Translate this page into:
Digestive α-amylase of Bacterocera oleae Gmelin (Diptera: Tephritidae): Biochemical characterization and effect of proteinaceous inhibitor
*Corresponding author. Tel.: +98 0131 6690485; fax: +98 0131 6690281 arash.zibaee@gmx.com (Arash Zibaee) arash.zibaee@guilan.ac.ir (Arash Zibaee)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Digestive α-amylase of Bacterocera oleae larvae was characterized and treated by an inhibitor to gain a better understanding of the degradation of nutritional molecules as a potential target for controlling the pest. Presence of α-amylase was confirmed in the gut of olive fruit fly through the use of a negative control in dinitrosalicylic acid procedure. An optimal pH of 5 was found for amylolytic activity in the gut. The enzyme had optimal activity in a broad range of temperatures 20–45 °C. Among used cations and specific inhibitors, Ca2+, phenylmethylsulphonyl fluoride (PMSF) and ethylene glycol-bis (β-aminoethylether) N,N,N′,N′-tetraacetic acid (EGTA) had statistical differences on amylolytic activity indicating the presence of amino acid triad and Ca2+ in active site of the enzyme. A proteinaceous α-amylase inhibitor was extracted from Polygonum persicaria, a medicinal plant, that widely grows in North of Iran. IC50 value of PPAI was 0.062 mg/ml (i.e. 0.062 mg/ml of extracted inhibitor inhibited 50% of amylolytic activity in the gut of B. oleae larvae) and was temperature and pH dependent. The use of enzyme inhibitors from different plant sources may serve as an important pest control strategy via plant breeding programs. Identification of genes responsible for these inhibitor proteins could be a first step to provide a resistant variety of olive.
Keywords
Bacterocera oleae
α-Amylase
Characterization
Plant origin inhibitor
1 Introduction
Dietary carbohydrates are one of the most important macromolecules for insects and play a major role in physiological processes. To utilize carbohydrate sources in plants, herbivorous insects have to disrupt plant cell walls through the use of several enzymes including b-glucanases, xylanases, and pectinases (Terra and Ferreira, 2005). When carbohydrates reach the midgut lumen, hydrolysis is initiated by the action of amylases and glycosidases. α-Amylases play a central role in carbohydrate metabolism of microorganisms, plants, and animals. α-Amylases (1,4-α-d-glucan-4-glucanohydrolase; EC 3.2.1.1) are endoglycosidases which catalyze the hydrolysis of internal α-1,4-d-glucosidic linkages in starch and dextrins, thereby generating smaller dextrins and oligosaccharides with a C1-OH group in the α-anomeric configuration (Terra and Ferreira, 2005). Understanding the digestive enzyme function is essential especially when developing methods of insect control such as those involving the use of enzyme inhibitors in transgenic plants. Comparatively less is known about the inhibitors of alpha-amylase which might, on the other hand, be equally attractive candidates for conferring pest resistance to transgenic plants since many of them inhibit both proteinases and alpha-amylase (Fei et al., 2008).
Insect α-amylases are the calcium-dependent enzymes and may be activated by chloride at optimal pH (Terra and Ferreira, 1994). The best known insect α-amylase has been studies in Tenebrio molitor L. (Coleoptera: Tenebrionidae) and has been shown to consist of three domains in which central domain (domain A) is a (b/a) 8-barrel that comprises core of the molecule and includes catalytic amino acid residues. Domains B and C are almost opposite to each other, on each side of domain A (Strobl et al., 1998). Evolutionary, food sources play a critical role on the abundance and activity of insect α-amylases so that feeding on wool and plant tissues causes the lowest and the highest amylolytic activity, respectively (Zibaee et al., 2008).
Olive fruit fly, Bacterocera oleae Gmelin (Diptera: Tephritidae) is the most destructive pest of olive in Africa, Southern Europe, India, western Asia and California (Richard et al., 2003). Larvae feed inside the fruits and cause fruit loss before harvesting, decreasing oil quality and allowing for penetration of plant pathogens. Females lay eggs into the skin of the olive using their ovipositor (Richard et al., 2003). Larvae intensively feed inside the fruit, pupate and adult flies exit to disperse and mate.
Olive fruit fly has been introduced to the North of Iran since 2004 and causes severe damages almost 100% in some plant varieties (Mirrahimi et al., 2008). Control tactics of olive fruit fly rely on using pheromone and bite trap as well as chemical spraying. One of the promising controls of insect pests might be using enzyme inhibitors of plant origin. These inhibitors are known to be widespread in nature and comprise of one of the most abundant classes of proteins in the world because of their role in plant defense against insect herbivory (Laskowski and Kato, 1980). Comparatively less is known about the inhibitors of alpha-amylase which might, on the other hand, be equally attractive candidates for conferring pest resistance to transgenic plants since many of them inhibit both proteinases and alpha-amylase (Fei et al., 2008). Before designing any molecular and ecological experiments to introduce proteinaceous inhibitors, characterization of digestive enzymes like α-amylase is mandatory for understanding the digestion of nutritional molecules and determining the effects of xenobiotic proteins on the digestive system of insects. Hence, the objectives of the current study were to characterize digestive α-amylase of B. oleae and evaluate the effect of proteinaceous extract of Polygonum persicaria L (Polygonaceae) on the enzyme. In detail, (i) the presence of the enzyme was confirmed by using a negative control, (ii) optimal pH and temperature were determined, (iii) nature of enzyme’s active site was determined by using cations and specific inhibitors and (iv) effective concentration of PPAI was determined on B. oleae α-amylase.
2 Material and methods
2.1 Insect rearing
The colony of B. oleae was established from field infested olives (variety Amigdalifolia) collected from Roudbar (North of Iran) in October, 2012. Larvae were reared on olive fruits (variety Amigdalifolia) at 25 ± 1 °C, 70% RH and 16L:8D in the laboratory. Third instar larvae were selected randomly for biochemical studies.
2.2 Sample preparation and enzyme assays
Third larval instars were separated from fruits and dissected under a stereomicroscope in saline solution (NaCl, 10 mM). Larval gut was removed and homogenized in pre-cooled homogenizer in distilled water (w/v). Homogenates were centrifuged at 13,000 rpm for 15 min at 4 °C. Supernatant was separated and kept at −20 °C for subsequent experiments as the enzyme source (Hosseinkhani and Nemat-Gorgani, 2003).
2.3 Determination of α-amylase activity
Activity of α-amylase was assayed according to a method described by Bernfeld (1955) using dinitrosalicilic acid (DNS) as the reagent and 1% (w/v) soluble starch as substrate with some modifications. Reaction mixture consisted of 50 μl Tris–HCl buffer1 (20 mM, pH 7), 20 μl of starch and 10 μl of enzyme sample. The reaction was stopped after 30 min by the addition of 80 μl DNS2 and heating in boiling water for 10 min prior to read absorbance at 545 nm. One unit of α-amylase activity was defined as the amount of enzyme required to produce 1 mg maltose in 30 min at 35 °C. The negative control contained all reaction mixtures with pre-boiled enzyme (for 15 min) to prove the enzyme presence in the samples.
2.4 Effect of pH and temperature on α-amylase activity
The effects of temperature and pH on α-amylase activity were evaluated by incubating enzyme reaction in different pH sets (3–12) for determination of optimal pH and different temperature ranges (20, 25, 30, 35, 40, 45, 50 and 60 °C). The procedure was continued as described above and absorbance was read at 545 nm.
2.5 Effect of different cations and inhibitors on α-amylase activity
Different concentrations of cations (0.5, 3 and 5 mM) and inhibitors (1, 3 and 5 mM) were assayed to find their possible effects on α-amylase activity in B. oleae. Ca2+, Cu2+, Fe2+, K+, Mg2+, Mn+, Na+ and Zn2+ were used as cations. Control consisted of substrate, buffer and enzyme. Inhibitors were ethylenediamide tetraacetic acid (EDTA), ethylene glycol-bis (β-aminoethylether) N,N,N′,N′-tetraacetic acid (EGTA), triethylenetetramine hexaacetic acid (TTHA), diethyldithiocarbamate (DTC), phenanthrolien and phenylmethylsulphonyl fluoride (PMSF). These compounds were added to the assay mixture, then incubation was done at 30 °C for 30 min, and absorbance was recorded at 545 nm.
2.6 Extraction of α-amylase inhibitor from Polygonum persicaria (PPAI)
PPAI was extracted from stems as described by Baker (1989) and Melo et al. (1999). Powdered stems (10 g each) were mixed with a solution of 0.1 M NaCl, stirred for 2 h, and then centrifuged at 5000 rpm for 20 min. The pellet was discarded, and the supernatant was incubated at 70 °C for 20 min to inactivate major endogenous enzymes. Fractionation of the supernatant was done using 60% concentration of ammonium sulfate followed by centrifugation at 5000 rpm for 20 min at 4 °C. The 60% pellet containing the highest fraction of α-amylase inhibitors was dissolved in ice-cold sodium phosphate buffer (0.02 M, pH 7.1) and dialyzed overnight against the same buffer. This dialyzed solution was used as a source of amylase inhibitors in enzyme assays.
2.7 Inhibition of α-amylase by different concentrations of PPAI
To find possible inhibition of the digestive α-amylase, 50 μl of Tris–HCl buffer (20 mM, pH 7), 20 μl of 1% starch and each concentration of PPAI separately (0, 0.05, 0.1, 0.3, 0.5, 1 mg/ml) were incubated for 5 min. Then, 10 μl of the enzyme was added and the reaction continued as described earlier. Control contains buffer, starch 1% and each concentration of PPAI) separately3. Absorbance was recorded at 545 nm.
2.8 Effect of pH and temperature on α-amylase inhibition by PPAI
Effect of pH on α-amylase inhibition by PPAI was determined at different pH values using Tris–HCl buffer (20 mM) with pH set from 4–10. The amylase activity was assayed after incubation of the reaction mixture containing Tris–HCl buffer, starch 1%, PPAI (IC50 concentration) and enzyme. Absorbance was recorded at 545 nm. To find the effect of temperature on α-amylase inhibition by PPAI, reaction mixture containing Tris–HCl (20 mM pH, 9), starch 1%, PPAI (IC50 concentration) and enzyme was incubated at different temperature sets 20, 25, 30, 35, 40, 45, 50 and 60 °C. Absorbance was recorded at 545 nm.
2.9 Protein assay and statistical analysis
Protein concentrations were assayed according to the method described by Lowry et al. (1951). Data were analyzed by one-way analysis of variance (ANOVA) followed by Tukey’s test where significant differences were found at P = 0.05. IC50 values were calculated by POLO-PC software.
3 Results
Presence of α-amylase has been proved in larvae of B. oleae by using a negative control. Then, properties of the enzyme were determined under biochemical conditions. Present study demonstrated an optimal pH of 5 and a temperature range of 20–45 °C for amylolytic activity in the gut of B. oleae (Fig. 1). Regarding optimal temperature, a wide range of amylolytic optimal activity was observed in a temperature range from 20–45 °C (Fig. 1).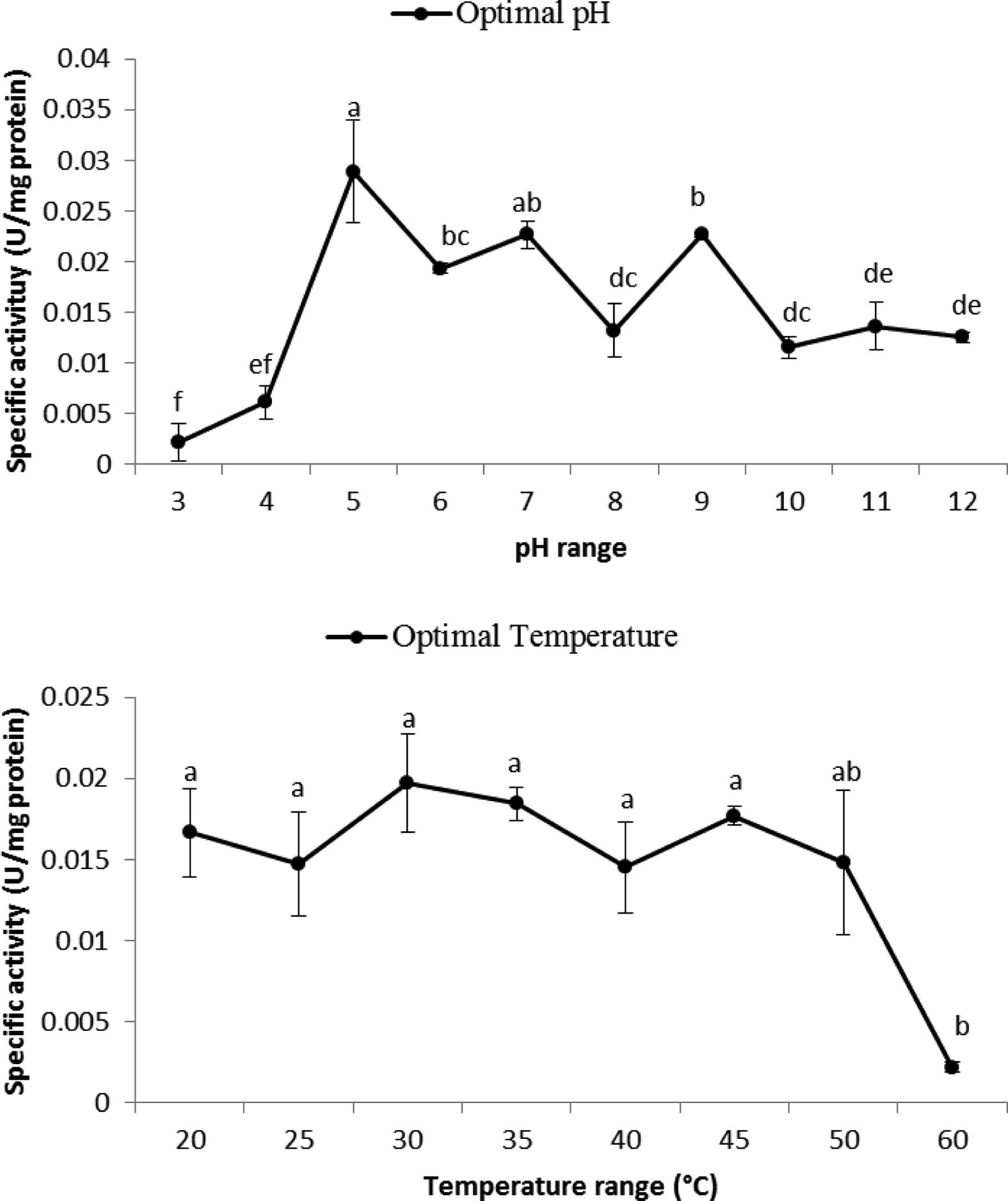
Optimal pH and temperature determination for digestive α-amylase of B. oleae larvae. Statistical differences have been shown by various letters (Tukey’s test, p ⩽ 0.05).
The present investigation revealed that Ca2+ at all concentrations and Mn+ (at 3 mM) significantly increased amylolytic activity in the midgut of B. oleae while other ions had no effect on α-amylase activity (Table 1). It was proved the presence of calcium in active site of the enzyme since chelating agent of calcium (EGTA) significantly decreased amylolytic activity (Table 2). Although, other chelating agents including DTC (for Cu2+) and TTHA (for Mg2+) have negative effects on enzymatic activity but their effect was not in accordance by ion assays (Table 2). Control consisted of substrate, buffer and enzyme. (F = 8.75, N = 30 and n = 3). Control consisted of substrate, buffer and enzyme. (F = 3.21, N = 30 and n = 3).
Compound
Concentration
Relative activity
Control
–
100
Ca
0.5
218.27*
3
174.73*
5
149.46*
Cu
0.5
74.70
3
60.58
5
0*
Fe
0.5
100.95
3
88.72*
5
75.29*
K
0.5
139.03
3
131.62
5
111.39
Mg
0.5
41.98*
3
89.72
5
39.27*
Mn
0.5
133.01
3
237.10*
5
139.62
Na
0.5
45.56*
3
59.29*
5
22.44*
Zn
0.5
57.99*
3
60.86*
5
64.34*
Compound
Concentration
Relative activity
Control
–
100
DTC
1
10.52*
3
44.95*
5
6.35*
Phenanthroline
1
15.57*
3
0*
5
0*
EGTA
1
50.65*
3
45.61*
5
4.16*
EDTA
1
106.57
3
114.25
5
97.36
PMSF
1
83.11
3
35.30*
5
10.52*
TTHA
1
1.75*
3
10.30*
5
0*
The present study employed α- amylase inhibitor extracted from the stems of P. persicaria (PPAI) with different concentrations I10, I30 and I50 determined at 0.027, 0.044 and 0.062%, respectively (Table 3). Different concentrations of proteinaceous extract inhibited α-amylase of B. oleae to 100% (Fig. 2). IC504 value of PPAI was calculated as 0.062 mg/ml. Although optimal pH of α-amylase was 5, PPAI produced the highest inhibition in the pH range of 8–10 (Fig. 3). Also, the highest inhibition of the enzyme was shown at 20 °C, this inhibition was not statistically different from temperature ranges of 20–35 °C (Fig. 3). IC stands for inhibitor concentration. N = 30.
IC10
IC30
IC50
Slope ± SE
X2
df
PPAI
0.027
0.044
0.062
3.6 ± 0.452
1.55
2
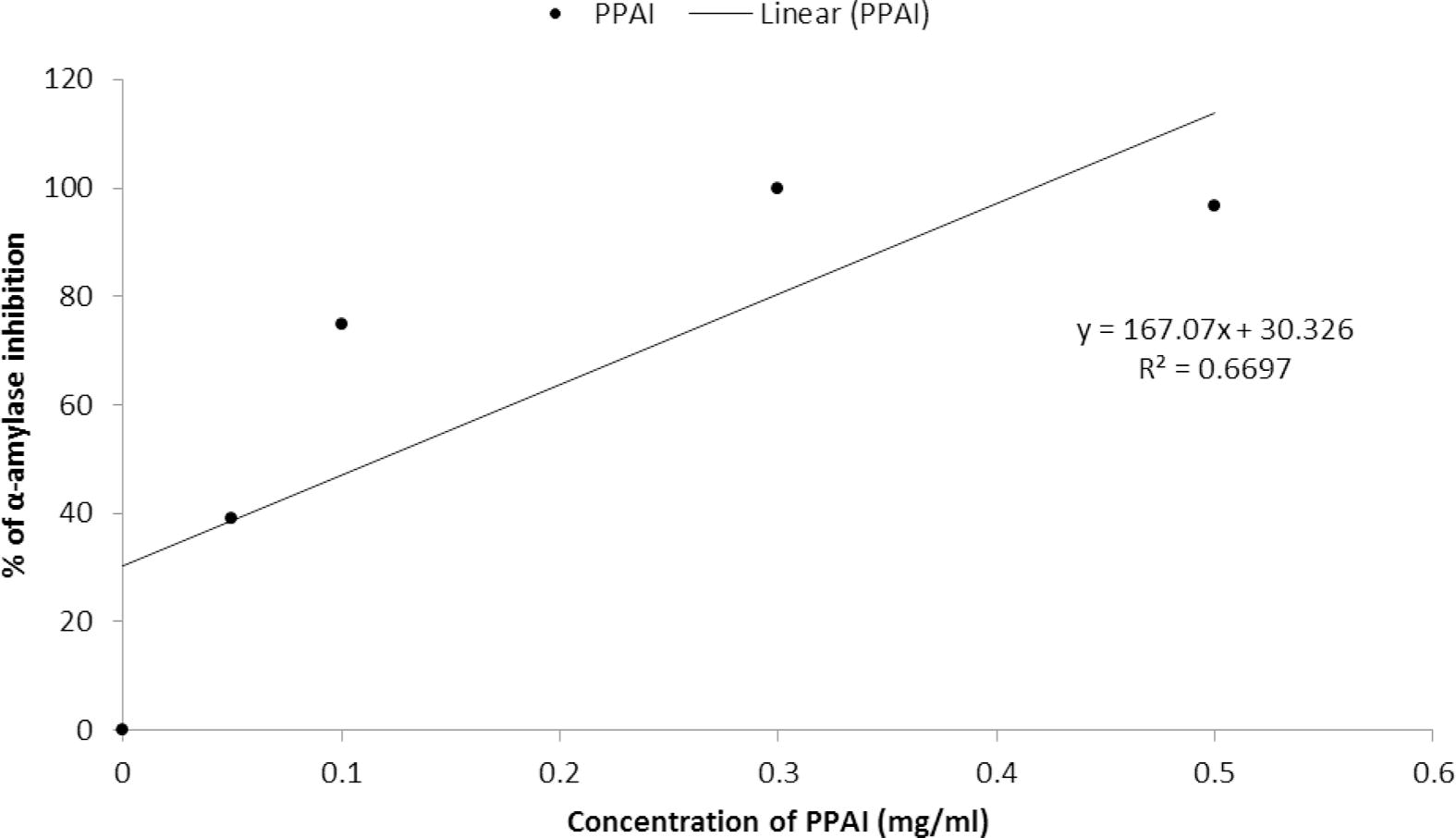
Inhibition of B. oleae α-amylase by different concentrations (mg/ml) of PPAI proteinaceous extract.
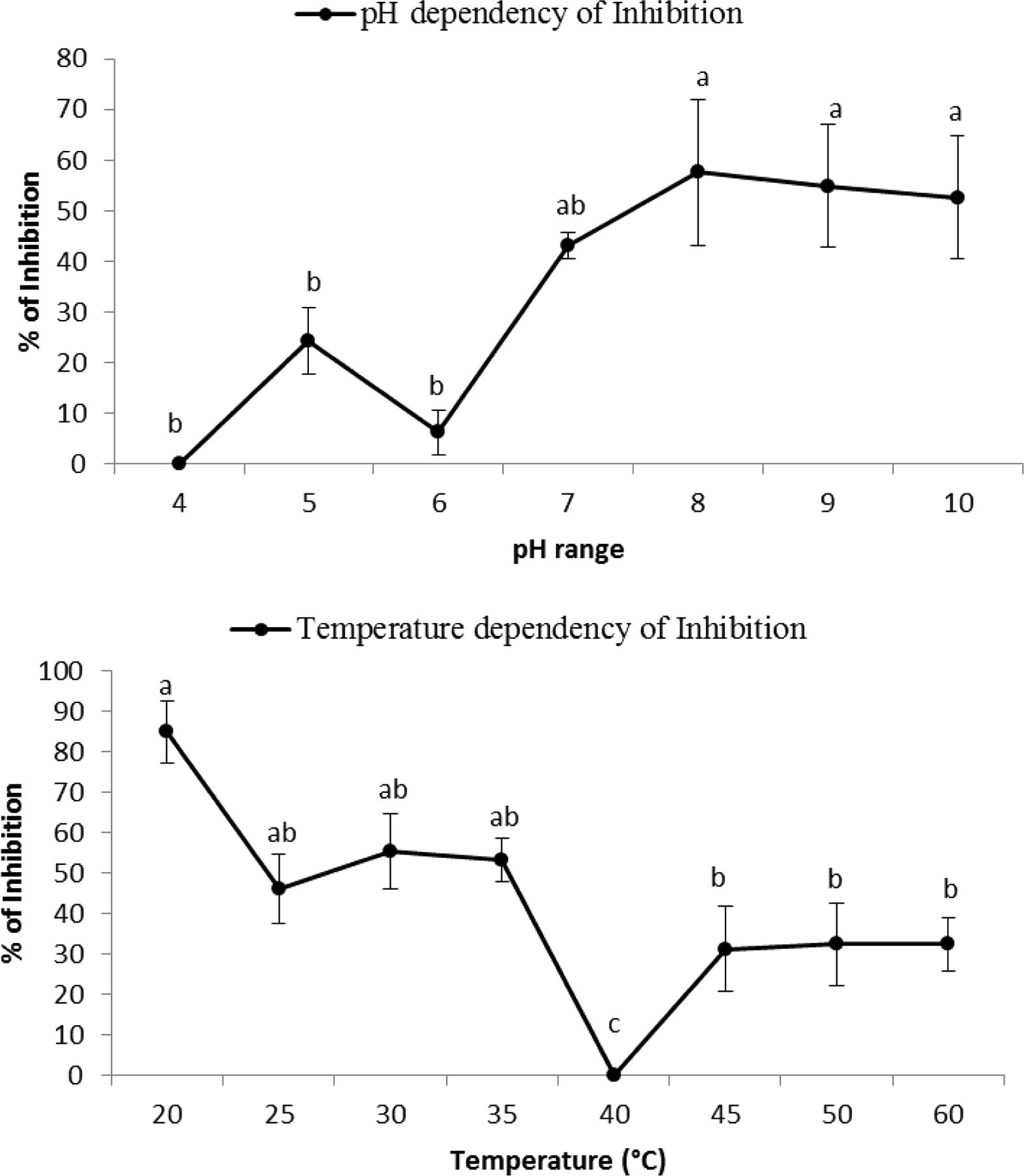
pH and temperature dependency of B. oleae α-amylase by PPAI. Statistical differences have been shown by various letters (Tukey’s test, p ⩽ 0.05).
4 Discussion
The present investigation clearly demonstrated the presence of a digestive α-amylase in the gut of B. oleae to digest polysaccharides like starch or glycogen. Similar findings have been reported on digestive α-amylase in Musca domestica L. (Diptera: Muscidae) (Espinoza-Fuentes and Terra, 1987), Phlebotomus papatasi (Scopoli) (Diptera: Psychodidae) (Jacobson and Schlein, 2001), Lutzomyia longipalpis L. (Diptera: Psychodidae) (Vale et al., 2012), Ceratitis capitata (Wiedemann) (Diptera: Tephritidae) (Darvishzadeh et al., 2012).
Temperature and pH are the two key factors affecting biochemical reactions. Studies have shown an optimal pH of 6–7 in dipterans such as Bigham et al. (2010), 7–8 (Darvishzadeh et al., 2012), 8.5 (Vale et al., 2012) and even more (Vale et al., 2012) for α-amylase activity at 40–50 °C in the midgut of L. longipalpis larvae. Usually, it is believed that the optimal pH of enzymes reflects prevailing pH conditions in the midgut from which the enzyme has been extracted. Variation in the pH of midgut is related to different feeding habits and sources. Also, chemistry of olive fruits is changed during development and maturation of fruit and therefore the physiological state of fruits might contribute to the variation in pH from 5–7. Although studies related to optimal temperature of α-amylase in dipterans are lacking, it is well known that temperature will increase the rate of enzyme-catalyzing reactions by increasing kinetic energy and collision frequency of the reacting molecules (Mohammadi et al., 2010).
Cations (ions) are one of the important components of enzyme’s site of activity. Terra and Ferreira (2005) believe that digestive α-amylases of insects are calcium-dependent enzymes and could be activated by chloride at optimal pH. In the current study, different concentrations of ions and some chelating agents were used to prove activation of α-amylase in B. oleae by calcium ion. Although some discrepancies were observed in case of Cu2+. In fact, this could be attributed to structural similarity of Cu2+ with two others. Meanwhile, phenanthroline (Metalloproteinase inhibitor) and PMSF (Inhibitor serine, histidine and aspartic acid triad) had negative effect on α-amylase of B. oleae. Importance of amino acids and Ca2+ in active site of α-amylase has been elucidated in amylase structure of T. molitor (Strobl et al., 1998). α-Amylase of T. molitor had a central domain that comprises the core of the molecule and have the catalytic amino acid residues (Aspartate, Glutamate and Aspartate). Domains B and C have been placed on each side of domain A. The substrate-binding site is located in a long V-shaped cleft between domains A and B. A calcium ion is placed in domain B and is coordinated by Asparagine, Arginine and Aspartate. This ion is important for the structural integrity of the enzyme and seems also to be relevant due its contact with Histidine (Strobl et al., 1998). Present results exhibit structural similarity of B. oleae α-amylase with found structure of insect α-amylase in involvement of amino acid triad (Inhibited by PMSF) and Ca2+ ion (Inhibited by EGTA).
Several classes of plant proteins have been discovered and characterized including lectins, ribosome-inactivating proteins (RIPs), protease inhibitors and α-amylase with demonstrated insecticidal effects against many insects (Franco et al., 2000). Inhibition of digestive α-amylase from larvae of B. oleae was achieved by using different concentrations of PPAI. Melo et al. (1999) reported inhibition of an α-amylase from Callosobruchus maculatus Fabricius (Coleoptera: Bruchidae) larvae by proteinaceous extract from cowpea seeds. Suzuki et al. (1994) extracted and purified two different α-amylase inhibitors from Proteus vulgaris. α-AI1 inhibits digestive α-amylases of C. maculatus and Cryptocarya chinensis and α-AI2 inhibits digestive α-amylase of Zabrotes subfasciatus Boheman (Coleoptera: Chrysomelliade) (Bonavides et al., 2007). Mehrabadi et al. (2010) determined dose-dependent inhibition of the α-amylase of Eurygaster integriceps Puton (Hemiptera: Scutelleridae) by triticale extract (T-αAI).
Although, information on other dipterans is lacking, lepidopteran amylases demonstrated the highest inhibition at alkaline pHs that shows pH dependency of enzymatic inhibition. Effect of temperature on the activity of α-amylase does not support an earlier finding of Marshall and Lauda (1975). There was 10-fold increase in activity of the α-amylase inhibitor with an increase in temperature from 25 to 37 °C as reported by Marshall and Lauda (1975). This variance could be attributed to the nature of the inhibitor and the enzyme and the stability of the tertiary structure at the desired temperatures. Also, inhibition of the enzyme increased from 45 to 60 °C. At these temperatures, enzyme is degraded. So, this inhibition could be attributed to enzyme denaturation rather than inhibitory mechanism.
In the current study, activity of α-amylase was measured by dinitrosalisylic acid procedure and using negative control. Also, different concentrations of PPAI showed inhibitory effect on α-amylase of B. oelae. Our study has shown a strong inhibitory effect of PPAI on α-amylase activity of olive fruit fly, B. oelae. IC50 value of PPAI was 0.062 mg/ml and was temperature and pH dependent. The use of enzyme inhibitors from different plant sources may serve as an important pest control strategy via plant breeding programs. Hence, it is mandatory to determine biological activities of these compounds against digestive enzymes of insects under optimal conditions, prior to cloning the relevant gene to new varieties of plants for conferring pest resistance.
References
- Interaction of partially-purified amylases from larval Anagasta kuehniella (Lepidoptera: Pyralidae) with amylase inhibitors from wheat. Comp. Biochem. Physiol. Part B. 1989;93:239-245.
- [Google Scholar]
- Effects of essential oil from Teucrium polium on some digestive enzyme activities of Musca domestica. Entomol. Res.. 2010;40:37-45.
- [Google Scholar]
- Molecular identification of four different α-amylase inhibitors from Baru (Dipteryx alata) seeds with activity toward insect enzymes. J. Biochem. Mol. Biol.. 2007;40:494-500.
- [Google Scholar]
- Identification and biochemical characterisation of α-amylase in the alimentary tract of Mediterranean fruit fly, Ceratitis capitata (Wiedemann) (Diptera: Tephritidae) Arch. Phytopathol. Plant Prot. 2012
- [CrossRef] [Google Scholar]
- Physiological adaptation for digesting bacteria. Water fluxes and distribution of digestive enzymes in Musca domestica larval midgut. Insect Biochem.. 1987;17:809-817.
- [Google Scholar]
- The crystal water affect in the interaction between the Tenebrio molitor alpha-amylase and its inhibitor. Bioinorg. Chem. Appl. 2008
- [CrossRef] [Google Scholar]
- Activity of wheat α-amylase inhibitors towards bruchid α-amylases and structural explanation of observed specificities. Eur. J. Biochem.. 2000;267:2166-2173.
- [Google Scholar]
- Partial unfolding of carbonic anhydrase provides a method for its immobilization on hydrophobic adsorbents and protects it against irreversible thermoinactivation. Enzyme Microb. Technol.. 2003;33:179-184.
- [Google Scholar]
- Phlebotomus papatasi and Leishmania major parasites express α-amylase and α-glucosidase. Acta Trop.. 2001;78:41-49.
- [Google Scholar]
- Protein measurement with the Folin phenol reagent. J. Biol. Chem.. 1951;193:265-275.
- [Google Scholar]
- Purification and properties of phaseolamin, an inhibitor of α-amylase, from the kidney bean Phaseolus vulgaris. J. Biol. Chem.. 1975;250:8030-8037.
- [Google Scholar]
- Inhibition of Sunn pest, Eurygaster integriceps, α-amylases by α-amylase inhibitors (T-αAI) from Triticale. J. Insect Sci.. 2010;10:179. Available online: insectscience.org/10.179
- [Google Scholar]
- Mirrahimi, S., Khalaghani, J., Nouri, H., 2008. Effect of time, direction and site of sampling on olive infestation by olive fruit fly. Proceedings of 18th Iranian Plant Protection Congress, p. 413.
- Activity and some properties of Helicoverpa armigera Hubner and Spodoptera exigua Hubner (Lep.: Noctuidae) midgut protease. Munis Entomol. Zool.. 2010;5:697-706.
- [Google Scholar]
- Olive fruit fly population in central and southern California. 2003;57
- [CrossRef]
- A novel strategy for inhibition of a-amylases: yellow mealworm a-amylases in complex with the Ragi bifunctional inhibitor at 2.5A resolution. Structure. 1998;6:911-921.
- [Google Scholar]
- CDNA sequence and deduced primary structure of an alpha-amylase inhibitor from a bruchid-resistant wild common bean. Biochem. Biophys. Acta. 1994;1206:289-291.
- [Google Scholar]
- Insect digestive enzymes: properties, compartmentalization and function. Comp. Biochem. Physiol. Part B. 1994;109:1-62.
- [Google Scholar]
- Biochemistry of digestion. In: Gilbert L.I., Iatrou K., Gill S.S., eds. Comprehensive Molecular Insect Science. Vol vol. 3. San Diego, California, USA: Elsevier; 2005. p. :171-224.
- [Google Scholar]
- Carbohydrate digestion in Lutzomyia longipalpis’ larvae (Diptera - Psychodidae) J. Insect Physiol.. 2012;58:1314-1324.
- [Google Scholar]
- Characterization of α-amylase in midgut and salivary glands of Chilo suppressalis Walker (Lepidoptera: Pyralidae), rice striped stem borer. J. Asia-Pacific Entomol.. 2008;11:201-205.
- [Google Scholar]







