Translate this page into:
Rodents in fire affected heather shrublands in Bale Mountains National Park, Ethiopia
*Corresponding author. Tel.: +251 11 6635302 balak212@yahoo.com (Balakrishnan Mundanthra)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.

Available online 28 May 2013
Abstract
A study on rodents in the fire affected ericaceous vegetation in the Web Valley of the Bale Mountains National Park was conducted during July 2008–March 2009. Five trapping girds were randomly selected based on the duration since the occurrence of fire such as 6-months, 2-years, 3-years, 4-years and Erica vegetation unaffected by fire. Full recovery of Erica vegetation was observed 4-years after fire. A total of 1088 individual rodents were trapped by Sherman live traps (990) and snap traps (98) during 4440 trap nights. The species and the relative abundance of live-trapped rodents were Lophuromys melanonyx (32.0%), Lophuromys flavopunctatus (25.4%), Arvicanthis blicki (18.1%), Stenocephalemys albocaudata (12.6%) and Otomys typus (11.9%). Tachyoryctes macrocephalus was observed in Erica vegetation affected by fire since 2–3-years, but was not trapped. L. flavopunctatus and O. typus were widely distributed in burned Erica vegetation and the habitat unaffected by fire. No rodent was recorded from 6-months post-fire Erica. Highest density and abundance of rodents were recorded in 2- and 3-years Erica post-fire and the least in the grid from unburned Erica vegetation. Biomass of rodents was also high in 3-year Erica post-fire habitat.
Keywords
Erica vegetation
Fire effect
Habitat association
Rodents
Web Valley
1 Introduction
Use of fire to control and manage vegetation communities has been a practice of human beings in rural areas for millennia (Angassa, 2007). Hunters have been using fire to drive game and to clear bush, where game can be seen more easily. Gatherers use fire to discourage the growth of unwanted species, and to smoke hives to chase away honey bees (Apis mellifera) (Heinseiman, 1973). Farmers, especially shifting cultivators and pastorals use fire to clear and fertilize land for plantation, to improve the quality and the quantity of grasses and to control pests during the summer months and in the beginning of new farming activities (Goldammer, 1990).
Fire plays an important role in the growth and development of vegetation and small mammal community composition in a given ecosystem (William et al., 2005). It helps to reduce dry grass and shrub vegetation during the peak summer months in savannah ecosystems (Kaufman et al., 1983), which would accelerate the growth of fresh offshoots at the beginning of the rainy season. However, the loss of cover, food and water results in changes in the community structure of small mammals (Afework and Corti, 1997). Bock and Bock (1978) described changes in small mammal communities in response to changes in the habitat of grassland in southeastern Arizona during post-fire period. Inter-specific differences in foraging behavior, like selection and use of microhabitats affected by fire, contribute to population fluctuations of small mammals in the habitat. The effect of fire on small mammals is a function of multi-trophic interactions like predation, availability of food and cover (William et al., 2005).
Fire can potentially affect species richness, diversity, relative abundance, reproductive behavior and population structure of small mammal communities (Cable, 1965). Forced emigration from, and immigration to the sites associated with fire were common in every ecosystem from heath to close forest (Kennedy, 2007), tropical biota (Goldammer, 1990) and grassland (Kaufman et al., 1990) during post-fire period. Post-fire emigration may be due to lack of vegetation cover and food in the affected area. Wildfires also cause a direct impact on small mammal communities due to the heat generated during fire (Clausnitzer, 2003), which has a high impact on ground nesting and burrowing rodents (Coulson and Franklin, 1968). The other impact of fire on small mammals is reduction in reproductive rates including delay in breeding, reduction in the number and size of young during the post-fire period (Begg et al., 1981; Hodieb and Ghobrial, 1982). According to intermediate disturbance hypothesis, high species diversity is a character of intermediate community succession stages due to the coexistence of competitive and opportunistic species (Connell, 1978; Dial and Roughgarden, 1988). Fox’s habitat accommodation model states that successional changes in vegetation derive the responses of small mammal species in post-fire conditions (Fox and Monamy, 2010). Investigations on the succession of small mammals during post-fire period showed inter-related complex features of the fire regime, and time since the occurrence of fire and the habitat association of species (Begg et al., 1981; Blanchard and Knight, 1990).
Uncontrolled and deliberate fire set in natural habitats is common in heather zones in most of the African highlands; but detailed studies on its effects on small mammal communities have not been conducted in Africa (Clausnitzer and Kityo, 2000). Pastoral communities are living in the Bale Mountains National Park permanently, and they are practicing Erica fire every year to promote re-growth of fresh grass for livestock. Therefore, the present investigation was carried out to test fire related disturbances on rodent populations in the ericaceous habitat in the Web Valley of the Bale Mountains National Park in Ethiopia.
2 The study area
The Bale Mountains National Park (BMNP) is situated in southeastern Ethiopia, along the eastern edge of the Rift Valley. Geographically, BMNP is located between 6o 29′–7o 10′N, and 39o 28′–39o 58′E (Fig. 1), about 400 km. by road from Addis Ababa. The temperatures vary widely in BMNP area ranging from 1.4 °C in January (minimum) to 18.4 °C in February (maximum) (Hillman, 1986). The rainfall in BMNP is bimodal, with heavy rains during July–October and short rains during March–June. The annual rainfall ranges from 1000 to 1400 mm.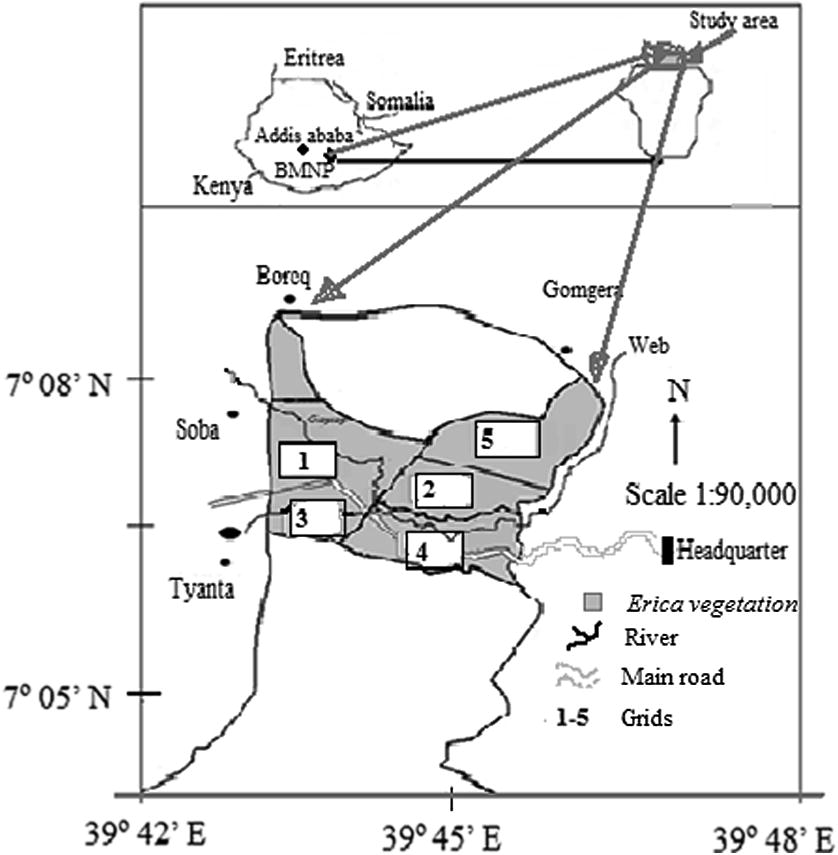
Location of the study area (in inset Ethiopia is shown in the left and the area of Bale Mountains National Park on the right).
In BMNP, afroalpine areas of altitude >3400 m asl have a vegetation composition of Erica arborea, Helichrysum spp., Alchemilla spp., short alpine grasses and giant Lobelia. The Web Valley consists of typical open habitats dominated by short tussock grasses, Helichrysum and heath moorlands (Sillero-Zubiri, 1994; Marino, 2003). The heath moorlands contain mainly Erica arborea, which is highly fire-prone and overgrazed by livestock (Sillero-Zubiri, 1994).
The Web Valley is walled to the west by the ridge that forms the western boundary of the BMNP, and to the east by 50–80 m cliffs of condensed lava. Most of the Web Valley is covered by Alchemilla pasture (Alchemilla abyssinica, Alchemilla rothii, and Alchemilla cyclophylla) dotted with Helichrysum shrubs (Helichrysum citrispinum, Helichrysum cymosum, Helichrysum gofense and Helichrysum splendidum) and Artemisia afra. Encircling the mountains between the afroalpine zone and the tree-line, there is a belt of ericaceous heathlands (Erica trimera). The cliffs and slopes are covered with Helichrysum/Artemisia afra scrub and Lobelia rhynchopetalum (Sillero-Zubiri, 1994). There are human settlements in BMNP area. Most of the settlers are pastoralists, and they burn Erica during the dry season. The present study sites included burned and unburned Erica habitats.
3 Methods
This investigation was made during July 2008–March 2009. A reconnaissance survey was conducted in the Web Valley area prior to the detailed investigation. During this survey, five study grids were identified and major vegetation types, climate and altitude of each of the grids were recorded (Table 1). Sherman live-traps and snap-traps were used to trap rodents. The vegetation in grid 1 was of an area burned 6-months prior to the investigation. This grid was located at an altitude of 3582 m asl, characterized by bare underground with dry standing Erica due to fire. Grid 2 was located at an altitude of 3586 m asl, consisted of vegetation characterized by Helichrysum sp. and annual herbs and grasses such as Koeleria and Aira spp. burned 2-years prior to the investigation. There was no regeneration of Erica vegetation in this grid due to the effects of fire and overgrazing by livestock. Grid 3 was located at an altitude of 3600 m asl, consisted of vegetation burned 3-years prior to the investigation. Regeneration of Erica was observed in this area, though overgrazed by livestock. This grid was characterized with smaller plant species such as Helichrysum, Alchemilla, Cerastium, grasses of the genus Cyperus and Scirpus and Lobelia rynchopetalum. Grid 4 had Erica vegetation burned 4-years prior to the investigation. This grid was located at an altitude of 3645 m asl. In this grid, Erica vegetation was recovered and the canopy cover was almost similar to that of the pre-fire habitat with thick undergrowth of grass as well as Helichrysum vegetation. Grid 5 was of Erica vegetation unaffected by fire, located at an altitude of 3647 m asl. Much of the vegetation of this grid was of heath scrub, 0.5–1.0 m high, dominated by Philippia sp. and Erica arborea.
Grids
Area (m2)
Altitudes (m asl.)
Fire effect
Vegetation types
1
70 × 70
3582
6-Months post-fire
Bare underground and dry standing Erica arborea trees
2
70 × 70
3586
2-Years post-fire
Helichrysum citrispinum, H. cymosum, H. gofense, H. splendidum, Koeleria sp. and Aira sp. No regeneration of Erica arborea
3
70 × 70
3600
3-Years post-fire
Helichrysum citrispinum, H. cymosum, H. gofense, Alchemilla abyssinica, A. rothii, A. cyclophylla, Scirpus falsus, S. ficinioides, giant Lobelia (Lobelia rynchopetalum) and regenerated Erica arborea.
4
70 × 70
3645
4-Years post-fire
Helichrysum citrispinum, H. cymosum and H. gofense, heath scrub vegetation (Philippa trimera and P. excels) and recovered Erica arborea, which reached pre-fire condition
5
70 × 70
3647
Unburned Erica vegetation
Heath scrub vegetation (Erica arborea, Philippa trimera, Philippa excels)
Five 70 × 70 m2 mammal trapping sites were randomly marked, one in each of the five Grids mentioned above, and used both during wet (July–September) and dry (December–February) seasons. Live-trap stations were located at every 10 m interval (with a 10 m external band to account for edge effects), marked with plastic tags. A total of 49 live-traps were set per grid for three consecutive days in two sessions per season, giving 12 days in each gird. There was a minimum of 45 day gap between the two trapping sessions of each season. Twenty-five snap-traps were set at a distance of 200 m away from live-trap stations of each grid at 20 m intervals (also for three consecutive days in two sessions per season) to trap rodents for information on body measurements, and number of embryos in the case of pregnant females. Data were collected during August 2008–March 2009.
Population estimation of rodents was done using capture-mark-recapture (CMR) (Lincoln–Peterson) method. Rodent biomass per hectare was calculated by multiplying the estimated population by the mean body weight of each of the species of rodents trapped. Species density was analyzed following Gotelli and Colwell (2011), taking into account the 10 m external band of the study grids. SPSS Version (13) computer program, and statistical methods such as Anova and Chi-square were used to analyze rodent community density and species abundance between habitats, respectively.
4 Results
In the study area, the Erica vegetation was overgrazed by livestock and hence dominated by shrubs, herbs and grasses. Six months post-fire habitat did not show any recovery of the effect of fire, where only burned and dry standing Erica trees were observed. The 2-years post-fire showed some levels of recovery with shrubs, herbs and grasses, but no Erica recovery. The 3-year post-fire habitat showed recovery, but with a significant qualitative difference in canopy cover compared to unburned Erica habitat. The 4-years post-fire Erica had vegetation recovered from the effect of fire, and reached similar canopy and height of pre-fire condition of the habitat type in unburned Erica.
A total of six species of rodents were recorded from burned and unburned Erica vegetation in the present study area. Among them, five were captured by live traps. The giant mole rat (Tachyoryctes macrocephalus) was observed in two and three year post-fire grids. A total of 990 rodents were live-trapped during the present investigation. The species of rodents live-trapped and their relative abundance were: Black-clawed mouse (Lophuromys melanonyx Petter, 1977) (32%), Harsh furred rat (Lophuromys flavopunctatus Thomas, 1988) (25.4%), Blick’s grass rat (Arvicanthis blicki Frick, 1914) (18.1), Ethiopian narrow-headed rat (Stenocephalemys albocaudata Frick, 1914) (12.6%) and Swamp rat (Otomys typus Heuqlin, 1877) (11.9%). Distribution of rodents in the present study area varied between habitats based on time gaps following fire in burned and unburned grids. L. flavopunctatus and O. typus were the two most widespread species, which were trapped from all study grids. L. melanonyx, S. albucaudata, A. blicki and T. macrocephalus were almost restricted in their distribution in 2- and 3-year post-fire grids. L. melanonyx was occasionally captured from 4-year post-fire grid. No rodent was recorded from 6-months post-fire grid (Table 2). (Lf = L. flavopunctatus, Lm = L. melanonyx, Ab = A. blicki, Sa = S. albocaudata, Ot = O. typus).
Species
Grids
Total
Relative abundance
6-Months post-fire
2-Years post-fire
3-Years post-fire
4-Years post-fire
Unburned
Lm
0
151(47.6)
164(51.7)
2(0.6)
0
317
32.0
Ab
0
73(40.8)
106(59.2)
0
0
179
18.1
Sa
0
56(44.8)
69(55.2)
0
0
125
12.6
Lf
0
14(5.6)
40(15.9)
114(45.4)
83(33.1)
251
25.4
Ot
0
3(2.5)
12(10.2)
70(59.3)
33(27.8)
118
11.9
Total
0
297
391
186
116
990
Out of the total number of individual rodents trapped, 688 (69.5%) were from 2- and 3-year post-fire grids. The remaining was from 4-year post-fire grid (187) (18.8%) and unburned Erica habitat (116) (11.7%). L. melanonyx was most abundant in 3-year post-fire grid (59.2%), followed by 2-year post-fire grid (40.8%) and was least in 4-year post-fire grid (0.6%). L. flavopunctatus was the second most abundant, which was trapped in all grids other than 6-months post-fire Erica. This species was trapped more from 4-year post-fire grid (45.4%), followed by unburned Erica habitat (33.1%). A. blicki and S. albocaudata were restricted in their distribution in 2- and 3-year post-fire grids. The second widely distributed species, O. typus was most abundant in 4-year post-fire grid (59.3%), followed by unburned Erica habitat (27.5%) and was least in 2-year post-fire grid, constituting only 2.5% of the total individuals trapped. The estimated population of rodents in the Web Valley was highest in 3-year post-fire habitat, both during wet and dry seasons, and the least was in unburned Erica habitat (Fig. 2). Trap success was highest in 3-year post-fire grid (66.7%), followed by 2-year post-fire, 4-year post-fire and unburned Erica grids.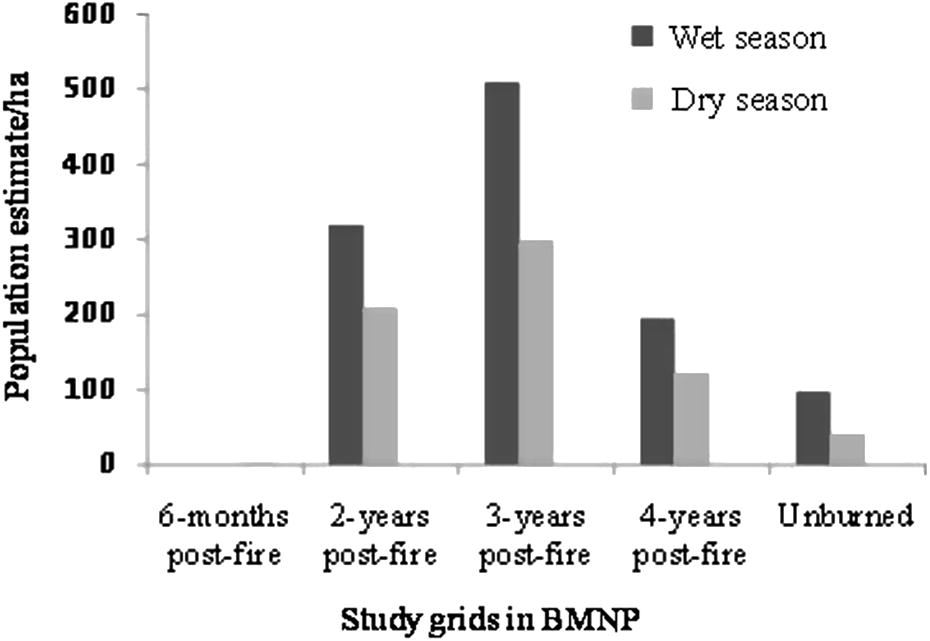
Population estimate of live-trapped rodents in different study grids (per hectare) in Bale Mountains National Park during wet and dry seasons (2008–2009).
The highest rodent community density was recorded in 3-years Erica post-fire during wet season (469.38/ha), while the least was recorded in unburned Erica during the dry season (100.00/ha). The rodent community density between the different years burned and unburned Erica vegetation was not significantly different (F = 0.083, P > 0.05). The differences in the abundance of specific rodent species in different grids were significant for A. blicki, L. melanonyx, L. flavopunctatus and O. typus (χ2 = 16.50, P < 0.001, χ2 = 151, P < 0.001, χ2 = 191.9, P < 0.001, and χ2 = 183, P < 0.001, respectively). However, the difference in the abundance of S. albocaudata between 2- and 3-years post-fire was not significant (χ2 = 2.41, P > 0.05) (Table 2). The highest beta diversity was recorded between 2- and 3-year Erica post-fire and unburned Erica habitats; the former harboring four species (A. blicki, L. melanonyx, S. albocaudata and T. macrocephalus). Comparatively high species density was recorded in 3-year post-fire Erica habitat (0.399/m2), followed by 2-year post-fire habitat (0.303/m2). The majority of rodent species was captured from 2- to 3-year post-fire Erica habitats, harboring five species, each. Lower number was recorded in 4-years post-fire and unburned Erica with 3 and 2 species, respectively (Table 3). (G1 = 6-months post-fire, G2 = 2-years post-fire, G3 = 3-years post-fire, G4 = 4-years post- fire, G5 = unburned Erica).
Grids
No. of species trapped
Individuals trapped
Species density
1
0
0
0
2
5
297
0.303
3
5
391
0.399
4
3
186
0.114
5
2
116
0.047
L. melanonyx and A. blicki were diurnal. T. macrocephalus was observed during the day time, and its nocturnal activity could not be ascertained by the present study. S. albocaudata was nocturnal. However, O. typus and L. flavopunctatus were captured both during the day and the night trappings. Higher proportion of pregnant females (n = 29) was trapped during the wet season than during the dry season (n = 15). Pregnant females were most common in L. flavopunctatus. The highest number of embryos (up to 5) was recorded in A. blicki. The highest biomass of rodents was recorded in 3-year post-fire grid during the wet season and the least biomass was in unburned Erica habitat during the dry season (Fig. 3).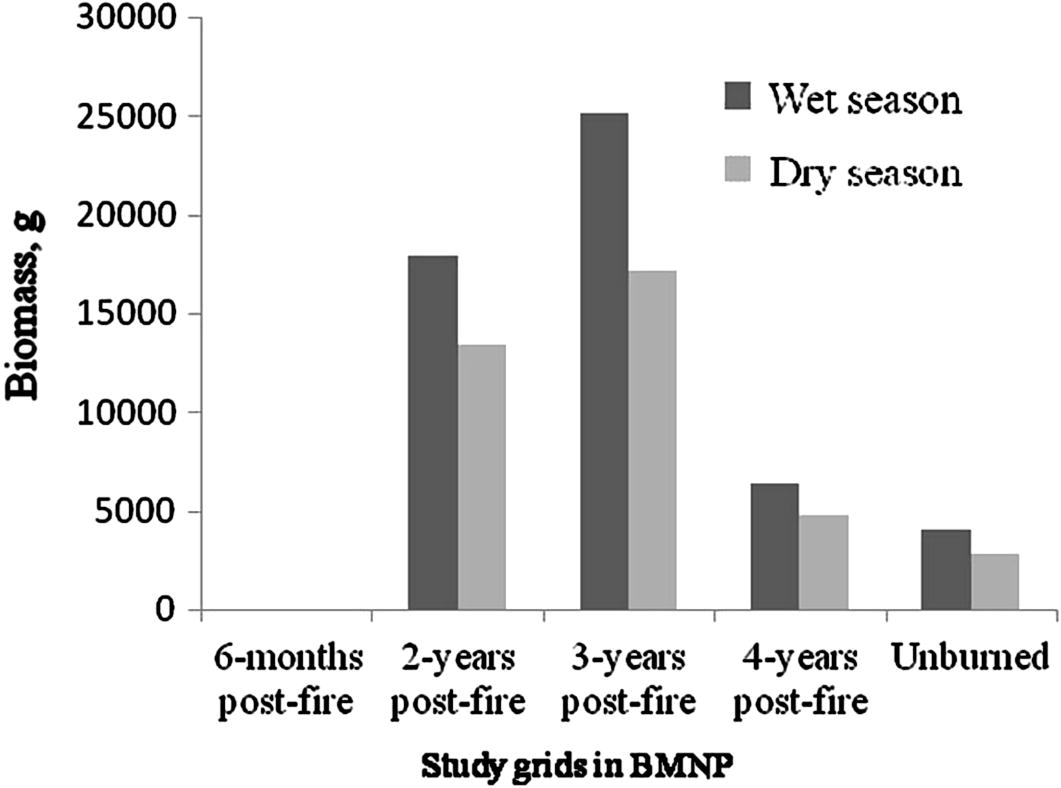
Biomass estimate of live-trapped rodents in different study grids (per hectare) in Bale Mountains National Park during wet and dry seasons (2008–2009).
5 Discussion
In Bale Mountains National Park, the incidence of fire is common, with 142 fire incidence burning 38,150 ha during the period 1999–2008. There were major fires in 2000 and in 2008 affecting large areas in the woodland, mountain forest, and in the ericaceous habitats during the dry months (January–March) (Abera and Kinahan, 2011). Accessibility was the main influencing factor reported for the occurrence of fire. A combination of factors such as vegetation type, accessibility and distance from human settlements influenced the occurrence and intensity of fires in the area. Abera and Kinahan (2011) have also reported that the ericaceous habitat in the Bale Mountains is preferentially burned by the local people.
In the present study in the Web Valley of the Bale Mountains in burned and unburned Erica vegetation, only six species of rodents were recorded. The presence of T. macrocephalus was confirmed through indirect observations of their larger conical shaped mounds (Yalden, 1988) and direct observations in two and three year post-fire grids. Out of the six recorded species, four (66.7%) (L. melanonyx, A. blicki, S. albocaudata and T. macrocephalus) were local endemics to the Eastern plateau of Ethiopia (Sillero-Zubiri et al., 1995). Even though the number of species recorded was less, the present results support the view that the eastern plateau of Ethiopia contains high rodent endemism and hence is a reservoir of unknown genetic diversity (Hillman, 1986; Kingdon, 1990; Cole et al., 1995).
The six species of rodents recorded from the present study area agree with the finding of Sillero-Zubiri et al. (1995), who have also obtained similar results at similar altitude ranges in afroalpine vegetation of the Web Valley. Low species composition, especially at higher altitudes can be attributed to the harsh climatic conditions, less vegetation composition and structures prevailing in the habitat, in addition to the result of the interactions between ecological and historical processes (Torre and Arrizabalaga, 2009).
In the present study, rodents were distributed unevenly in burned and unburned ericaceous vegetation. For instance, A. blicki, S. albocaudata, L. melanonyx and T. macrocephalus were restricted to 2- and 3-year post-fire Erica habitats. However, L. melanonyx was also rarely recorded from 4-year Erica post-fire grid. O. typus and L. flavopunctatus were widely distributed in all study grids as described earlier by Clausnitzer and Kityo (2000), who have recorded L. flavopunctatus and O. typus in different habitats of afroalpine zone of Mt. Elgon though their relative abundance was high in dense vegetation. In the present study, they were recorded from both burned and unburned ericaceous vegetation except from the freshly burnt site (6-months post-fire). L. melanonyx was the most abundant species in the present study area, in spite of its being mainly restricted in distribution in open Helichrysum vegetation in 2- and 3-year Erica post-fire habitat with rare trapping in 4-year post-fire gird. This is due to the expansion of open habitats as a result of fire (Sillero-Zubiri et al., 1995; Hillman, 1993a, b; Yalden, 1988).
The population estimate of rodents in the study area is high as reported earlier (Randall et al., 2011). The highest estimate was in 3-year Erica post-fire habitat. Species richness of rodents varied between the different habitats studied. High species density and abundance of rodents were also recorded in the 3-year post-fire habitat. Begg et al. (1981) also reported high richness of small mammals in three year post-fire area in little Nourlangie Rock, Australia. Shenko et al. (2012) compared small mammal community composition between disturbed and undisturbed habitats in the New Jersey Pinelands, USA and found that disturbance by fire, soil disruption and logging had effects on occurrence and distribution of small mammals.
Clausnitzer (2003) has reported high diversity of rodents in Mount Elgon in relation to the increased growth of annual and perennial forbs in burned habitats. In the present study, grasses of the genus Cyperus, Scirpus and Koeleria and scrub vegetation such as Helichrysum, Alchemilla and Cerastum provided micro-habitats for rodents for shelter and food (Afework et al., 2003; Workneh et al., 2004). Among the grids in which rodents were present, the least abundance and density were recorded in the unburned Erica vegetation. Only two species of rodents (L. flavopunctatus and O. typus) were captured from this grid. Yalden (1988) also recorded only few species of rodents in old ericaceous vegetation in BMNP area. Four year Erica post-fire habitat was similar to unburned Erica vegetation in its species composition of rodents except that L. melanonyx occurred rarely during the wet season. Both grids of 4-year post-fire and unburned Erica habitats had similar canopy cover also. The present finding is comparable with the intermediate community succession hypothesis, which states high species diversity as a feature of intermediate succession stages due to the coexistence of competitive and opportunistic species (Dial and Roughgarden, 1988). The possession of three unique rodent species in intermediate levels (2–3-years post-fire Erica) might be the result of dynamics of intermediate community succession, which support coexistence of different species (Roxburgh et al., 2004).
There were no rodents captured from six months Erica post-fire grid. Fire results in mortality, and emigration of rodents to nearby habitats. Post-fire emigration of small mammals is attributed to lack of vegetation for food and shelter (Clausnitzer and Kityo, 2000). This shows that habitat quality is important to sustain rodent populations in their natural habitat.
During the present study, population structure and relative abundance in relation to burned and unburned ericaceous vegetation were made only for L. flavopunctatus as this species was widely distributed in most of the study grids. The relative abundance of L. flavopunctatus between burned and unburned ericaceous vegetation was significantly different. High relative abundance was recorded in 4-year Erica post-fire habitat, which had dense vegetation (Clausnitzer, 2003). This finding agrees with Fox’s habitat accommodation model that relates relative abundances of small mammal species to heath land vegetation regeneration following fire. Fox and Monamy (2010) attribute a high relative abundance of Rattus lutreolus in a late seral stage of heathland post-fire to dense vegetation requirement for cover. The population structure of L. flavopunctatus was not significantly different between grids of burned and unburned ericaceous vegetation. Waser (1984) and Kennedy (2007) have earlier revealed no significant difference in population structure of Merriam’s kangaroo rats (Dipodomys merriami) in burned and unburned habitats.
The data on trap success of the present study revealed variation between burned and unburned vegetation with the overall trap success of 67.3%. The highest trap success was recorded in 3-year Erica post-fire grid and the least in unburned Erica habitat. Afework (1996) recorded different trap success rates in different habitats, as an outcome of vegetation composition and structure for shelter and food as well as availability of water in the habitat. Similarly, Clausnitzer (2003) reported high trap success in disturbed habitats than in undisturbed habitats.
In the present study, the number of embryos observed in pregnant females snap-trapped from burned and unburned ericaceous vegetation did not vary significantly. Hodieb and Ghobrial (1982) revealed fire affected reproduction of Dasyurus hallucatus by delaying breeding and reducing the number as well as the size of the embryos. On the other hand, William et al. (2005) reported similar number and size of embryos among adult females in habitats affected and unaffected by fire.
The relatively high biomass of rodent community at high elevations in the present study is characteristic of afroalpine grassland habitats of BMNP that peaks in the present study sites in Web Valley as revealed earlier by Randall et al. (2011), which they attributed to high rainfall and vegetation density in the BMNP. The variation in biomass of rodents between seasons was attributed to availability of food, cover and water. In natural habitats, these essential resources are available in plenty during the wet season (Clausnitzer, 2003), and hence the population of rodents and their biomass would be higher during this season. The decreased biomass of rodents during the dry season would reflect on the shortage of food and shelter, when rodents may be easily subjected for predation. Shortage of food, water and shelter would also affect the population abundance by affecting reproduction (Afework et al., 2003).
BMNP is established mainly for the conservation of the Mountain nyala (Tragelaphus buxtoni) and the Ethiopian wolf (Canis simensis); both are endemic and seen only in certain afroalpine habitats of Ethiopia. Rodents form major food of the Ethiopian wolf, and the BMNP habitat is known for its high rodent density (Randall et al., 2011). Conservation of the Ethiopian wolf depends upon the habitat quality with sufficient prey populations. The present study shows that fire affected areas provide higher biomass in 2–3 years of time interval, revealing that the intermediate succession stages of the post-fire habitats support high species richness and abundance of rodents in comparison to the early colonization stages and final climax stages (Mammides et al., 2008). The theory of intermediate succession stages shows that the intermediate stages possess those species both from early stages and climax community (Whittaker, 1975; Roxburgh et al., 2004). This can be a consequence of intermediate cover of re-sprouting shrubs and perennial herbs. This indicates that controlled fire may support better rodent populations as evidenced by the higher abundance and density of them in 2–3 year post-fire grids. Therefore, controlled fire at a frequency of every 3–4 years in the ericaceous vegetation, leaving unburned habitat patches in between to support immigrants from the burned areas and during early stages of habitat succession would provide suitable habitats for afroalpine specialist rodents such as A. blicki, S. albocaudata, L. melanonyx and T. macrocephalus, which form high proportion of the prey of the Ethiopian wolf.
Livestock and fire are not new to the Bale Mountains habitats, but, the current problem is the increased number of herders and their correspondingly increased number of livestock. If properly controlled and managed, fire can be used as an effective tool to control vegetation structure and growth and maintain better rodent biomass in BMNP, which would eventually support the populations of the endangered Ethiopian wolf.
Acknowledgements
We are thankful to the four anonymous reviewers for their critical comments, which helped us to improve the quality of this manuscript. We are also thankful to the Addis Ababa University and the Ethiopian Wolf Conservation Program for funds and facilities.
References
- Factors affecting fire extent and frequency in the Bale Mountains National Park. Walia Spec. Ed. Bale Mountains 2011:146-157.
- [Google Scholar]
- Population dynamics of the Ethiopian endemic rodent Proamys albipes in the Menagesha state forest. J. Zool. (London). 1996;238:1-12.
- [Google Scholar]
- Forest blocks and altitude as indicators of Myomys albipes (Rüppell, 1842) (Mammalia: Rodentia) distribution in Ethiopia. Trop. Zool.. 1997;10:287-293.
- [Google Scholar]
- Composition of rodents and damage estimates on Maize farms at Ziway, Ethiopia. In: Singleton G.R., Hinds L.A., Krebs C.J., Spratt D.M., eds. Rats, Mice and People: Rodent Biology and Management. Canberra, Australia: Australian Center for International Agricultural Research; 2003. p. :262-263.
- [Google Scholar]
- Angassa, A., 2007. The Dynamics of Savanna Ecosystems and Management in Borana, Southern Ethiopia, Ph.D. Thesis, Norwegian University of Life Sciences, Aas, Norway. pp. 35–68.
- The small mammals of little Nourlangie rock, Northern territory V. The effects of fire. Austr. Wildl. Res.. 1981;8:515-527.
- [Google Scholar]
- Reactions of grizzly bears (Ursus arctos) to wildfire in Yellowstone National Park. Wyoming. Can. Field Nat.. 1990;104:592-594.
- [Google Scholar]
- Response of birds, small mammals, and vegetation to burning Sacaton grasslands in South eastern Arizona. J. Range Manage.. 1978;31:296-300.
- [Google Scholar]
- Damage to mesquite, lehmann, lovegrass and black gram by a hot June fire. J. Range Manage.. 1965;18:326-329.
- [Google Scholar]
- Rodents of Mt. Elgon, Uganda: ecology, biogeography, and the significance of fire. Ecotrop. Monogr.. 2003;3:1-176.
- [Google Scholar]
- Ecology of the high-altitude environment on Mt. Elgon, Uganda/Kenya. Climate, vegetation and the impact of fire. Ecol. Monogr.. 2000;56:546-578.
- [Google Scholar]
- Conservation implication of introduced game birds in high elevation Hawaiian shrubland. Conserv. Biol.. 1995;9:306-313.
- [Google Scholar]
- Theory of marine communities: the intermediate disturbance hypothesis. Ecology. 1988;79:1412-1424.
- [Google Scholar]
- Response of two species of heathland rodents to habitat manipulation: vegetation density thresholds and the habitat accommodation model. Austral Ecol.. 2010;35:334-347.
- [Google Scholar]
- Fire in the Tropical Biota, Ecosystem Processes and Global Challenges: Ecological Studies. New York, USA: Springer Verlag, Berlin-Heidelberg; 1990. (p. 497)
- Estimating species richness. In: Magurran A.E., McGill B.J., eds. Frontiers in Measuring Biodiversity. New York, USA: Oxford University Press; 2011. p. :39-54.
- [Google Scholar]
- Fire in the virgin forests of the boundary Waters Canoe area, Minnesota. Quatern. Res.. 1973;3:329-382.
- [Google Scholar]
- Bale Mountains National Park Management Plan. Vol vol. 1. Addis Ababa, Ethiopia: Ethiopian Wildlife Conservation Organization (EWCO); 1986. (p. 72)
- Ethiopia: Compendium of Wildlife Conservation Information. Vol vol. I. Addis Ababa, Ethiopia: Wildlife Conservation in Ethiopia, Ethiopian Wildlife Conservation Organization; 1993. (p. 454)
- Ethiopia: Compendium of Wildlife Conservation Information. Vol vol. II. Addis Ababa, Ethiopia: Information of Wildlife Conservation Areas, Ethiopian Wildlife Conservation Organization; 1993. (p. 451)
- Seasonal variations in breeding of the Nile rat (Arvicanthis niloticus) J. Mamm.. 1982;46:319-333.
- [Google Scholar]
- Effects of fire on rodents in tallgrass Prairie of the Flint Hills region of Eastern Kansas. Prairie Nat.. 1983;15:49-56.
- [Google Scholar]
- Small mammals and grassland fires. In: Kaufman G.A., Finck E.J., eds. Fire in North American Tallgrass Prairies. Oklahoma, USA: University of Oklahoma Press; 1990.
- [Google Scholar]
- Kennedy, M.S., 2007. Prescribed Fire on Fraser Island: Small Mammal Responses and Their Underlying Ecological Processes. Ph.D. Dissertation, University of Queensland, Queensland, Australia, (p. 402).
- Island Africa. Princeton, USA: Princeton University Press; 1990. (p. 287)
- Effects of habitat disturbance and food supply on population densities of three primate species in the Kakamega forest. Kenya. Afr. J. Ecol.. 2008;47:87-96.
- [Google Scholar]
- Marino, J., 2003. Spatial Ecology of Ethiopian Wolf, Canis Simensis, Ph.D. Dissertation, University of Oxford, Oxford., UK, (p. 243).
- Ethiopian Wolf Monitoring in the Bale Mountains from 2001-2004. Walia Spec. Ed. Bale Mountains 2011:28-36.
- [Google Scholar]
- The intermediate disturbance hypothesis: Patch dynamics and mechanisms of species coexistence. Ecology. 2004;85:359-371.
- [Google Scholar]
- Effects of disturbance on small mammal community structure in the New Jersey pinelands, USA. Integrat. Zool.. 2012;7:16-29.
- [Google Scholar]
- Sillero-Zubiri, C., 1994. Behavioral Ecology of the Ethiopian Wolves (Canis simensis), Ph.D. Thesis, University of Oxford, Oxford, UK, (p. 283).
- Bale Mountains rodent communities and their relevance to the Ethiopian Wolf. Afr. J. Ecol.. 1995;33:301-320.
- [Google Scholar]
- Species richness and abundance of small mammals along an elevational gradient of a Mediterranean mountain. Life Environ.. 2009;59:203-212.
- [Google Scholar]
- The relative abundance of species: post fire changes in a coastal sage scrub rodent community. Ecology. 1984;65:1161-1169.
- [Google Scholar]
- Communities and Ecosystems (second ed.). New York, USA: Macmillan; 1975. (p. 352)
- The effects of a low-intensity fire on small mammals and lizards in a logged, burnt forest. Wildl. Res.. 2005;30:477-486.
- [Google Scholar]
- Microhabitat choice and diet of small mammals in Maynugus irrigation field. Ethiopia. Afr. J. Ecol.. 2004;42:315-321.
- [Google Scholar]







