Translate this page into:
Sublethal effects of diazinon, fenitrothion and chlorpyrifos on the functional response of predatory bug, Andrallus spinidens Fabricius (Hem.: Pentatomidae) in the laboratory conditions
*Corresponding author. Tel.: +98 131 6690009; fax: +98 131 6690281 b_gh.chitgar60@yahoo.com (Moloud GholamzadehChitgar) mchitgar@phd.guilan.ac.ir (Moloud GholamzadehChitgar)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Available online 19 September 2013

Abstract
The sublethal effects of diazinon, fenitrothion and chlorpyrifos on the functional response of predatory bug, Andrallus spinidens Fabricius (Hem.: Pentatomidae), a potential biological control agent, were studied on 5th-instar nymphs. The experiment was conducted in varying densities (2, 4, 8, 16, 32 and 64) of last instars larvae of Chilo suppressalis Walker (Lepidoptera: Pyralidae) as prey at 25 ± 2 °C, 60% ± 10% relative humidity (RH) and a photoperiod of 16:8 h (L: D). The results of logistic regressions revealed a type II functional response in the control and all insecticide treatments. Comparison of functional response curves revealed that tested insecticides markedly decreased the mean of preys consumed by A. spinidens. Among them, functional response curve of A. spinidens in chlorpyrifos treatment was significantly lower than the other treatments. In this study, application of insecticides caused a decrease in the attack rate and an increase in the handling time of exposed bugs compared with the control. The longest handling time (3.97 ± 0.62) and the lowest attack rate (0.023 ± 0.007) were observed in chlorpyrifos and fenitrothion treatments, respectively. The results suggested that the adverse effect of these insecticides on A. spinidens should be considered in integrated pest management programs (IPM).
Keywords
Attack rate
Handling time
IPM
1 Introduction
Rice is an important crop and is cultivated mainly in the north of Iran at Mazandaran, Guilan and Golestan provinces. It is attacked by very destructive pests like Chilo suppressalis Walker (Pyralidae), Naranga aenescens Moore (Noctuidae) and Mythimna unipunctata Haworth (Noctuidae). Among them, the rice striped stem borer, C. suppressalis is one of the most serious pests of rice. Khan et al. (1990) reported that the stem borers are major pests in all rice ecosystems. The pests attack the rice plant in different developmental stages causing symptoms like dead heart and white head (Rubia-Sanchez et al., 1997). The chemical control has been a prevalent tool for controlling these lepidopterous pests. Diazinon, fenitrothion and chlorpyrifos have been used extensively in rice fields (Ghassempour et al., 2002). More than 60% of chemical pesticides were used in Northern provinces of Iran against rice pests. In these regions, pesticides are applied 2–4 times during the rice cropping season (Noorhosseini, 2010). The extensive and repeated use of pesticides could cause serious problem such as possible toxicity in humans and animals. Further, side effects of pesticides on non-target organisms, secondary pest outbreaks, development of insecticide resistance and environmental pollution are also of concern (Talebi et al., 2011). For example, the residue of diazinon which is commonly used to control C. suppressalis was detected in the soil and surface-water of rice fields in the north of Iran. The studies have also shown that several useful soil microorganisms failed to grow on a medium containing diazinon (Ghassempour et al., 2002). In the rice ecosystem, natural enemies include predators and parasitoids, which are considered as important biological agents for controlling various insect pests. Conservation of natural enemies in the rice fields may suppress the pest populations, which in turn will reduce the rate of insecticide application (Jadhao, 2011).
Andrallus spinidens Fabricius is a non-specific predator on lepidopterous larvae in rice fields (Manley, 1982). Second to fifth instar nymphs and adults of A. spinidens have predatory activity on caterpillar pests of rice like C. suppressalis, N. aenescens and M. unipunctata (Nageswara Rao, 1965; Manley, 1982; Mohaghegh and Najafi, 2003; Behera and Prakash, 2004). This pentatomid bug has a critical role in the regulation of rice pest’s population (Najafi-Navaee et al., 1998). There are three factors which should favor A. spinidens as a potentially useful biological control agent of rice pests: relatively short life cycle, aggressive feeding behavior and ability to feed continually for several hours (Manley, 1982). This natural enemy may be affected by insecticide sprays in rice fields via direct contact with residues, or indirectly through contaminated food. Integrating the application of biocontrol agents and insecticides for Integrated Pest Management (IPM) in rice ecosystem requires knowledge about impact and selectivity of the insecticides on natural enemies (Croft, 1990; Dent, 1995).
The control of a pest by a predator depends strongly on the predator–prey interaction such as the predator’s numerical and functional responses (Holling, 1959). Functional response tests show the potential of parasitoid/predator ability to suppress the different densities of prey/host. In fact, it describes the way a natural enemy responds to the changing densities of its prey and it is a commonly measured attribute of natural enemies. Holling (1959, 1966) proposed three types of functional responses. In type I, number of killed host/prey rises as linear to a plateau; type II, a curvilinear rise to a plateau which then levels off under the influence of handling time or satiation and in type III predator/parasitoid response by a sigmoid increase in prey/hosts attacked (Hassell, 2000; Mills and Lacan, 2004). Many factors such as pesticides influence the functional response of a predator (Murdoch and Oaten, 1975). Several studies provided a strong evidence that insecticides affect the functional response of natural enemies (e.g. Gu, 1991; Jebanesan, 1998; Wang and Shen, 2002; Claver et al., 2003; Deng et al., 2007; Ambrose et al., 2008, 2010; Rafiee-Dastjerdi et al., 2009; Rezac et al., 2010; Abedi et al., 2012). The functional response of A. spinidens to different densities of larvae of N. aenescens has been studied by Javadi and Sahragard (2005). However, there is no data about the investigation of insecticides on functional response of the predatory bug. In this study, we examined the sublethal effects of three insecticides, diazinon, fenitrothion and chlorpyrifos on the functional response of A. spinidens. Such information can be used to predict the potential of these pesticides in combination with A. spinidens in controlling rice pests.
2 Materials and methods
2.1 Insect rearing
The adults and nymphs of A. spinidens were collected from rice fields in Amol, Mazandaran province (north of Iran), in late September 2012. These insects were reared on last larval instar of Galleria melonella Linnaeus (Lep.: Pyralidae) in the laboratory conditions (25 ± 2 °C, 60% ± 10% RH and a photoperiod of 16:8 h L: D) h. The second generation of A. spinidens was used for experiments. Also, pupae of C. suppressalis were collected from the rice field in late may 2012 and kept in rearing chamber as above. After the emergence of adults and subsequent copulation and egg laying, the hatched larvae were reared on the rice seedling Oryza sativa L. (Taroum variety). The last larval instar of C. suppressalis was used as prey for functional response experiment of A. spinidens.
2.2 Pesticides
The pesticides used in this study were technical material of diazinon (Gyah Corporation, Iran, 99.8% purity), fenitrothion (Pesticides and Agriculture Research Center, Iran, 99.8% purity) and chlorpyrifos (ACO, USA, 99.9% purity).
2.3 Bioassay
Initially, preliminary bioassays were conducted to determine the effective concentrations caused between 10% and 90% mortality. The insecticides were bioassayed at serial concentrations in ranges of 1000–3500, 200–800 and 200–950 ppm a.i., for diazinon, fenitrothion and chlorpyrifos, respectively. These insecticides were diluted in acetone (Merck Company, Germany) and 1 μl of each concentration was applied topically using a microapplicator on the thoracic dorsum of newly molted 5th-instar nymphs of A. spinidens. Control treatment received 1 μl of acetone alone. Forty nymphs of A. spinidens were used for each concentration and the control. Mortality was assessed 24 h after treatment and the LC30 value of each insecticide was estimated.
2.4 Functional response assay
In order to evaluate the searching efficiency of A. spinidens, the functional response of this predator to different densities of C. suppressalis was studied. Twenty-four hours after treatment with the sublethal concentration, LC30 of each insecticide, the surviving nymphs were kept separately without food for 12 h. Then, they were individually transferred to Petri dishes (60 mm in diameters) and were fed with different densities (2, 4, 8, 16, 32, and 64) of last instar larvae of C. suppressalis. After 24 h, the predators were removed and the number of consumed prey (Na) was evaluated. Each concentration was replicated five times.
2.5 Data analysis
The LC30 values and 95% confidence intervals were calculated from probit regressions using the POLO-PC computer program (Leora Software, 1987). The type of the functional response was determined by logistic regression analysis (SAS/STAT, CATMOD pro cedure) of the proportion of prey killed (Ne) in relation to initial prey density (N0) (Trexler and Travis, 1993). The data were fitted to the logistic regression which describes the relationship between Na/N0 and N0 (Juliano, 1993): where P0, P1, P2, and P3 are the intercept of linear, quadratic and cubic coefficients, respectively, and estimated using the method of maximum likelihood. If the linear parameter P1 is negative, a type II functional response is evident, whereas a positive linear parameter indicates density-dependent predation and thus a type III functional response (Juliano, 1993).
After the determination of the shape of the curve, the handling times and attack coefficients of a Type II response were estimated using Holling’s disk equation (Williams and Juliano, 1985). Statistical analysis of the functional response was performed using the SAS software (SAS Institute, 2002).
3 Results
The LC30 values of each insecticide on A. spinidenis are presented in Table 1. The rank order of the toxicity, from the highest to the lowest, was fenitrothion > chlorpyrifos > diazinon.
Insecticide
Na
LC30b (95% confidence limit)
Slope ± SE
χ2 (df)
Diazinon
40
1343.51(1132.4–1515.5)
4.35 ± 0.55
1.84(5)
Fenitrothion
40
287.66(243.5–324.5)
4.33 ± 0.52
2.21(5)
Chlorpyrifos
40
312.7(250.7–365)
3.33 ± 0.44
3.31(5)
The relationship between number of prey density and number of prey consumed for all treatments is illustrated in Fig. 1. In this figure, the response curve rises in a negatively accelerating manner to a plateau that showed type II of functional response. The proportion of preys consumed by a predator with a type II functional response decreases exponentially as the prey density increases. Type II is curvilinear and the saturation level is reached in a gradual manner. Comparison of functional response curves revealed that functional response curve of A. spinidenis in chlorpyrifos treatment was significantly lower than the other treatments. The mean number of prey consumed at the density of 64 larvae was significantly different among the treatments (F = 7.48; df = 3; P < 0.0024).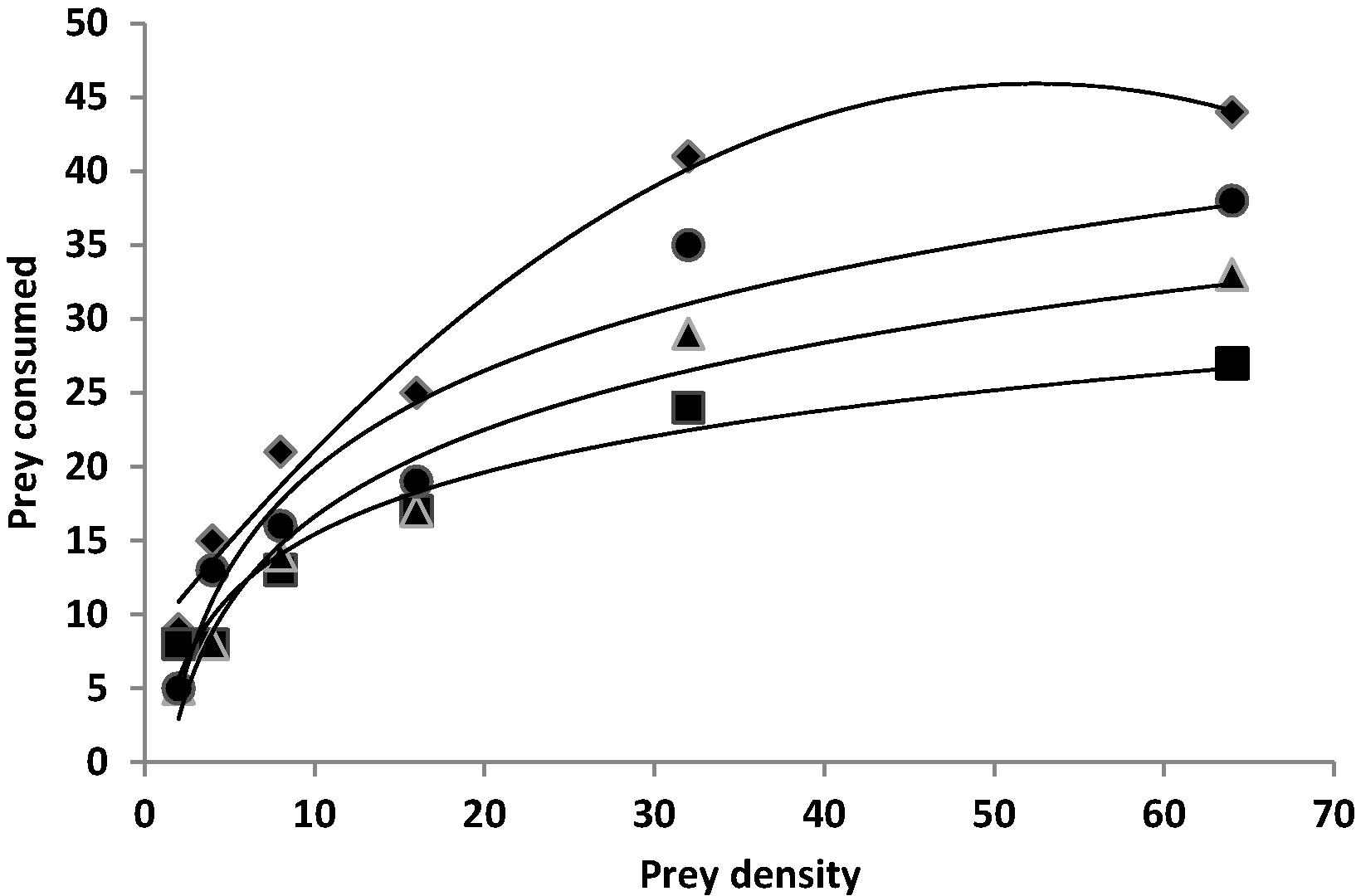
Type II functional response of A. spinidens exposed to the LC30 of different insecticides and control to densities of last larval instar of C. suppressalis. Chlorpyrifos (■), fenitrothion (
), diazinon (●) and Control (♦).
Parameter estimates for logistic regressions of all treatments are presented in Table 2. In the logistic regressions, if linear parameter P1 was negative, it could show a type II functional response, whereas a positive linear parameter could indicate density-dependent predation and thus a type III functional response.
Treatment
Parameters
Estimate
SE
χ2
p
Control
Constant
2.3126
0.6294
13.5
0.0002
Linear
−0.3370
0.0940
12.84
0.0003
Quadratic
0.0102
0.00352
8.46
0.0036
Cubic
−0.00009
0.000035
7.32
0.0068
Diazinon
Constant
1.0364
0.5521
3.52
0.0605
Linear
−0.2320
0.0878
6.98
0.0083
Quadratic
0.00709
0.00337
4.42
0.0354
Cubic
−0.00007
0.000033
3.86
0.0495
Fenitrothion
Constant
0.2915
0.5490
0.28
0.5954
Linear
−0.1587
0.0886
3.21
0.0731
Quadratic
0.00451
0.00343
1.73
0.1882
Cubic
−0.00004
0.00034
1.44
0.2295
Chlorpyrifos
Constant
0.8446
0.5560
2.31
0.1287
Linear
−0.2334
0.0911
6.56
0.0104
Quadratic
0.00673
0.00355
3.59
0.0580
Cubic
−0.00006
0.000035
2.9
0.0886
The values of searching efficiency (a′) and handling time (Th), estimated by Rogers random attack equation are depicted in Table 3. The longest handling time (3.97 ± 0.62) and the lowest attack rate (0.023 ± 0.007) were observed in chlorpyrifos and fenitrothion treatments, respectively. Th – handing time; a – maximum attack rates; SE – standard error
Treatment
Parameters
Estimate
SE
95% CI
Lower
Upper
Control
a′ (h−1)
0.0495
0.0155
0.0176
0.0813
Th (h)
2.3193
0.2808
1.8160
2.9666
Diazinon
a′ (h−1)
0.0304
0.00916
0.0117
0.0492
Th (h)
2.5778
0.3712
1.8174
3.3382
Fenitrothion
a′ (h−1)
0.0231
0.00786
0.00701
0.0392
Th (h)
2.8947
0.5201
1.8293
3.9602
Chlorpyrifos
a′ (h−1)
0.0297
0.0118
0.00553
0.0538
Th (h)
3.9709
0.6218
2.6973
5.2445
4 Discussion
The negative values for the linear parameters (P1 < 0) obtained in this study confirm type II functional response for all treatments, in which the proportion of preys consumed by a predator decreases exponentially as the prey density increases, hence the linear term is negative. The logistic regression model thus can be recommended as a tool for further analyzing functional response curves. The type II and III functional responses are common among arthropod predators (Hassell et al., 1977). The results of this study are consistent with the results of Javadi and Sahragard (2005), who reported that the functional response of A. spinidens on various densities of N. aenescens was type II. Similarly, this type of functional response has also been reported for coccinellid predators like; Cheilomenes sexmaculata Fabricius, Propylea dissecta Mulsant, Coccinella transversalis Fabricius (Coleoptera: Coccinellidae) (Pervez and Omkar, 2005) and Rhynocoris marginatus Fabricius (Hem.: Reduviidae) (Ambrose et al., 2010). Comparison of functional response curves revealed that the three tested insecticides markedly decreased the mean of prey consumed by A. spinidens. Among them, functional response curve of A. spinidens in chlorpyrifos treatment was significantly lower than the other treatments (Fig. 1). Rezac et al. (2010) reported the spider Philodromus cespitum Walckenaer (Araneae: Philodromidae) exposed to NeemAzal® and Dimilin® had a significantly lower asymptote compared to the control, which showed a reduction by about one half.
Functional response manifests two important parameters including a′ and Th used to evaluate the effectiveness of predators and parasitoids (Hassel and Waage, 1984). The rate of these parameters and the type of functional response in predators are influenced by different factors. One of them is the sublethal concentrations of insecticides (Rafiee-Dastjerdi et al., 2009; Ambrose et al., 2010). In this study, sublethal effects of diazinon, fenitrothion and chlorpyrifos on functional response parameters of A. spinidens showed different searching efficiency and handling time compared to control. Our findings demonstrated that the bugs exposed to insecticides had a higher handling time (Table 3), in which the highest Th occurred in the chlorpyrifos treatment. Moreover, effects of insecticides on a′ revealed that the value of this parameter decreased with insecticide application and the lowest value was observed in the bugs exposed to fenitrothion (0.0231 ± 0.00786). The attack rate or instantaneous rate of discovery or searching efficiency (a′) is the proportion of the total area searched by a predator/unit of searching time. It determines how rapidly the functional response curve approaches the upper plateau. Moreover, it is a function of (1) maximum distance at which the predator can perceive the prey, (2) speed of movement of predator that can perceive the prey, (3) proportion of attacks that are successful (Holling, 1965, 1966). Li et al. (2006) reported that acarophagous thrips, Scolothrips takahashii Priesner (Thysanoptera: Thripidae) females exposed to mancozeb showed prolonged handling time. Similarly, fenpropathrin and abamectin caused significantly lower attack rates and prolonged handling time in both males and females. Perturbation of predation by pesticides may drastically reduce the efficiency of natural enemies.
Pesticides used in this study were organophosphorus (OP) insecticides that have been widely used to control rice lepidopter pests in Iran. It is reported that organophosphates have low selectivity to natural enemies (Fernandes et al., 2010). For example, the insecticide chlorpyrifos used for the control of the coffee leaf miner Leucoptera coffeella Guérin-Méneville (Lep.: Lyonetiidae) was not selective to vespid predators in coffee plantations. Galvan et al. (2002) found similar results for wasps, Protonectarina sylveirae Saussure (Hymenoptera:Vespidae), Brachygastra lecheguana Perty (Hym.:Vespidae) and P. exigua de Saussure (Hym.:Vespidae) for the insecticide fenitrothion. The highest toxicity of the organophosphates to predators may be associated with the pro-insecticide activity of this group. When these compounds penetrate organisms, they suffer reactions and become more toxic. Another factor possibly related to the toxicity of organophosphates is the lipophilic characteristic of some insecticides associated with thickness and lipid composition of the insect cuticle. Such relation is accountable for the penetration of the product into the insect cuticle and the translocation to target of site (Fernandes et al., 2010). Organophosphates are highly lipid-soluble agents and absorbable, therefore, in this study when insecticides were topically applied on the thoracic dorsum of A. spinidens nymphs they could be easily absorbed and translocated to the target site. This might have been the reason for high toxicity of used compounds. On the other hand, the basic mechanism of action of organophosphate insecticides is based on acetylcholinesterase (AChE) inhibition. They are subsequently accumulated in neuromediator acetylcholine at the cholinergic synapses, either peripheral or central. They could cause cholinergic hyper stimulation and development of symptoms of poisoning such as hyperexcitation, restlessness, incoordination, prostoration and paralysis. It is reported that the behavioral alterations in motility of natural enemy include lack of motor coordination, tremors, downfalls, abdomen tucking and rotational movement for abdomen cleaning may increase after application of insecticides (Suchail et al., 2001). In the present study we observed leg and proboscis tremor on A. spinidens after pesticide treatment, within minutes to hours. This case may affect the feeding behavior of exposed predators and explain the reduced capture of A. spinidenis in pesticide treatments compared with the control. In addition, as the abnormal behavior increased, the time that takes for a predator to encounter and consume a single prey may be prolonged. According to the handling time, time-consuming activities may prolong handling time and cause delayed predatory acts. Deng et al. (2007) found that contact with organophosphorus compounds resulted in increased handling time in Hylyphantes graminicola Sundevall (Araneae: Linyphiidae). A similar observation was reported by Ambrose and George (1998) in monocrotophos-treated Acanthaspis pedestris Stal (Hem.: Reduviidae). The negative effects of insecticides on functional response have been reported in many natural enemies. Ambrose et al. (2010) studied the impact of Synergy-505® (chlorpyrifos 50% and cypermethrin 5% E.C), on functional response of R. marginatus and reported that this chemical compound caused a less pronounced type II functional response with reduced numbers of prey killed, attack rate, searching time, and prolonged handling time in 4th and 5th nymphal instars, adult males and females reflecting reduced predatory potential. Rafiee-Dastjerdi et al. (2009) demonstrated that profenofos, thiodicarb, hexaflumuron and spinosad had a negative effect on functional response of Habrobracon hebetor Say (Hym.: Braconidae). They reported that the wasps exposed to insecticides had higher handling time and among the pesticides, spinosad caused the most negative effect on searching efficiency. In addition, Abedi et al. (2012) showed that the application of cypermethrin on H. hebetor caused longest handling time and lowest attack rate. In other study, the functional response of the spider, P. cespitum was significantly affected after application of SpinTor®, NeemAzal®, and Dimilin® (Rezac et al. 2010). Furthermore, the effect of cypermetrin on the functional response of A. pedestris indicated that the pesticide negatively affected the functional response events such as attack ratio, handling time and rate of discovery and also reduced the predatory efficiency (Claver et al., 2003). However, most of the studies reported negative effects of insecticides on the functional response of natural enemies. However, a positive effect on predation rate has been observed for some low-dose of pesticides. For example, a stronger functional response was seen in wolf spiders, Pardosa pseudoannulata Boes. and Strand (Wang et al., 2006) and increased consumption was seen in Mallada signatus Schneider (Neuroptera: Chrysopidae) (Qi et al., 2001). These results can be explained by the general theory of hormoligosis (the stimulation of reproductive physiology by sublethal doses of pesticides) (Luckey 1968; Stebbing, 1982) or by the disruption of predator avoidance ability of prey.
5 Conclusion
In an integrated control program, it is necessary to utilize some insecticides with minimal toxicity to natural enemies of pests. In this study, the sublethal effects of diazinon, fenitrothion and chlorpyrifos were evaluated on the functional response of A. spinidens. These pesticides are organophosphate compounds that are widely used for controlling rice lepidopterous pests in north of Iran. Among the pesticides, fenitrothion and chlorpyrifos caused considerable effects on the functional response of A. spinidens nymphs compared to diazinon pesticide. The results showed that the attack rate and handling time in exposed bugs were affected by fenitrothion and chlorpyrifos more than diazinon. Diazinon is commonly used to control pests in all rice fields of Iran. Since, the pesticide has extensive use compared to two other pesticides it seems that bugs exposed to diazinon are more compatible with this pesticide. In addition, data obtained from LC30 values confirmed low toxicity of this pesticide on A. spinidens nymphs compared with other pesticide treatments. In the present study, although the used concentrations were lower than the recommended field rate for rice pest control, our results indicated that these concentrations had adverse effects on functional response of the predatory bug. According to our finding, diazinon, fenitrothion and chlorpyrifos are not suitable for use in the IPM of rice pests with A. spinidens. These insecticides markedly reduced the functional response of A. spinidens and potentially limited its biocontrol potentiality. It is recommended that where the use of these pesticides is essential for control of rice pests, they should be used by caution. In this way, at times of the highest population of A. spinidens in the rice field in June and July, the extensive use of pesticides should be avoided.
References
- Effects of azadirachtin, cypermethrin, methoxyfenozide and pyridalil on functional response of habrobracon hebetor Say (Hym.: Braconidae) J. Plant Prot. Res.. 2012;52:353-358.
- [Google Scholar]
- Comparative toxicological effects of monocrotophos to the third nymphal instars and the adults of Acanthaspis pedestris Stal, a potential biocontrol agent (Insecta: Heteroptera: Reduviidae) Indian J. Environ. Sci.. 1998;2:105-111.
- [Google Scholar]
- Impact of insecticide Synergy-505 on the functional response of a nontarget reduviid predator Rhynocoris marginatus (Fabricius) (Heteroptera: Reduviidae) feeding on Spodoptera litura (Fabricius) (Lepidoptera: Noctuidae) J. Biol. Control. 2008;22:283-290.
- [Google Scholar]
- Impacts of Synergy-505 on the functional response and behavior of the reduviid bug, Rhynocoris marginatus. J. Insect Sci.. 2010;10:1-10.
- [Google Scholar]
- Indian insect predators on insect pests of rice. Indian Insect Predators Biol. Control 2004:275-296.
- [Google Scholar]
- Impact of cypermethrin on the functional response, predatory and mating behaviour of a non-target potential biological control agent Acanthaspis pedestris (Stal) (Het., Reduviidae) J. Appl. Entomol.. 2003;127:18-22.
- [Google Scholar]
- Arthropod Biological Control Agents and Pesticides. New York: John Wiley; 1990. p. 723
- Effects of methamidophos on the predating behavior of Hylyphantes graminicola (Sundevall) (Araneae: Linyphiidae) Environ. Toxicol. Chem.. 2007;26:478-482.
- [Google Scholar]
- Integrated Pest Management. London: Chapman and Hall; 1995.
- Impact and selectivity of insecticides to predators and parasitoids. Entomo Bras.. 2010;3:1-10.
- [Google Scholar]
- Selectivity of eight insecticides to predators of citrus caterpillars. Pesqui Agropecu Bras.. 2002;37:117-122.
- [Google Scholar]
- Monitoring of the pesticide Diazinon in soil, stem and surface water of rice fields. Anal. Sci.. 2002;18:779-783.
- [Google Scholar]
- Influence of sublethal dose of insecticides on the foraging behavior of Diaeretiella rapae. Acta Ecol. Sin.. 1991;11:324-329.
- [Google Scholar]
- Sigmoid functional response by invertebrate predators and parasitoids. J. Anim. Ecol.. 1977;46:249-262.
- [Google Scholar]
- The Spatial and Temporal Dynamics of Host Parasitoid Interactions. London, UK: Oxford University Press; 2000.
- Some characteristics of simple types of predation and parasitism. Can. Entomol.. 1959;7:385-399. XCI
- [Google Scholar]
- The functional response of predators to prey density and its role in mimicry and population regulation. Can. Entomol.. 1965;91:385-398.
- [Google Scholar]
- Functional response of invertebrate predators to prey density. Mem. Entomol. Soc. Can.. 1966;48:1-87.
- [Google Scholar]
- A preliminary study of the predatory natural enemy complex of rice ecosystem in Vidarbha region of Maharashtra, India. Int. Ref. Res. J. 2011:25-27.
- [Google Scholar]
- The functional response of Andrallus spinidens F. (Hem.: Pentatomidae) to densities of Naranga aenescens (Lep.: Noctuidae) in laboratory. J. Agric. Sci. Nat. Resour.. 2005;12(2):111-117.
- [Google Scholar]
- Sublethal effect of etofenprox (Trebon) on the predation of Culex quinquefasciatus (Say) by Diplonychus indicus (Venk. and Rao.) Indian J. Environ. Toxicol.. 1998;8:33-34.
- [Google Scholar]
- Nonlinear curve fitting: predation and functional response curves. In: Scheiner S.M., Gurevitch J., eds. Design and Analysis of Ecological Experiments. New York: Chapman and Hall; 1993. pp 159–182
- [Google Scholar]
- LeOra Software., 1987. POLO-PC: A user guide to probit or logit 786 analysis. LeOra software, Berkeley, California.
- Effects of pesticides on the functional response of predatory thrips, Scolothrips takahashii to Tetranychus viennensis. J. Appl. Entomol.. 2006;130:314-322.
- [Google Scholar]
- Biology and life history of the rice field predator Andrallus spinidens F. (Heteroptera: Pentatomidae) Entomol. News. 1982;93:19-24.
- [Google Scholar]
- Ratio dependence in the functional response of insect parasitoids: evidence from Trichogramma minutum foraging for eggs in small host patches. Ecol. Entomol.. 2004;29:208-216.
- [Google Scholar]
- Predation capacity of Andrallus spinidens (F.) (Het.: Pentatomidae) on Naranga aenescens Moore (Lep.: Noctuidae) under semi-field and field conditions. Appl. Entomol. Phytopathol.. 2003;71:57-68.
- [Google Scholar]
- Andrallus (Audinetia) spinidens Fabr., as predator on rice pests. Oryza. 1965;2:179-181.
- [Google Scholar]
- Najafi-Navaee, A., Saeb, H., Osco, T., 1998. Biology and ecology of Andrallus spinidens F. as the predator of rice, cotton and maize pests. In: 13th Iranian Plant Protection Congress.
- Decline of pesticides application by using biological control: the case study in North of Iran. Middle-East J. Sci. Res. 2010:166-169.
- [Google Scholar]
- Functional responses of coccinellid predators: an illustration of a logistic approach. J. Insect. Sci. 2005:6.
- [Google Scholar]
- Effects of neem-fed prey on the predacious insects Harmonia conformis (Boisduval) (Coleoptera: Coccinellidae) and Mallada signatus (Schneider) (Neuroptera: Chrysopidae) Biol. Control.. 2001;22:185-190.
- [Google Scholar]
- Effects of some insecticides on functional response of ectoparasitoid, Habrobracon hebetor (Say) (Hym.: Braco nidae) J. Entomol.. 2009;6(3):161-166.
- [Google Scholar]
- The negative effect of some selective insecticides on the functional response of a potential biological control agent, the spider Philodromus cespitum. BioControl. 2010;55:503-510.
- [Google Scholar]
- White stem borer damage and grain yield in irrigated rice in WEST Java, Indonesia. Crop Prot.. 1997;16:665-671.
- [Google Scholar]
- SAS Institute, 2002. SAS/STAT user’s guide. SAS Institute Inc., Cary, NC Inc.
- Hormesis—the stimulation of growth by low levels of inhibitors. Sci. Total Environ.. 1982;22:213-234.
- [Google Scholar]
- Discrepancy between acute and chronic toxicity induced by imidacloprid and its metabolites in Apis mellifera. Environ. Toxicol. Chem.. 2001;20:2482-2486.
- [Google Scholar]
- Ecological impacts of pesticides in agricultural ecosystem. In: Stoytcheva M., ed. Pesticides in the Modern World – Risks and Benefits. Rijeka, Croatia: In Tech Open Access Publisher; 2011. p. :143-168.
- [Google Scholar]
- Effects of sublethal doses of insecticides on predation of multicolored Asian ladybird, Harmonia axyridis (Pallas) Acta. Ecol. Sin.. 2002;22:2278-2284.
- [Google Scholar]
- Functional response and searching behavior to the brown planthopper, Nilaparvata lugens by the wolf spider, Pardosa pseudoannulata under low-dose chemical pesticides. Acta Entomol. Sin.. 2006;49:295-301.
- [Google Scholar]
- Further difficulties in the analysis of functional response experiment and a resolution. Can. Entomol.. 1985;117(5):631-640.
- [Google Scholar]







