Translate this page into:
Triphenylphosphine: An efficient catalyst for the synthesis of 4,6-diphenyl-3,4-dihydropyrimidine-2(1H)-thione under thermal conditions
*Corresponding author. Tel.: +91 9944093020 smansoors2000@yahoo.co.in (S. Sheik Mansoor)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Available online 14 May 2013

Abstract
An efficient and direct procedure for the synthesis of 4,6-diphenyl-3,4-dihydropyrimidine-2(1H)-thione derivatives by condensation of 1,3-diaryl-2-propen-1-ones (chalcones) and thiourea in ethanol at 65 °C using triphenylphosphine (PPh3) as a catalyst is reported. The method gave good yields of 4,6-diphenyl-3,4-dihydropyrimidine-2(1H)-thione derivatives in short reaction times in comparison with earlier methods. The catalyst is recycled without loss of activity. Using non-toxic and inexpensive materials, simple work-up, short reaction times and high yields of the products are the advantages of this method.
Keywords
4,6-diphenyl-3,4-dihydropyrimidine-2(1H)-thione
Triphenylphosphine
1,3-Diaryl-2-propen-1-one
Chalcones
1 Introduction
3,4-Dihydropyrimidin-2(1H)-ones (DHPM) and their sulfur analogs have been reported to possess remarkable pharmacological properties including antiviral, antitumor, antibacterial, anti-inflammatory and antioxidant activities (Kappe, 1993, 2000a,b; Russowsky et al., 2006; Stefani et al., 2006; Chitra et al., 2011). Dihydropyrimidinones have also exhibited important therapeutic and pharmacological properties as the integral backbones of several calcium channel blockers, calcium channel modulators, antihypertensive agents and α-la-adrenergic receptor antagonists (Atwal et al., 1990; Rovnyak et al., 1995; Nagarathnam et al., 1999). Several alkaloids isolated from marine sources also exhibit interesting biological activities, molecular structures of which contain the dihydropyrimidinone moiety. In particular, the batzelladine alkaloids have been found to be potent HIV gp-120-CD4 inhibitors (Patil et al., 1995; Snider et al., 1996). Therefore, in consequence of their valuable biological activities, great interest has been established in the preparation of pyrimidin-2(1H)-ones.
The first protocol to prepare compounds of this type was presented by Biginelli in 1893 and involved a three-component, one-pot condensation of benzaldehyde, ethyl acetoacetate and urea under strongly acidic conditions (Biginelli, 1893). However, this reaction often requires harsh conditions and long reaction times and affords low yields, particularly when substituted aromatic and aliphatic aldehydes are employed. The scope of the original Biginelli reaction was gradually extended by the variation of all three building blocks, allowing access to a large number of multi-functionalized dihydropyrimidineone derivatives.
The most direct procedure for the preparation of dihydropyrimidinones and thiones is through condensation of β-dicarbonyl compounds with an aromatic aldehyde and urea or thiourea in the presence of Lewis and Brønsted acid promoters such as silica immobilized nickel complex (Sharma and Rawat, 2012), cellulose sulfuric acid (Rajack et al., 2013), bioglycerol-based sulfonic acid functionalized carbon (Konkala et al., 2012), perchloric acid doped silica (Narahari et al., 2012), melamine trisulfonic acid (Shirini et al., 2011), sulfonated carbon (Moghaddas et al., 2012) and so on. The derivatives of 3,4-dihydro-4,6-diarylpyrimidin-2(1H)-one are a class of pyrimidin-2(1H)-one skeletons which were prepared via two more common procedures: through the reaction of α, β-unsaturated ketones and urea/thiourea (Safaei-Ghomi and Ghasemzdeh, 2011) or through condensation reaction of acetophenone derivatives, aldehydes and urea/thiourea using various Brønsted and Lewis acid catalysts (Pourghobadi and Derikvand, 2010; Khosropour et al., 2006; Heravi et al., 2008; Sharghi and Jokar, 2009; Lei et al., 2009; Wang et al., 2010; Oskooie et al., 2011; Liu et al., 2013; Chitra et al., 2011). Some of these newer reported methods, however, exhibit drawbacks such as unsatisfactory yields, cumbersome product isolation procedures, and environmental pollution. Moreover, the main disadvantage of almost all existing methods is that the catalysts are destroyed in the work-up procedure and cannot be recovered or reused. There is still a need, therefore, for versatile, efficient, simple and environmentally friendly processes for the formation of DHPM derivatives.
Since Lewis acids have already been used as catalysts for the synthesis of 4,6-diarylpyrimidine-2(1H)-one derivatives, it was thought to develop the synthesis of 4,6-Diphenyl-3,4-dihydropyrimidine-2(1H)-thione derivatives using a Lewis base catalyst. In this respect, it is previously reported that the Biginelli condensation can be easily achieved with a catalytic amount of triphenylphosphine as a Lewis base (Mansoor et al., 2011) and this method is very flexible and also enables the preparation of a large number of 3,4-dihydropyrimidin-2-ones/thiones/imines.
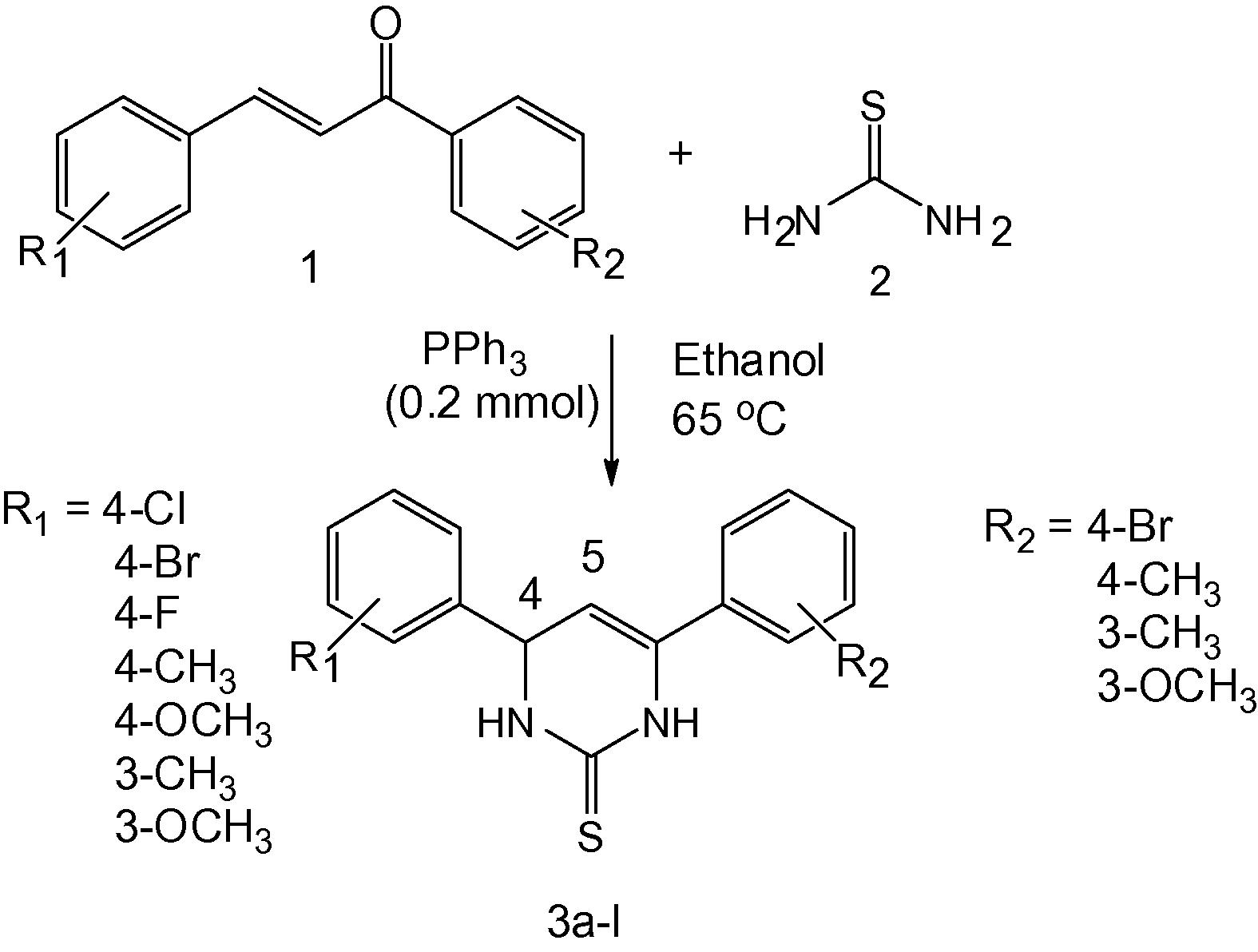
Considering the potential of developing new routes to the synthesis of heterocyclic compounds (Mansoor et al., 2013; Mansoor et al., 2012a,b; Ghashang et al., 2013), the present investigation aimed toward the synthesis of 4,6-diphenyl-3,4-dihydropyrimidine-2(1H)-thione derivatives using condensation reaction of 1,3-diaryl-2-propen-1-one derivatives and thiourea in the presence of triphenylphosphine as catalyst.
2 Materials and methods
2.1 Apparatus and analysis
Commercially available laboratory grade chemicals with high purity were used. Triphenylphosphine is used as catalyst. The benzaldehydes used were with substituents H, p-Cl, p-Br, p-CH3, p-OCH3, p-F, m-CH3, and m-OCH3. The solid aldehydes were used as such and the liquid aldehydes were used after vacuum distillation. Acetopheones with substituents H, m-CH3, m-OCH3, p-Br, and p-CH3, and thiourea were used in the synthesis of various 4,6-diphenyl-3,4-dihydropyrimidine-2(1H)-thiones. The solvents like methanol, ethanol, acetonitrile, 1,4-dioxane and cyclohexane were used to study the optimization of solvent. Analytical thin-layer chromatography was performed with E. Merck silica gel 60F glass plates. 1H-NMR (500 MHz) and 13C-NMR (125 MHz) spectra were obtained using Bruker DRX- 500 Avance at ambient temperature, using TMS as internal standard. Fourier-transform infrared spectra were obtained as KBr disks on Shimadzu spectrometer. Mass spectra were determined by employing Varion – Saturn 2000 GC/MS instrument while elemental analyses were measured by Perkin Elmer 2400 CHN elemental analyzer flowchart. All yields refer to isolated products unless otherwise stated.
2.2 General procedure for the preparation of pyrimidine-2-thione derivatives (3a–l)
A mixture of 1,3-diaryl-2-propen-1-one (2 mmol), thiourea (3 mmol) and PPh3 (0.2 mmol) was refluxed with stirring at 65 °C in ethanol as solvent and the progress of the reaction monitored by TLC. Subsequently, the reaction mixture was left overnight, concentrated under reduced pressure, the residue collected, washed with water and recrystallized from ethanol to afford the pure product. The catalyst is removed by filtration and the recovered catalyst can be washed consequently with an aliquot of fresh CH2Cl2 (2 × 10 mL), water and then acetone. After drying, it can be reused without noticeable loss of reactivity. All the products obtained were fully characterized by spectroscopic methods such as IR, 1H-NMR, 13C-NMR, mass spectroscopy and elemental analysis and have been identified by the comparison of the spectral data with those reported.
3 Results
We have prepared a series of 4,6-diphenyl-3,4-dihydropyrimidine-2(1H)-thione derivatives from a mixture of 1,3-diaryl-2-propen-1-one derivatives (2 mmol) and thiourea (3 mmol) and our results are presented here.
Among various catalyst tested, PPh3 was found to be an effective catalyst wherein the product was obtained in good yields under mild conditions (Table 1). With PPh3 as catalyst, next the effect of various solvents such as methanol, ethanol, acetonitrile, 1,4-dioxane and cyclohexane and also solvent-free condition was screened (Table 2; entries 1–5). The reaction was completed within 5 h and the expected product was obtained in 94% yield in ethanol. Under the optimized set of reaction conditions a number of 1,3-diaryl-2-propen-1-one derivatives (1) were allowed to react with thiourea (2) in the presence of PPh3 (0.2 mmol) in ethanol at 65 °C (Scheme 1). The results are given in Table 3.
Entry
Catalyst
Amount of catalyst (mmol)
Time (h)
Yield (%)b
1
None
–
8
20
2
AlCl3
1
6
38
3
ZnCl2
1
6
40
4
FeCl3
1
6
52
6
BiCl3
0.2
6
72
7
BiBr3
0.2
6
76
11
PPh3
0.4
5
86
12
PPh3
0.2
5
94
13
PPh3
0.1
5
86
14
PPh3
0.06
5
80
Entry
Solvent
Amount of catalyst (mmol)
Time (h)
Yield (%)b
1
Methanol
0.2
5
85
2
Ethanol
0.2
5
94
3
Acetonitrile
0.2
5
65
4
1,4-Dioxane
0.2
5
60
5
Cyclohexane
0.2
5
52
6
None
0.2
5
74
Synthesis of various 4,6-diphenyl-3,4-dihydropyrimidine-2(1H)-thione derivatives.
Entry
Chalcones
Product
Time (h)
Yield (%)b
Mp (oC)
Found
Reportedc
1
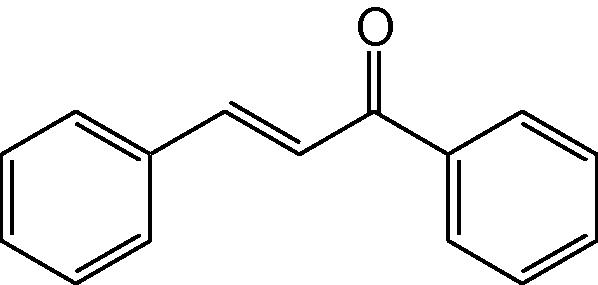
3a
5.0
94
180–182
182–184
2
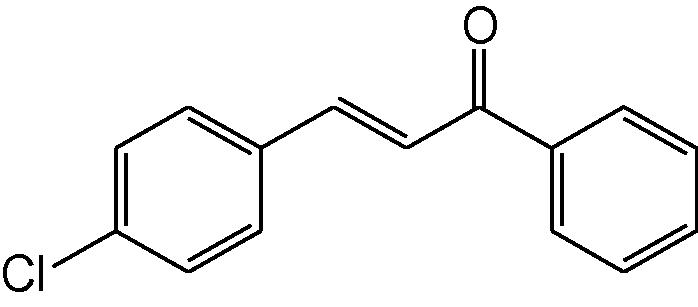
3b
4.5
96
156–158
–
3
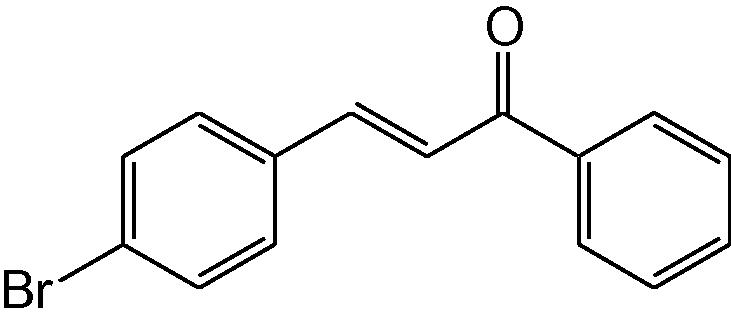
3c
4.5
95
144–146
–
4
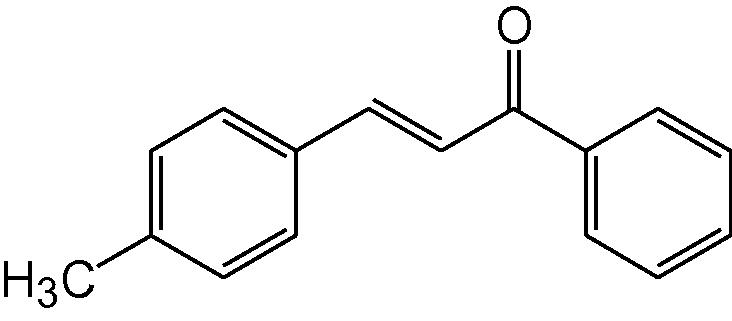
3d
5.5
86
197–199
198–200
5
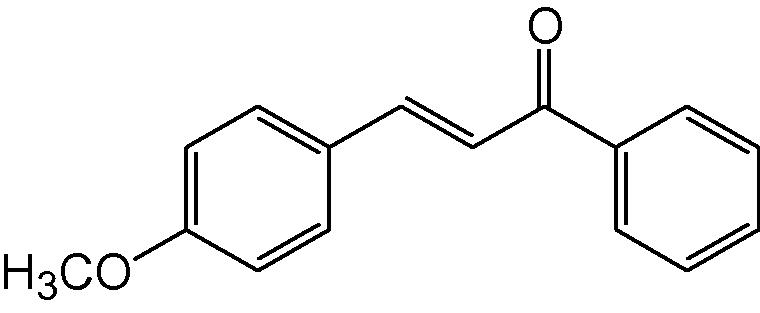
3e
5.5
88
124–126
123–124
6
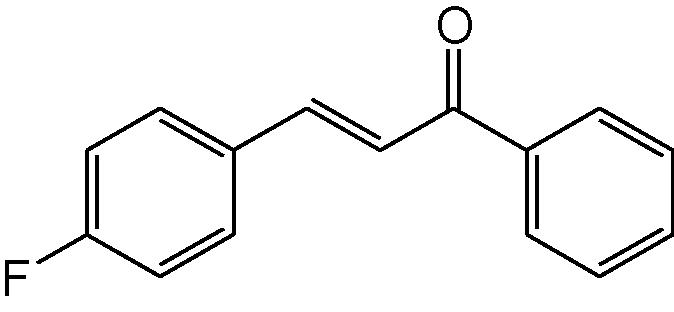
3f
4.5
92
186–188
–
7
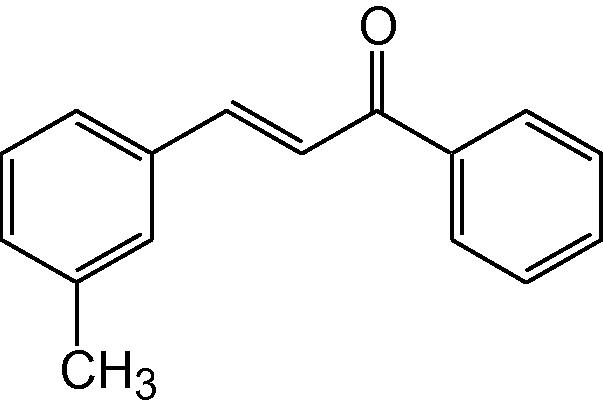
3g
5.5
89
182–184
183–185
8
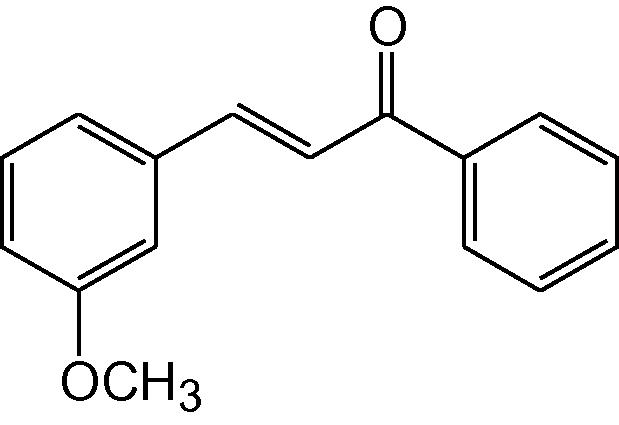
3h
5.5
90
140–142
–
9
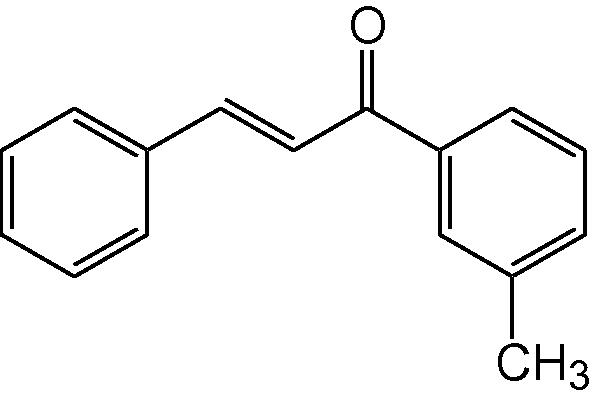
3i
5.0
88
178–180
–
10
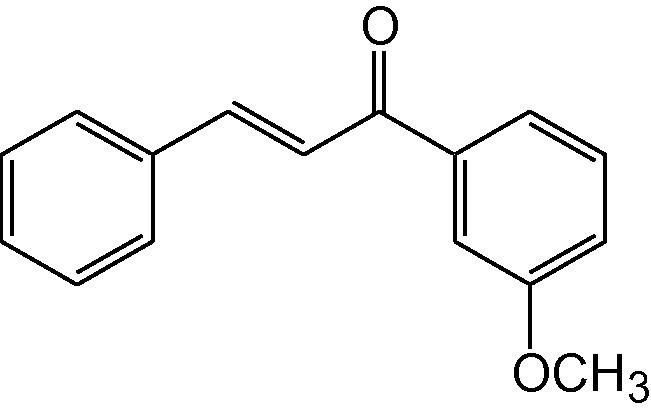
3j
5.0
86
166–168
–
11
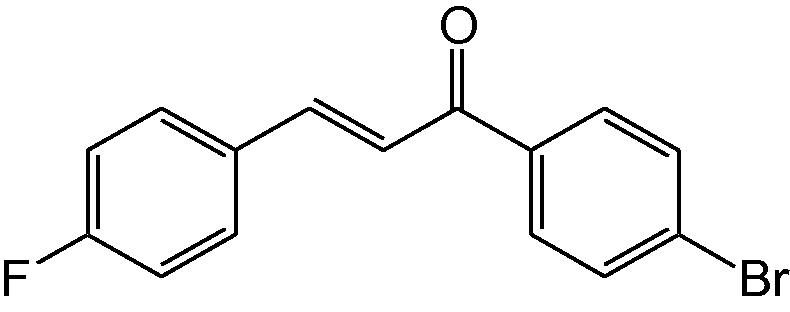
3k
4.0
93
196–198
–
12
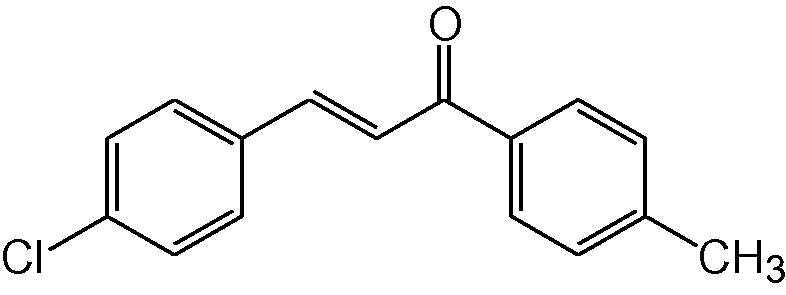
3l
4.5
91
180–182
–
3.1 The spectroscopic and analytical data for the synthesized compounds are presented below (3a−l)
3.1.1 4,6-Diphenyl-3,4-dihydropyrimidine-2(1H)-thione (3a)
IR (KBr, cm−1): 3173 (NH), 1644 (C⚌N), 1544, 1470 (C⚌C), 1183 (C⚌S). 1H-NMR (500 MHz, DMSO-d6) δ: 4.90 (1H, d, J = 5.0 Hz, 4-CH), 5.22 (1H, d, J = 5.0 Hz, 5-CH), 6.92–7.44 (10H, m, Ar–H), 8.80 (1H, bs, NH), 9.66 (1H, bs, NH) ppm; 13C-NMR (125 MHz, DMSO-d6) δ: 54.9, 102.1, 126.0, 126.8, 127.4, 128.5, 129.4, 129.8, 133.6, 134.5,144.4, 176.0 ppm; MS(ESI): m/z 267 (M + H)+; Anal. Calcd for C16H14N2S: C, 72.18; H, 5.26; N, 10.52%. Found: C, 72.13; H, 5.22; N, 10.52%.
3.1.2 4-(4-Chlorophenyl)-6-phenyl-3,4-dihydropyrimidine-2(1H)-thione (3b)
IR (KBr, cm−1): 3152 (NH), 1655 (C⚌N), 1548, 1472 (C⚌C), 1180 (C⚌S). 1H-NMR (500 MHz, DMSO-d6) δ: 4.88 (1H, d, J = 5.0 Hz, 4-CH), 5.28 (1H, d, J = 5.0 Hz, 5-CH), 7.03–7.55 (9H, m, Ar–H), 8.75 (1H, bs, NH), 9.71 (1H, bs, NH) ppm; 13C-NMR (125 MHz, DMSO-d6) δ: 50.6, 56.2, 100.4, 111.3, 121.3, 126.2, 126.7, 128.5, 129.1, 129.5, 132.1, 133.6,134.9, 155.5, 177.2 ppm; MS(ESI): m/z 301.45 (M + H)+; Anal. Calcd for C16H13ClN2S: C, 63.90; H, 4.33; N, 9.32%. Found: C, 63.88 H, 4.31; N, 9.31%.
3.1.3 4-(4-Bromophenyl)-6-phenyl-3,4-dihydropyrimidine-2(1H)-thione (3c)
IR (KBr, cm−1): 3145 (NH), 1646 (C⚌N), 1540, 1472 (C⚌C), 1182 (C⚌S). 1H-NMR (500 MHz, DMSO-d6) δ:4.87 (1H, d, J = 5.0 Hz, 4-CH), 5.19 (1H, d, J = 5.0 Hz, 5-CH), 7.08–7.3 (9H, m, Ar-H), 8.88 (1H, bs, NH), 9.77 (1H, bs, NH) ppm; 13C-NMR (125 MHz, DMSO-d6) δ: 50.2, 55.9, 100.3, 111.5, 121.1, 126.3, 126.9, 128.4, 129.1, 129.2, 132.5, 133.7,134.4, 155.5, 178.0 ppm; MS(ESI): m/z 345.9 (M + H)+; Anal. Calcd for C16H13BrN2S: C, 55.66; H, 3.77; N, 8.12%. Found: C, 55.64; H, 3.74; N, 8.10%.
3.1.4 4-(4-Methylphenyl)-6-phenyl-3,4-dihydropyrimidine-2(1H)-thione (3d)
IR (KBr, cm−1): 3188(NH), 1652 (C⚌N), 1560, 1484 (C⚌C), 1184 (C⚌S). 1H-NMR (500 MHz, DMSO-d6) δ: 2.03 (3H, s, CH3), 4.82 (1H, d, J = 5.0 Hz, 4-CH) 5.16 (1H, d, J = 5.0 Hz, 5-CH), 6.96–7.42 (9H, m, Ar–H), 8.83 (1H, bs, NH), 9.64 (1H, bs, NH) ppm; 13C-NMR (125 MHz, DMSO-d6) δ: 21.2, 56.0, 102.5, 127.3, 127.7,128.6, 129.5, 130.5, 130.4, 134.9, 138.6, 142.4, 178.5 ppm; MS(ESI): m/z 281 (M + H)+; Anal. Calcd for C17H16N2S: C, 72.85; H, 5.71; N, 10.00%. Found: C, 72.81; H, 5.70; N, 10.01%.
3.1.5 4-(4-Methoxyphenyl)-6-phenyl-3,4-dihydropyrimidine-2(1H)-thione (3e)
IR (KBr, cm−1): 3156 (NH), 1658 (C⚌N), 1564, 1488 (C⚌C), 1186 (C⚌S). 1H-NMR (500 MHz, DMSO-d6) δ: 3.60 (3H, s, –OCH3), 4.89 (1H, d, J = 5.0 Hz, 4-CH) 5.19 (1H, d, J = 5.0 Hz, 5-CH), 6.88–7.44 (9H, m, Ar–H),8.74 (1H, bs, NH), 9.72 (1H, bs, NH) ppm; 13C-NMR (125 MHz, DMSO-d6) δ: 50.2, 56.0, 100.4, 111.4, 121.1, 126.4, 128.6, 129.1, 129.6, 132.1, 135.1, 156.0,178.0 ppm; MS(ESI): m/z 297 (M + H)+; Anal. Calcd. for C17H16N2OS: C 68.92, H 5.41, N 9.46%. Found: C 68.68, H 5.38, N 9.40%.
3.1.6 4-(4-Fluorophenyl)-6-phenyl-3,4-dihydropyrimidine-2(1H)-thione (3f)
IR (KBr, cm−1): 3144 (NH), 1650 (C⚌N), 1558, 1482 (C⚌C), 1190 (C⚌S). 1H-NMR (500 MHz, DMSO-d6) δ: 4.92 (1H, d, J = 5.0 Hz, 4-CH) 5.22 (1H, d, J = 5.0 Hz, 5-CH), 6.93–7.33 (9H, m, Ar–H), 8.85(1H, bs, NH), 9.81 (1H, bs, NH) ppm; 13C-NMR (125 MHz, DMSO-d6) δ: 51.4, 55.8, 100.3, 111.5, 121.1, 125.8, 126.7, 128.7, 129.3, 129.8, 132.3, 133.6,134.7, 155.3, 177.7 ppm; MS(ESI): m/z 285 (M + H)+; Anal. Calcd for C16H13FN2S: C, 67.60; H, 4.57; N, 9.86%. Found: C, 67.50; H, 4.54; N, 9.84%.
3.1.7 4-(3-Methylphenyl)-6-phenyl-3,4-dihydropyrimidine-2(1H)-thione (3g)
IR (KBr, cm−1): 3177 (NH), 1638 (C⚌N), 1566, 1477 (C⚌C), 1194 (C⚌S). 1H-NMR (500 MHz, DMSO-d6) δ: 2.08 (3H, s, CH3), 4.83 (1H, d, J = 5.0 Hz, 4-CH) 5.24 (1H, d, J = 5.0 Hz, 5-CH), 6.83–7.31 (9H, m, Ar–H), 8.80 (1H, bs, NH), 9.74 (1H, bs, NH) ppm; 13C-NMR (125 MHz, DMSO-d6) δ: 55.5, 101.5, 124.2, 126.4, 127.6,127., 128.7, 129.2, 129.6, 133.5, 134.6, 138.2, 144.8, 178.0 ppm; MS(ESI): m/z 281 (M + H)+; Anal. Calcd for C17H16N2S: C, 72.85; H, 5.71; N, 10.00%. Found: C, 72.83; H, 5.69; N, 10.02%.
3.1.8 4-(3-Methoxyphenyl)-6-phenyl-3,4-dihydropyrimidine-2(1H)-thione (3h)
IR (KBr, cm−1): 3152 (NH), 1656 (C⚌N), 1555, 1479 (C⚌C), 1188 (C⚌S). 1H-NMR (500 MHz, DMSO-d6) δ: 3.60 (3H, s, –OCH3), 4.87 (1H, d, J = 5.0 Hz, 4-CH), 5.14 (1H, d, J = 5.0 Hz, 5-CH), 6.90–7.20 (9H, m, Ar–H), 8.79(1H, bs, NH), 9.82 (1H, bs, NH) ppm; 13C-NMR (125 MHz, DMSO-d6) δ: 50.8, 56.5, 100.3, 111.8, 121.3, 126.6, 126.9, 128.5, 129.1, 129.5, 131.9, 133.5,134.5, 155.4, 176.9 ppm; MS(ESI): m/z 297 (M + H)+; Anal. Calcd. for C17H16N2OS: C 68.92, H 5.41, N 9.46%. Found: C 68.88, H 5.40, N 9.44%.
3.1.9 4-Phenyl-6-(3-methylphenyl)-3,4-dihydropyrimidine-2(1H)-thione (3i)
IR (KBr, cm−1): 3152 (NH), 1662 (C⚌N), 1540, 1482 (C⚌C), 1192 (C⚌S). 1H-NMR (500 MHz, DMSO-d6) δ: 2.11 (3H, s, –CH3), 4.89 (1H, d, J = 5.0 Hz, 4-CH), 5.20 (1H, d, J = 5.0 Hz, 5-CH), 6.99–7.46 (9H, m, Ar–H), 8.81(1H, bs, NH), 9.66 (1H, bs, NH) ppm; 13C-NMR (125 MHz, DMSO-d6) δ: 50.5, 56.3, 100.1, 111.3, 121.1, 126.2, 126.6, 128.4, 129.1, 129.6, 132.1, 133.6,134.6, 155.5, 177.5 ppm; MS(ESI): m/z 281 (M + H)+; Anal. Calcd for C17H16N2S: C, 72.85; H, 5.71; N, 10.00%. Found: C, 72.80; H, 5.67; N, 10.04%.
3.1.10 4-Phenyl-6-(3-methoxyphenyl)-3,4-dihydropyrimidine-2(1H)-thione (3j)
IR (KBr, cm−1): 3148 (NH), 1661 (C⚌N), 1550, 1470 (C⚌C), 1191 (C⚌S). 1H-NMR (500 MHz, DMSO-d6) δ: 3.60 (3H, s, –OCH3), 4.90 (1H, d, J = 5.0 Hz, 4-CH), 5.21 (1H, d, J = 5.0 Hz, 5-CH),7.06–7.38 (9H, m, Ar–H), 8.73(1H, bs, NH), 9.79 (1H, bs, NH) ppm; 13C-NMR (125 MHz, DMSO-d6) δ: 50.6, 55.9, 101.3, 110.8, 120.8, 126.7, 127.1, 128.8, 129.1, 129.4, 132.4, 133.4,134.6, 155.1, 177.9 ppm; MS(ESI): m/z 297 (M + H)+; Anal. Calcd. for C17H16N2OS: C 68.92, H 5.41, N 9.46%. Found: C 68.90, H 5.38, N 9.43%.
3.1.11 4-(4-Fluorophenyl)-6-(4-bromophenyl)-3,4-dihydropyrimidine-2(1H)-thione (3k)
IR (KBr, cm−1): 3161 (NH), 1654 (C⚌N), 1554, 1473 (C⚌C), 1187 (C⚌S). 1H-NMR (500 MHz, DMSO-d6) δ: 4.94 (1H, d, J = 5.0 Hz, 4-CH), 5.19 (1H, d, J = 5.0 Hz, 5-CH), 6.92–7.32 (8H, m, Ar–H), 8.91 (1H, bs, NH), 9.76 (1H, bs, NH) ppm; 13C-NMR (125 MHz, DMSO-d6) δ: 50.6, 56.4, 101.5, 111.0, 121.0, 126.5, 126.9, 128.4, 129.4, 129.8, 132.1, 133.8,134.7, 155.0, 177.9 ppm; MS(ESI): m/z 363.9 (M + H)+; Anal. Calcd for C16H12BrFN2S: C, 52.90; H, 3.30; N, 7.71%. Found: C, 52.88; H, 3.28; N, 7.68%.
3.1.12 4-(4-Chlorophenyl)-6-(4-methylphenyl)-3,4-dihydropyrimidine-2(1H)-thione (3l)
IR (KBr, cm−1): 3146 (NH), 1658 (C⚌N), 1549, 1475 (C⚌C), 1184 (C⚌S). 1H-NMR (500 MHz, DMSO-d6) δ: 2.16 (3H, s, CH3) 4.92 (1H, d, J = 5.0 Hz, 4-CH), 5.18 (1H, d, J = 5.0 Hz, 5-CH), 6.97–7.37 (8H, m, Ar–H), 8.78 (1H, bs, NH), 9.82 (1H, bs, NH) ppm; 13C-NMR (125 MHz, DMSO-d6) δ: 49.8, 55.8, 100.0, 111.3, 121.3, 126.5, 127.3, 128.9, 129.3, 129.7, 132.4, 133.9,134.7, 155.2, 174.3 ppm; MS(ESI): m/z 315.45 (M+ H)+; Anal. Calcd for C17H15ClN2S: C, 64.87; H, 4.77; N, 8.90%. Found: C, 64.82; H, 4.73; N, 8.88%.
The structures of isolated products 3a-l were deducted by physical and spectroscopic data such as: IR, 1H-NMR and 13C-NMR spectroscopy, mass and elemental analysis. In IR spectra, the stretching frequency of NH is formed in the region between ν = 3144–3188 cm−1. The stretching vibration of C–N appeared in the region between ν = 1638–1655 cm−1. The stretching frequency of C⚌S is formed in the region between ν = 1180–1194 cm−1. In the 1H-NMR spectra of compound 3a, the two signals around δ = 8.80 and δ = 9.66 ppm correspond to two NH groups at N−1 and N−3 in the product. Presence of these two signals confirmed the formation of desired products in the reaction.
4 Discussion
A novel and efficient procedure for the synthesis of 4,6-diphenyl-3,4-dihydropyrimidine-2(1H)-thione derivatives by the reaction of 1,3-diaryl-2-propen-1-one derivatives and thiourea in the presence of PPh3 as a catalyst is described. Triphenyl phosphine has been widely used, however as an effective catalyst for the synthesis via multicomponent reaction of 1,4-dihydropyridine derivatives (Debache et al., 2009), 3,4-dihydropyrimidine derivatives (Debache et al., 2008), γ-butyrolactone derivatives and highly substituted enones (Bayat et al., 2010) and silyl esters (Liu et al., 2007).
Initially, the cyclo-condensation reaction of 1,3-diaryl-2-propen-1-one (1) and thiourea (2) as a model substrate was investigated to optimize the reaction conditions. At the beginning, the model reaction was carried out to represent a selection of Lewis acids, including AlCl3, ZnCl2, FeCl3, BiCl3, BiBr3, and PPh3 in ethanol at 65 °C (Table 1). It was noted that the best yields are obtained when 0.2 mmol of PPh3 was used (Table 1).
It was noted that solvents played an important role in the synthesis of 4,6-diphenyl-3,4-dihydropyrimidine-2(1H)-thione derivatives. It was found that the best results were obtained in ethanol with 0.2 mmol of PPh3 as catalyst at 65 °C (Table 2; entry 2).
Generally aldehydes bearing both electron-donating and electron-withdrawing groups resulted in the corresponding product in good to excellent yields. The advantage of the present protocol is the use of PPh3 which can be easily recovered and reused several times without any loss in its activity (Table 4) (Fig. 1). The reaction is also characterized by its operational simplicity and high yield of products.
Entry
Cycle
Time (h)
Yield (%)b
1
0
5.0
94
2
1
5.0
92
3
2
5.0
90
4
3
5.0
87
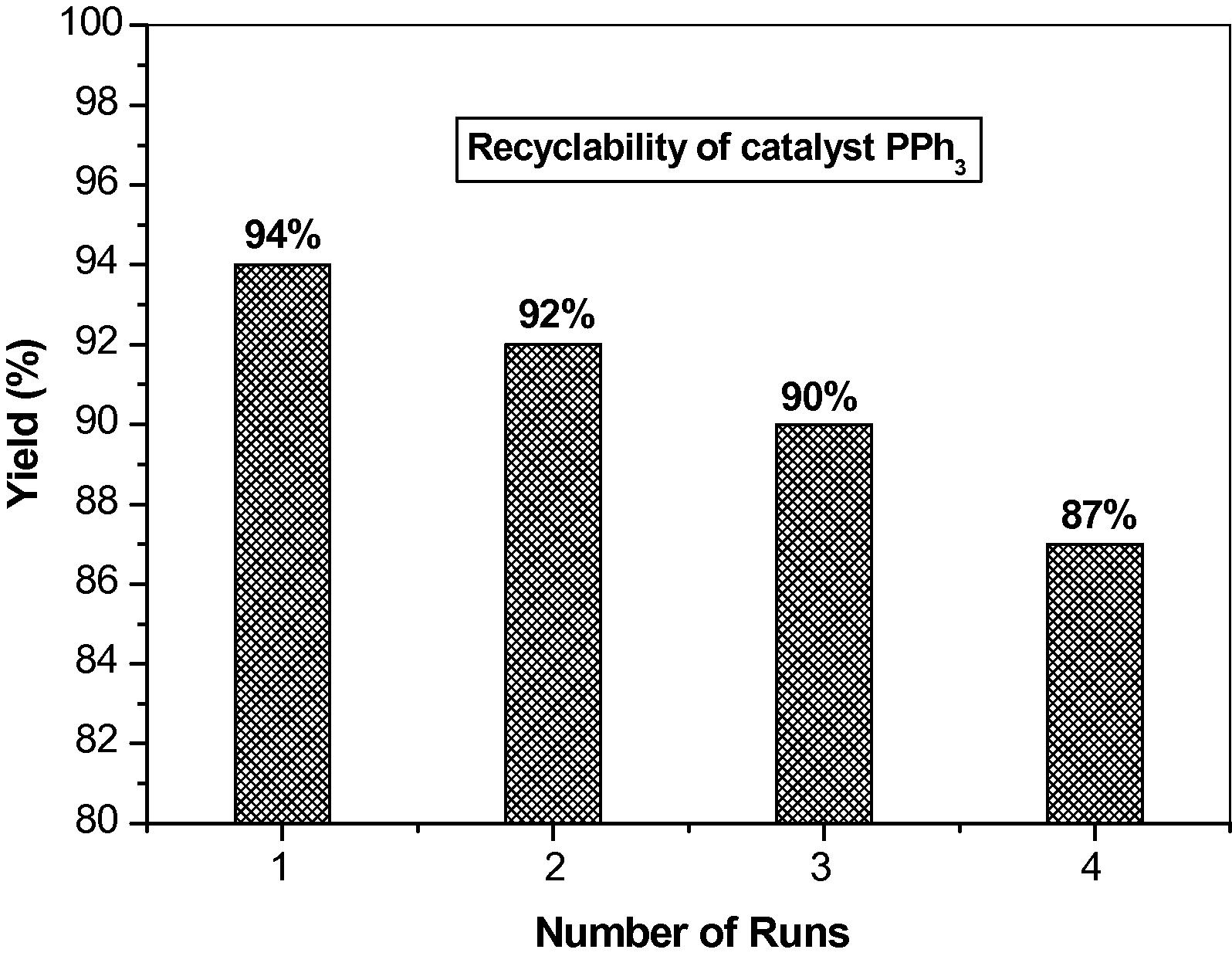
Recyclability of PPh3 for the synthesis of 4,6-diphenyl-3,4-dihydropyrimidine-2(1H)-thione. Yield (%) of the product and number of runs of catalyst PPh3 are shown.
5 Conclusion
In conclusion, an efficient process has been developed for the synthesis of 4,6-diphenyl-3,4-dihydropyrimidine-2(1H)-thione derivatives from the reaction of 1,3-diaryl-2-propen-1-one derivatives and thiourea in the presence of PPh3 (0.2 mmol) at 65 °C in ethanol. All the synthesized compounds were fully characterized by spectroscopic methods such as IR, 1H-NMR, 13C-NMR, mass spectroscopy and elemental analysis. The simplicity of this procedure, together with the eco-friendly nature represents the main advantages of this method. We expect this method will find extensive applications in the field of drug discovery.
The catalyst can be easily recovered, regenerated and reused without loss of its activity, thus providing an economic and environmentally friendly method for other organic reactions.
Acknowledgement
The authors gratefully acknowledge University Grants Commission, Government of India, New Delhi for financial support (Major Research Project: F. No. 40-44 / 2011(SR)).
References
- Dihydropyrimidine calcium channel blockers. II. 3-Substituted-4-aryl-1,4-dihydro-6-methyl-5-pyrimidinecarboxylic acid esters as potent mimics of dihydropyridines. J. Med. Chem.. 1990;33:2629-2635.
- [Google Scholar]
- Triphenylphosphine-catalysed one-pot synthesis of γ-butyrolactone derivatives and highly substituted enones via reaction of dimethyl acetylenedicarboxylate and aryl aldehydes. Tetrahedron Lett.. 2010;51:1873-1875.
- [Google Scholar]
- Aldehyde – urea derivatives of aceto- and oxaloacetic acids. Gazz. Chim. Ital.. 1893;23:360-413.
- [Google Scholar]
- Synthesis and in vitro microbiological evaluation of novel 4-aryl-5-isopropoxycarbonyl-6-methyl-3,4-dihydropyrimidinones. Eur. J. Med. Chem.. 2011;45:367-371.
- [Google Scholar]
- A one-pot Biginelli synthesis of 3,4-dihydropyrimidin-2-(1H)-ones/thiones catalyzed by triphenylphosphine as Lewis base. Tetrahedron Lett.. 2008;49:6119-6121.
- [Google Scholar]
- An efficient one-step synthesis of 1,4-dihydropyridines via a triphenylphosphine-catalyzed three-component Hantzsch reaction under mild conditions. Tetrahedron Lett.. 2009;50:5248-5250.
- [Google Scholar]
- An eco-friendly catalytic route for one-pot synthesis of 2-amino-6-(2-oxo-2Hchromen-3-yl)-4-arylnicotinonitrile derivatives by silica supported perchloric acid (HClO4-SiO2) under solvent-free conditions. Res. Chem. Intermed. doi 2013
- [CrossRef] [Google Scholar]
- Three-component one-pot synthesis of 4,6-diarylpyrimidin-2(1H)-ones under solvent-free conditions in the presence of sulfamic acid as a green and reusable catalyst. Mol. Divers.. 2008;12:191-196.
- [Google Scholar]
- 100 years of the biginelli dihydropyrimidine synthesis. Tetrahedron. 1993;49:6937-6963.
- [Google Scholar]
- Biologically active dihydropyrimidones of the Biginelli-type-a literature survey. Eur. J. Med. Chem.. 2000;35:1043-1052.
- [Google Scholar]
- Recent advances in the Biginelli dihydropyrimidine synthesis. New tricks from an old dog. Acc. Chem. Res.. 2000;33:879-888.
- [Google Scholar]
- Bi(TFA)3 immobilized in [nbpy]FeCl4: an efficient catalyst system for the one-pot synthesis of 4,6-diarylpyrimidin-2(1H)-ones. Catal. Commun.. 2006;7:713-716.
- [Google Scholar]
- Revisit to the Biginelli reaction: a novel and recyclable bioglycerol-based sulfonic acid functionalized carbon catalyst for one-pot synthesis of substituted 3,4-dihydropyrimidin-2-(1H)-ones. Tetrahedron Lett.. 2012;53:1968-1973.
- [Google Scholar]
- Polymer-supported 4-aminoformoyldiphenylammonium triflate (PS-AFDPAT): an effective and recyclable catalyst for the Biginelli reaction. Synth. Commun.. 2009;39(3):475-483.
- [Google Scholar]
- Triphenylphosphine-catalyzed dehydrogenative coupling reaction of carboxylic acids with silanes – A convenient method for the preparation of silyl esters. Adv. Synthesis Catalysis. 2007;349(6):807-811.
- [Google Scholar]
- Microwave-assisted and iodine-catalyzed synthesis of dihydropyrimidin-2-thiones via Biginelli reaction under solvent-free conditions. Synth. Commun.. 2013;43(1):139-146.
- [Google Scholar]
- An efficient one-pot synthesis of 3,4-dihydropyrimidines via a Lewis base catalyzed three-component Biginelli-type reaction under solvent-free conditions. Arab. J. Chem. 2011
- [CrossRef] [Google Scholar]
- Melamine trisulfonic acid: a new, efficient and reusable catalyst for the synthesis of some fused pyranopyrrole derivatives. J. Saudi Chem. Soc 2012 http://dx.doi.org/10.1016/j.jscs.2012.12.009
- [Google Scholar]
- An efficient one-pot multi component synthesis of polyhydroquinoline derivatives through Hantzsch reaction catalysed by Gadolinium triflate. Arab. J. Chem 2012 http://dx.doi.org/10.1016/j.arabjc.2012.10.017
- [Google Scholar]
- Melamine Trisulfonic Acid as an efficient catalyst for the synthesis of 2,6-Dimethyl-4-substituted-1,4-dihydropyridine-3,5-diethyl/dimethylcarboxylate derivatives via Hantzsch reaction in solvent free condition. J. King Saud University – Sci.. 2013;25(3):191-199.
- [Google Scholar]
- Sulfonated carbon catalyzed Biginelli reaction for one-pot synthesis of 3,4-dihydropyrimidin-2(1H)-ones and -thiones. Chin. J. Catal.. 2012;33(4):706-710.
- [Google Scholar]
- Design and synthesis of novel α1a adrenoceptor-selective antagonists. 1. Structure–activity relationship in dihydropyrimidinones. J. Med. Chem.. 1999;42:4764-4777.
- [Google Scholar]
- Synthesis of dihydropyrimidinones via Biginelli multi-component reaction. Tetrahedron Lett.. 2012;53:1543-1545.
- [Google Scholar]
- FeCl3 immobilized in Al-MCM 41: an efficient catalyst system for the Biginelli reaction. Synth. Commun.. 2011;41:826-831.
- [Google Scholar]
- Novel alkaloids from the sponge Batzella sp.: inhibitors of HIV gpl20-human CD4 binding. J. Org. Chem.. 1995;60:1182-1188.
- [Google Scholar]
- H3PMo12O40 catalyzed three-component one-pot synthesis of 4,6-diarylpyrimidin-2(1H)-ones under solvent-free conditions. Chin. Chem. Lett.. 2010;21:269-272.
- [Google Scholar]
- A facile synthesis of 3,4-dihydropyrimidinones/thiones and novel N-dihydro pyrimidinone-decahydroacridine-1,8-diones catalyzed by cellulose sulfuric acid. J. Mole. Catal. A Chem.. 2013;370:197-204.
- [Google Scholar]
- Calcium entry blockers and activators: conformational and structural determinants of dihydropyrimidine calcium channel modulators. J. Med. Chem.. 1995;38:119-129.
- [Google Scholar]
- Synthesis and differential antiproliferative activity of Biginelli compounds against cancer cell lines: monastrol, oxo-monastrol and oxygenated analogues. Bioorg. Chem.. 2006;34:173-182.
- [Google Scholar]
- Ultrasound-assisted synthesis of dihydropyrimidine-2-thiones. J. Serb. Chem. Soc.. 2011;76(5):679-684.
- [Google Scholar]
- Al2O3/MeSO3H: a novel and recyclable catalyst for one-pot synthesis of 3,4-dihydropyrimidinones or their sulfur derivatives in Biginelli condensation. Synth. Commun.. 2009;39(6):958-979.
- [Google Scholar]
- Silica immobilized nickel complex: an efficient and reusable catalyst for microwave-assisted one-pot synthesis of dihydropyrimidinones. Inorg. Chem. Commun.. 2012;17:58-63.
- [Google Scholar]
- Melamine trisulfonic acid: a new, efficient and recyclable catalyst for the synthesis of 3,4-dihydropyrimidin-2(1H)-ones/thiones in the absence of solvent. Chin. Chem. Lett.. 2011;22:318-321.
- [Google Scholar]
- Synthesis of the Tricyclic Portions of Batzelladines A, B and D. Revision of the Stereochemistry of Batzelladines A and D. Tetrahedron Lett.. 1996;37:6977-6980.
- [Google Scholar]
- Dihydropyrimidin-(2H)-ones obtained by ultrasound irradiation: a new class of potential antioxidant agents. Eur. J. Med. Chem.. 2006;41:513-518.
- [Google Scholar]
- Efficient, green, solvent-free synthesis of 3,4-dihydropyrimidin-2(1H)-ones via Biginelli reaction catalyzed by Cu(NO3)2·3H2O. Synth. Commun.. 2010;40:1115-1122.
- [Google Scholar]







