoids, characteristically present in the resin of Commiphora mukul, might be significant chemotaxonomic markers to identify plants of genus Commiphora (Shen et al., 2012b).
Commiphora opobalsamum (L.) Engl., locally known as Gafal (Sudan), is an ancient herb used in Arabian folk medicine for the treatment of various diseases including sore throat, cough, laryngitis, chronic bronchitis and inflammations due to rheumatism and arthritis (Al-Howiriny et al., 2005). It is widely distributed in the western Sudan through Kordofan and Darfur states. The plant produces soft, lightweight and aromatic wood that is used locally to make household utensils (cups and pots), furniture (stools) and tools (hammers).
The resins but not leaves, barks and stems of C. opobalsamum are the most commonly investigated target product for potential bioactive compounds and the presence of cycloartane-type triterpenoids, an aliphatic alcohol glycoside, and eudesmane-, guaiane-, germacrane-, and cadinane-type sesquiterpenoids in the resinous exudates have been established (Yang and Shi, 2012). The present paper describes isolation and structure elucidation of two new prenylated flavonoids along with four known flavonoids from stem wood acetone extract of locally grown C. opobalsamum.
2 Materials and methods
2.1 General
Proton and Carbon-13 nuclear magnetic resonance (NMR) were recorded at 200 and 50 MHz, respectively using a Mercury-200BB apparatus with tetramethylsilane (TMS) as internal reference. A low resolution mass spectrum (LR-MS) was produced on a Finnigan SSQ 700 mass spectrometer. Infrared (IR) spectra were recorded on FTIR-8400 spectrophotometer and UV spectra were measured on a Shimadzu UV-240, following Mabry et al. (1970). Column chromatography was carried out using Sephadex LH-20 and silica gel (230–400 mesh). Analytical TLC and PTLC were performed on precoated Merck F254 silica gel plates and visualized first under vis–UV light (254 and 366 nm), then sprayed with vanillin–H2SO4 and heated at 105 °C (Harborne, 1998).
2.2 Plant material and sampling site
The North kordofan region lies between latitude 12° 43− – 13° 42− N and longitude 30° 14− – 31° 55− E. It is characterized by a dry, hot climate, typically tropical continental with a relatively short rainy season. Plant material was collected from Wad al Baga, a rainy forest about 15 km south of El-Obeid city, capital of the region (Fig. 1) between August and September 2009. Taxonomical identification was determined using the available relevant African Flora (El Amin, 1990; Maydell, 1990) and by means of a comparison with herbarium specimens conserved in the Herbaria of Soba Forests Research Centre, and voucher specimen was deposited in the Herbarium of Botany Department, University of Kordofan (voucher no. B01185).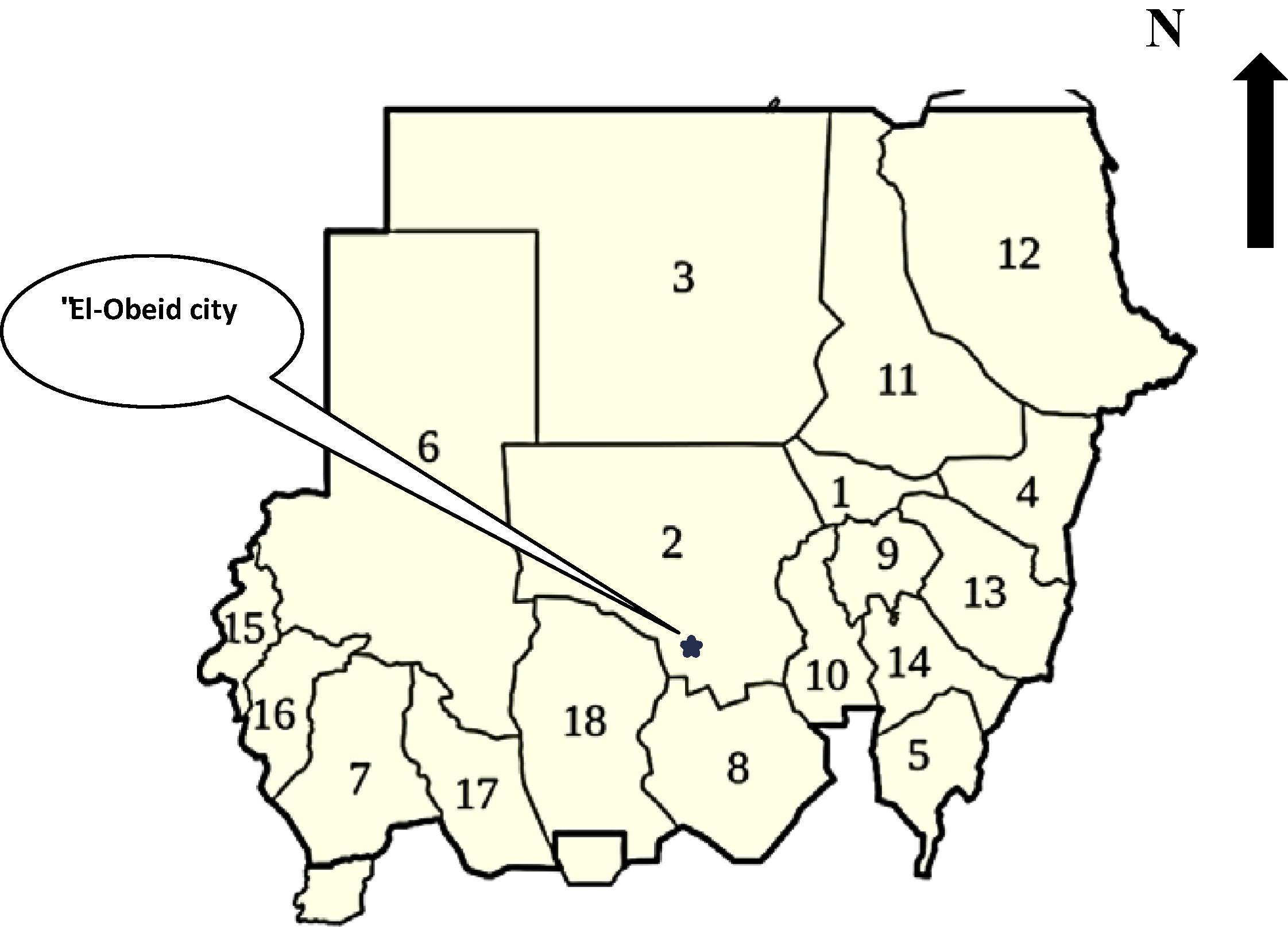
Location map of Northern kordofan, Sudan. 1: Khartoum state; 2: North Kordofan state; 3: Northern state; 6: Northern Darfur state; 8: South Kordofan state; 10: White Nile; 18: East Kordofan.
2.3 Extraction, isolation and characterization
Shade dried stem wood was milled into powder (1300 g) and successively extracted in a Soxhlet with hexane, dichloromethane (DCM), acetone, and methanol (Harborne, 1998). Acetone extract was evaporated in a rotatory evaporator, dried (14 g) and subjected to chromatography on silica gel eluted with hexane–EtOAc and EtOAc–MeOH solvent systems. The 25 column fractions obtained were combined according to their TLC profile into 14 major fractions (A1 to A14). Further fractionation led to the isolation of two new prenylated flavonoids along with four known flavonoids. Their structures were determined by analysis of their spectroscopic data (UV, IR, MS, 1H- and 13C-NMR) in comparison with those reported in the literature.
2.4 Compound 1 (6-(3,3-dimethylallyl)-2,3-diyhdrokaempferol)
Collected as colorless crystals (20 mg) by repeated crystallization of the residue obtained from fraction A3; m.p. 200–202 °C. UV/VIS data are shown in Table 1. IR (KBr) cm−1: 3552 (OH), 1616 (C⚌O), 1521. 1H-NMR (Acetone-d6), δ: 7.40 (2H, m, H-2′/6′), 6.89 (2H, m, H-3′/5′), 6.01 (1H, s, H-8), 4.64 (1H, d, J = 11.6 Hz, H-3), 5.06 (1H, d, J = 11.6 Hz, H-2), 5.23(1H, m), 3.26 (2H, d, J = 6.8 Hz), 1.75 and 1.64 (s each, all –CH3). 13C-NMR (Acetone-d6), δ: 83.7 (C-2), 72.5 (C-3), 161.3 (C-5), 108.7 (C-6), 164.6 (C-7), 94.8 (C-8), 158.2 (C-9), 130.8 (C-2′/6′), 115.2 (C-3′/5′), 158.1 (C-4′), 17.2(CH3, C-4‴), 25.2 (CH3, C-5‴), 122.7 (CH, C-2‴), 20.9 (CH2, C-1‴), 130.7 (C-3‴). LR-MS (EI, 70 eV): m/z (%) = 356.14 (68), [M]•+.
Solvent/reagent
1
2
3
4
5
6
MeOH
295, 328sh
270, 322sh, 365
290, 328sh
257, 269sh, 302sh, 370
285, 328sh
285, 320sh
NaOMe
245, 329
280, 320, 410
245, 325
247sh, 345, 410
250sh, 288, 345
Not done
NaOAc
300sh, 329
275, 305, 385
250sh, 285sh, 325
255sh, 275, 329, 385
288, 315sh, 345
NaOAc + H3BO3
295, 332sh
267, 295sh, 320sh, 375
292, 330sh
259, 303sh, 385
285, 345sh
AlCl3
317, 370
269, 305sh, 350sh, 426
315, 375
272 (IIb)
312, 405
ALCl3 + HCl
317, 370
269, 272, 305sh, 348sh, 425
312, 375
265 (IIb), 305sh, 365 (Ib), 425 (Ia)
315, 403
2.5 Compound 2 and 3
A precipitate filtered out from fractions (A4 and A5) was separated on a Sephadex LH-20 eluted with DCM/MeOH (9:1) and the obtained fractions were further purified by preparative TLC using DCM/EtOAc (7:3) as developing solvent to yield compound 2 and 3.
2.5.1 Compound 2 (kaempferol)
Obtained as yellow powder (22 mg); m.p. 271–273 °C. UV/VIS data are shown in Table 1. IR (KBr) cm−1: 3294 (OH), 1658 (C⚌O), 1616 (C⚌C), 1508. 1H-NMR (Acetone-d6), δ: 6.27 (1H, d, J = 1.8 Hz, H-6), 6.54 (1H, d, 1.8 Hz, H-8), 8.16 (2H, d, J = 8.8 Hz, H-2′/6′), 7.01 (2H, d, J = 8.8 Hz, H-3′/5′), 12.19 (s, 5-OH). 13C-NMR (Acetone-d6), δ: 176.6 (C-4), 164.9 (C-7), 162.3 (C-5), 160.1 (C-4′), 157.8 (C-9), 141.1 (C-2), 136.6 (C-3), 130.5 (C-2′/6′), 123.3 (C-1′), 116.3 (C-3′/5′), 104.2 (C-10), 99.1 (C-6), 94.5 (C-8). LR-MS (EI, 70 eV): m/z (%) = 286.11 (100), [M]•+.
2.5.2 Compound 3 (aromadendrin)
Obtained as white powder (48 mg); m.p. 218–220 °C. UV/VIS data are shown in Table 1. IR (KBr) cm−1: 3442 (OH), 1637 (C⚌O), 1521. 1H-NMR (Acetone-d6), δ: 7.42 (2H, m, H-2′/6′), 6.90 (2H, m, H-3′/5′), 5.94 (2H, d, J = 2.2 Hz, H-6), 5.99 (2H, d, J = 2.2 Hz, H-8); 5.08 (1H, d, J = 11.8 Hz, H-2), 4.66 (1H, d, J = 11.8 Hz, H-3). The 13C-NMR (Acetone-d6), δ: 84.3 (C-2), 73.1 (C-3), 198.2 (C-4), 164.9 (C-5), 97.04 (C-6), 167.8 (C-7), 95.9 (C-8), 130.3 (C-2′/6′), 158.8 (C-9), 115.9 (C-3′/5′), 101.9 (C-10), 158.8 (C-4′), 129.1 (C-1′). LR-MS (EI, 70 eV): m/z (%) = 288.12 (63), [M]•+.
2.6 Compound 4 (quercetin)
Major fractions A6, A7 and A8 were repeatedly chromatographed on Sephadex LH-20 eluted with DCM/MeOH (8:2 and 1:1) then purified by preparative TLC to give compound 4. This compound was obtained as a yellowish-green powder (15 mg); m.p. 310–312 °C. UV/VIS data are shown in Table 1. IR (KBr) cm−1: 3394 (OH), 1654 (C⚌O), 1514, 1560. 1H-NMR (Acetone-d6), δ: 7.82 (1H, d, J = 2.1 Hz, H-2′), 7.70 (1H, dd, J = 2.1 and 8.4 Hz, H-6′), 6.99 (1H, d, J = 8.4 Hz, H-5′), 6.25 (1H, d, J = 2.1 Hz, H-6), 6.52 (1H, d, J = 2.1 Hz, H-8), 12.18 (s, 5-OH). LR-MS (EI, 70 eV): m/z (%) = 302.12 (100), [M]•+.
2.7 Compounds 5 and 6
Major fractions A9, A10, A11 and A12 were re-grouped (750 mg) and subjected to a silica gel column eluted with gradient of hexane/EtOAc (7:3, 1:1, 3:7, 1:9 and 100% EtOAc) to afford sixty fractions. Fractions 33–38 and 41–55 were combined to give sub-fractions f1 (120 mg) and f2 (300 mg), respectively.
2.7.1 Compound 5 (6-(3,3-Dimethylallyl)-2,3-dihydrokaempferol-3-β-O-glycoside)
Further purification of sub-fraction f2 on Sephadex LH-20 eluted with DCM/MeOH (19:1 and 9:1) followed by preparative TLC analysis yielding 12 mg of compound 5. Its UV/VIS data are shown in Table 1. IR (KBr) cm−1: 3419 (OH), 1635 (C⚌O), 1519, 1579, 1444, 2925, 1070. 1H-NMR (Acetone-d6), δ: 5.23 (1H, m), 3.22 (2H, m), 1.75 (s, CH3), 1.62 (s, CH3), 5.07 (1H, m, H-3), 5.10 (1H, m, H-2), 6.27 (1H, s, H-8), 7.42 (2H, m, H-2′/6′), 6.90 (2H, m, H-3′/5′). 13C-NMR (Acetone-d6), δ: 84.4 (C-2), 73.3 (C-3), 160.9 (C-5), 111.3 (C-6), 161.9 (C-7), 95.1 (C-8), 158.8 (C-9), 130.3 (C-2′/6′), 115.9 (C-3′/5′), 158.8 (C-4′), 101.2 (C-1″), 74.5 (C-2″), 77.8 (C-3″), 71.1 (C-4″), 77.9 (C-5″), 62.4 (C-6″) 21.8 (C-1‴), 123.5 (C-2‴), 131.4 (C-3‴), 17.9 (C-4‴), 25.8 (C-5‴). LR-MS (EI, 70 eV): m/z (%) = 354.22 (62), [M-glc]•+.
2.7.2 Compound 6 (6-(3,3-Dimethylallyl) naringenin-7-O-β-glucoside)
Obtained as a pale pink amorphous solid (10 mg), from sub-fraction f1 while eluting the Sephadex LH-20 column with DCM/MeOH (19:1). Its UV/VIS data are shown in Table 1. IR (KBr) cm−1: 3438 (OH), 1635 (C⚌O), 1519, 1438 (2CH3). 1H-NMR (Acetone-d6), δ: 5.24 (1H, q), 3.22 (2H, m), 1.75 (s, CH3), 1.62 (s, CH3), 5.07 (1H, m, H-3), 2.75 (2H, m, H-3), 6.29 (1H, s, H-8), 7.39 (2H, m, H-2′/6′), 6.90 (2H, m, H-3′/5′). 13C-NMR (Acetone-d6), δ: 80.02 (C-2), 43.6 (C-3), 162.2 (C-5), 110.9 (C-6), 164.3 (C-7), 94.9 (C-8), 161.2 (C-9), 128.8 (C-2′/6′), 116.2 (C-3′/5′), 158.8 (C-4′), 101.3 (C-1″), 74.6 (C-2″), 77.8 (C-3″), 71.1 (C-4″), 77.9 (C-5″), 62.5 (C-6″) 21.8 (C-1‴), 123.6 (C-2‴), 131.4 (C-3‴), 17.9 (C-4‴), 25.8 (C-5‴). LR-MS (EI, 70 eV): m/z (%) = 340.27 (100), [M-glc]•+.
3 Results and discussion
The four known flavonoids (1–4), were identified as: 6-(3,3-Dimethylallyl)-2,3-diyhdrokaempferol (1) (Ingham et al., 1986), kaempferol (2) (Jiang et al., 2013; Lin et al., 2014), aromadendrin (3) (Han et al., 2007) and quercetin (4) (Zi et al., 2011; Lin et al., 2014).
3.1 Compound 5
The UV spectrum of this amorphous yellow powder demonstrated a similar profile as compound 1, and indicated the presence of a flavanonol skeleton with 5,7-dihydroxy groups. The IR spectrum exhibited absorptions at 3419 cm−1 (OH), 1635 cm−1 (C⚌O), 1519 and 1579 cm−1 for aromatic structure, 1444 cm−1 for gem-dimethyl groups and broad bands at 2925 and 1070 cm−1 demonstrated the glycosidic nature. The 1H- and 13C-NMR spectra (Tables 2 and 3) showed a typical pattern to compound 1, except the position of H-3 (5.07 ppm) which indicated the acylation of C3–OH and this was confirmed further from the absence of substantial chemical shift effects for the aromatic ring protons of compound 5 relative to 1 which according to Moco et al. (2006) suggested attachment of the glucose moiety to the OH group at C-3. The 13C-NMR signals at 101.2 (C-1″), 74.5 (C-2″), 77.8 (C-3″), 71.1 (C-4″), 77.9 (C-5″) and 62.4 (C-6″) along with resonances at 3.5–3.2 ppm and coupling constant 7.2 Hz of H-1‴ proton in 1H-NMR confirmed the presence of the O-sugar moiety in β-configuration. The molecular ion peak at m/z 354.22 corresponding to M+–C6H11O6 (glucose moiety) and the 13C-NMR, DEPT-135, HMBC and HSQC analysis confirmed the molecular formula C26H30O12. Thus, compound 5 was identified as 6-(3,3-Dimethylallyl)-2,3-dihydrokaempferol-3-β-O-glycoside (Fig. 2) and it was the first report of its isolation from natural sources.
Position
Compounds
1
5
6
2
5.06 (1H, d, J = 11.6)
5.10 (1H, m)
5.24 (1H, q)
3
4.64 (1H, d, J = 11.6)
5.07 (1H, m)
2.75 (2H, m)
6
–
–
–
8
6.01 (1H, s)
6.27 (1H, s)
6.29 (1H, s)
2′/6′
7.40 (2H, m)
7.42 (2H, m)
7.39 (2H, m)
3′/5′
6.89 (2H, m)
6.90 (2H, m)
6.90 (2H, m)
Position
Compounds
1
5
6
2
83.7
84.4
80.02
3
72.5
73.3
43.6
4
198.0
199.2
198.2
5
161.3
160.9
162.2
6
108.7
111.3
110.9
7
164.6
161.9
164.3
8
94.8
95.1
94.9
9
158.2
158.8
161.2
10
97.1
102.6
104.2
1′
129.6
129.04
129.1
2′/6′
130.8
130.3
128.8
3′/5′
115.2
115.9
116.2
4′
158.1
158.8
158.8
1″
…..
101.2
101.3
2″
…..
74.5
74.6
3″
…..
77.8
77.8
4″
…..
71.1
71.1
5″
…..
77.9
77.9
6″
…..
62.4
62.5
1‴
20.9
21.8
21.8
2‴
122.7
123.5
123.6
3‴
130.7
131.4
131.4
4‴
17.2
17.9
17.9
5‴
25.2
25.8
25.8
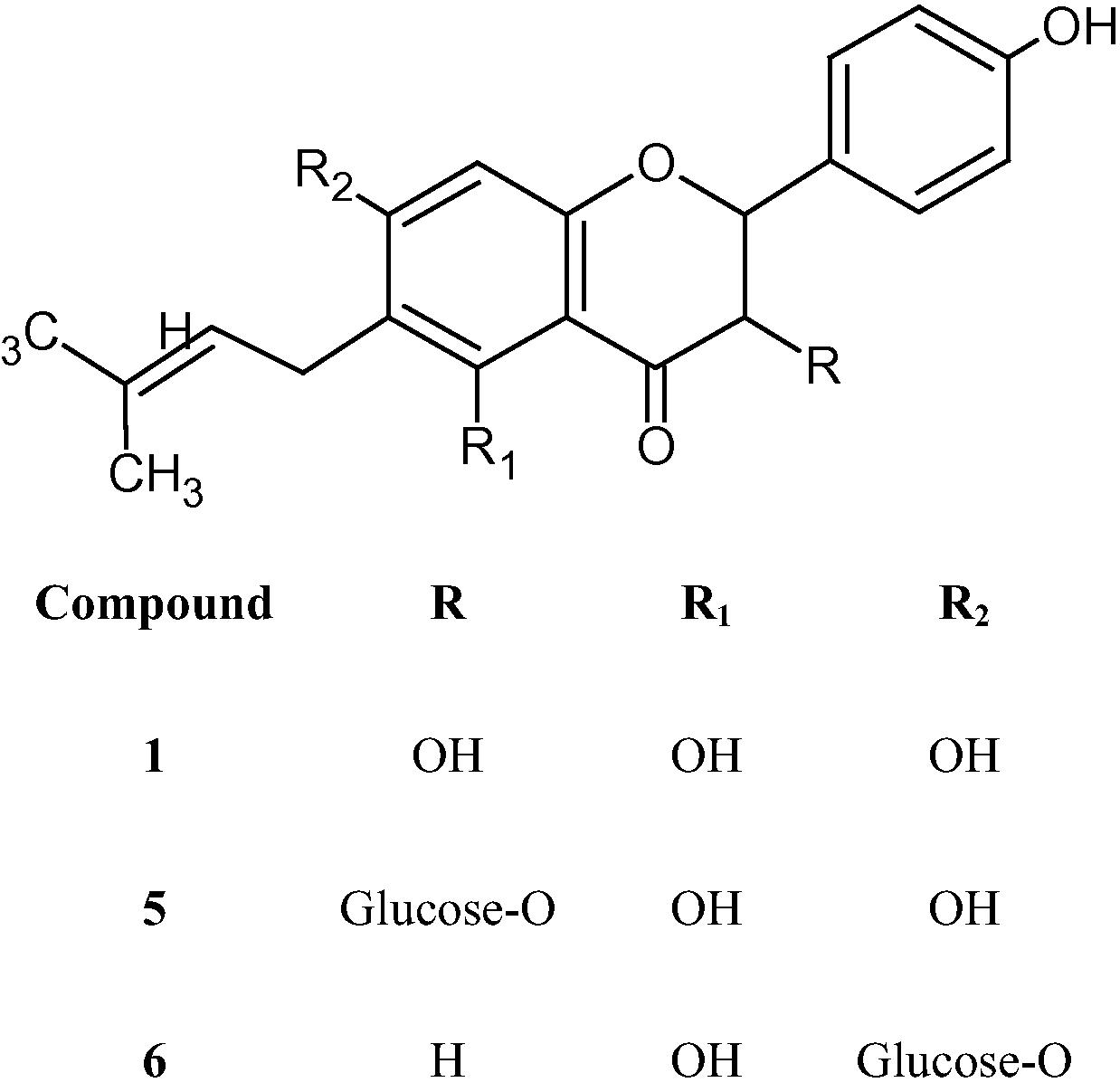
Structures of prenylated flavonoids isolated from Commiphora opobalsamum.
3.2 Compound 6
The UV spectrum (MeOH) of this new prenylated flavanone showed one major peak with a shoulder at 285 and 320 nm corresponding to flavanones (Mabry et al., 1970). The IR spectrum indicated the presence of OH (3438 cm−1), C⚌O (1635 cm−1), an aromatic structure (1519 cm−1) and dimethyl groups (1438 cm−1). The 1H- and 13C-NMR (Tables 2 and 3) showed spectral data similar to those of compounds 1 and 5, except the higher shift of C-3 to 43.6 which indicated the absence of OH at that position. This was supported by both the higher shift up field of H-3 (2.75 ppm) relative to that of compound 5 and the presence of cross peak of C-3 with the protons at 2.75 and 3.21 ppm in the HSQC spectra. Low shift of C-7 to 164.3 (∼2.4 ppm) relative to that of compound 5 indicated the acylation of the C7–OH by the glucose moiety which was further confirmed by downfield shift of H-8 to 6.29 ppm relative to 5.9–6.1 ppm, the typical position in 5,7-dihydroxyflavanons (Markham, 1982). The LR-MS analysis showed a base peak at m/z 340.27 (100%) corresponding to M+-glucose moiety and suggested the molecular formula C26H30O11 which was confirmed from 13C-, 1H-HSQC and HMBC spectra. Thus, compound 6 was identified as 6-(3,3-Dimethylallyl) naringenin-7-O-β-glucoside (Fig. 2) and to the best of our knowledge it was isolated for the first time from a natural source.
In conclusion, fractionation of the acetone extract of C. opobalsamum stem wood afforded six compounds, two new prenylated flavonoids along with known flavonols (kaempferol and quercetin) and flavanonols (6-[3,3-Dimethylallyl]-2,3-diyhdrokaempferol and aromadendrin). To the best of our knowledge all the known isolated flavonoids except quercetin (Abbas et al., 2007) are reported from this plant species for the first time.
Acknowledgements
I would like to express my thanks to Prof. Saad Mohamed Hussein Ayoub (University of Medical Sciences and Technology, Sudan) for his continuous encouragement during the work and assistance with identification of compounds, and Mr. El Taib A. Lisieg, Department of Botany, University of Kordofan, Sudan for the identification and collection of plant species.
References
- Phytochemical and biological studies on Saudi Commiphora opobalsamum L. Nat. Prod. Res.. 2007;21:383-391.
- [Google Scholar]
- Effect of Commiphora opobalsamum (L.) Engl. (Balessan) on experimental gastric ulcers and secretion in rats. J. Ethnopharmacol.. 2005;98:287-294.
- [Google Scholar]
- Two octanordammarane triterpenes from Commiphora kua. Phytochemistry. 2002;59:399-403.
- [Google Scholar]
- Furanosesquiterpenes from Commiphora sphaerocarpa and related adulterants of true myrrh. Fitoterapia. 2002;73:48-55.
- [Google Scholar]
- Trees and Shrubs of the Sudan. Exeter, UK: Ithaca Press; 1990.
- Muscanone: a 3-O-(1″, 8″, 14″-trimethyl-hexadecanyl) naringenin from Commiphora wightii. Phytochemistry. 2003;62:1251-1255.
- [Google Scholar]
- Bioactive terpenoids and gugglustreroids from Commiphora mulkul gum resin of potential anti-inflammatory interest. Chem. Biodivers.. 2004;1:1842-1853.
- [Google Scholar]
- Anti-inflammatory, antioxidant and antifungal furanosesquiterpenoids isolated from Commiphora erythraea (Ehrenb.) Engl. resin. Fitoterapia. 2011;82:654-661.
- [Google Scholar]
- Monoamine oxidase inhibitory components from Cayratia japonica. Arch. Pharm. Res.. 2007;30:13-17.
- [Google Scholar]
- Phytochemical Methods: A Guide to Modern Techniques of Plant Analysis (third ed.). UK: Springer; 1998.
- New 3-hydroxyflavanone (dihydroflavonol) phytoalexins from the paoilionate legume Shuteria vestita. J. Nat. Prod.. 1986;49:631-638.
- [Google Scholar]
- Identification of a novel phenolic compound in litchi (Litchi chinensis Sonn.) pericarp and bioactivity evaluation. Food Chem.. 2013;136:563-568.
- [Google Scholar]
- Plant Resins: Chemistry, Evolution, Ecology and Ethnobotany. Portland, Cambridge: Timber Press; 2003.
- Production of quercetin, kaempferol and their glycosidic derivatives from the aqueous-organic extracted residue of litchi pericarp with Aspergillus awamori. Food Chem.. 2014;145:220-227.
- [Google Scholar]
- The Systematic Identification of Flavonoids. New York: Springer-Verlag; 1970.
- Technique of Flavonoids Identification. London: Academic Press; 1982.
- Trees and Shrubs of the Sahel, Their Characteristics and Uses. Germany: GTZ; 1990.
- Inhibition of LPS-induced NO production by the oleogum resin of Commiphora wightii and its constituents. Phytochemistry. 2003;62:213-218.
- [Google Scholar]
- Building-up a comprehensive database of flavonoids based on nuclear magnetic resonance data. Chromatographia. 2006;64:503-508.
- [Google Scholar]
- Secondary metabolites from Commiphora opobalsamum and their antiproliferative effect on human prostate cancer cells. Phytochemistry. 2007;68:1331-1337.
- [Google Scholar]
- The genus Commiphora: a review of its traditional uses, phytochemistry and pharmacology. J. Ethnopharmacol.. 2012;142:319-330.
- [Google Scholar]
- Steroids from Commiphora mukul display antiproliferative effect against human prostate cancer PC3 cells via induction of apoptosis. Bioorg. Med. Chem. Lett.. 2012;22:4801-4806.
- [Google Scholar]
- Four new sesquiterpenes from Commiphora myrrha and their neuroprotective effects. Fitoterapia. 2012;83:801-805.
- [Google Scholar]
- Cycloartane-type triterpenoids and sesquiterpenoids from the resinous exudates of Commiphora opobalsamum. Phytochemistry. 2012;76:124-132.
- [Google Scholar]
- Metabolism of quercetin by Cunninghamella elegans ATCC 9245. J. Biosci. Bioeng.. 2011;112:360-362.
- [Google Scholar]
Appendix A
Supplementary data
LR-MS and NMR (1H, HMBC, HSQC and DEPT-135) spectra of compounds 5 and 6. Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.jksus.2014.04.005.
Appendix A
Supplementary data
Supplementary data 1
Supplementary data 1
LR-MS and NMR (1H, HMBC, HSQC and DEPT-135) spectra of compounds 5 and 6.







