Translate this page into:
In vitro oxidation of aldehyde oxidase from rabbit liver: Specificity toward endogenous substrates
*Tel.: +218 916 395 449 khaledk630@yahoo.co.uk (Khaled S. Al salhen)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Available online 17 September 2013

Abstract
The endogenous vitamins such as pyridoxal (vitamin B6) and all-trans retinaldehyde (vitamin A) are metabolized to more or less toxic metabolites by drug-metabolizing enzymes including aldehyde oxidase (AO; EC 1.2.3.1). To better understand this function, the specificity of the rabbit liver aldehyde oxidase enzyme toward endogenous vitamins was quantitatively studied. Therefore, the present study showed the kinetic parameters of AO for the oxidation of vitamin B6 and vitamin A were measured in partially purified rabbit liver fraction. Km values of AO endogenous vitamin were observed with pyridoxal (21 ± 6.4 μM) and all-trans-retinal (46 ± 9.1 μM) respectively for partially purified rabbit liver fraction. AO from rabbit liver fraction showed high Vmax with vitamin B6 and vitamin A (1.84 ± 0.2 and 1.28 ± 0.1 nmol/min/mg protein, respectively). Therefore, the present study showed the kinetic parameters of AO for the oxidation of vitamin B6 and vitamin A were measured in partially purified rabbit liver fraction. A high affinity and low Km values of AO endogenous vitamin were observed with pyridoxal (21 ± 6.4 μM) and all-trans-retinal (46 ± 9.1 μM), respectively for partially purified rabbit liver fraction. Pyridoxal and all-trans-retinal oxidized to their metabolites (25.2 ± 12.7 and 13.3 ± 4.1 nmol/min/mg protein, respectively) by partially purified rabbit liver aldehyde oxidase. These results confirmed that the hydrophobicity enhances affinity of pyridoxal and all-trans-retinal (aromatic aldehyde) toward AO as excellent substrates. It is concluded these results presented serve as a guide for predicting the susceptibility of endogenous to oxidation by rabbit liver AO.
Keywords
Pyridoxal (vitamin B6)
All-trans retinaldehyde (vitamin A)
Aldehyde oxidase
Molybdo-flavoenzymes
1 Introduction
Xenobiotics are compounds that are foreign to the body, which include drugs, pollutants and other substances that are not normally present in the body and are potentially toxic. Xenobiotic metabolism is the series of metabolic reactions that change the chemical structure of xenobiotics; generally acting to detoxify the toxic chemical compounds. Sometimes, however, the product of xenobiotic metabolism can be the cause of toxic effects (Hodgson and Smart, 2001). Of the biotransformations that occur in animals oxidation plays a major role in the metabolism of foreign compounds. Although the microsomal cytochrome P-450 mono-oxygenase system is of major importance in this respect, enzymes present in the cytosol also contribute to this process. This study is concerned with the enzyme aldehyde oxidase (AO; EC 1.2.3.1) which is a molybdo-flavoenzyme found in nearly every organism from bacteria to humans (Beedham, 2001; Garattini et al., 2003, 2008, 2009; Garattini and Terao, 2011). AO catalyzes the oxidation of many different N-heterocyclic compounds as well as aliphatic and aromatic aldehydes to their corresponding lactam and carboxylic acids respectively (Beedham, 2001; Garattini et al., 2003, 2008; Garattini and Terao, 2011, 2012). Although AO catalyzes the biotransformation of several endogenous compounds, the absolute primary physiological function of AO is yet to be determined. The physiological importance of aldehyde oxidase’s role in aldehyde oxidation is in question due to the fact that the Michaelis constant (Km) for AO is higher for aliphatic aldehydes than is that of another mammalian enzyme, aldehyde dehydrogenase (ALDH) [EC; 1.2.1.3] (Jakoby and Ziegler, 1990; Panoutsopoulos et al., 2004). Two notable endogenous substrates for AO include retinaldehyde and pyridoxal (Beedham, 2001; Garattini et al., 2003, 2008; Garattini and Terao, 2011, 2012; Huang et al., 1999; Kitamura et al., 2006). Retinaldehyde is the principle component of visual pigments and for this reason it has been suggested that aldehyde oxidase may play an important part in the overall visual process since it catalyzes the biotransformation of this aldehyde to its corresponding carboxylic acid, and retinoic acid, which is the active form of vitamin A (Calzei et al., 1995; Garattini et al., 2008; Garattini and Terao, 2011, 2012; Huang et al., 1999; Stanulovic and Chaykin, 1971). The involvement of AO in all-trans retinaldehyde oxidation to all-trans retinoic acid was first seen in rabbit liver cytosol, where it was observed that a fraction of the oxidizing activity did not require an addition of NAD+ and was due to a molybdo-flavoenzyme (Garattini et al., 2008; Tomita et al., 1993; Tsujita et al., 1994). As well as its ability to catalyze the biotransformation of vitamin A metabolite (Fig. 1) AO also converts vitamin B6 (pyridoxal) to 4-pyridoxic acid (Fig. 1) (Tomita et al., 1993). Vitamin B6 is a water-soluble compound that contains a pyridine ring. Vitamin B6 is present in nature in several different forms such as pyridoxal (PL), pyridoxine (PN), pyridoxamine (PM) and their active form pyridoxal 5′-phosphate (PLP) (Fitzpatrick et al., 2007). PLP is the coenzymatically active form of vitamin B6 and plays an important role in maintaining the biochemical homeostasis of the body (Meister, 1990). There are more than 100 PLP-dependent enzymes in a cell that perform essential roles in various metabolic pathways including amino acid metabolism (such as amino acid synthesis and degradation), fatty acid metabolism (such as synthesis of polyunsaturated fatty acids) and carbohydrate metabolism (such as breakdown of glycogen) (Mooney et al., 2009). The preferred degradation route from PLP to 4-pyridoxic acid involves the dephosphorylation of PLP by phosphatase (Jang et al., 2003) followed separately by the actions of aldehyde oxidase and β-nicotinamide adenosine dinucleotide-dependent dehydrogenase (Schwartz and Kjeldgaard, 1951; Stanulovic et al., 1976). In mice Garattini et al. (2008) reported that pyridoxal can be oxidized by purified mouse aldehyde oxidase AOX1 and AOH1, although it is not an efficient substrate in the case of AOH2. Although a wealth of data is available on endogenous substrates of AO they are still being sought (Garattini et al., 2009).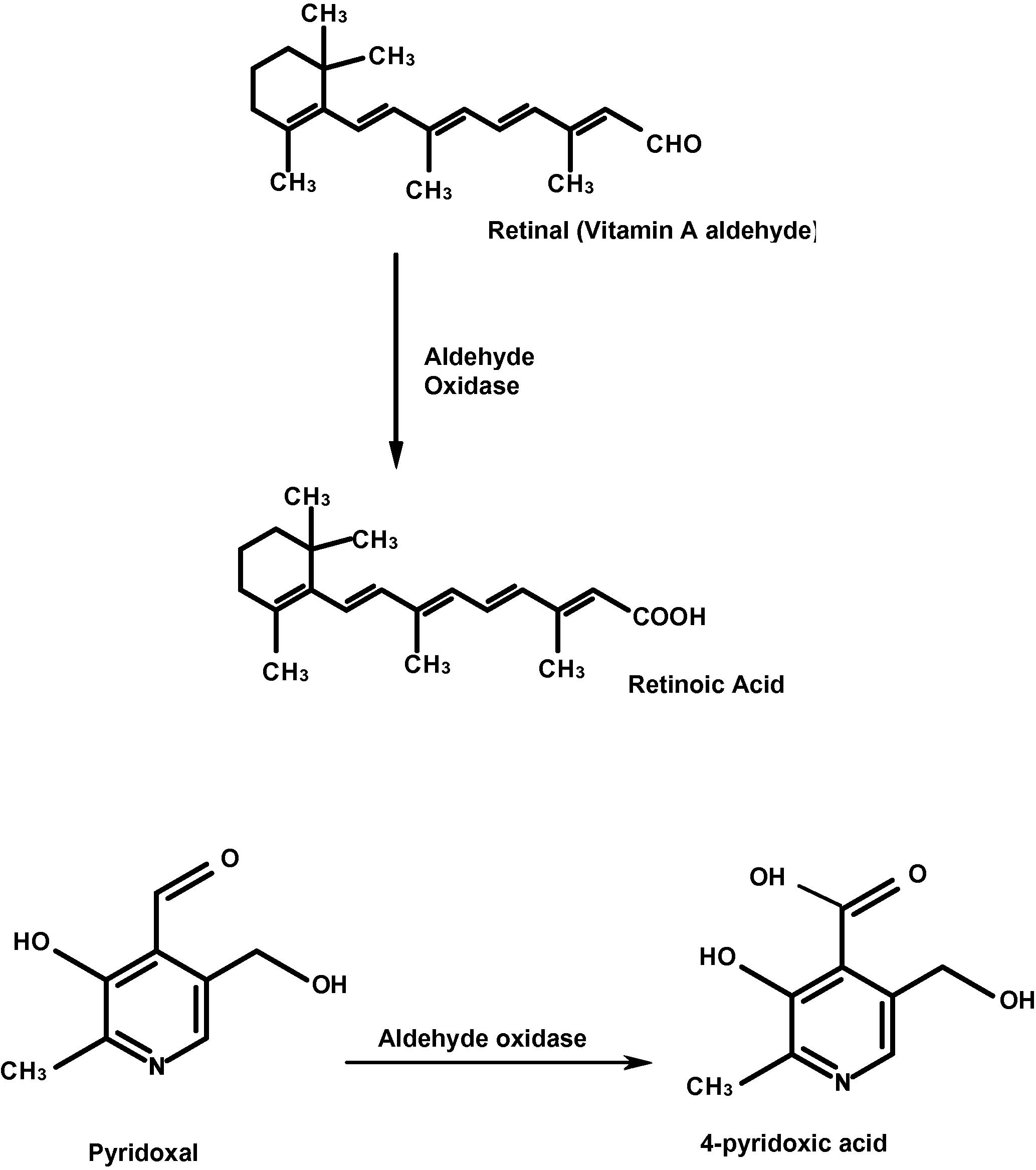
Conversion of retinal (vitamin A) to its corresponding carboxylic acid (retinoic acid) and oxidation of vitamin B6 (pyridoxal) to its corresponding carboxylic acid (4-pyridoxic acid) catalyzed by aldehyde oxidase. Based on Macrae et al., 1984; Tomita et al., 1993.
The activity of AO between animal species varies depending on the substrate considered. Sugihara et al. found the activity of AO in monkeys is higher than in humans when using N1-methylnicotinamide and benzaldehyde as substrates (Sugihara et al., 2006). Klecker et al. (2006) found the activity of AO is highest in mouse toward zebularine substrate than in monkeys and humans (Klecker et al., 2006). Species differences have also been found when using cinchonidine as a substrate where rabbits have higher AO activity than monkeys (Fukiya et al., 2010).
Therefore, the present study investigates the role of AO in the metabolism of pyridoxal and all-trans retinaldehyde in partially purified rabbit liver fractions. The specificity of liver rabbit AO for these substrates was quantitatively explored by determining kinetic constants for a variety of endogenous compounds.
2 Materials and methods
2.1 Chemicals
All chemicals and reagents were obtained from Fisher Scientific and Sigma/Aldrich Chemical Company Ltd., Poole, UK. Mobile phase reagents and solvents were obtained from various companies but were all for HPLC grade purity.
2.2 Preparation of aldehyde oxidase fractions
The New Zealand white male rabbit liver sample was isolated, apportion was taken, weighed, chopped and placed immediately in 3–4 volumes of ice-cold isotonic potassium chloride solution (1.15% KCl w/v) containing 0.1 mM EDTA and homogenized on ice in a homogenizer fitted with a Teflon pestle for 1–2 min at 4 °C. The resulting homogenate was then heated at 55–57 °C for 10 min on a steam bath and ammonium sulfate precipitation as described by Beedham et al. (1995). Rabbit liver fraction was stored in liquid nitrogen until used for spectrophotometric and HPLC analyses.
2.3 Spectrophotometric measurement of enzyme activity
All spectrophotometric aldehyde oxidase assays were conducted using a microplate reader spectrophotometer (BioTek). All assays were carried out in triplicate in 100 μl reaction volumes. All cytosol samples were frozen and thawed only once, and the spectrophotometric data were collected at 5 s intervals for 3–5 min using Gen5™ software on a Windows XP PC connected to the microplate reader spectrophotometer (BioTek). Enzyme activity of partially purified fractions was monitored using all-trans-retinal and pyridoxal. Oxidation of 0.1 mM pyridoxal was then monitored at 388 nm and of 0.1 mM all-trans-retinal was monitored at 380 nm as substrates in the presence of molecular oxygen as the electron acceptor using the molar extinction coefficients for pyridoxal and all-trans-retinal which are 4900 M−1 cm−1 and 43,400 M−1 cm−1, respectively (Peterson and Sober, 1954; Jäger et al., 1996). All reactions were carried out in phosphate buffer saline, pH 7.4 at 37 °C.
2.4 Protein determination
The amount of protein in each sample was calculated using a modification of the method described by Smith et al. using bovine serum albumin (BSA) as standard (Smith et al., 1985). The concentration of protein in initial rabbit liver fraction was 38.9 ± 9.6 mg/ml. The bicinchoninic acid (BCA) based assay is available as a kit from Sigma–Aldrich Co.
2.5 Incubations with partially purified aldehyde oxidase fractions
Pyridoxal and all-trans-retinal (0.1 mM) were incubated with 0.1 ml partially purified rabbit liver fractions at 37 °C in a total volume of 1 ml 67 mM phosphate buffer saline, pH 7.4. Incubations were performed in 1 ml closed vials which were placed in a Dri-Block heater (DB-2D, Techne, UK) and pre-warmed to 37 °C. Aliquots (200 μl) were removed at 5, 10 and 15 min and added to either 100 μl of 20% trichloroacetic acid to terminate the reaction. Samples were centrifuged in a Microfuge 16 Centrifuge (Beckman Coulter) at 10,000 rpm for 1 min and the supernatants were subsequently analyzed by HPLC. In all in vitro assays no spontaneous oxidation of any substrate was observed when control incubations were carried out without cytosol.
2.6 HPLC analysis of all-trans retinal and pyridoxal oxidation
HPLC analysis was carried out using a system supplied by Beckman system gold™, with solvent modules (127 pumps) and a programmable diode array detector (module 168). Chromatographic separation was achieved using a LiChrospher® RP-18 column (C18; 250 mm × 4 mm I.D.5 μM) protected with a guard column (μBondapak C18 Guard). All mobile phases and HPLC methods for the above compounds are summarized in Table 1. Oxidized metabolites were identified by comparison of their HPLC retention times and UV spectra with those of authentic standards. (dH2O): Distilled water. (CH3COONH4): Ammonium acetate. (Na2 HPO4): Di-sodium hydrogen phosphate.
Analyte
How analyte dissolved
Chromatograph column
Mobile phases
Gradient/Isocratic
Flow rate ml/min
Wavelength
References
Pyridoxal
dH2O sonicating
Kromasil 5 μm (25 cm × 4.6 mm, C18) with guard column 5 μm
A: 60 mM Na2HPO4,pH 6.5 B: methanol 25% v/v.
Isocratic
0.8
315 nm UV detector
(Talwar et al., 2003)
All-trans-retinal
Absolute ethanol sonicating
LiChrosphere® 5 μm (25 cm × 4.0 mm, C18) with guard column 5 μm
A: 30 mM CH3COONH4, pH 4.5 B: acetonitrile 70% v/v.
Isocratic
1.0
378 nm diode array detector
Tsujita et al. (1994)
2.7 Determination of kinetic constants for oxidation of all-trans retinal and pyridoxal by aldehyde oxidase
UV Determination: Km (Michaelis–Menten constant) and Vmax (maximum initial velocity) values for the oxidation of all-trans retinal and pyridoxal with rabbit liver fractions were determined spectrophotometrically using a method similar to that described by Beedham et al. (1990). Kinetic constant values were determined using molecular oxygen as an electron acceptor. At least eight different substrate concentrations were used in the range of Km values in 67 mM phosphate buffer saline, pH 7.4. The spectrophotometric method was determined by measurement of the gradient of the line recorded on the curve and expressed as the change in absorbance/time. An Eadie Hofstee V/[S] versus [S] was then plotted. The intercept on the ordinate axis is Vmax; the slope is Km. The line of best fit through the points on the plot was determined using linear regression by least squares method using Microsoft Excel (Microsoft Office).
3 Results and discussion
Activities of the molybdenum hydroxylase fraction from rabbit liver were measured spectrophotometrically using 0.1 mM final substrate concentrations of all-trans retinal and pyridoxal specific substrates of aldehyde oxidase (Tomita et al., 1993; Macrae et al., 1984). The ratio of oxidation rates of all-trans retinal and pyridoxal was calculated to reflect the relative aldehyde oxidase activity in the rabbit liver investigated (Table 2). The results demonstrate the high aldehyde oxidase activity of rabbit liver toward pyridoxal was much higher than all-trans retinal substrate (Table 2). In order to determine that there was definitely activity present in the rabbit liver for these endogenous substrates a HPLC assay was performed with these substrates. This is shown in Fig. 2, where the product (4-pyridoxic acid and retinoic acid) was detected even after extended incubation periods. In agreement with the present result Schwartz and Kjeldgaard, 1951 found that pyridoxal, the vitamin B6 precursor, is oxidized to 4-pyridoxic acid by the human enzyme (Schwartz and Kjeldgaard, 1951), pyridoxal was rapidly oxidized to 4-pyridoxic acid by the liver (28.1 ± 19.3 nmol/min/mg protein) (Merrill et al., 1994) and by the two mouse liver aldehyde oxidases (Garattini et al., 2009). Pyridoxal is currently the sole example of a substrate that shows a certain degree of selectivity for a specific aldehyde oxidase protein as it is not recognized by purified mouse (Terao et al., 2009). These findings are consistent with Huang and Ichikawa (1994) and Tsujita et al. (1994) who first observed the role of AO in the oxidation of all-trans-retinaldehyde to retinoic acid without NAD+ in rabbit by liver AO cytosol (Huang and Ichikawa, 1994; Tsujita et al., 1994). The values are expressed as means ± SD.
Enzyme fraction
All-trans retinal oxidase activity (nmol/min/mg protein) (0.1 mM)
Pyridoxal oxidase activity (nmol/min/mg protein) (0.1 mM)
Rabbit liver aldehyde oxidase (N = 6)
13.3 ± 4.1
25.2 ± 12.7
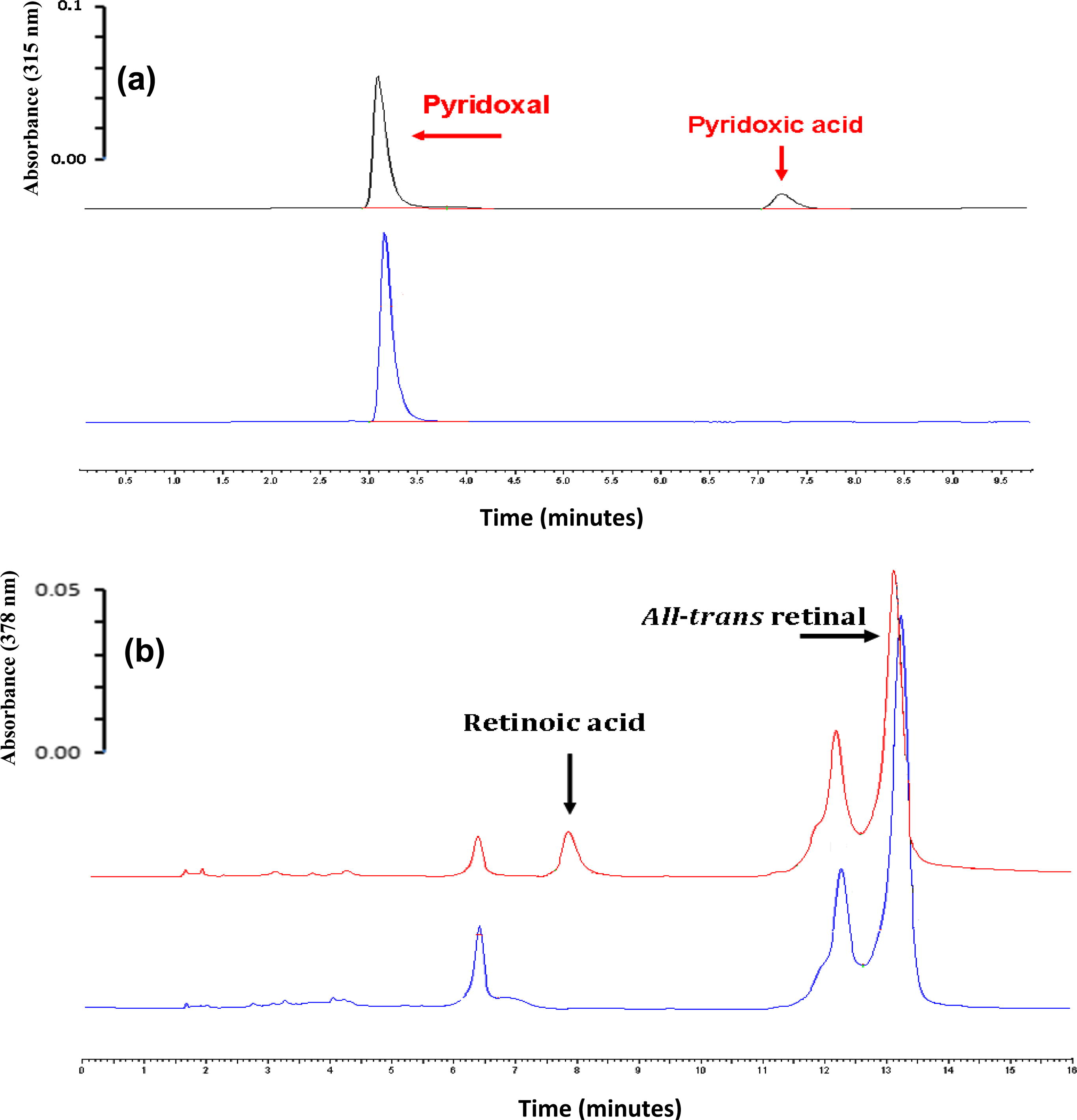
HPLC-UV analysis of the in vitro biotransformation of endogenous compounds (pyridoxal and all-trans retinal) by partially purified rabbit liver fraction. HPLC chromatograms are offset on the vertical axis to allow comparison between different incubation times. (a) Incubation rabbit liver fraction with pyridoxal at 37 °C for 0 (blue line) and 15 min (red line) (b) Incubation rabbit liver fraction with all-trans retinal. Blue line is a zero minute incubation and red line for 15 min incubation.
Km and Vmax values for the formation of 4-pyridoxic acid and retinoic acid metabolites, also determined spectrophotometrically as described in Methods, by rabbit enzyme fractions are tabulated in Table 3 and Figs. 3 and 4. The kinetic parameters of pyridoxal and all-trans-retinal were found to be: Km 21 ± 6.4 and 46 ± 9.1 μM, respectively and Vmax 1.84 ± 0.2 and 1.28 ± 0.1 nmol/mg/min, respectively (Mean ± Standard deviation (SD), N = 6) for rabbit liver aldehyde oxidase using molecular oxygen as an electron acceptor although, rabbit liver enzyme gave the lowest km value for 4-pyridoxic acid formation (Table 3). The lowest Km value for an AO substrate was found with pyridoxal and Km value for pyridoxal was ∼2 lower than that for all-trans-retinal as a substrate. The results of this study indicated that pyridoxal is a better substrate of rabbit AO than all-trans-retinal (Table 3) which has been discovered in rabbit liver cytosol (Tomita et al., 1993) and confirmed using purified preparations of mouse liver AOX1(Huang et al., 1999; Vila et al., 2004). In other hands, retinaldehyde has been one of the best substrates not only of mouse AO, but also of aldehyde dehydrogenases (Duester, 2000; Vasiliou and Nebert, 2005) which have long been known to catalyze the oxidation of retinaldehyde and to play a critical role in the morphogenetic activity of retinoic acid during the development of the vertebrate embryo (Duester, 2008). A similar finding of pyridoxal oxidase has been made in detailed studies of the enzyme purified from rabbit liver, which had higher activity (Choi et al., 1983). This activity agrees well with the pharmacokinetics of vitamin B6 metabolism in humans (Wozenski et al., 1980) and other animals (Colombini and McCoy, 1970; Johansson et al., 1974). Furthermore, this study confirmed that hydrophobicity enhances affinity of pyridoxal and all-trans-retinal (aromatic aldehyde) toward AO as excellent substrates (Beedham, 2001; Garattini and Terao, 2013; Panoutsopoulos et al., 2004). The values are expressed as means ± SD.
Enzyme fraction
Substrates
Km (μM)
Vmax (nmol/min/mg) protein
Rabbit liver aldehyde oxidase (N = 6)
Pyridoxal
21 ± 6.4
1.84 ± 0.2
All-trans-retinal
46 ± 9.1
1.28 ± 0.1
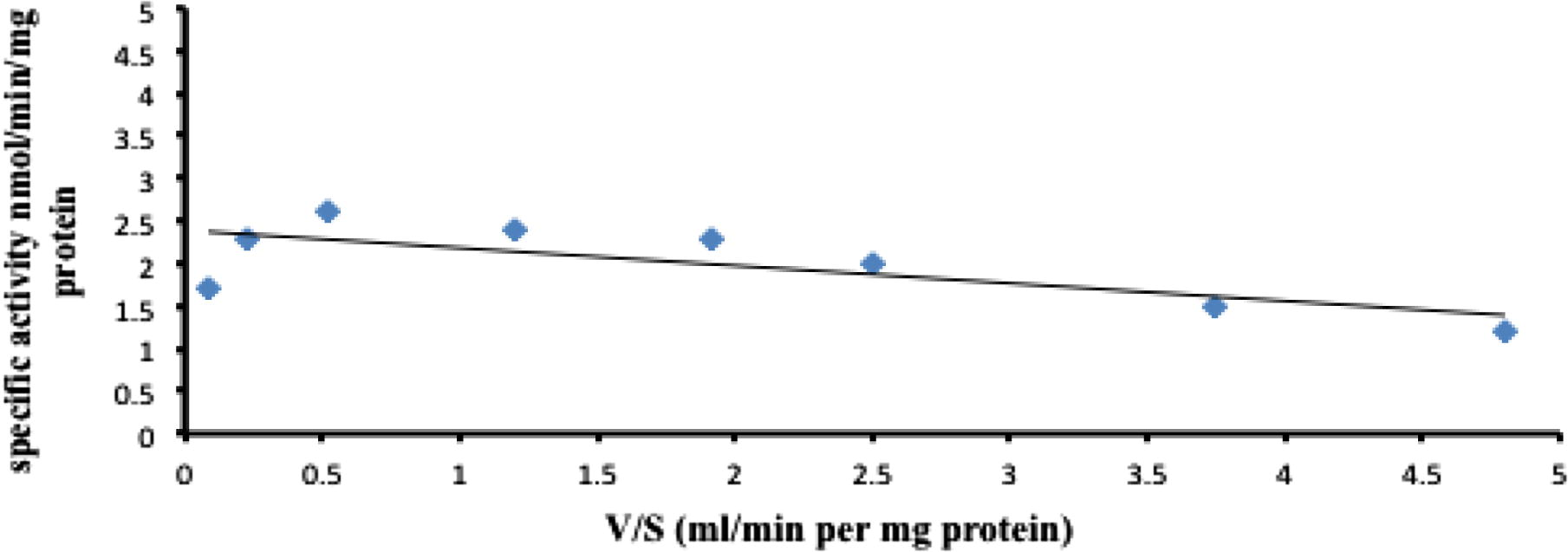
Eadie-Hofstee plot for the oxidation of pyridoxal by rabbit liver aldehyde oxidase. The substrate concentrations were between 25–100 μM.
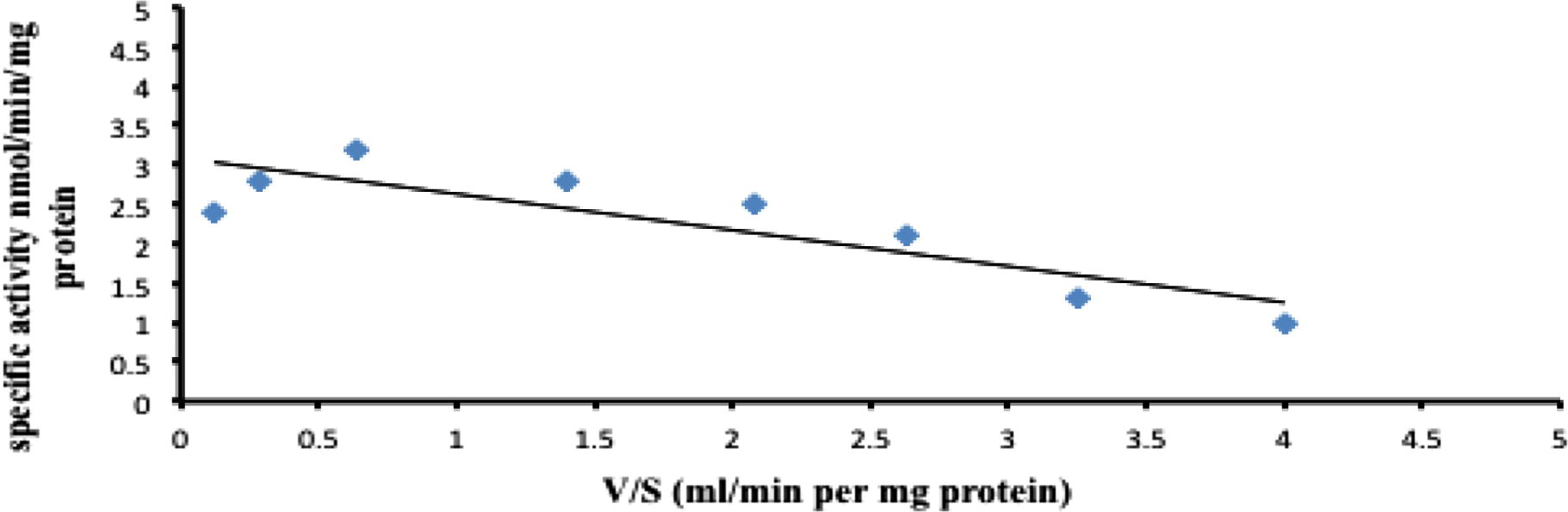
Eadie-Hofstee plot for the oxidation of all-trans-retinal by rabbit liver aldehyde oxidase. The substrate concentrations were between 25–100 μM.
However, species variation in the levels of aldehyde oxidase has been reported (Beedham, 1987; Rashidi and Nazemiyeh, 2010). This marked variation in substrate specificity of aldehyde oxidase would make it difficult to extrapolate the results obtained from one animal to another. For instance, carbazeran is a good substrate for guinea-pig, baboon and human liver aldehyde oxidase, whereas it does not serve as a substrate for rabbit enzyme (Beedham, 1987). Conversely, methotrexate is a more efficient substrate for rabbit liver aldehyde oxidase than that of other species (Kitamura et al., 1999). Furthermore, the site of substrate oxidation catalyzed by aldehyde oxidase may be species-dependent. N1-methylnicotinamide is oxidized to two metabolites, a 2-pyridone (N1-methyl-2-pyridone-5-carboxamide) and a 4-pyridone (N1-methyl-4-pyridone-3-carboxamide) by liver aldehyde oxidase from various species; however, the ratio of the 4-pyridone to the 2-pyridone metabolite differs from one species to another (Stanulovic and Chaykin, 1971).
It is concluded that difference in activities of endogenous substrates metabolizing aldehyde oxidase may be controlled by a number o factors such as gene sequence, protein structure and hormonal regulation of AO activity (Beedham, 2001 and Beedham, 2010). Overall, the data presented serve as a guide for predicting the susceptibility of endogenous to oxidation by rabbit liver aldehyde oxidase, because the species variation and substrate specificity of this enzyme indicates that this enzyme has a relatively large active/binding site with a marked flexibility from one animal to another (Beedham, 1987; Garattini and Terao, 2013; Rashidi and Nazemiyeh, 2010).
Conflict of interest
Declared none.
Acknowledgment
Declared none.
References
- Molybdenum hydroxylases: biological distribution and substrate-inhibitor specifcity. In: Ellis G.P., West G.B., eds. Progress in Medicinal Chemistry. Amsterdam, New York, Oxford: Elsevier Science Publishers (Biomedical Division); 1987. p. :8-127. (24)
- [Google Scholar]
- Molybdenum hydroxylase. In: Ioannides C., ed. Enzyme Systems that Metabolise Drugs and Other Xenobiotics. John Wiley and Sons Ltd.; 2001.
- [Google Scholar]
- Beedham, C., 2010. Xanthine oxidoreductase and aldehyde oxidase. (second ed. Chapter 4.10): Comprehensive Toxicology. Copyright © 2010 Elsevier Ltd. All rights reserved.
- 1-Substituted phthalazines as probes of the substrate-binding site of mammalian molybdenum hydroxylases. Biochem. Pharmacol.. 1990;39:1213-1221.
- [Google Scholar]
- Substrate specificity of human liver aldehyde oxidase toward substituted quinazolines and phthalazines: a comparison with hepatic enzyme from guinea pig, rabbit, and baboon. Arch. Biochem. Biophys.. 1995;319:481-490.
- [Google Scholar]
- Purification, cDNA cloning, and tissue distribution of bovine liver aldehyde oxidase. J. Biol. Chem.. 1995;270:31037-31045.
- [Google Scholar]
- Kinetic properties of pyridoxamine (pyridoxine)-5′-phosphate oxidase from rabbit liver. J. Biol. Chem.. 1983;258:840-845.
- [Google Scholar]
- Vitamin B6 metabolism. Utilization of [14C] pyridoxine by the normal mouse. Biochemistry. 1970;9:533-538.
- [Google Scholar]
- Families of retinoid dehydrogenases regulating vitamin A function: production of visual pigment and retinoic acid. Eur. J. Biochem.. 2000;267:4315-4324.
- [Google Scholar]
- Retinoic acid synthesis and signaling during early organogenesis. Cell. 2008;134:921-931.
- [Google Scholar]
- Two independent routes of de novo vitamin B6 biosynthesis: not that different after all. J. Biochem.. 2007;407:1-13.
- [Google Scholar]
- A single amino acid substitution confers high cinchonidine oxidation activity comparable with that of rabbit to monkey aldehyde oxidase 1. Drug Metab. Dispos.. 2010;38:302-307.
- [Google Scholar]
- Increasing recognition of the importance of aldehyde oxidase in drug development and discovery. Drug Metab. Rev.. 2011;43:374-386.
- [Google Scholar]
- The role of aldehyde oxidase in drug metabolism. Expert Opin. Drug Metab. Toxicol.. 2012;8:487-503.
- [Google Scholar]
- Aldehyde oxidase and its importance in novel drug discovery: present and future challenges. Expert. Opin. Drug Discov.. 2013;8:641-654.
- [Google Scholar]
- Mammalian molybdo-flavoenzymes, an expanding family of proteins: structure, genetics, regulation, function and pathophysiology. Biochem. J.. 2003;372:15-32.
- [Google Scholar]
- Mammalian aldehyde oxidases: genetics, evolution and biochemistry. Cell Mol. Life Sci.. 2008;65:1019-1048.
- [Google Scholar]
- Introduction to Biochemical Toxicology (third ed.). Canada: Wiley-Interscience; 2001. (pp. 1–9)
- Two different enzymes are primarily responsible for retinoic acid synthesis in rabbit liver cytosol. Biochem. Biophys. Res. Commun.. 1994;205:1278-1283.
- [Google Scholar]
- Molecular cloning of retinal oxidase/aldehyde oxidase cDNAs from rabbit and mouse livers and functional expression of recombinant mouse retinal oxidase cDNA in Escherichia coli. Arch. Biochem. Biophys.. 1999;364:264-272.
- [Google Scholar]
- Opsin/all-trans-retinal complex activates transducin by different mechanisms than photolyzed rhodopsin. Biochemistry. 1996;35:2901-2908.
- [Google Scholar]
- Human pyridoxal phosphatase. Molecular cloning, functional expression, and tissue distribution. J. Biol. Chem.. 2003;278:50040-50046.
- [Google Scholar]
- Metabolic interconversions of different forms of vitamin B6. J. Biol. Chem.. 1974;249:6040-6046.
- [Google Scholar]
- Variation of hepatic methotrexate 7-hydroxylase activity in animals and humans. IUBMB Life. 1999;48:607-611.
- [Google Scholar]
- Drug-metabolizing ability of molybdenum hydroxylases. Drug Metab. Pharmacokinet.. 2006;21:83-98.
- [Google Scholar]
- Zebularine metabolism by aldehyde oxidase in hepatic cytosol from humans, monkeys, dogs, rats, and mice: influence of sex and inhibitors. Bioorg. Med. Chem.. 2006;14:62-66.
- [Google Scholar]
- Chromatographic determination of vitamins in dairyproducts. In: RSC Proceedings, Challenges in Dairy Analytical Techniques. Reading, UK: Royal Society of Chemistry; 1984. p. :155-166.
- [Google Scholar]
- Vitamin B6: a long known compound of surprising complexity. Molecules. 2009;14:329-351.
- [Google Scholar]
- Contribution of aldehyde oxidase, xanthine oxidase, and aldehyde dehydrogenase on the oxidation of aromatic aldehydes. Chem. Res. Toxicol.. 2004;17:1368-1376.
- [Google Scholar]
- Preparation of crystalline phosphorylated derivatives of vitamin B6. J. Amer. Chem. Soc.. 1954;76:169-173.
- [Google Scholar]
- Inhibitory effects of flavonoids on molybdenum hydroxylases activity. Expert Opin. Drug Metab. Toxicol.. 2010;6:133-152.
- [Google Scholar]
- The enzymic oxidation of pyridoxal by liver aldehyde oxidase. Biochem. J.. 1951;48:333-337.
- [Google Scholar]
- Aldehyde oxidase: catalysis of the oxidation of N1-methylnicotinamide and pyridoxal. Arch. Biochem. Biophys.. 1971;145:27-34.
- [Google Scholar]
- New pathway of conversion of pyridoxal to 4-pyridoxic acid. Enzyme. 1976;21:357-369.
- [Google Scholar]
- Estimation of aldehyde oxidase activity in vivo from conversionratio of N1-methylnicotinamide to pyridones, and intraspecies variation of the enzyme activity in rats. Am. Soc. Pharmacol. Exp. Ther.. 2006;34:208-212.
- [Google Scholar]
- Optimization and validation of a sensitive high-performance liquid chromatography assay for routine measurement of pyridoxal 5-phosphate in human plasma and red cells using pre-column semicarbazide derivatization. J. Chromatogr., B: Anal. Technol. Biomed. Life Sci. 2003;792:333-343.
- [Google Scholar]
- Role of the molybdoflavoenzyme aldehyde oxidase homolog 2 in the biosynthesis of retinoic acid: generation and characterization of a knockout mouse. Mol. Cell Biol.. 2009;29:357-377.
- [Google Scholar]
- Characteristic properties of retinal oxidase (retinoic acid synthase) from rabbit hepatocytes. Biochim. Biophys. Acta. 1994;1204:108-116.
- [Google Scholar]
- Analysis and update of the human aldehyde dehydrogenase (ALDH) gene family. Hum. Genomics. 2005;2:138-143.
- [Google Scholar]
- Regulation and biochemistry of mouse molybdo-flavoenzymes. The DBA/2 mouse is selectively deficient in the expression of aldehyde oxidase homologues 1 and 2 and represents a unique source for the purification and characterization of aldehyde oxidase. J. Biol. Chem.. 2004;279:8668-8683.
- [Google Scholar]







