Translate this page into:
Adsorption of 2,4,6-trichlorophenol (TCP) onto activated carbon
*Corresponding author. Tel.: +60 88 320000x3222; fax: +60 88 320348 anis_zaman@ums.edu.my (S.M. Anisuzzaman)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.

Available online 17 October 2012
Abstract
The adsorption of 2,4,6-trichlorophenol (TCP) by activated carbon was carried out at 30 °C with initial concentrations of 100–600 mg/L. The adsorption capacity of TCP was 457.9 mg/g at 30 °C. The adsorption isotherm and kinetics of TCP by activated carbon are investigated. The equilibrium isotherms of TCP/acetone mixtures were determined using a conventional method. Pseudo-Ideal adsorption model was used to analyze the liquid phase adsorption equilibrium data of TCP. As a result, the adsorption isotherm followed the Langmuir class (L type) and fits the experimental data well. The adsorption isotherm constant in this work also was compared with other researcher’s work showing the comparable values.
Keywords
Adsorption
2,4,6-Trichlorophenol
Activated carbon
Langmuir isotherm
1 Introduction
Phenolic derivatives belong to a group of common environmental contaminants. The presence of the phenolic derivatives even at low concentrations can be an obstacle to the use of waste water. Phenolic derivatives can cause unpleasant taste and odor of drinking water and can exert many negative effects on different biological processes. 2,4,6-Tricholrophenol (TCP) is one of the phenolic derivatives which is recognized as a toxic carcinogen (Podkoscielny et al., 2003). 2,4,6-TCP contains three chlorine atoms (at position 2,4 and 6) attached to the phenol ring. It is normally found in the industrial wastewater such as from paint, pharmaceutical, pesticide, wood, pulp and paper industries as well as water disinfecting process (Gao and Wang, 2007; Hammed, 2007).
2,4,6-TCP is a toxic, mutagenic and carcinogenic pollutant. 2,4,6-TCP is a weak acid which can easily permeate into the human skin in vitro and is readily absorbed by the gastro-intestinal tract (Hameed et al., 2009). It is also very harmful to the aquatic life if it is discharged into the river, lake and sea as well. The stable carbon-chlorine bond and the position of chlorine atoms relative to the hydroxyl group are responsible for their toxicity, carcinogenic properties, structural stabilization and persistence in the environment, making the removal of 2,4,6-TCP from the environment very crucial (Vidic et al.,1993). Besides, 2,4,6-TCP has been classified as one of the primary pollutants as enacted by the Department of Environment (DOE), Malaysia in Environmental Quality Act 1979 (Sewage and Industrial Effluent) which should be treated to be less than 1 ppm for inland water discharge. Therefore, suitable and ideal treatment which can effectively remove a large amount of 2,4,6-TCP in wastewater is critical since the demand for better quality treated wastewater effluent is increasing.
Several methods have been proposed in the literature on techniques for removal of 2,4,6-TCP compounds from wastewater such as photocatalytic, microbial degradation, chemical-biological oxidation, catalytic oxidation process, ion exchange resins and others. However, the adsorption process appears to be the most applicable method for removing trace amount of contaminant from wastewater effluent (Podkoscielny et al., 2003). TCP removal by activated carbon from coconut husk and palm fruit bunch was reported (Tan et al., 2008a,b 2009a,b).
Adsorption on activated carbon, which is one of the adsorption techniques, is the most effective and widely used technique in treating high strength and low volume phenolic wastewaters, besides it has fast adsorption kinetics and simplicity of design as compared to the other treatments (Hameed et al., 2009; Tan et al., 2009a,b). Activated carbon possesses perfect adsorption ability for relatively low-molecular-weight organic compounds such as 2,4,6-TCP (Podkoscielny et al.,2003). It also provides a large surface area, high adsorption capacity and high degree of surface reactivity (Singh et al., 2007). Activated carbon can be manufactured in such a way that a highly fractal material is obtained, which is roughly structured with each magnification and with pores of any width.
Adsorption process is broadly used for removal of odor, oil, colors and organic contaminants especially from a liquid-phase system. The potential of granular and powdered activated carbon has been proven as an effective adsorbent used in adsorption technology over the century. Therefore, study of adsorption equilibrium and adsorptivity needs to be done to further understand about adsorption kinetics on the activated carbon.
2 Materials and methods
2.1 Chemical reagents and apparatus
Reagents used in this experiment were 2,4,6-TCP as component (1) and acetone as component (2). The pure sample of acetone (solvent) used in this work was obtained from Merck. Meanwhile 2,4,6-TCP (adsorbate) was obtained from Sigma Aldrich with 98% purify which is in solid form. The adsorbent used to study the adsorption process was activated carbon Norit 97876 types purchased from Sigma Aldrich which is in powder form (BET surface area: 800 m2; average pore size: 50 nm). Gas Chromatography (Techcomp Ltd, GC 1000 II, Hong Kong) was used for composition analysis by using a Thermal Conductivity Detector (TCD). The gas chromatography method for detection of 2,4,6-TCP is shown in Table 1.
Detector
Thermal conductivity detector (TCD)
Column
Capillary column, 30 m L × 0.25 ID × 0.25 μm film thickness
Initial oven temperature
60 °C
Initial temperature duration
2 min
Temperature ramp
39 °C/min
Final oven temperature
240 °C
Final time duration
5 min
Injector temperature
250 °C
Detector temperature
250 °C
Injection volume
0.4 μL
Injection technique
Manual injection
Carrier gas
Helium gas (99.99% purity)
Carrier gas pressure
40 psi
2.2 Adsorption experiment
The measurement of excess adsorption of 2,4,6-TCP was performed by using a conventional method (Bono, 1989; Farhadpour and Bono, 1996a, 1996b), where 2 g of activated carbon, W0, was added into initially weight percentage, w% of 2,4,6-TCP on 20 g total weight of liquid 2,4,6-TCP and acetone mixture W, in a volumetric flask. Ten samples of liquid 2,4,6-TCP and acetone mixture were prepared ranging initially from 100–600 mg/L.
Before the activated carbon being used in the experiment, the activated carbon was dried first in the oven with temperature of 50 °C and left overnight. Then the activated carbon was placed in desiccators to cool it before being used. This is to prevent any moisture in the activated carbon which affects the adsorption result.
The volumetric flask then was put into the water bath shaker and shaken at 100 rpm at a constant temperature of 30 °C for 2 days. After 2 days, the solution was left for equilibrium to allow the activated carbon particle to precipitate at the bottom of the flask. When all the activated carbon particles had precipitated at the bottom of the flask, it was filtered (using microfilter) to prevent fine activated carbon particles entering the GC column during injection. Then all the samples of 2,4,6-TCP and acetone mixtures were injected by using a micro syringe manually with a volume of 0.4 μL into gas chromatography to determine the peak area fraction of 2,4,6-TCP in the mixture.
2.3 Measurement of excess adsorption
After the completion of analysis part on the gas chromatography, excess adsorption of 2,4,6-TCP onto activated carbon was calculated. The adsorption equilibrium was calculated using a conventional method (Bono, 1989; Farhadpour and Bono, 1996a, 1996b) and the equation is:
2.4 Analysis of excess isotherm
In order to optimize the design of an adsorption system, it is important to establish the most appropriate correlation. For the interpretation of the adsorption equilibrium in this study, the Pseudo ideal adsorption model was used.
2.4.1 Pseudo ideal adsorption model
The Pseudo ideal adsorption model is basically based on the Langmuir model. However, it is extended in the analysis by consideration of Eq. (2) for the interpretation of adsorption equilibrium (Bono, 1989; Farhadpour and Bono, 1996a, 1996b, 1988; Bono et al., 2008). The adsorption isotherms for 2,4,6-TCP onto activated carbon were analyzed using Eq. (2) while the individual isotherms for 2,4,6-TCP onto activated carbon (n1s) mixtures were calculated based on Eq. (3). Finally the selectivity of adsorption for the 2,4,6-TCP onto activated carbon (x1s) was calculated based on the Eq. (4).
3 Result and discussion
3.1 Adsorption equilibrium curve
From the excess adsorption data, adsorption equilibrium curve is plotted with the excess isotherm on the y-axis and the equilibrium mole fraction on the x-axis like has been shown in Fig. 1. Fig. 1 shows excess isotherms of 2,4,6-TCP onto activated carbon at constant temperature of 30 °C over entire concentration range. We can see from Fig. 1 that there were no negative adsorptions occurred in the mixture. From Fig. 1 it can also be observed that the adsorption of 2,4,6-TCP onto activated carbon increases at increasing concentrations of 2,4,6-TCP.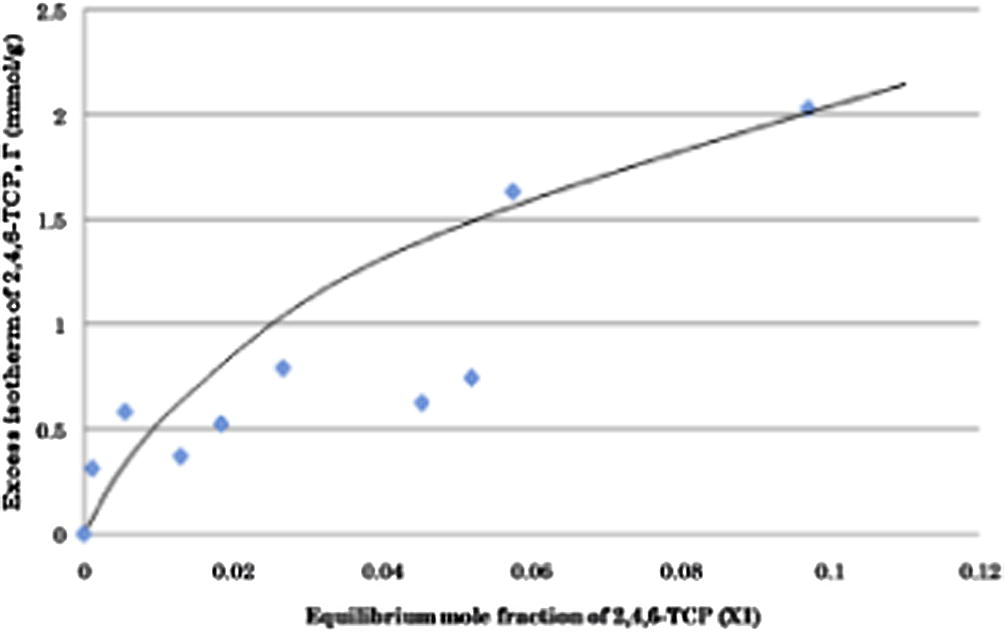
Adsorption isotherm of 2,4,6-TCP onto activated carbon at constant temperature of 30 °C.
3.2 Adsorption isotherm analysis using the Pseudo-Ideal adsorption model
Equation (2) has been used in the analysis of the experimental data for the adsorption of 2,4,6-TCP onto activated carbon at 30 °C. The analysis of experimental data for correspondence with the Eq. (2) is carried out by plotting (x1x2/Γ) vs. X1 as shown in Fig. 2. From Fig. 2, we can see a straight line at entire concentration range with equation of y = 0.3721x + 0.0087 and regression coefficient of 0.9767. It indicated that the adsorption of 2,4,6-TCP onto activated carbon obeyed Eq. (2) well and found to correlate well by the Pseudo-Ideal adsorption model at entire concentration range.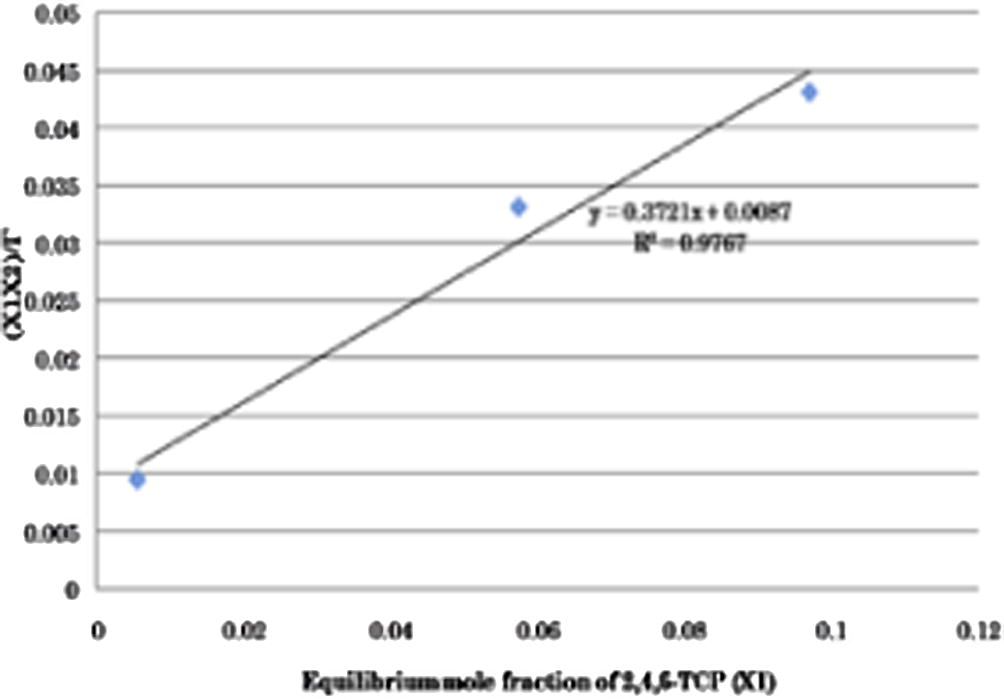
Monolayer adsorption of 2,4,6-TCP onto activated carbon at 30 °C according to the Pseudo-Ideal adsorption model.
The value of Ns and K can be obtained from Fig. 2 by manipulating Eq. (2) with the straight line equation, the value is shown in Table 2. Fig. 3 depicts the individual adsorption phase of 2,4,6-TCP onto activated carbon at 30 °C according to the Equation (3). The adsorption isotherm curve follows the Langmuir class (L type) which is widespread in the case of adsorption of phenolic compounds from water and it was characterized by an initial region. The L type suggests that the aromatic ring absorbs and no strong competition exists between the adsorbate and the solvent to occupy the adsorption sites. We also can see that the shape of the curve fits the ‘favorable isotherm’ with non-linear characteristics. ‘Favorable isotherm’ permits higher solid loadings at lower solution concentrations. Hence, this prove that the adsorption of 2,4,6-TCP onto activated carbon occurs immediately even at lower concentrations of 2,4,6-TCP in the solution mixture.
Ns, mmol/g
2.32
K
111.56
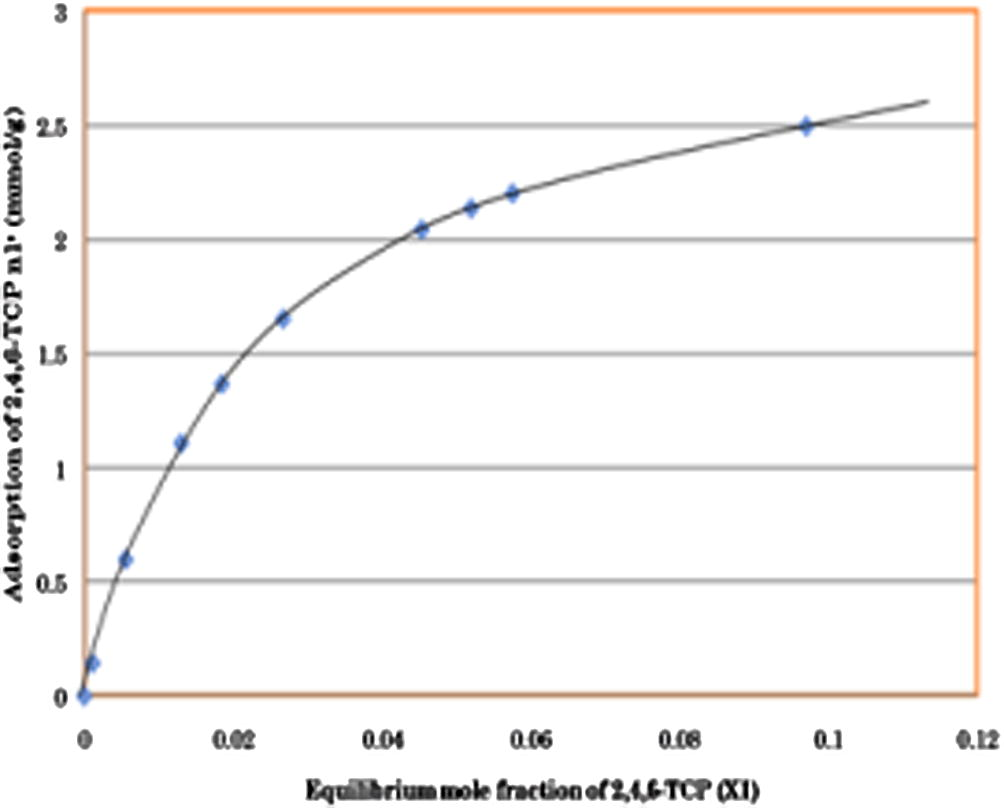
Individual adsorption isotherms of 2,4,6-TCP onto activated carbon at 30 °C.
The isotherm curve clearly showed that the 2,4,6-TCP adsorption rate on the activated carbon was fast and rapid even at lower concentrations of 2,4,6-TCP. This phenomenon was due to the high molecular weight and low solubility characteristics of 2,4,6-TCP making it to become easily absorbed by the activated carbon. 2,4,6-TCP also was adsorbed fast due to its high affinity of the interacting groups on the surface of the activated carbon (Hameed and El-Khaiary, 2008).
Fig. 4 shows the selectivity adsorption of 2,4,6-TCP onto activated carbon based on Eq. (4). From Fig. 4, we can see that the degree of selectivity of 2,4,6-TCP onto activated carbon is high at increasing concentrations of 2,4,6-TCP. This is because of the characteristics of the 2,4,6-TCP itself such as its high affinity, high molecular weight and low solubility causing it selectivity to be adsorb on activated carbon is high.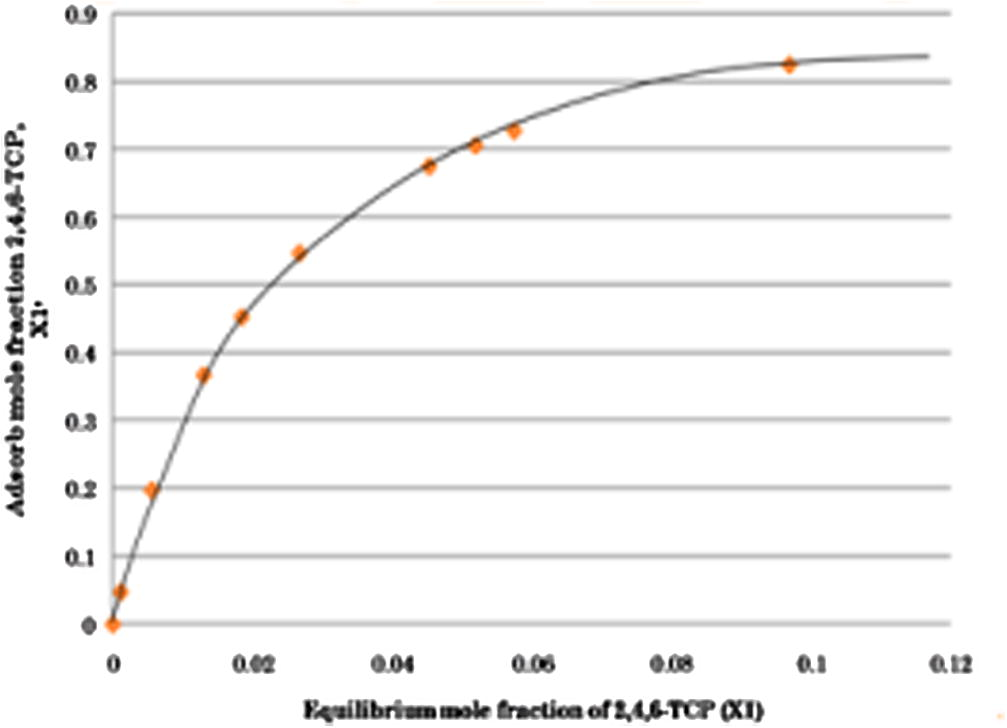
Selectivity of 2,4,6-TCP onto activated carbon at 30 °C.
3.3 Comparison of Pseudo-Ideal adsorption model constant Ns and K
Table 3 shows the Pseudo-Ideal adsorption model constant related to adsorption capacity value of 2,4,6-TCP onto activated carbon, obtained from this work and has been compared with other previous works done by other researches. In this study, the constant obtained only to show significant difference and are comparable with other researchers work proving that the adsorption of 2,4,6-TCP onto activated carbon follow favorable isotherm. These also proved that adsorption isotherm approach using the Pseudo-Ideal adsorption model was convenient for the characterization of adsorption equilibrium data as the other researchers are using the Freundlich model for their analysis. Table 3 also shows the regression coefficient R2 values obtained from this work and other researcher’s values. Thus proving the Pseudo-Ideal adsorption model is suitable to describe the adsorption equilibrium of 2,4,6-TCP on the activated carbon in this research.
Authors
Type of activated carbon
Ns, mmol/g
K
R2
Dabrowski et al. (2005)
Commercial activated carbon
2.50
155.10
Not stated
Tan et al. (2009a,b)
Activated carbon from coconut husk
1.25
22.04
0.998
Hameed et al., (2008)
Activated carbon from rice straw
1.20
14.20
0.994
Maarof et al. (2003)
Norit granular activated carbon NAC 1240
2.38
166.67
0.92
This work
Norit activated carbon 97876
2.32
111.56
0.967
4 Conclusion
The excess adsorption isotherm of 2,4,6-TCP onto activated carbon correlated well by the Pseudo-Ideal adsorption model and conformed as ‘favorable isotherm’ with non-linear characteristics and followed the Langmuir class (L type) adsorption. Comparison of adsorption isotherm constant and regression coefficient constant with other researchers’ (Dabrowski et al., 2005; Tan et al., 2009a,b; Hameed et al., 2008; Maarof et al., 2003) work has been proved that the values are comparable and showed a significant difference proving that the adsorption of 2,4,6-TCP onto activated carbon follows favorable isotherm.
Acknowledgments
The authors wish to acknowledge the help rendered by Mr. Fadzlan Y. Yahya for carrying out the experiments.
References
- Bono, A., 1989. Sorptive Separation of Simple Water Soluble Organics. Dissertation submitted for the Degree of Doctor of Philosophy, Chemical and Process Engineering Department, University of Surrey, England.
- Effect of ultrasound on liquid phase adsorption of azeotropic and non-azeotropic mixture. Catalysis Today. 2008;131:472-476.
- [Google Scholar]
- Adsorption of phenolic compound by activated carbon- a critical review. Chemosphere. 2005;58:1049-1070.
- [Google Scholar]
- Adsorption from solution of nonelectrolytes by croporous crystalline solids: ethanol—water/silicalite system. Journal of Colloid and Interface Science. 1988;124:209-227.
- [Google Scholar]
- Separation of Ethanol–Water mixtures with a bi-dispersed hydrophobic molecular sieve. silicalite: measurement and interpretation of column dynamics. Chemical Engineering Programme. 1996;35:157-168.
- [Google Scholar]
- Sorptive separation of ethanol–water mixture with a bi-dispersed hydrophobic molecular sieve silicalite: determination of the controlling mass transfer mechanism. Chemical Engineering Process. 1996;35:141-155.
- [Google Scholar]
- Effect of pH and temperature on isotherm parameters of chlorophenol biosorption to anaerobic granular sludge. Journal of Hazardous Materials. 2007;145:398-403.
- [Google Scholar]
- Kinetics and equilibrium studies of malachite green adsorption on rice straw-derived char. Journal of Hazardous Materials. 2008;153:701-708.
- [Google Scholar]
- Preparation of oil palm empty fruit bunch-based activated carbon for removal of 2,4,6-trichlorophenol: optimization using response surface methodology. Journal of Hazardous Materials. 2009;164:1316-1324.
- [Google Scholar]
- Equilibrium and kinetics studies of 2,4,6-trichlorophenol adsorption onto activated clay. Colloids and Surfaces, A: Physicochemical and Engineering Aspects. 2007;307:45-52.
- [Google Scholar]
- Aqueous phase adsorption of phenolic compound on activated carbon. Malaysia: School of Engineering, Universiti Sains Malaysia; 2003.
- Heterogeneity of active carbons in adsorption of phenol aqueous solutions. Applied Surface Science. 2003;205:297-303.
- [Google Scholar]
- Liquid-phase adsorption of phenols using activated carbons derived from agricultural waste material. Journal of Hazardous Materials. 2007;150:626-641.
- [Google Scholar]
- Adsorption of basic dye on high surface area activated carbon prepared from coconut husk: equilibrium, kinetic and thermodynamic studies. Journal of Hazardous Materials. 2008;154:337-346.
- [Google Scholar]
- Preparation of activated carbon from coconut husk: Optimization study on removal of 2,4,6-trichlorophenol using response surface methodology. Journal of Hazardous Materials. 2008;153:709-717.
- [Google Scholar]
- Fixed-bed adsorption performance of oil palm shell-based activated carbon for removal of 2,4,6-trichlorophenol. Bioresource Technology. 2009;100:1494-1496.
- [Google Scholar]
- Adsorption isotherms, kinetics, thermodynamics and desorption studies of 2,4,6-trichlorophenol on oil palm empty fruit bunch-based activated carbon. Journal of Hazardous Materials. 2009;164:473-482.
- [Google Scholar]
- Oxidative coupling of phenols on activated carbon: impact on adsorption equilibrium. Environmental Science and Technology. 1993;27:2079-2085.
- [Google Scholar]







