Translate this page into:
Biomimetic synthesis of silver nanoparticles using the amphibious weed ipomoea and their application in pollution control
*Corresponding author abbasi.cpee@gmail.com (S.A. Abbasi)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Use of aqueous extracts of leaves, stems, and roots of the pernicious aquatic weed ipomoea (Ipomoea carnea) drawn from different locations was explored in the biomimetic extracellular synthesis of silver nanoparticles (SNPs). It was found that despite the natural variability in the chemical content of ipomoea growing in different locations, certain extract–metal stoichiometries can be identified which give strikingly reproducible results in terms of the size and the shape of the SNPs. This is one of the first reports of its type in which possible role of natural variability in the chemical composition of a given botanical species on nanoparticle synthesis involving that species has been assessed.
The use of the SNPs was explored in the degradation of typical organic pollutants—the dyes Alizarin Red S and Remazol Brilliant Blue R. The SNPs were found to speed up the dye degradation.
Keywords
Biomimetic synthesis
Silver nanoparticles
Ipomoea
Alizarin Red S
Remazol Brilliant Blue R
1 Introduction
These authors (Abbasi et al., 2012a,b) have recently established the feasibility of using aqueous extracts of the highly pernicious and dominant weed ipomoea (Ipomoea carnea) in generating silver nanoparticles (SNPs). It is a biomimetic procedure because it mimes the way in which chemicals present in the body of ipomoea reduce metal ions to nanoparticles and then stabilize those nanoparticles by forming a coating around them. Whereas in nature this happens within the plant cells, these authors achieve it extracellularly by first bringing those chemicals into an aqueous extract of the relevant plant part and then make portions of the extract react with Ag (I). Unlike several other bottom-up approaches of nanoparticle generation which are based either on chemical synthesis or the use of microorganisms, plant-based biomimetic methods consume very little energy, generate very little pollution (if any), and mostly operate under conditions of normal temperature and pressure (Anuradha et al., 2010, 2011a; Awwad et al., 2013; Bhat et al., 2013; Dubey et al., 2013; Kumar et al., 2012, 2013; Kotakadi et al., 2014; Nazeruddin et al., 2014). The state-of-the-art has been summarized from time to time (Anuradha et al., 2011, 2014). Plant-based methods also do not require sophisticated controls in maintaining the bioagent as are necessitated in the case of microorganisms. In this respect they represent an advantage similar to the other ‘green’ procedures that are emerging, such as microwave-assisted nanoparticle synthesis (Dahl et al., 2007; Raspolli Galletti et al., 2008, 2009, 2010, 2012, 2013). Moreover, by using ipomoea as the main bioagent, the authors have achieved several additional advantages:
-
Ipomoea is a freely available weed which has no beneficial uses at present. Hence this manner of utilization of ipomoea does not compete with its any other use. In contrast, most past attempts to biomimetically synthesize SNPs have relied on botanical species which have numerous other beneficial uses as a food item (Antony et al., 2013), a source of cosmetics/medicines (Anuradha et al., 2011b; Gavhane et al., 2012, or an ornamental plant (Kumar et al., 2013; Dubey et al., 2013).
-
Any process which can gainfully utilize ipomoea has the great advantage that it would contribute to the mechanical removal of ipomoea, thereby effecting some control over the weed’s spread. At present, in the absence of such use, no one is interested in harvesting ipomoea resulting into its increasing colonization of land surface and wetlands (Chari et al., 2005).
-
When it gets a chance to spread unchecked as it has in India and several other parts of the world (Meira et al., 2012; Gorniak et al., 2010), ipomoea becomes a very harmful plant in the sense that it causes toxicity to the mammals that graze upon it. It also generates toxic exudates (Ikeda et al., 2003; Hueza et al., 2005) which make the soil unfit for the growth of several other species. The weed exerts allopathic effect—it is able to discourage and prevent the growth of several species of plants in its habitat. Apart from these particularly harmful attributes, ipomoea’s infestation also generates all the other negative impacts associated with the dominance of any single species: it destroys biodiversity, and it monopolizes the use of water, soil, and associated nutrients (Chari and Abbasi, 2004, 2005). When ipomoea plants die, they degrade in the open generating global warming gases CO2 or CO2–CH4 mixtures, depending on whether the degradation occurs under aerobic or anaerobic conditions.
In this paper, a major step is reported which has been taken toward possible large-scale utilization of ipomoea in generating SNPs by (a) assessing how reproducible the process can be, given the natural variability that occurs in the chemical composition of any plant species that grows at different locations; and (b) assessing how best each plant part—leaves, stems, roots—can be used for the purpose so that the entire ipomoea biomass can be gainfully utilized in the process.
2 Materials and method
All glassware was thoroughly cleaned with liquid detergents and washed liberally with tap water followed by rinsing with deionized distilled water and drying in hot air oven before use. All chemicals used were of analytical reagent grade, or equivalent, unless otherwise specified.
Whole plants of ipomoea were collected from different locations in and around Puducherry. The plants were washed with tap water, saline (9% NaCl) water, and deionized distilled water. The adhering water was removed using blotting paper and the randomly picked plant parts were cut into 1–2 cm pieces and weighed. Their dry weight was determined by keeping them at 110 °C in an oven till they reached a constant weight. The total solid (TS) content was computed.
The extracts of leaves, stems, and roots were separately prepared. For this weighted quantities of the relevant plant parts were heated with measured volumes of water to ∼100 °C for about 5 min in sterile distilled water in a water bath. The contents were cooled to room temperature and filtered through a nylon mesh and then by a Whatman No. 42 filter paper. The extracts thus obtained were stored at 4 °C, and used within 7 days.
A 10−3 M stock solution of silver nitrate was prepared and kept in amber color bottle wrapped in black plastic sheet to keep off light. Other stock solutions were prepared as needed. All ipomoea quantities as reported are on dry weight (TS) basis.
2.1 Nanoparticle synthesis
The extra-cellular biomimetic synthesis achieved by us evidently occurs in two-steps which take place in quick succession—in the first step certain biomolecules present in the plant reduce the monovalent silver ion to uncharged atoms. Then, as these atoms aggregate to reach nano-size, other biomolecules from the plant envelope or ‘cap’ them to prevent their further aggregation. This mechanism was proposed by the early workers in this field (Shankar et al., 2003, 2004) and nothing else has been reported so far to suggest it may not be true.
Different proportions of metal ion solutions and plant extracts, formed with the extracts of 1000, 2000, 4000, 6000, and 10,000 mg/l concentrations and metal ions at 134, 134, 134, 120, and 84 mg/l levels, respectively, (to yield metal–extract ratios of 1:7.5, 1:15, 1:30, 1:50, and 1:120, respectively), were employed to determine the effect of various stoichiometric combinations on the shapes and sizes of the resulting SNPs. This is a crucial input to process development. The reactions between the metal ion solutions and the plant extracts were all carried out at room temperature (30 ± 3 °C) without any stirring. Hence the use of energy was minimal. SNP formation was signaled by the appearance of a brownish yellow color. The progress of SNP formation was monitored spectrophotometrically over the wavelength range 190–1100 nm.
The peak wavelength (λmax), the values of the absorbance (O.D.), the manner of change in the color of the reaction mixture, etc., were noted at different time intervals. These findings were then linked to HR-SEM and TEM images, FTIR spectra, EDAX, and other studies to generate information on the shapes and the sizes of the SNPs.
2.2 Catalytic reduction of typical dyes by silver nanoparticles
To study the role of SNPs in catalyzing dye-degradation, 7 × 10−4 M and 3 × 10−4 M solutions of Alizarin Red S and Remozal Brilliant Blue R, respectively, were prepared. These concentrations were employed as Beer’s law was seen to be followed in the ranges that began with these concentrations and went onto 10−5 M and lesser. Both stock solutions were stored in the dark. To a mixture containing 1 ml of either dye solution and 1 ml of sodium borohydride (0.001 M), 1 ml of SNP suspension was added in a reaction tube. The volume of the mixture was made up to 4 ml with water. The progress of the reaction was monitored spectrophotometrically by recording the optical density of the dye’s absorption maxima. For the uncatalyzed reaction 1 ml of SNPs was replaced by an equal amount of water. In another set of studies for each dye, hydrogen peroxide (0.1 M) was used instead of sodium borohydride for the dye degradation with or without SNP catalyst.
3 Results and discussion
3.1 Effect of natural variability in the chemical content of ipomoea on the SNP formation
The spectral characteristics of the SNPs formed—as influenced by the variety of ipomoea, reaction time, and the stoichiometry—for ipomoea leaves are summarized in Table 1. As may be seen, at lower extract–metal ratios, SNP formation commences in 4 h in ipomoea drawn from locations I, II, and IV while in ipomoea belonging to location III it occurs in 2 h. Hence, it can be said, that in general, and independent of the ipomoea source, SNP formation by this process would commence in about 4 h from the time of mixing the reactants.
Locations
Reaction duration (hrs)
Metal–extract proportions
1:7.5
1:15
1:30
1:50
1:120
λmax
Abs
λmax
Abs
λmax
Abs
λmax
Abs
λmax
Abs
I
0th
–
–
–
–
–
–
–
–
–
–
2nd
–
–
–
–
–
–
–
–
–
–
4th
440
0.98
440
1.38
–
–
–
–
–
–
6th
441
1.21
442
1.66
–
–
–
–
–
–
24th
449
2.26
459
2.68
–
–
–
–
–
–
II
0th
–
–
–
–
–
–
–
–
–
–
2nd
–
–
–
–
–
–
–
–
–
–
4th
439
0.90
–
–
–
–
–
–
–
–
6th
439
1.10
–
–
–
–
–
–
–
–
24th
441
1.75
450
0.92
–
–
–
–
–
–
III
0th
–
–
–
–
–
–
–
–
–
–
2nd
439
0.86
437
0.99
–
–
–
–
–
–
4th
442
1.33
442
1.61
–
–
–
–
–
–
6th
447
1.57
443
1.79
–
–
–
–
–
–
24th
447
2.52
443
2.48
450
0.77
–
–
–
–
IV
0th
–
–
–
–
–
–
–
–
–
–
2nd
–
–
–
–
–
–
–
–
–
–
4th
429
0.34
440
0.74
442
1.10
–
–
–
–
6th
432
0.47
443
0.95
445
1.38
–
–
–
–
24th
440
0.97
450
1.67
448
2.08
443
1.04
–
–
Extracts prepared from the roots of ipomoea plants derived from different locations also gave fairly reproducible results, especially in terms of positions of the absorption peaks (Table 2); similar were the findings when stem extract was used, with the exception that no SNP formation was seen at 1:30 and higher Ag(I)-extract concentrations (Table 3).
Locations
Reaction duration (hrs)
Metal–extract proportions
1:7.5
1:15
1:30
1:50
1:120
λmax
Abs
λmax
Abs
λmax
Abs
λmax
Abs
λmax
Abs
I
0th
–
–
–
–
–
–
–
–
–
–
2nd
447
0.71
450
1.28
435
0.98
–
–
–
–
4th
451
0.91
457
1.55
440
1.29
–
–
–
–
6th
451
1.00
456
1.71
443
1.44
–
–
–
–
24th
460
1.57
457
2.45
456
2.28
–
–
–
–
490
2.32
II
0th
–
–
–
–
–
–
–
–
–
–
2nd
–
–
–
–
–
–
–
–
–
–
4th
444
0.65
447
0.92
–
–
–
–
–
–
6th
445
0.84
449
1.15
–
–
–
–
–
–
24th
447
1.49
444
1.91
–
–
–
–
–
–
III
0th
–
–
–
–
–
–
–
–
–
–
2nd
–
–
444
0.64
–
–
–
–
–
–
4th
–
–
448
1.09
–
–
–
–
–
–
6th
435
0.50
456
1.34
–
–
–
–
–
–
24th
442
0.81
449
2.13
441
1.07
–
–
–
–
IV
0th
–
–
–
–
–
–
–
–
–
–
2nd
–
–
–
–
–
–
–
–
–
–
4th
429
0.34
440
0.74
442
1.10
–
–
–
–
6th
432
0.47
443
0.95
445
1.38
–
–
–
–
24th
440
0.97
450
1.67
448
2.08
443
1.04
–
–
Location
Reaction duration (in hrs)
Metal–extract proportions
1:7.5
1:15
1:30
1:50
1:120
λmax
Abs
λmax
Abs
λmax
Abs
λmax
Abs
λmax
Abs
I
0th
–
–
–
–
–
–
–
–
–
–
2nd
–
–
–
–
–
–
–
–
–
–
4th
446
0.85
–
–
–
–
–
–
–
–
6th
449
1.01
–
–
–
–
–
–
–
–
24th
461
1.63
490
1.26
–
–
–
–
–
–
II
0th
–
–
–
–
–
–
–
–
–
–
2nd
–
–
–
–
–
–
–
–
–
–
4th
443
0.18
–
–
–
–
–
–
–
–
6th
445
1.40
–
–
–
–
–
–
–
–
24th
447
1.98
470
1.41
–
–
–
–
–
–
III
0th
–
–
–
–
–
–
–
–
–
–
2nd
465
0.88
–
–
–
–
–
–
–
–
4th
461
1.17
–
–
–
–
–
–
–
–
6th
459
1.36
–
–
–
–
–
–
–
–
24th
456
1.96
465
1.39
–
–
–
–
–
–
IV
0th
–
–
–
–
–
–
–
–
–
–
2nd
–
–
–
–
–
–
–
–
–
–
4th
433
0.29
–
–
–
–
–
–
–
–
6th
435
0.42
–
–
–
–
–
–
–
–
24th
440
0.87
456
0.68
–
–
–
–
–
–
The spectra of SNPs formed in 1:7.5 and 1:15 metal–extract combinations were remarkably similar in terms of peak position and strength in all locations, even as there were differences in the rate and characteristics of SNPs formed by different plant parts (Table 4). This indicates that the shapes and sizes of SNPs can be controlled and reproduced by controlling metal–extract proportions, and choosing appropriate plant parts, irrespective of the natural variability in the chemical content of ipomoea.
Aspect
SNPs synthesized using leaf extract
SNPs synthesized using stem extract
SNPs synthesized using root extract
Reproducibility
The metal–extract combinations of 1:7.5 and 1:15 gave reproducible results in terms of peak position and strength for all samples
The metal–extract combinations of 1:7.5 and 1:15 gave reproducible results in terms of peak position and strength for all the samples
The metal–extract combinations of 1:7.5 and 1:15 gave reproducible results in terms of peak position and strength for all samples
Peak position (λmax) and intensity (absorbance)
The λmax was in the range 432–459 nm and the absorbance was in the range 0.617–2.678
The λmax was in the range 446–465 nm and the absorbance was in the range 0.849–1.968
The λmax was in the range 429–452 nm and the absorbance was in the range 0.339–2.125
Bioreduction of Ag+ to Ago
The formation of SNPs started at the 4th hr
In ipomoea of two locations, SNP formation started earlier than the ipomoea of the other two locations
The formation of SNPs, started at the 4th hr
Nature of SNPs
Narrow SPR band occurred in leaf extract indicating that the SNPs were monodispersed and spherical
Broad SPR band occurred in stem extract indicating that the SNPs were polydispersed
Initially a broad SPR band occurred indicating that nanoparticles were polydispersed. With time the SNPs the SPR band become narrow indicating that the SNPs gradually became monodispersed
The rate of nanoparticle formation was greater when compared to SNPs formed by extracts of stem and root
The concentration and the rate of nanoparticle formation were lesser than the values for leaf and root
The concentration and the rate of nanoparticle formation were more when compared with stem but lesser when compared to leaves
Moreover, the shape of the spectra remained more or less unchanged with time (Fig. 1). This reveals that the patterns of polydispersion of nanoparticles and their shape isotropy did not change with time or with the source of ipomoea. Once the optical density at the λmax had peaked, it remained constant for several days indicating that nanoparticles responsible for the peak had remained stable.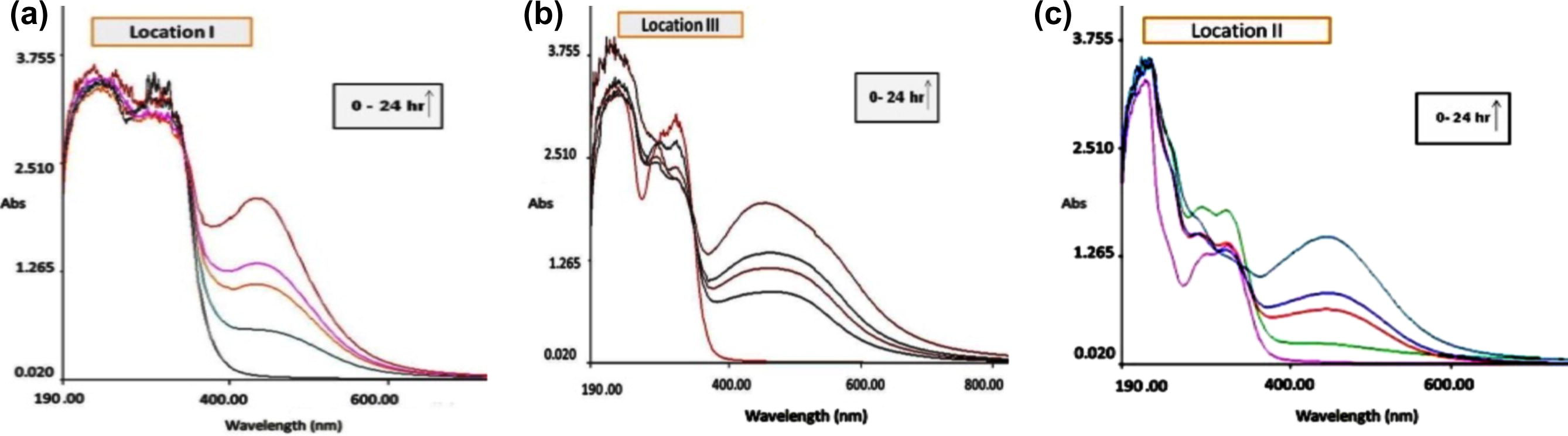
Typical UV–visible spectra of SNPs derived from the extracts of ipomoea (a) leaves, (b) stem and (c) roots, as a function of time.
3.2 Characteristics of the SNPs
The synthesized SNPs were subjected to high-resolution scanning electron microscopy (HR-SEM) and transmission electron microscopy (TEM). Typical TEM and HR-SEM images are reproduced in Figs. 2 and 3, respectively.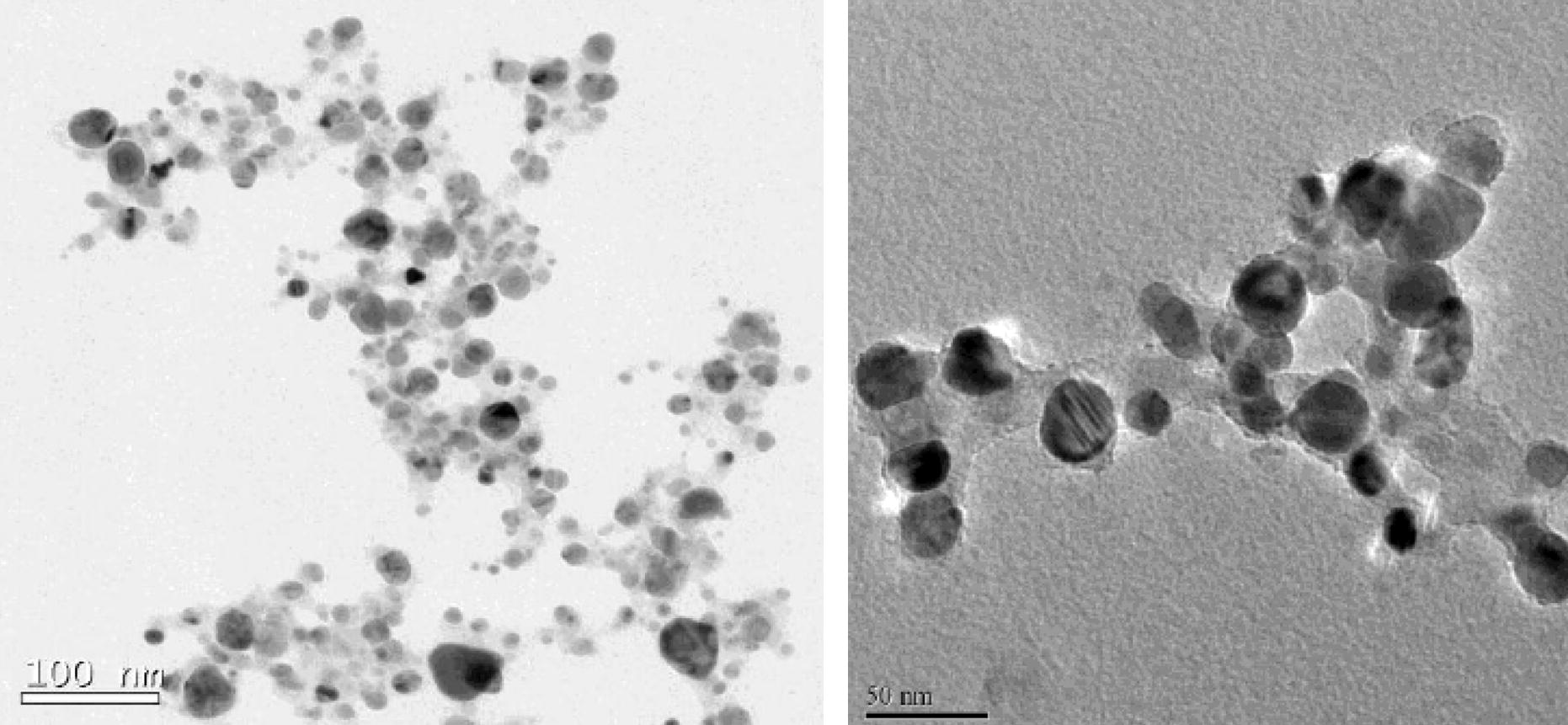
Spherical nanoparticles of fairly uniform (spherical) shape, but different, sizes formed by the extracts of ipomoea leaves (left) and of stem (right).
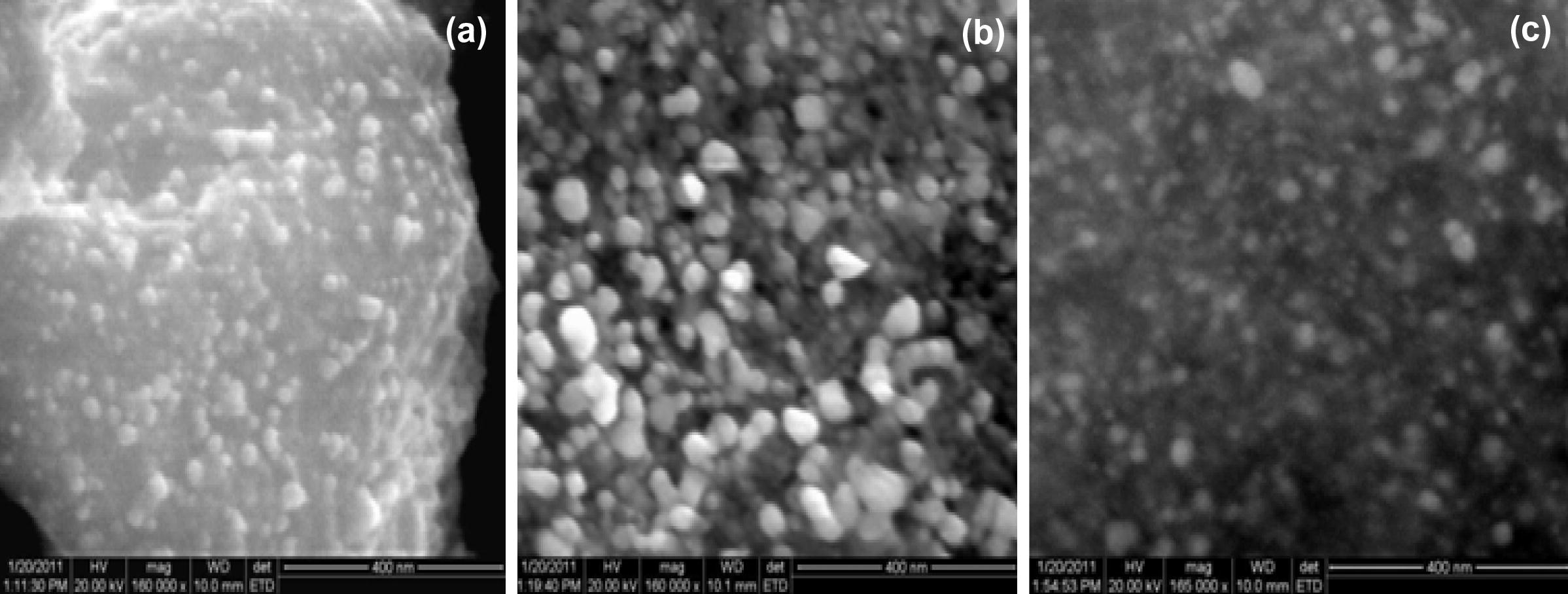
HR-SEM micrographs of SNPs generated using extracts of ipomoea (a) leaves, (b) stem and (c) root.
X-ray diffractometry revealed the crystalline nature of the nanoparticles. Intense peaks were seen corresponding to (1 1 1), (2 0 0) and (2 2 0) Bragg’s reflection based on the fcc crystal structure (Fig. 4).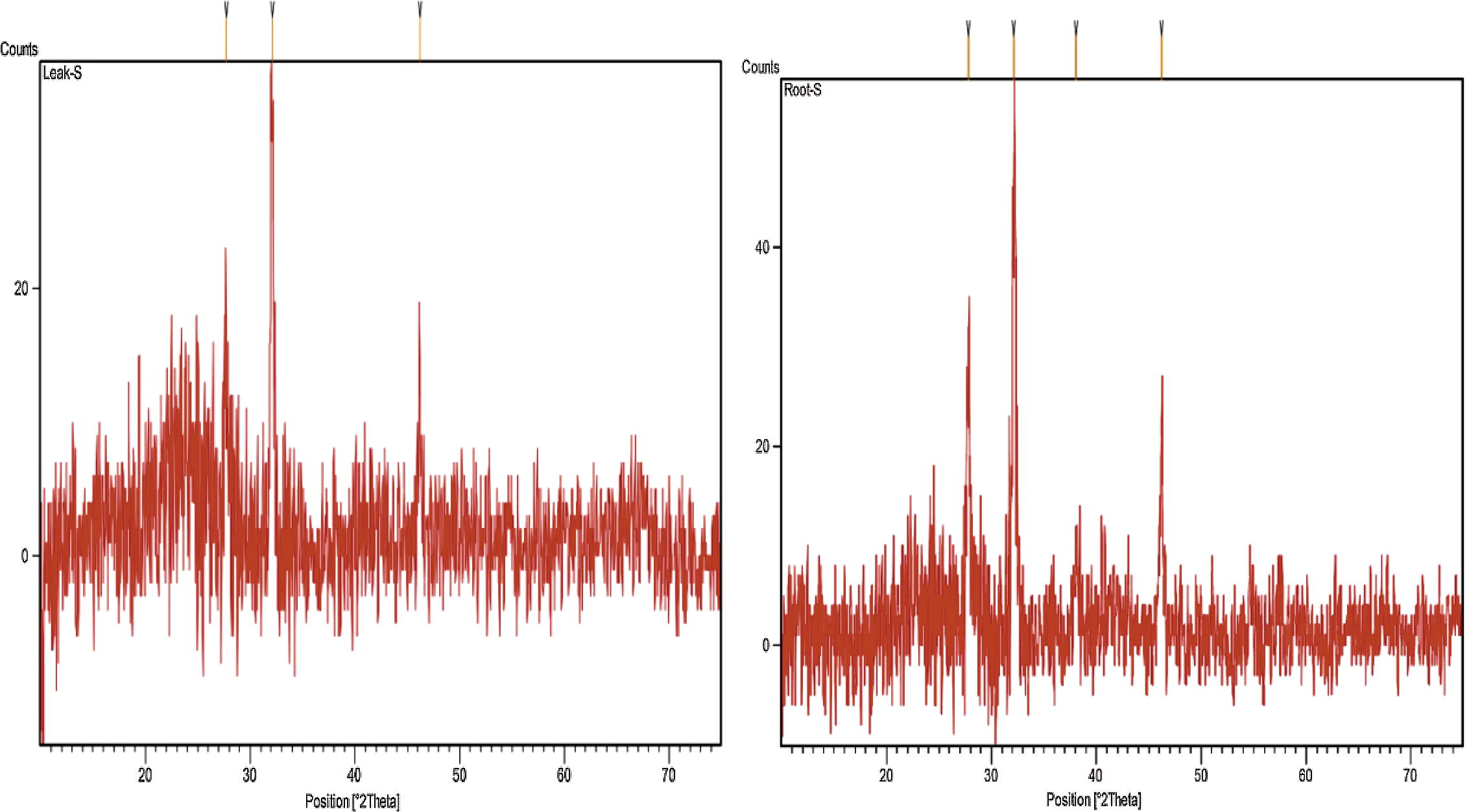
XRD images of SNPs generated using extracts of ipomoea leaf (left) and of root (right).
Fourier transform infrared (FTIR) measurements revealed a strong band at 1654 cm−1 which corresponds to the stretching vibrations of amide C⚌O bands of proteins/polypeptides (Fig. 5). The bands at 1590 cm−1 and 1385 cm−1 correspond to carboxylates and C–N stretching of aromatic amino groups, respectively (Narayanan and Sakthivel, 2008). The bands at 1607 cm−1 and 1385 cm−1 correspond to C⚌C groups/aromatic rings (Das et al., 2010). Apparently the SNPs were stabilized by amide groups of proteins and the phenolic groups. Moreover, the fact that SNPs derived from different parts of ipomoea have similar FTIR profiles, indicates that similar biomolecules have been responsible for the reduction of silver ions and the stabilization of the resulting SNPs.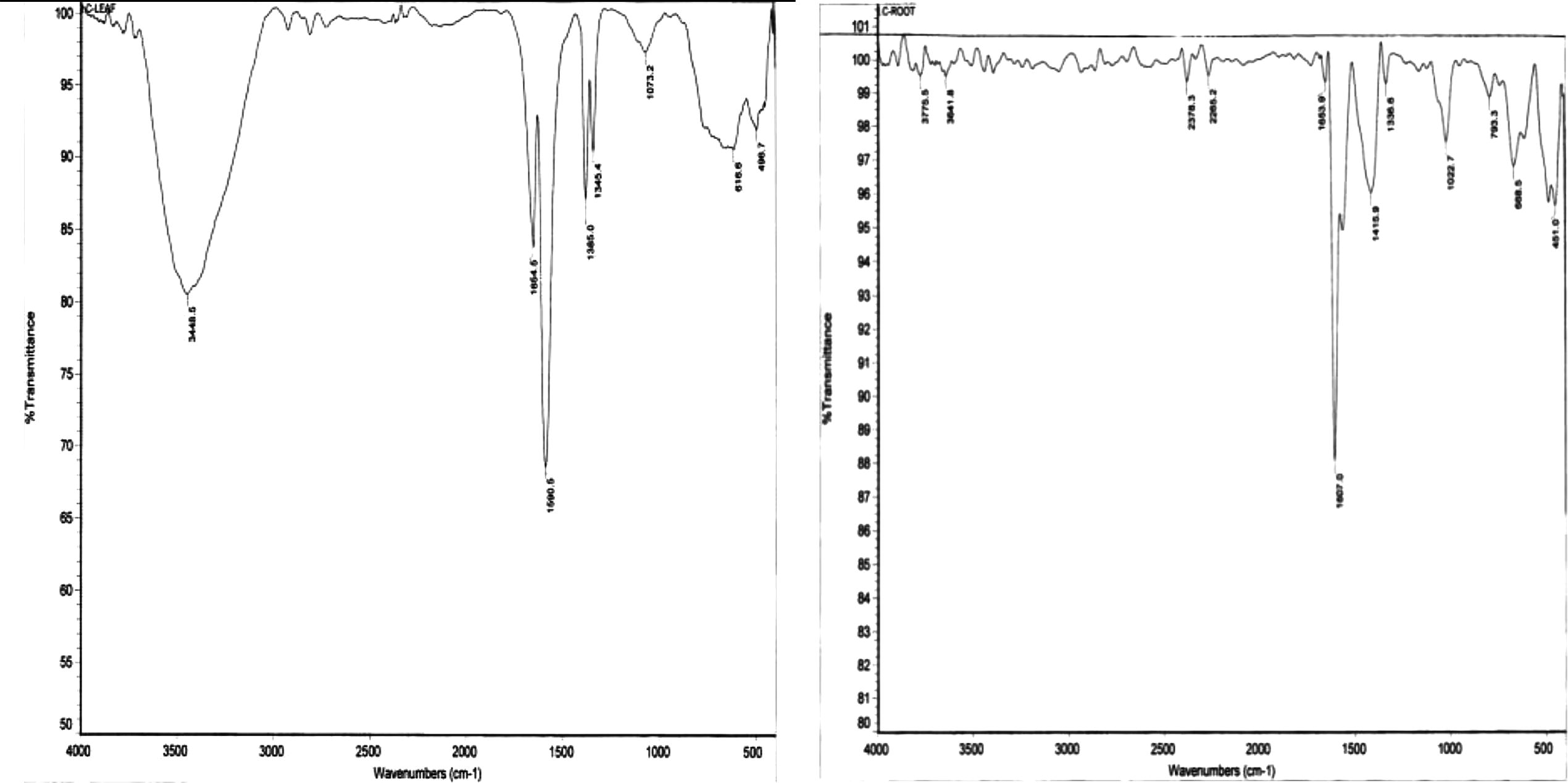
FTIR images of SNPs generated using extracts of ipomoea leaves (left) and of root (right).
The energy dispersive X-ray (EDAX) spectra (Fig. 6) showed strong signals for silver, along with weak signals from few other elements like C and O. These signals could have arisen from proteins and enzymes that had stabilized the nanoparticles.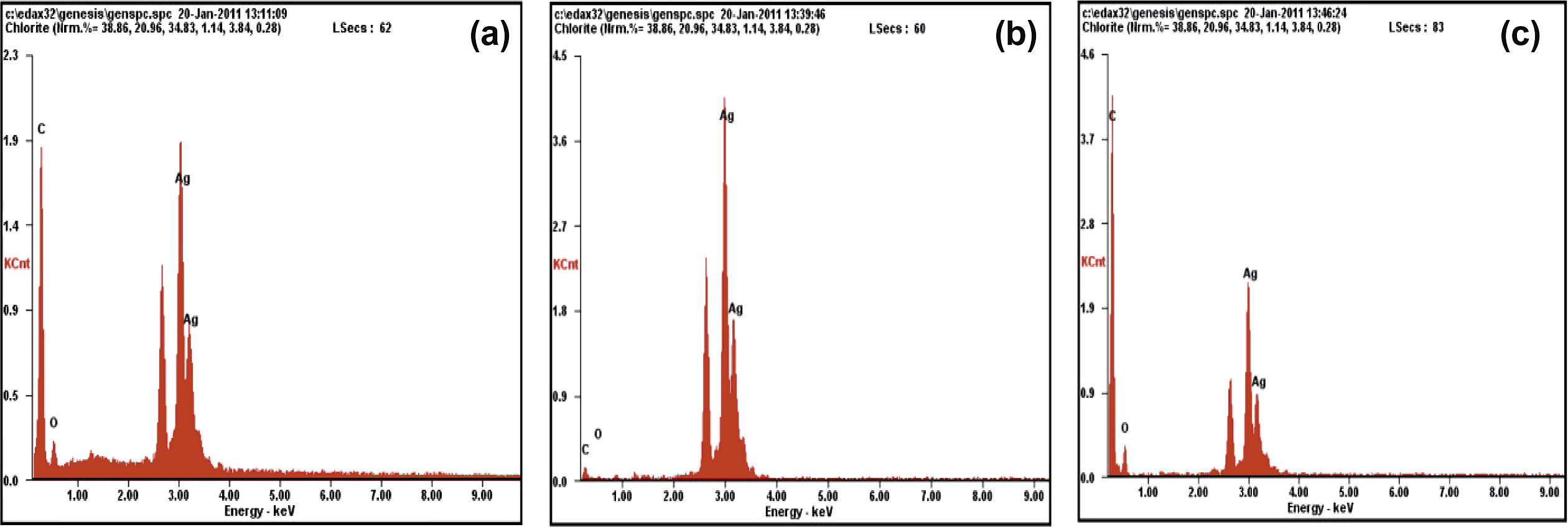
EDAX images of SNPs generated using extracts of ipomoea (a) leaves, (b) stem and (c) root.
3.3 Effect of SNPs in catalyzing degradation of typical organic pollutants
The rate of degradation of Alizarin Red S and Remazol Brilliant Blue R dyes by NaBH4 or H2O2 was monitored in the presence and absence of SNPs spectrophotometrically. SNPs are seen to catalyze the degradation, albeit slowly, under the conditions explored by us (Tables 5 and 6). This is reflected in faster lowering of dyes’ absorbance in reactants containing the SNPs. The results establish the proof-of-concept.
Reactants
Absorbance, at minutes
0
20
40
60
Dye + NaBH4
1.25
1.00
0.94
0.94
Dye + NaBH4 + SNPs
0.93
0.80
0.78
0.77
Dye + H2O2
1.60
1.60
1.58
1.56
Dye + H2O2 + SNPs
1.16
1.14
1.31
1.21
Reactants
Absorbance, at minutes
0
15
30
45
60
90
Dye + NaBH4
0.43
0.41
0.40
0.38
0.38
0.36
Dye + NaBH4 + SNPs
0.40
0.37
0.33
0.33
0.32
0.32
Dye + H2O2
0.44
0.42
0.42
0.42
0.42
0.42
Dye + H2O2 + SNPs
0.41
0.38
0.37
0.37
0.39
0.36
4 Summary and conclusion
In perhaps the first-ever study of its type, effect of natural variability in the chemical composition of a botanical species on the synthesis of nanoparticles employing that specie’s aqueous extract was explored. The plant species happened to be ipomoea (I. carnea) which is a major weed of the tropical and sub-tropical world, including India. The metal chosen was silver owing to the high demand of silver nanoparticles (SNPs) in several branches of technology (Abbasi et al., 2012a,b; Ghaffari-Moghaddam and Hadi-Dabanlou, 2014; Wang et al., 2014).
It was seen that, by-and-large, it is possible to identify metal–extract concentrations with which highly reproducible results vis a vis the shapes and sizes of SNPs can be achieved. Different plant parts—leaves, stem, and roots—give similar SNPs at certain metal–extract stoichiometries and dissimilar at some other. In this respect, too, fairly reproducible results were achieved when ipomoea drawn from different locations was used.
Proof-of-concept that SNPs can be used to catalyze degradation of complex organic pollutants was demonstrated with the examples of two dyes.
Acknowledgements
TA and SAA thank University Grants Commission (UGC), New Delhi for support in the form of a Major Research Project and SUG thanks the UGC for the award of Maulana Azad National Fellowship.
References
- A process for synthesis of bimetallic silver gold nanoparticles from weeds. Official Journal of the Patent Office. 2012;3:944.
- [Google Scholar]
- Gainful use of highly invasive terrestrial weed Ipomoea carnea for rapid and ‘green’ synthesis of silver nanoparticles. International Journal of Current Science. 2012;2:57-62.
- [Google Scholar]
- ‘Green’ synthesis of gold nanoparticles with aqueous extracts of neem (Azadirachta indica) Research Journal of Biotechnology. 2010;5(1):75-79.
- [Google Scholar]
- Biomimetic synthesis of gold nanoparticles using Aloe vera. International Journal of Environmental Science and Engineering Research. 2011;2(1):01-05.
- [Google Scholar]
- Rapid and reproducible ‘green’ synthesis of silver nanoparticles of consistent shape and size using Azadirachta indica. Research Journal of Biotechnology. 2011;6:69-70.
- [Google Scholar]
- Use of plants in biomimetic synthesis of gold nanoparticles. Journal of Nanoscience 2014 in press
- [Google Scholar]
- In vivo antitumor activity of biosynthesized silver nanoparticles using Ficus religiosa as a nanofactory in DAL induced mice model. Colloids and Surfaces B. 2013;108:185-190.
- [Google Scholar]
- Green synthesis of silver nanoparticles using carob leaf extract and its antibacterial activity. International Journal of Industrial Chemistry. 2013;4:29.
- [Google Scholar]
- Rapid biosynthesis of silver nanoparticles using Areca nut (Areca catechu) extract under microwave-assistance. Journal of Cluster Science. 2013;24:107.
- [Google Scholar]
- Comprehensive Environmental Impact Assessment of Water Resources Projects. Vol Volume 1. New Delhi: Discovery Publishing House; 2005. xvi+580 pages
- A study of the aquatic and amphibious weeds of Oussudu lake. Hydrology Journal. 2005;28:89-98.
- [Google Scholar]
- Implications of environmental threats on the composition and distribution of fishes in a large coastal wetland. Hydrology Journal. 2004;27:85-93.
- [Google Scholar]
- Chemical Reviews. 2007;107:2228-2269.
- Green synthesis of gold nanoparticles using ethanolic extract of Centella asiatica. Material Letters. 2010;64:1445-1447.
- [Google Scholar]
- Protocol for development of various plants leaves extract in single-pot synthesis of metal nanoparticles. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy. 2013;103:134-142.
- [Google Scholar]
- A novel microwave assisted process for the synthesis of nanostructured ruthenium catalysts active in the hydrogenation of phenol to cyclohexanone. Applied Catalysis, A: General. 2008;350:46-52.
- [Google Scholar]
- Innovative process for the synthesis of nanostructured ruthenium catalysts and their catalytic performance. Topics in Catalysis. 2009;52(8):1065-1069.
- [Google Scholar]
- An easy microwave-assisted process for the synthesis of nanostructured palladium catalysts and their use in the selective hydrogenation of cinnamaldehyde. Applied Catalysis, A: General. 2010;386(1–2):124-131.
- [Google Scholar]
- New palladium catalysts on polyketone prepared through different smart methodologies and their use in the hydrogenation of cinnamaldehyde. Applied Catalysis, A: General. 2012;447–448:49-59.
- [Google Scholar]
- Novel microwave-synthesis of Cu nanoparticles in the absence of any stabilizing agent and their antibacterial and antistatic applications. Applied Surface Science. 2013;280:610-618.
- [Google Scholar]
- Synthesis of silver nanoparticles using extract of neem leaf and Triphala and evaluation of their antimicrobial activites. International Journal of Pharma and Bio Sciences. 2012;3:88-100.
- [Google Scholar]
- The effects of Ipomoea carnea on neonate behavior: a study in goats. Toxicology Letters. 2010;196:S186.
- [Google Scholar]
- Plant mediated green synthesis and antibacterial activity of silver nanoparticles using Crataegus douglasii fruit extract. Journal of Industrial and Engineering Chemistry. 2014;20(2):739-744.
- [Google Scholar]
- The role of alkaloids in Ipomoea carnea toxicosis: a study in rats. Experimental and Toxicologic Pathology. 2005;57(1):53-58.
- [Google Scholar]
- Alkaloids from the poisonous plant Ipomoea carnea: effects on intracellular lysosomal glycosidase activities in human lymphoblast cultures. Journal of Agricultural and Food Chemistry. 2003;51(26):7642-7646.
- [Google Scholar]
- Biofabrication of silver nanoparticles using Andrographis paniculata. European Journal of Medicinal Chemistry. 2014;73:135-140.
- [Google Scholar]
- Green synthesis of silver nanoparticles with Zingiber officinale extract and study of its blood compatibility. BioNanoScience 2012
- [Google Scholar]
- Biobased green method to synthesise palladium and iron nanoparticles using Terminalia chebula aqueous extract. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy. 2013;102:128-133.
- [Google Scholar]
- Review of the genus Ipomoea: traditional uses, chemistry and biological activities. Brazilian Journal of Pharmacogenesis. 2012;22:682-713.
- [Google Scholar]
- Coriander leaf mediated biosynthesis of gold nanoparticle. Materials Letters. 2008;62:4588-4590.
- [Google Scholar]
- Extracellular biosynthesis of silver nanoparticle using Azadirachta indica leaf extract and its anti-microbial activity. Journal of Alloys and Compounds. 2014;583:272-277.
- [Google Scholar]
- Bioreduction of chloroaurate ions by geranium leaves and its endophytic fungus yields gold nanoparticles of different shapes. Journal of Materials Chemistry. 2003;13:1822-1826.
- [Google Scholar]
- Immobilization of biogenic gold nanoparticles in thermally evaporated fatty acid and amine thin films. Journal of Colloid and Interface Science. 2004;274:69-75.
- [Google Scholar]
- Green synthesis of Fe nanoparticles using eucalyptus leaf extracts for treatment of eutrophic wastewater. Science of the Total Environment. 2014;466–467:210-213.
- [Google Scholar]







