Translate this page into:
Screening and characterization of antimicrobial secondary metabolites from Halomonas salifodinae MPM-TC and its in vivo antiviral influence on Indian white shrimp Fenneropenaeus indicus against WSSV challenge
*Corresponding author. Mobile: +91 9994273822; fax: +91 4652253078 citarasu@gmail.com (T. Citarasu)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.

Available online 21 March 2013
Abstract
Antimicrobial secondary metabolites from extremophiles play a significant role in the pharmacological industry due to their stable and strong activity and it is used in the treatment of microbial infections. In the present work, Halomonas salifodinae MPM-TC (M. Peter Marian-T. Citarasu) was isolated from the solar salt works in India and identified by 16S rRNA sequencing. The secondary metabolites were extracted from H. salifodinae MPM-TC and tested for antibacterial activity against aquatic bacterial pathogens such as Vibrio harveyi, Vibrio parahaemolyticus, Pseudomonas aeruginosa and Aeromonas hydrophila isolated from infected fish/shrimp, and it effectively controlled them with more than 10 mm of zone of inhibition. The metabolites were purified through silica column chromatography and in vitro antiviral activity was performed against White Spot Syndrome Virus (WSSV) using different fractions. Among the different tested fractions, fraction-III (F-III) was able to suppress WSSV replication. Shrimps challenged with a WSSV inoculum incubated with F-III and treated Fenneropenaeus indicus survived around twice as many as the controls. Gas chromatography–mass spectroscopic (GC–MS) analysis revealed that the antiviral active fraction contains around eight compounds including Perfluorotributylamine, Cyclopentane, 1-butyl-2-ethyl and 1,1′-Biphenyl]-3-amine. Further the active fraction F-III was incorporated in the artificial diets at the concentration of 200 (HS1), 400 (HS2) and 800 (HS3) μg kg−1 and fed to F. indicus for 30 days. After 30 days of culture, shrimps were challenged with virulent WSSV and studied for WSSV VP 28 gene expression, biochemical, haematological and immunological changes. Surprisingly, groups treated with lower concentrations of fraction F-III (HS1 or HS2) significantly (P < 0.05) suppressed the viral replication. Different levels of protein and glucose, improved total haemocyte count (THC), coagulase activity and oxyhaemocyanin level all were comparable to controls. Also, immunological parameters such as prophenol oxidase and intracellular superoxide anion production were significantly increased (F = 97.18; P ⩽ 0.001 and F = 5.70; P ⩽ 0.05) in the groups treated with the three test concentrations. The presence of antiviral and immunostimulant active principles in the F-III fraction effectively suppressed the WSSV load and boosted F. indicus’s immune system. This research will help to develop novel antiviral drugs from plants against aquatic important pathogens.
Keywords
Halomonas salifodinae
Antiviral drugs
WSSV
16S rRNA sequencing
1 Introduction
Halophilic microbes are found in three domains of life such as Archaea, Bacteria, Eukarya and adapt to moderate and high salt concentrations. They grow in the saline range between 3% and 25% NaCl, w/v, but the true halophilic archaea grow only in extreme saline environments (Litchfield, 2002). Hypersaline environments originate by the evaporation of sea water and are also called thalassic environments (Oren, 2002).
Microbes from marine sources have a rich potential of antimicrobial active principles (Burgess et al., 1999). Due to the rich potential bioactive metabolites in the marine microbes, it may be used as drugs directly or used as lead structures for drug discovery (Proksch et al., 2002). Around 2500 new metabolites (MNPs) were reported from marine organisms (1977–1987) ranging from microbes to fish, which accounts for less than 1% of the total marine organisms. Salt pans are extreme environments which inhabit organisms that thrive on high salinities, temperatures and withstand severe solar radiations. Such organisms are capable of producing interesting metabolites which may benefit the mankind (Kamat and Kerkar, 2011).
Microbes from salt pans are yet to be fully explored as potential producers of antimicrobial agents. However, few reports are available on the antimicrobial potential of microorganisms in Indian salterns. Dhanasekaran et al. (2005) have reported the antibacterial potential of salt pan actinomycetes from Tamil Nadu, India. Kamat and Kerkar (2004) have carried out studies on a marine salt pan bacterium, producing a broad spectrum of antibiotic, from Goa, India. Pharmacological activities including antibacterial, antiviral, antifungal, anticoagulant, cardiatonic and antitumour were well understood from the marine microbial metabolites (Molinski, 1993) and it will help developing novel drugs (Bernan et al., 1997).
The killer pathogen, White Spot Syndrome Virus (WSSV) is causing high mortalities and severe damages leading to heavy economical losses in the shrimp aquaculture industry. Once there is a disease outbreak in the cultured shrimps, all shrimps will succumb to death within 3–10 days (Lightner, 1996). The methods of current disease treatment against WSSV are cost effective, less effective and are creating so many undesirable side effects (Citarasu et al., 2006). Even though antibiotics and synthetic drugs are giving positive effects, they cannot be recommended due to their residual effects, resistant strain development and other environmental hazards (Citarasu, 2010). In order to consider the health and environmental issues against WSSV infection, the culture management should be focused on environmentally safer methods such as developing novel antimicrobial secondary metabolite drugs of halophilic origin. The current research work focuses on controlling WSSV at in vitro and in vivo levels by delivering antiviral secondary metabolites isolated from Halomonas salifodinae.
2 Material and methods
2.1 Bacterial sampling and phenotypic identification
Condenser sample with salinity of 230‰ collected from the Thamaraikulam solar salt works (Lat. 8° 11′ N and Long. 77° 29′ E), Kanyakumari district, Tamil Nadu, India. Water samples were serially diluted from 10−1 to 10−8 in sterile saline water and 100 μl of each dilution was spread onto sterile Halophilic Agar plates (Hi Media, India). The plates were incubated at 37 °C until the colony grows. The dominant pink colonies were identified by morphology, physiology and biochemical confirmations following Holt et al. (1994).
2.2 Identification at genomic level by 16S rRNA sequencing
One hundred nano grams of genomic DNA was extracted in the dominant bacterial colony and the 16S rRNA gene was amplified using the universal primer with the standard PCR protocol. The PCR products were purified by a Gel extraction kit (Medox Biotech India Pvt. Ltd) and sequenced (Chromos Biotech Pvt. Ltd, Bangalore). The nucleotides of the 16S rRNA sequence were matched with the other microbes in the NCBI database using the BLAST program. The construction of phylogenetic tree was carried out by Geneious Basic software and evolutionary history inferred using the neighbour-joining method (Sneath and Sokal, 1973).
2.3 Extraction of antimicrobial secondary metabolites
The strain H. salifodinae MPM-TC was inoculated in halophilic broth (Hi Media, India) and incubated for 7 days in a shaker (2000 g) at 37 °C. The bacterial culture after incubation was spun at 5000 g for 30 min and the supernatant filtered through a 0.22 μm membrane filter. The filtrates were extracted with the equal volume of ethyl acetate. Extraction process was repeated for four times and then the extract was concentrated in a rotary evaporator and lyophilized. The crude extracts were stored at 4 °C.
2.4 In vitro antibacterial screening
In vitro antibacterial activity was performed by the extracted metabolites against aquatic bacterial pathogens (Vibrio harveyi, Vibrio parahaemolyticus, Pseudomonas aeruginosa and Aeromonas hydrophila) using agar diffusion following the method of Baur et al. (1966).
2.5 Purification of antimicrobial secondary metabolites
The preparative silica column chromatography was used to purify the crude metabolites (50–80 μm particle size; 30 cm column length; 0.5 ml elution flow rate and three bed volume elution). Different proportions of the mobile phases such as hexane/ethyl acetate and ethyl acetate/methanol were used for eluting the active compounds. The different eluates were collected, concentrated in a rotary evaporator and stored at 4 °C. The fractions were spotted on silica gel plates GF254 (Merck), 20 × 20 cm, 1 mm thick and the chromatogram was developed using, hexane: ethyl acetate (8:2) as mobile phase. The plates were visualized under short UV wavelength.
2.6 Determination of antiviral activity
Shrimps infected with WSSV with prominent white spots were collected from the intensive shrimp farm located near Nellore, Andhra Pradesh, India. The infected shrimp Penaeus monodon were bled, the haemolymph samples pooled and spun at 3000 g for 20 min at 4 °C. Then the supernatant was re-spun by 8000 g for 30 min at 4 °C and the supernatant was filtered by 0.22 μm membrane filters for isolating WSSV particles. This supernatant contains a high quantity of WSSV particles. After protein quantification (Lowry et al., 1951), isolated WSSV was stored at −20 °C for further studies. Also, 1000 μg of purified metabolite fractions (FI–V) were dissolved in 1000 μl of NTE buffer (0.2 M of NaCl, 0.02 M of Tris–HCl and 0.02 M of EDTA and adjusted to pH 7.4) as stock for further bioassay studies. A purified WSSV suspension (10 μl) was mixed with 10 μl of H. salifodinae MPM-TC secondary metabolite fractions, independently and incubated at 29 °C for 3 h in a thermostat incubator. After 3 h, 10 μl of the mixture was injected intramuscularly into the second abdominal segment of Fenneropenaeus indicus (weight = 8.0 ± 1 g). Mortalities were recorded daily in a day up to 10 days after challenge. Control shrimps were also maintained as described for the animals treated with the fractions. The haemolymph samples were collected from the challenged shrimps, extracted for genomic DNA and WSSV diagnostic PCR performed (Takahashi et al., 1996).
2.7 Structural characterization of the active fractions
Based on the initial screening results, the fraction III (H-6;EA-4), had higher antiviral activity against WSSV. The same fraction was used for the structural characterization by Gas Chromatography–Mass Spectroscopy (GC–MS) analysis. GC–MS was performed with a machine HP5890 model and a column Rtx-502.20 was used. The column size was 60 m, 0.25 mm, 1.4 μm. Flow rate was (Helium) 1 ml/min and the temperature of injector was 250 °C. Mass spectra were recorded for a mass range of 40–550 amu. NIST 98 library software was used for identifying compounds.
2.8 Experimental diet preparation
Ingredients and formulation of the basal diet were described by Boonyaratpalin (1993). H. salifodinae secondary metabolites were incorporated to artificial diets at the concentration of 200 (HS1), 400 (HS2), and 800 (HS3) μg kg−1. Control diet (C), was also prepared devoid of metabolites. The basal feed ingredients were thoroughly mixed with 4% gelatin solution containing appropriate concentration of secondary metabolites along with oil ingredients. In order to adjust the pH (7 ± 0.1), saturated sodium hydroxide solution was added to the ingredients and mixed thoroughly for 15 min. After that, the dough was extruded by a pelletiser to needed size, air dried and stored in air tight containers.
2.9 Culture set-up and WSSV challenge
WSSV free F. indicus (8.2 ± 1 g) were collected from the Manakudy estuary of Tamilnadu, India. They were acclimatized to laboratory conditions and transferred into individual experimental fibre glass tanks (1000 l capacity) with continuous flow-through water and constant aeration. Each treatment and control groups was done in triplicate (n = 50 × 3 = 150). Water quality parameters such as temperature (27 ± 1.0 °C), salinity (28 ± 1.5‰), and pH (8.2 ± 0.1) were monitored daily. The shrimps were fed three times a day at 8.00, 13.00 and 18.00 h at 10% of the mean body weight. After 30 days of feeding experiment, 100 shrimps from each experimental groups and control were injected intramuscularly with a WSSV filtrate prepared from infected shrimps (30 μg of total protein per animal) in the second abdominal segment. Meanwhile, 10 shrimps from each group were similarly injected with 0.01 ml saline per shrimp as the control ‘blank group’. The percentage of cumulative mortality was monitored at least for 10 days after challenge
2.10 Molecular diagnosis of WSSV
Haemolymph samples collected from both the experimental and control groups were checked for WSSV infection using a nested PCR using the primers designed by Takahashi et al. (1996). The DNA extraction and PCR amplification were carried out by following the method described by Chang et al. (1999). Haemolymph samples from treatment and control shrimps were tested by the first step PCR. The negative samples detected in the first step were further subjected to second step PCR analysis. In each group, 10 surviving shrimp samples were individually tested.
2.11 Biochemical and haematological changes
Total protein (Lowry et al., 1951) and glucose levels (Malik and Singh, 1980), were analysed in haemolymph samples of all groups after WSSV challenge.
Capillary method was adopted to determine the coagulation time of the haemolymph (Sachdev, 1983). Burker haemocytometer was used to count the Total Haemocyte Count (THC) (cells ml−1) from the haemolymph (Le Moullac et al., 1997). For measuring oxyhaemocyanin, 100 μl of haemolymph was mixed with 900 μl of distilled water in a 10-mm quartz cuvette. The absorbance was measured at 335 nm using a Hitachi U-2000 spectrophotometer (Hitachi, Tokyo, Japan). In order to assess the concentration, extinction coefficient (EmM (1 cm)) of 17.26, was calculated from E1% (1 cm) = 2.83 (Nickerson and van Holde, 1971) on the basis of a functional subunit of 74,000 (Antonini and Brunori, 1974; Hagerman,1983).
2.12 Immunological parameters
Phenol oxidase activity in haemolymh was determined using l-dihydroxyphenylanine (l-DOPA) as a substrate (Söderhäll, 1983). Superoxide anion production was quantified following the method of Song and Hsieh (1994). One hundred micro litres of haemolymph from each groups was centrifuged at 800 g for 5 min and the pellets washed three times with HBSS. Further the haemocytes were stained with NBT solution (0.3%, 100 ml) for 30 min at 37 °C. The reaction was terminated by adding absolute methanol and washed with 70% methanol for three times. After drying, 120 ml of 2-M KOH and 140 ml of DMSO were added into the haemocytes for dissolving the cytoplasmic formazan. Dissolved formazan was read at 630 nm on the generation of in all the tested and control groups.
2.13 Statistical analysis
One way and Two way Analysis of Variance (ANOVA) were carried out using SPSS statistics data package and Ky plot respectively. Means were compared at 0.05 and 0.001% levels for One way ANOVA and Two way ANOVA respectively.
3 Results
3.1 Identification of H. salifodinae
The phenotypic confirmation such as morphological, biochemical and physiological tests revealed that H. salifodinae MPM-TC was very similar to H. salifodinae sp. The MPM-TC strain is motile, with gram negative rods that grow well in 15% NaCl concentration in the growth media (Table 1). Phylogenetic and evolutionary analysis of the 16S rRNA sequence revealed that, H. salifodinae shared more than 90% similarity to other Halomonas sp. such as Himantura pacifica PL51; Halomonas sp. MML1959 and FIB 29–19 (Fig. 1). The sequence was deposited in NCBI database and strain name and GenBank accession number were H. salifodinae MPM-TC; JQ315254.1.
Sl. No.
Test
H. salifodinae MPM-TC
H. salifodinae*
1
Gram staining
Negative
Negative
2
Cell shape
Rods
Long rods
3
NaCl concentration
15%
0–20%
4
Motility
Motile
Motile
5
Indole
Negative
Negative
6
Methyl red
Negative
Positive
7
VP
Positive
Positive
8
Citrate
Positive
Positive
9
Oxidase
Positive
Positive
10
Catalase
Positive
Positive
11
Nitrate
Positive
Negative
12
Urease
Positive
Positive
13
Gelatin hydrolysis
Positive
Negative
14
Starch hydrolysis
Positive
Positive
15
CHO fermentation
Glucose
Positive
Positive
Sucrose
Positive
Positive
Galactose
Negative
Negative
Lactose
Positive
Negative
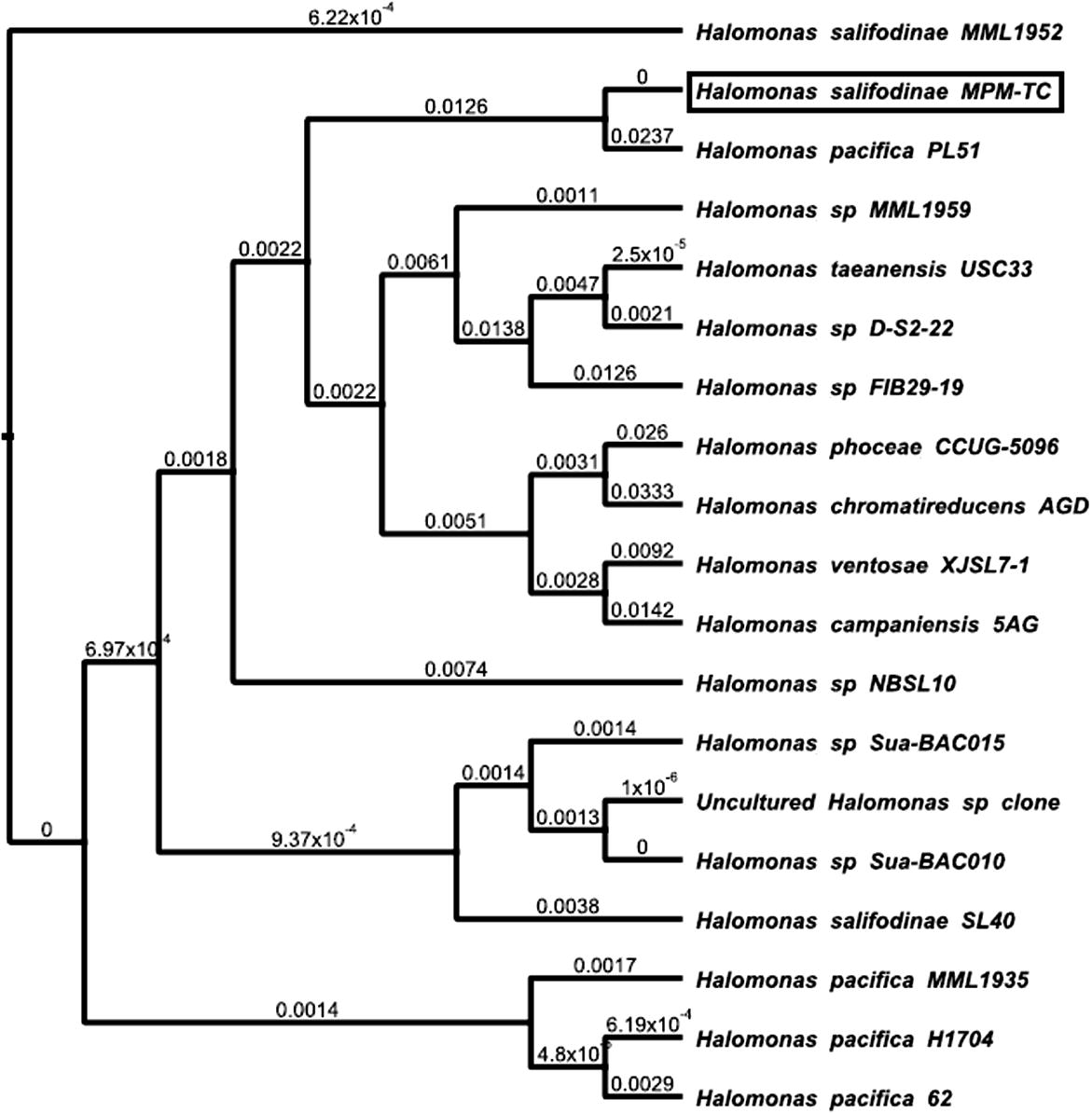
Phylogenetic relationship of H. salifodinae MPM-TC with other Halomonas sp. by neighbour joining method constructed by Geneious software analysis.
3.2 In vitro antibacterial screening
In vitro antibacterial screening results of the H. salifodinae MPM-TC antimicrobial secondary metabolites against bacterial aquatic pathogens are presented in Table 2. Secondary metabolites effectively suppressed the different bacterial pathogens such as A. hydrophila, V. parahaemolyticus, V. harveyi and P. aeruginosa with inhibition zones of 17.11, 16.06, 15.5 and 14 mm, respectively.
Sl. No.
Bacterial pathogens
Antibacterial activity (mm of zone of inhibition)
1
V. harveyi
15.5 ± 0.43
2
V. parahaemolyticus
16.06 ± 0.18
3
P. aeruginosa
14.10 ± 0.52
4
A. hydrophila
17.11 ± 0.33
3.3 Antiviral screening with purified H. salifodinae secondary metabolites fractions
A 100% mortality was observed in F. indicus injected with WSSV alone and WSSV incubated H. salifodinae MPM-TC antiviral secondary metabolite fraction F I. 80% cumulative mortality was observed in F II and F V fractions respectively and 70% mortality observed in F IV treated F. indicus. Surprisingly, the FIII treated F. indicus achieved only 30% cumulative mortality after 10 days of WSSV challenge. This was the lowest percentage of cumulative mortality among the different fractions treated F. indicus due to the antiviral principles. Two way ANOVA revealed that, the values are significantly different from each other (F = 19.48; P ⩽ 0.001) (Fig. 2).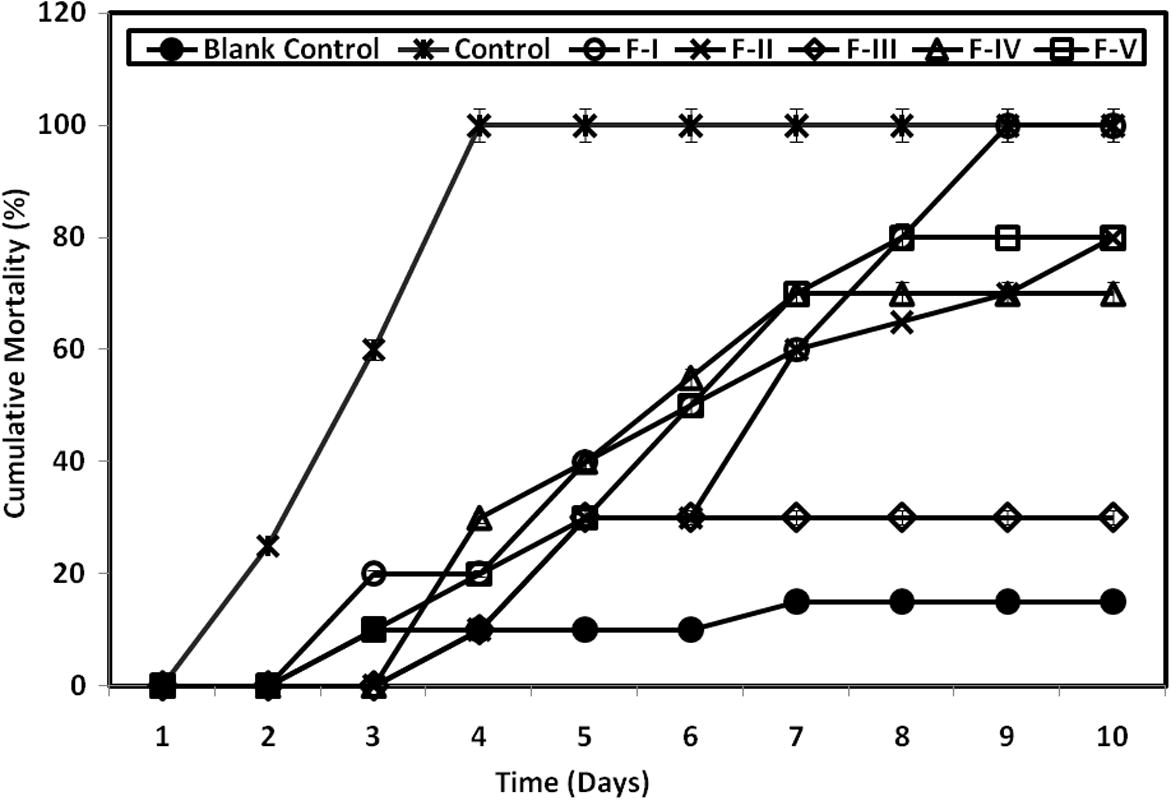
Cumulative mortality of F. indicus injected with WSSV incubated partially purified fractions of antiviral secondary metabolites of H. salifodinae MPM-TC. The values significantly differed from each other (F = 19.48; P ⩽ 0.001) – Two Way ANOVA.
3.4 Partial characterization of the antiviral secondary metabolites
The elution of F-III by 60% hexane and 40% ethyl acetate through the silica column chromatography revealed that the compounds may have mild polar properties. The higher intensity peaks and their quality in F-III showed that the compounds are Perflurotributylamine (43%), Cyclopentane, 1-butyl-2-ethyl-(60%), 1,1’-Biphenyl]-3-amine (27%), Pyridine, 4-(phenylmethyl)- (45%), Hexadecane (25%), 2-methyl-, Nonadecane (35%) and Phytol (74%) by GC–MS analysis (Table 3).
Sl. no.
Retention time
Name of the compounds
Molecular formula
Molecular weight
Quality (%)
1
17.15
Perfluorotributylamine
C12F27N
671.5209
43
2
19.33
Cyclopentane, 1-butyl-2-ethyl-
C11H22
154.2924
60
3
20.24
1,1′-Biphenyl]-3-amine
C12H11N
169.224
27
4
20.52
Pyridine, 4-(phenylmethyl)-
C12H11N
169.224
45
5
21.84
Hexadecane, 2-methyl-
C17H36
240.4677
25
6
25.03
Nonadecane
C19H40
268.5209
35
7
25.30
Phytol
C20H40O
296.5310
74
3.5 Influence of antiviral secondary metabolites on F. indicus
3.5.1 Molecular diagnosis by the expression VP 28 gene
There is no PCR positive signals observed in the blank control whereas the control group had 100% PCR positive by first and second step detection. A strong PCR amplification was observed in shrimps fed with the HS1 diet. The WSSV PCR signal weakened in HS2 and no WSSV amplification was observed in shrimps treated with the HS3 feed. This may be due to the increasing concentrations of antiviral secondary metabolites. In HS1 group, among the tested individual shrimps 40% were PCR positive during first step detection, 25% positive in second step detection and 65% positive in overall detection after WSSV challenge. Shrimps treated with HS2 showed no WSSV-positive signal after first step PCR but 12% were WSSV-positive after second and overall PCR step. None of the shrimps treated with HS3 were detected WSSV-positive after second step PCR amplification. The number of WSSV-positive animals was significantly different (P < 0.05) among the groups (Fig. 3).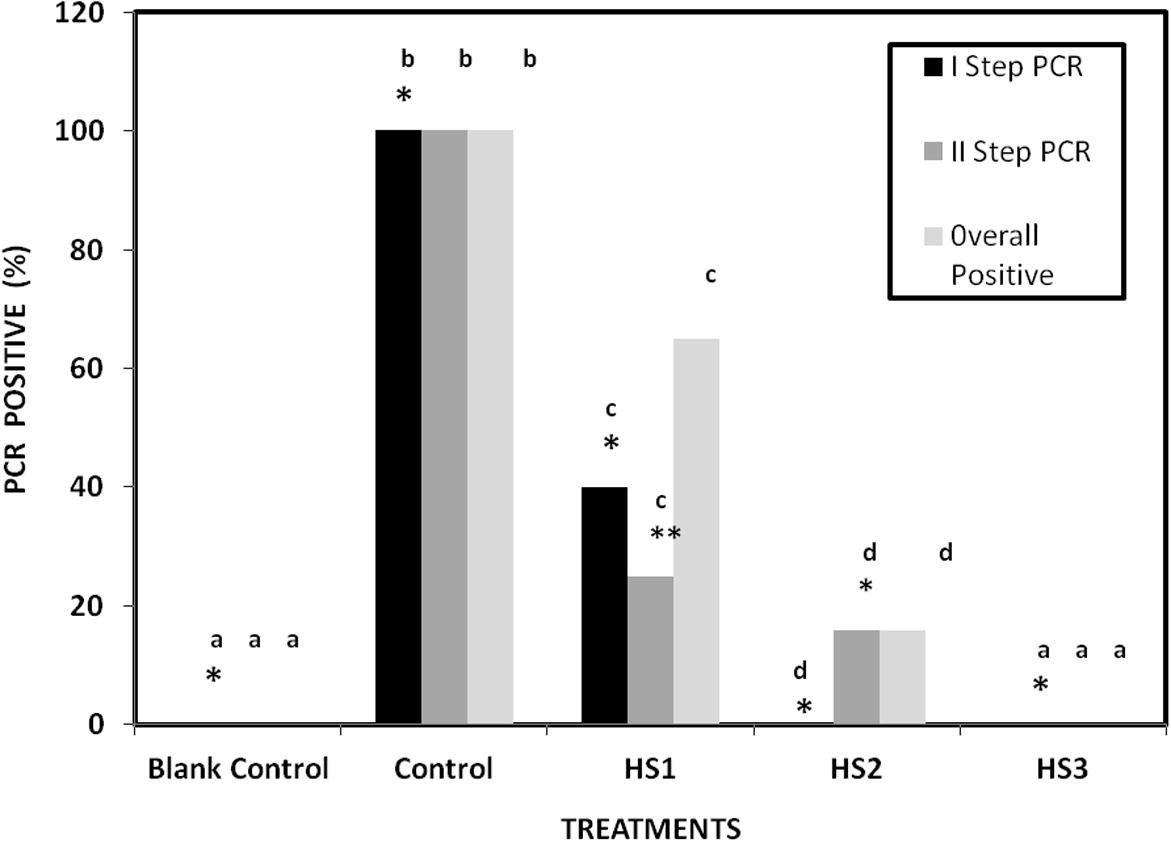
Percentage PCR detection of F. indicus fed with antiviral secondary metabolites of H. salifodinae MPM-TC incorporated diets after challenged with WSSV. Means with the same superscripts (a–d) do not differ from each other (P < 0.05) – One Way ANOVA; ∗n = 20; ∗∗n = 12.
3.5.2 Biochemical and haematological characterization
Total protein value was 113.56 μg ml−1 in haemolymph of the blank control. Total protein value in control shrimps after WSSV challenge increased significantly (P < 0.05) to 121.1 μg ml−1 due to enriched WSSV load. Further the protein level significantly decreased to (P < 0.05) 118.1 and 115 μg ml−1 in HS1 and HS2 groups respectively from the control group. The H. salifodinae MPM-TC antiviral secondary metabolites probably helped to reduce the WSSV load levels in the experimental treatments which were reflected by a decrease of the total protein levels. The lowest glucose level of 99.2 μg ml−1 was observed in the control group and this significantly increased (P < 0.05) to 101, 104.2 and 109 μg ml−1 in HS1, HS2 and HS3 groups, respectively (Table 4).
Treatments
Biochemical parameters
Haematological parameters
Protein (μg/ml)
Glucose (μg/ml)
Total Haemocyte (×106 cells ml−1)
Coagulase activity (S)
Oxyhaemocyanin (m mol l−1)
Blank control
113.56a ± 1.24
105.0a ± 0.81
35.34a ± 1.24
120.0a ± 1.69
1.31a NS ± 0.02
Control
121.1b ± 1.17
99.2b ± 0.72
23.56b ± 1.24
218.43b ± 0.56
0.75a NS ± 0.03
HS1
118.1c ± 0.65
101.0c ± 0.351
27.13c ± 1.63
175.2c ± 0.43
1.43b NS ± 0.04
HS2
115.0d ± 0.4
104.2a ± 0.52
33.0a ± 1.69
130.56d ± 0.49
1.57c NS ± 0.01
HS3
112.0a ± 0.47
109.0d ± 1.24
36.65a ± 1.63
121a ± 1.69
1.61a NS ± 0.02
Control shrimp F. indicus had a total haemocyte count (THC) of 35.34 × 106 cells ml−1. The THC drastically decreased to 23.56 × 106 cells ml−1 in the control group and this was increased significantly (P < 0.05) by HS1, HS2 and HS3 of 27.13, 33 and 36.65 × 106 cells ml−1, respectively. Haemolymph took 218.43 s for coagulation after WSSV challenge when no antiviral secondary metabolite was given. In contrast, coagulation time significantly (P < 0.05) decreased to 175, 130 and 121 s in the HS1, HS2 and HS3 groups, respectively. The lowest oxyhaemocyanin level (0.75 mmol l−1) was measured in shrimp fed without antiviral secondary metabolite incorporated diet. The levels of oxyhaemocyanin increased to 1.43, 1.57 and 1.61 mmol l−1 in HS1, HS2 and HS3 groups, respectively (Table 4). The values were not statistically different to those of control (P > 0.05).
3.5.3 Immunological improvement
The prophenol oxidase activity (pro PO) was higher in animals treated with HS3 (1.074 after 240 min) whereas the control group had only an activity of 0.164. Groups HS1 and HS2 showed an increase in the PO activity compared to the control (0.44 and 0.71 vs. 0.164, respectively). Two-way ANOVA revealed that these values were significantly different to each other (F = 97.18; P ⩽ 0.001) (Fig. 4a). The intracellular superoxide anion
production had a value of 0.02 in the control group at 4 days post challenge. Treatment groups HS1, HS2 and HS3 showed a significant increase (P < 0.0001) to 0.05, 0.079 and 0.81, respectively. Two-way ANOVA showed that the values were significantly different to each other (F = 5.70; P ⩽ 0.05) (Fig. 4b).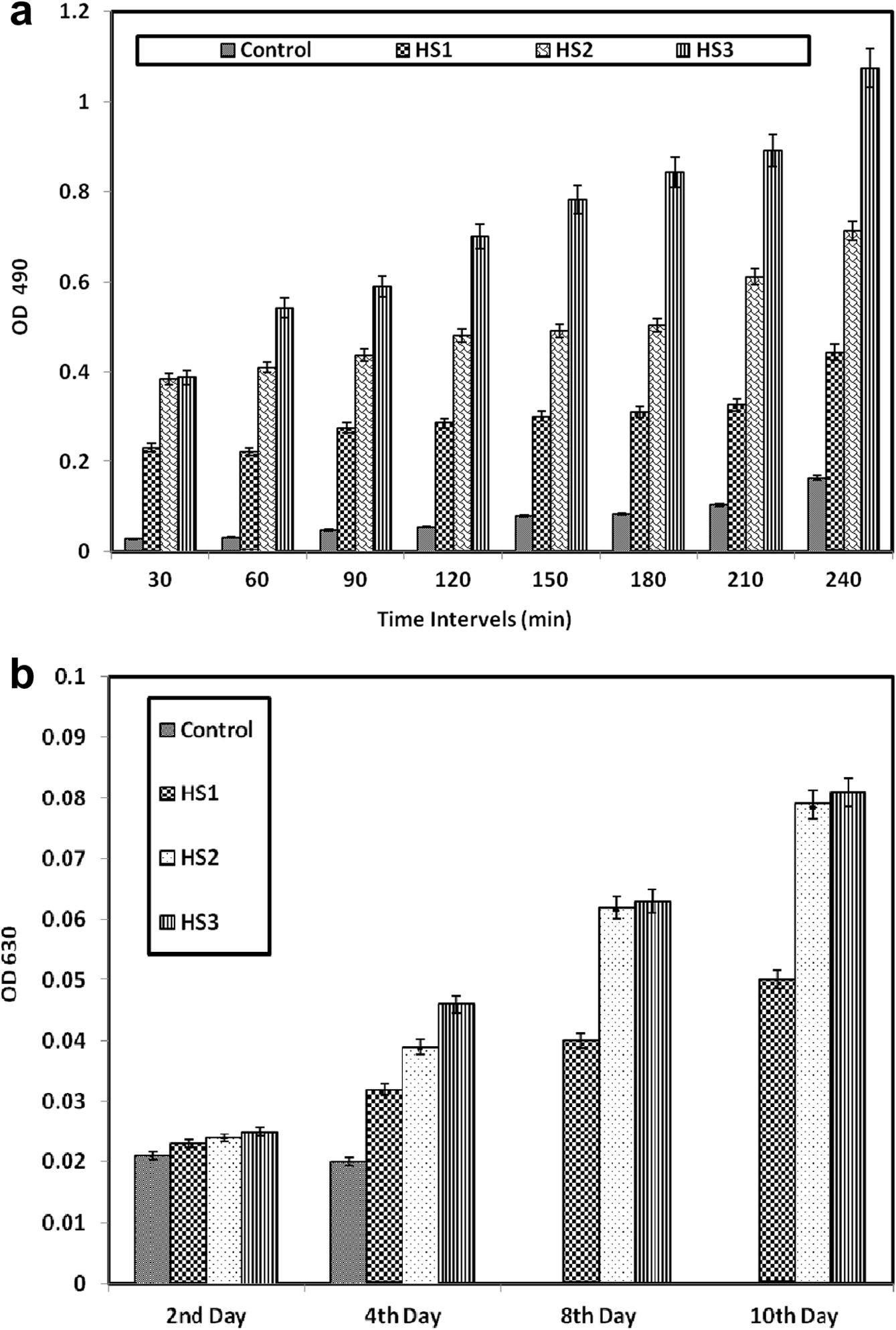
a and b Immunological improvement, Phenol Oxidase (a-top) and Superoxide anion production (b-lower) of F. indicus fed with antiviral secondary metabolites of H. salifodinae MPM-TC incorporated diets after challenged with WSSV. The values significantly differed from each others (F = 97.18; P ⩽ 0.001 – Fig. 6a) and (F = 5.70; P ⩽ 0.05 – Fig. 6b) – Two Way ANOVA.
4 Discussion
Nowadays antibiotic resistance is a serious problem in microbial control and the microbes are developing resistance against commercial antibiotics. The rate of discovery of new compounds from the terrestrial microbes is declining when compared with the rate of discovery of new secondary metabolites from the microbes of marine and halophilic origin. In the present study, secondary metabolites of H. salifodinae MPM-TC were able to inhibit the bacterial growth, suppress the WSSV multiplication and boost the immune system in F. indicus against the WSSV infection. The results of the present study showed that metabolites from H. salifodinae MPM-TC are a feasible alternative to commercially banned antibiotics and also may help to develop new antiviral drugs against shrimp viruses such as WSSV. Secondary metabolites with various biological activities can be induced as a result of the complicated marine environment, some of which represent a valuable resource waiting to be discovered for the treatment of infectious diseases (Ronica, 2011). Novel microbial products extracted from marine microbes exhibited antibacterial, antifungal, antiviral, anticoagulant, cardioactive and antitumor properties (Austin, 1989).
In the present study, antimicrobial secondary metabolites of H. salifodinae MPM-TC were able to inhibit the growth of aquatic pathogens such as Vibrio sp., P. aeruginosa and the fresh water aquatic pathogen A. hydrophila as they produced an inhibition zone of more than 10 mm. Saccharopolyspora salina VITSDK4 isolated from salt pan soil of Marakkanam coast of the Bay of Bengal, India had antimicrobial activity against various bacteria and fungi (Suthindhiran and Kannabiran 2009). Kamat and Kerkar (2004) have reported a halotolerant Acinetobacter sp. from salt pans of Ribandar, Goa producing antibacterial compound. In our previous works, the biosurfactants of halophilic Bacillus sp. and Halomonas sp. isolated from same solar salt works were able to suppress the growth of pathogenic bacteria as well as fungi at in vitro levels (Ronica, 2011). Purified biosurfactants isolated from marine Bacillus circulans exhibited enhanced surface tension and antimicrobial activities (Mukherjee et al., 2009). Acidic extracts of the molluscs, Cerastoderma edule, Ruditapes philippinarum, Ostrea edulis, Crepidula fornicata and Buccinum undatum had antiviral activity against Herpes simplex virus type 1 (Defer et al., 2009). Aqueous extract of the mangrove plant Ceriops tagal had anti WSSV activity in the P. monodon culture (Sudheer et al., 2011).
Secondary metabolite fractions from H. salifodinae MPM-TC arrest the WSSV transcription and translation that lead to no viral multiplication and reduced cumulative mortality in F. indicus. As there was a un-availability of WSSV cell lines commercially, the crude type of antiviral screening was performed by incubating the WSSV suspension with secondary metabolites. The cumulative mortality data of F. indicus treated with different H. salifodinae MPM-TC metabolite fractions may have arrested WSSV transcription and translation leading to inhibition of viral replication which was reflected in F. indicus cumulative mortality. Herbal extracts having antiviral and immunostimulant properties when incubated with WSSV and injected to the shrimp P. monodon, effectively suppressed the WSSV and reflected in the improved shrimp survival after WSSV challenge (Citarasu et al., 2006; Balasubramanian et al., 2008; Yogeeswaran et al., 2012). The PCR detection at in vivo levels also proved that the WSSV suppression was significant. The increasing concentrations of the F-III (800 mg kg−1) in the diets highly suppressed the viral load at 100% level after second step PCR detection. The F-III, antiviral secondary metabolite fraction of H. salifodinae MPM-TC contained the active compounds of potent antiviral activities. This was also supported by Citarasu et al. (2006) who stated that by treating P. monodon with herbal extracts then challenging with WSSV had only 25% PCR positive.
GC–MS analysis revealed that, F-III contains the active compounds of Perflurotributylamine, Cyclopentane, 1-butyl-2-ethyl, 1,1′-Biphenyl]-3-amine, Pyridine, 4-(phenylmethyl), Hexadecane, 2-methyl-, Nonadecane and Phytol. These compounds may be responsible for suppressing the WSSV transcription. Our previous study (Ronica, 2011), also revealed that the antiviral activity against WSSV using the biosurfactant isolated from halophilic Bacillus sp. BS3 from the same solar salt works reduced WSSV multiplication. Virmani et al. (1983) pointed out that, perfluorotributylamine (Oxypherol) influenced to stimulate the immune functions such as phagocytes in rabbit and human blood exposed to in vivo and in vitro conditions and improve Neutrophil superoxide (O−2) (Virmani et al., 1984). The presence of perfluorotributylamine in F III, boosted the immune system in F. indicus leading to an arrest of the multiplication of WSSV. Cyclopentane and cyclopentene P2-motifs inhibited the hepatitis C virus NS3 protease (Johansson et al., 2006; Bäck et al., 2007). The FIII also contains Cyclopentane, 1-butyl-343 2-ethyl- at the molecular weight of 154.29. The presence of this compound in FIII might contribute to inhibit WSSV at the translational level in vivo. The derivatives of 3-(4′-bromo-[1,1′-biphenyl]-4-yl)-3-(4-X-phenyl)-N,N-dimethyl-2-propen-1-amine (5a–m) had the potent antibacterial activity against M. tuberculosis and other Mycobacterium sp. (de Souza et al., 2001) and antifungal activities against Candida albicans, Candida parapsilosis, Criptococcus neoformans, Tricophyton verrucosum, Trichophyton rubrum, Microsporum gypseum and Aspergillus fumigates (Castellano et al., 2003). Also pytol had the molecular weight of 296.53 at high quality in the F III able to suppress the multiplication of WSSV. Pytol and its derivatives were reported as the immunostimulants in mice (Chowdhury and Ghosh, 2012) and act as antibacterial agents (Inoue et al., 2005). Based on the presence of immunostimulant, antiviral, antibacterial and antifungal compounds in the F III, it effectively controlled the WSSV multiplication.
The H. salifodinae MPM-TC secondary metabolites helped to decrease the protein level in WSSV challenged F. indicus by arresting the viral multiplication. Generally high total protein levels were seen in infected crustaceans due to the huge bacterial or viral loads and the heavy load reflects the increased protein content. The delivery of antiviral or immunostimulant compounds may help to decrease the load after infection (Citarasu et al., 2006). The total protein level observed was 121.1 μg ml−1 in the control group. In this study, the experimental groups helped to decrease the load significantly from the control group due the presence of antiviral compounds. The compounds might have suppressed the transcription and translation of WSSV that lead to the failure of multiplication and effected as decreased protein level. Earlier study by Citarasu et al. (2006) proved that the delivery of plant antiviral/immunostimulant extracts when treated to infected shrimp showed a reduced protein level. Manduca sexta larvae infected with polydnavirus had abundant viral protein in the haemolymph as reported by Harwood et al. (1994). Sahul Hameed et al. (1998) supported that, there was a higher WSSV viral protein level observed by the western blot analysis in different tissues and haemolymph of the WSSV infected shrimp. PCR analysis revealed that there is a presence of high concentration of WSSV in the haemolymph of infected shrimp (Lo et al., 1997). Herbal immunostimulants along with inactivated WSSV vaccines also helped to decrease the protein level in infected P. monodon leading to arrest of the WSSV multiplication (Yogeeswaran et al., 2012). Stressed or infected animals had found high levels of glucose and total carbohydrate in haemolymph. This is due to the transportation of glucose and carbohydrate from hepatopancreas and muscle to haemolymph (Yoganandhan et al., 2002). The present study showed the lowest glucose level (99.2 μg ml−1) in the control group and this parameter significantly increased in the HS3 treated group (109.0 μg ml−1).
The heavy WSSV load in haemolymph affected haematological parameters such as reduced THC, prolonged coagulation time and reduced oxyhaemocyanin levels (Citarasu et al., 2006). This was reflected in the present work with the values of 23.56 × 106 cells ml−1 (THC), 218.43 s of coagulation time and 0.75 mmol l−1 of oxyhaemocyanin level in the control group, respectively. The groups treated with antiviral secondary metabolites showed an increase in THC and oxyhaemocyanin levels and a reduction in coagulation time. Coagulation time and THC significantly differed between F. indicus infected with WSSV and those un- infected (Yoganandhan et al., 2002). Total haemocyte count was found to decrease in shrimp infected with a penaeid rod-shaped DNA virus (Maeda et al., 1997). Ratcliffe and Rowley (1979) and Sahul Hameed (1989) reported that, a decline in the level of THC in infected shrimps is due to the accumulation of haemocytes on the injection site for wound healing and phagocytosis of foreign bodies. Budding of the virus or virus induced apoptosis is also responsible for declining THC during viral infections (Cohen, 1993). Hagerman (1986) reported that, moulting cycle, nutritional and stress conditions affected the haemocyanin levels. The decreased level of oxyhaemocyanin was observed when F. indicus was devoid of antiviral secondary metabolite treatment and it was increased by the delivery of metabolites. It is concluded that, the H. salifodinae MPM-TC secondary metabolites helped to recover from the infections and improved the haematological parameters.
In the present experiment, proPO activity and production were higher in the experimental groups when compared to the control and those parameters seem to act as stimulators of shrimp immune system against the WSSV infection. Johansson et al. (2000) described that, phagocytosis, nodule formation, encapsulation, and haemocyte locomotion were activated by the proPO activating system. Song and Hsieh (1994) pointed out that, the increased disease resistance response is by production of extra bactericidal substances, such as H2O2 and superoxide anion ( ) from activated haemocytes. Both activities were observed at the decreased level when devoid of H. salifodinae MPM-TC antiviral secondary metabolite delivery. It was gradually increased with the increasing concentrations of metabolites in the diets. Phenoloxidase (PO) activity was significantly (P < 0.05) enhanced in black tiger shrimp haemolymph treated with Brewer’s yeast β-glucans compared with control shrimp (Suphantharika et al., 2003). Takahashi et al. (2000) found that boosted proPO activating system helps to control the virus by oral administration of LPS. P. monodon fed with 1–3% polysaccharide gel (PG) supplemented diet achieved higher survival percentage, increased THC and prophenoloxidase activity against the WSSV challenge (Pholdaeng and Pongsamart, 2010). Sarlin and Philip (2011) studied the efficacy of marine yeasts Debaryomyces hansenii (S8) and Candida tropicalis (S186) to stimulate the immune system in F. indicus against WSSV infection. The secondary metabolites of the thermophilic bacteria, Anoxybacillus kamchatkensis, improved the immunological parameters like serum SOD, lysozyme, bactericidal activity and phagocytic activity in common carp, Cyprinus carpio against A. hydrophila (Wang et al., 2011). P. monodon brooder treated with beta glucan for 24 days, helped to improve the relative in vivo intracellular production of haemocytes by 15.7 times. The antiviral secondary metabolites of H. salifodinae MPM-TC had the better antiviral and immunostimulating effects in F. indicus against WSSV infection. The findings of the present study concluded that, the secondary metabolites of H. salifodinae MPM-TC were able to suppress the WSSV multiplication at the in vivo level and boost F. indicus’s immune system against WSSV infection. This approach will help to develop novel antiviral drugs against fin and shell fish virus from extremophilic source.
References
- Transport of oxygen: respiratory proteins. In: Hayaishi O., ed. Molecular Oxygen in Biology: Topics in Molecular Oxygen Research. Amsterdam: North Holland; 1974. p. :219-274.
- [Google Scholar]
- A review: novel pharmaceutical compounds from marine bacteria. J. Appl. Bacteriol.. 1989;67:461-470.
- [Google Scholar]
- Novel potent macrocyclic inhibitors of the hepatitis C virus NS3 protease: use of cyclopentane and cyclopentene P2-motifs. Bioorg. Med. Chem.. 2007;15(22):7184-7202.
- [Google Scholar]
- Studies on the immunomodulatory effect of extract of Cyanodon dactylon in shrimp, Penaeus monodon, and its efficacy to protect the shrimp from white spot syndrome virus (WSSV) Fish Shellfish Immunol.. 2008;25(6):820-828.
- [Google Scholar]
- Antibiotics susceptibility testing by a single disc method. Am. J. Clin. Pathol.. 1966;45:493.
- [Google Scholar]
- Marine microorganisms as a source of new natural products. Adv. Appl. Microbiol.. 1997;43:57-90.
- [Google Scholar]
- Boonyaratpalin, M., 1993. Nutritional requirements of grouper Epinephelus. In: The Proceedings of Grouper Culture, National Institute of Coastal Aquaculture, Department of Fisheries, Thailand, pp. 50–55.
- Microbiol antagonism: a neglected avenue of natural products research. J. Biotechnol.. 1999;70(1–3):27-32.
- [Google Scholar]
- Synthesis and antimicrobial properties of 3-aryl-1-(1,1′-biphenyl-4-yl)-2-(1H-imidazol-1-yl) propanes as ‘carba-analogues’ of the N-arylmethyl-N-[(1,1′-biphenyl)-4-ylmethyl])-1H-imidazol-1-amines, a new class of antifungal agents. Il Farmaco. 2003;58(8):563-568.
- [Google Scholar]
- Effect of dietary β-1,3-glucan on resistance to white spot syndrome virus (WSSV) in postlarval and juvenile Penaeus monodon. Dis. Aquat. Organ.. 1999;36:163-168.
- [Google Scholar]
- Herbal biomedicines – a new opportunity to aquaculture industry. Aquacult. Int.. 2010;18:403-414.
- [Google Scholar]
- Influence of selected Indian immunostimulant herbs against white spot syndrome virus (WSSV) infection in black tiger shrimp. Fish Shellfish Immunol.. 2006;21:372-384.
- [Google Scholar]
- Synthesis, antimycobacterial activities and cytotoxicity on V79 of 3-[4′-Y-(1,1′-biphenyl)-4-yl]-N, N-dimethyl-3-(4-X-phenyl)-2-propen-1-amine derivatives. Eur. J. Med. Chem.. 2001;36(10):843-850.
- [Google Scholar]
- Screening for antibacterial and antiviral activities in three bivalve and two gastropod marine mollusks. Aquaculture. 2009;293:1-7.
- [Google Scholar]
- Screening of salt pans actinomycetes for antibacterial agents. Internet J. Microbiol.. 2005;1:2.
- [Google Scholar]
- Haemocyanin concentration of juvenile lobster (Homarus gammarus) in relation to molting cycle and feeding conditions. Mar Biol.. 1983;77:11-17.
- [Google Scholar]
- Haemocyanin concentration in the shrimp Crangon crangon (L) after exposure to moderate hypoxia. Comp. Biochem. Physiol.. 1986;85A:721-724.
- [Google Scholar]
- An abundantly expressed haemolymph glycoprotein isolated from newly parasitised Manduca sexta larvae in polydnavirus gene product. Virology. 1994;205:393-405.
- [Google Scholar]
- Bergey’s Manual of Determinative Bacteriology (9th ed.). Baltimore: Williams and Wilkins; 1994.
- Biphasic effects of geranylgeraniol, teprenone, and phytol on the growth of Staphylococcus aureus. Antimicrob. Agents Chemother.. 2005;49(5):1770-1774.
- [Google Scholar]
- Potent inhibitors of the hepatitis C virus NS3 protease: use of a novel P2 cyclopentane-derived template. Bioorg. Med. Chem.. 2006;14(15):5136-5151.
- [Google Scholar]
- Kamat, T., Kerkar, S., 2004. Studies on a bioactive compound produced by a halotolerant salt pan isolate. In: Conference on Microbiology of the Tropical Seas (COMITS), National Institute of Oceanography, Goa. MB 10p.
- Bacteria from salt pans: a potential resource of antibacterial metabolites. Recent Res. Sci. Technol.. 2011;3(9):46-52.
- [Google Scholar]
- Haematological and phenoloxidase activity changes in the shrimp Penaeus stylirostris in relation with the moult cycle: protection against vibriosis. Fish Shellfish Immunol.. 1997;7:227-234.
- [Google Scholar]
- A handbook of pathology and diagnostic procedures for diseases of penaeid shrimp. Baton Rouge, Louisiana, USA: World Aquacultrue Society; 1996. 304pp
- Detection and tissue tropism of white spot syndrome baculovirus (WSSV) in captured brooders of Penaeus monodon with special emphasis on reproductive organs. Dis. Aquat. Org.. 1997;30:53-72.
- [Google Scholar]
- Protein measurement with the Folein phenol reagent. J Biol Ctem.. 1951;193:265-275.
- [Google Scholar]
- Maeda, M., Itami, T., Kondo, M., Henning, O., Takahashi, Y., Hirono, I., 1997. Characteristics of penaeid rod shaped DNA virus of kuruma shrimp. NRIA international workshop. In: New Approaches to Viral Disease of Aquatic Animals. National Research Institute of Aquaculture, Nansei, Mie, Japan, pp. 218–228.
- Plant Enzymology and Histoenzymology. New Delhi: Kalyani Publishers; 1980. p. 278
- Marine pyridoacridine alkaloids: structure, synthesis, and biological chemistry. Chem. Rev.. 1993;93:1825-1838.
- [Google Scholar]
- Antimicrobial biosurfactants from marine Bacillus circulans: extracellular synthesis and purification. Lett. Appl. Microbiol.. 2009;48(3):281-288.
- [Google Scholar]
- A comparison of molluscan and arthropod haemocyanin. I. Circular dichroism and absorption spectra. Comp. Biochem. Physiol.. 1971;39B:855-872.
- [Google Scholar]
- Molecular ecology of extremely halophilic archaea and bacteria. FEMS Microbial. Ecol.. 2002;39:1-7.
- [Google Scholar]
- Studies on the immunomodulatory effect of polysaccharide gel extracted from Durio zibethinus in Penaeus monodon shrimp against Vibrio harveyi and WSSV. Fish Shellfish Immunol.. 2010;28:555-561.
- [Google Scholar]
- Drugs from the seas current status and microbiological implications. Appl. Microbial Biotechnol.. 2002;59:125-134.
- [Google Scholar]
- Role of haemocytes in defense against biological agents. In: Gupta A.P., ed. Insect Haemocytes E Development Forms, Functions and Techniques. Cambridge, UK: Cambridge University Press; 1979. p. :331-414.
- [Google Scholar]
- Ronica, S.F.A., 2011. Screening and characterization of novel biosurfactants from halophilic bacteria for biomedical application, M. Sc dissertation, Manonmaniam Sundaranar University, Tirunelveli, India.
- Clinical Pathology and Bacteriology. New Delhi: Jaypee Brothers; 1983. p. 133
- Sahul Hameed, A.S., 1989. Studies on the pathobiology of penaeid larvae and post larvae. Ph.D. thesis, Cochin University of Science and Technology, Cochin, India. p. 243.
- Studies on the pathogenicity of systemic ectodermal and mesodermal baculovirus (SEMBV) and its detection in shrimps by immunological methods. Aquaculture. 1998;160:31-45.
- [Google Scholar]
- Efficacy of marine yeasts and baker’s yeast as immunostimulants in Fenneropenaeus indicus: a comparative study. Aquaculture. 2011;321:173-178.
- [Google Scholar]
- Numerical Taxonomy. San Francisco: Freeman; 1973.
- Beta-1,3 glucan enhancement of protease activity in crayfish haemocyte lysate. Comp. Biochem. Physiol.. 1983;74:221-224.
- [Google Scholar]
- Immunostimulation of tiger shrimp (Penaeus monodon) haemocytes for generation of microbiocidal substances: analysis of reactive oxygen species. Dev. Comp. Immunol.. 1994;18:201-209.
- [Google Scholar]
- In vivo screening of mangrove plants for anti WSSV activity in Penaeus monodon and evaluation of Ceriops tagal as a potential source of antiviral molecules. Aquaculture. 2011;311:36-41.
- [Google Scholar]
- Preparation of spent brewer’s yeast beta-glucans with a potential application as an immuno-stimulant for black tiger shrimp, Penaeus monodon. Bioresour. Technol.. 2003;88:55-60.
- [Google Scholar]
- Cytotoxic and antimicrobial potential of Actinomycete species Saccharopolyspora saline VITSDK4 isolated from the Bay of Bengal coast of India. Am. J. Infect. Dis.. 2009;5(2):90-98.
- [Google Scholar]
- Polymerase chain reaction (PCR) amplification of bacilliform virus (RV-PJ) DNA in Penaeus japonicus Bate and systemic ectodermal and mesodermal baculovirus (SEMBV) DNA in Penaeus monodon Fabricius. J. Fish Dis.. 1996;19:399-403.
- [Google Scholar]
- Enhancement of disease resistance against penaeid acute viraemia and induction of virus-inactivating activity in haemolymph of kuruma shrimp, Penaeus japonicus by oral administration of Pantoea agglomerans lipopolysaccharide (LPS) Fish Shellfish Immunol.. 2000;10:555-558.
- [Google Scholar]
- Effects of perfluorochemical on phagocytic function of leukocytes. Transfusion. 1983;23(6):512-515.
- [Google Scholar]
- Effect of perfluorochemical blood substitutes on human neutrophil function. Transfusion. 1984;24(4):343-347.
- [Google Scholar]
- Halomonas salifodinae sp. nov., a halophilic bacterium isolated from a salt mine in China. Int. J. Syst. Evol. Microbiol.. 2008;58:2855-2858.
- [Google Scholar]
- Immunomodulatory effects of secondary metabolites from thermophilic Anoxybacillus kamchatkensis XA-1 on carp, Cyprinus carpio. Fish Shellfish Immunol.. 2011;30:1331-1338.
- [Google Scholar]
- Biochemical, physiological and hematological changes in white spot syndrome virus infected shrimp, Penaeus indicus. Aquaculture. 2002;62:1-11.
- [Google Scholar]
- Protection of Penaeus monodon against white spot syndrome virus by inactivated vaccine with herbal immunostimulants. Fish Shellfish Immunol.. 2012;32(6):1058-1067.
- [Google Scholar]







