Translate this page into:
Influence of single walled carbon nanotubes at subtoxical concentrations on cell adhesion and other cell parameters of human epithelial cells
*Corresponding author. Tel.: +41 71 274 76 89; fax: +41 71 274 76 94 jean-pierre.kaiser@empa.ch (J.-P. Kaiser),
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Available online 4 August 2012
Peer review under responsibility of King Saud University.
Abstract
The production of SWCNT has increased tremendously in the last decade, due to high expectations of their benefits. Despite all precautions SWCNT may come into contact with organisms and may be taken up by them. The aim of the present study was to assess and elucidate the effects of SWCNT bundles (purified SWCNT) and SWCNT raw material on the behaviour of human epithelial cells (A549). When cells were exposed to SWCNT (bundles and raw material) cell adhesion was significantly affected. The effects of SWCNT were dependent on the SWCNT purity (purified SWCNT or SWCNT raw material), the SWCNT concentration and the incubation period. SWCNT raw material affected cell adhesion more than the SWCNT bundles did. Beside effects on cell adhesion, cell spreading and the development of focal adhesion points and the actin skeleton were affected, however to a much lesser extent. Cells grown in the presence of SWCNT showed an elevated amount of reactive oxygen species (ROS). The effect was concentration dependent.
Other cell parameters, such as cell viability, cell death (apoptosis and necrosis), activation of cell signalling proteins, such as focal adhesion kinase (FAK) and mitogen activated protein kinase (MAPK-Erk1/2), as well as the gap junctional intercellular communication proteins (connexin-43 and β-catenin) were not seriously affected when cells were grown in the presence of SWCNT.
Keywords
Single walled carbon nanotubes (SWCNT)
Cell-adhesion
Cell-signalling
Cytoskeleton organization
- SWCNT
-
single walled carbon nanotubes
- Total-FAK
-
focal adhesion kinase
- p-FAK
-
phosphorylated focal adhesion kinase (activated FAK)
- Total-Erk1/2
-
mitogen-activated protein kinases
- p-Erk1/2
-
phosphorylated mitogen-activated protein kinases (activated Erk1/2)
- ROS
-
Reactive oxygen species
Abbreviations
1 Introduction
Particles in the nanoscale range and among them the single walled carbon nanotubes (SWCNT) possess new chemical, physical and electrical properties, which differ from the properties of bulk materials. Therefore the global production of carbon nanotubes has increased tremendously within the last decade. Carbon nanotubes are industrially produced by several technological processes, such as arc discharge (Walters et al., 1999), laser ablation (Guo et al., 1995) and chemical vapour deposition (Cassell et al., 1999). Unpurified SWCNT (raw material) contain quite a large amount of by-products, such as amorphous and soot like carbon, catalyst metal particles and supporting materials (Donaldson et al., 2006). The cytotoxicity of SWCNT is dependent on the type and concentration of SWCNT, as well as on the composition of the raw material.
Despite all precautions SWCNT may come into contact with organisms and may be taken up by them. Nowadays it is well documented that different cell types e.g. mesothelioma cells (MSTO-211H), primary neurons and glial cells, heart cells and co-cultures consisting of human epithelial lung cells (A549), human monocyte-derived macrophages, monocyte-derived dendritic cells (MDDC) are not severely affected when exposed to short SWCNT (Belyanskaya et al., 2009; Helfenstein et al., 2008; Kaiser et al., 2008; Muller et al., 2010; Wick et al., 2007).
All methods used in this study were reliable and suitable for analysing the different cell parameters at subtoxic concentrations (Belyanskaya et al., 2007). Subtoxic concentrations were defined as SWCNT concentrations up to 30 μg/ml. Exposure of epithelial cells to SWCNT up to this concentration range did not severely affect important cell parameters (Belyanskaya et al., 2007, 2009; Muller et al., 2010; Wick et al., 2007). This guarantees that the obtained data concerning effects of SWCNT on cell adhesion was conducted with cell cultures with a high number of living cells.
Proteins and amino acids from the culture medium as well as extra-cellular matrix proteins, which are excreted from the cells adsorb to surfaces, e.g. to the cell culture plastic of petri dishes. Surface properties of the materials influence the composition of the adsorbed protein layers, which subsequently regulate a variety of cell behaviours, such as attachment, viability, spreading, migration and differentiation (Webb et al., 2000). Cells adhere to the underlying surface through transmembrane-proteins, e.g. integrins. However, cells do not make direct contact with the underlying surface. Transmembrane-proteins like integrins bind to extra-cellular matrix proteins, which are adsorbed to the underlying substrate. The extra-cellular matrix contains a lot of proteins, e.g. fibronectin, which include several RGD-peptide sequences (arginine, glycine, and aspartate). These small peptide sequences are the binding sites for the integrins. The integrins are further attached to the cytoskeleton of the cells through linker proteins (Bershadsky et al., 1996; Owen et al., 2005). These linker proteins influence the actin cytoskeleton of the cells. Vinculin is one of these linker proteins and vinculin has direct interactions with cell proteins as well as the cell membrane.
Transmembrane signals that affect cell proliferation, differentiation and cell survival are generated by the adhesion of the cells to the extra-cellular matrix. These signals are triggered by interactions between the transmembrane-proteins (e.g. integrins) and the extra-cellular matrix and involve tyrosine phosphorylation of specific proteins, the assembly of focal adhesion and actin bundles (Bershadsky et al., 1996).
Cell adhesion to the extra-cellular matrix influences many cellular functions, such as apoptosis, viability and migration (Schwartz and Ginsberg, 2002). However, focal adhesions are not only responsible for the anchoring of the intra-cellular cytoskeleton to the underlying surface (substrate). They are also involved in biochemical signalling of many regulatory pathways. Therefore the regulation of the assembly and disassembly of focal adhesion is a critical mechanism for controlling cell functions (Geiger and Bershadsky, 2002; Schwartz and Ginsberg, 2002). Cell spreading and cell morphology have been correlated with changes in cell survival, cell viability, and cellular differentiation (Bershadsky et al., 1996; Chen et al., 1997). It was found that the surface properties of a material influence the composition of the adsorbed protein layer, which subsequently regulates a variety of cell behaviours of MC3T3-E1 osteoblast, such as cell attachment efficiency, spread cell area or cell morphology, cytoskeleton organization and cell migration rate. Changes in cell spreading (morphology) and migration rate are therefore an indicator of cell physiology. This phenomenon (cell behaviour) can be used as an indicator for toxicological effects.
The aim of this study was to investigate the effects of SWCNT (bundles and raw material) at subtoxic concentrations (15 μg/ml and 30 μg/ml) on other cell parameters than cell viability and apoptosis/necrosis; cell responses such as reactive oxygen species (ROS), activation of cell signalling proteins, distribution of cell communication proteins (connexin-43, β-catenin), cell adhesion, cytoskeleton organization, cell shape and cell spreading, and development of focal adhesion points to understand more the mechanics of CNT – cell interactions.
2 Materials and methods
2.1 Material
The investigated SWCNT raw material was purchased from Yangtse Nanotechnology, Shanghai, China. SWCNT raw material was produced by the conventional arc-discharged evaporation of graphite rode filled with nickel and yttrium powder as catalysts. The procedure for solubilization and purification of SWCNT raw material as well as the characterization by infra-red spectroscopy (NIR), inductive coupled plasma optical emission spectrometry (ICP, OES) and scanning electron (SEM) as well as transmission electron microscopy (TEM) has been described in detail by Wick et al., (Wick et al., 2007).
The amount of metallic impurities was analysed by inductive coupled plasma optical emission spectroscopy (ICP OES). The content of Fe, Co, Ca, and Mg in the SWCNT raw materials was under the detection limit (Fe < 130 μg/ml, Co < 40 μg/ml, Ca < 340 μg/ml, Mg < 40 μg/ml). The SWCNT were sterilized for 3 h at 160 °C before they were suspended in distilled water containing 20 μg/ml polyoxyethylene sorbitol monoleate (Tween 80) to a final stock suspension of 250 μg/ml. The SWCNT were sonicated in an ultra sound bath for 2 times for 15 min. and were prepared by a centrifugation step (10’000g for 10 min.) in order to separate the suspended carbon nanotubes (SWCNT bundles) from the non-tube fraction. The physical parameters of SWCNT were examined by SEM and TEM analysis. The SWCNT bundles consisted of 10 to 20 parallel aligned tubes of around 50 nm with an approximate length of 0.5–3.0 μm (Belyanskaya et al., 2009), whereas the SWCNT raw material contains aggregates with diameter in the μm-range (Belyanskaya et al., 2007).
2.2 Cells
The behaviour of human lung epithelial cells (cell line, A549, European Collection of Cell Cultures (ECACC), Salisbury, UK) was investigated. The cells were cultured in the RPMI-1640 medium (Sigma, Buchs, CH), with 10% heat inactivated foetal calf serum (FCS) (Life Technologies, Basel, CH), 1% PSN-solution (Life Technologies, Basel, CH) and 1% glutamine solution (Life Technologies, Basel, CH) under cell culture conditions (5% CO2, 95% air and 37° C). The cultures were passaged once a week. Cells were exposed to SWCNT (bundles and raw material) at concentrations of 15 μg/ml and 30 μg/ml.
2.3 Cell viability
Epithelial cells were cultivated for 3 and 5 days in the presence of 15 μg/ml (39 ng/mm2, 92 pg/cell) and 30 μg/ml (78 ng/mm2, 184 pg/cell) SWCNT (bundles and raw material) on petri dishes with diameter of 35 mm. Then the cells were trypsinized and collected and the amount of cells was estimated by counting the cells using a Neubauer cell chamber. Trypan blue was used for staining the dead cells.
2.4 Apoptosis
Apoptosis in cell cultures was quantitatively analysed by flow cytometric analysis (FACS) based on the binding of annexin V fluorescein to phosphatidyl serine in cells, which were in an early apoptotic stage and the binding of propidium iodide to DNA in cells, which were in a late apoptotic stage. The staining procedure was performed according to the user protocol (PF032) annexin V binding with adherent cells of Calbiochem, D. For the different chromophores the following excitation and emission wavelengths were used: annexin V-FITC (ex. 488 nm; em. 528 nm), propidium iodide (ex. 488 nm; em. 635 nm). 10,000 cells were counted in each flow cytometric analysis.
Killer Trail and staurosporine were used in positive control cultures to induce apoptosis/necrosis. After exposure of epithelial cells for 6 h to 50 ng/ml KillerTrail, cells in the early apoptotic stage were analysed in these cultures. After incubating epithelial cell cultures in the presence of 4 μM staurosporine for 6 h, cells in the late apoptotic/necrotic stage were analysed in these cultures.
2.5 Reactive oxygen species (ROS)
Reactive oxygen species (ROS) were estimated with the reactive dye 2′,7′-dichlorodihydrofluorescein-diacetate (H2DCF-DA) and 3-morpholinosydnonimine hydrochloride (5 μM) was used as positive control according to Limbach et al., (Limbach et al., 2007). The release of ROS was analysed after an exposure time of 1 h, which corresponds to the first accumulative burst of ROS after SWCNT exposure.
2.6 Distribution of connexin-43 and β-catenin
The distribution of connexin-43 and β-catenin in the cells was analysed by staining the proteins with fluorescent dyes and thereafter microscopically analysed. The following antibodies were used: connexin-43: rabbit polyclonal anti-connexin-43 (Cat. No. C6219, Sigma, CH), β-catenin: mouse anti-β-catenin (Cat. No. 610154, BD Transduction Laboratories, CH).
2.7 Cell adhesion
Analysis of cell adhesion was performed by measuring centrifugal force according to the description of Koo et al., (Koo et al., 2002). Epithelial cells were cultivated for 1, 3 and 5 days in the presence of 15 and 30 μg/ml SWCNT (bundles and raw material) on petri dishes with diameter of 90 mm. The dishes with the adherent cells were horizontally rotated nine times for 1 min. After each consecutive time the spin was increased by augmentation of the rotation speed. After each spin detached cells were collected and counted with a flow cytometer (Schaerfe, Reutlingen, D). Cell adhesion was estimated by measuring the centrifugal force necessary to detach epithelial cells in the control culture, which were not exposed to SWCNT, as well as to SWCNT bundles (15 and 30 μg/ml) and SWCNT raw material (15 and 30 μg/ml) after 1, 3 and 5 days of incubation.
In addition cell adhesion of cell cultures, which were preincubated in the presence of a soluble peptide containing a RGD-sequence in a serum-free media was investigated. Epithelial cells were preincubated for 18 h on petri dishes (90 mm) in a serum free RPMI 1640 media with 1 mg/ml BSA and 30 μg/ml of a small peptide consisting of 7 amino acids (GRGDSPK) including a RGD-sequence (JPT Peptide Technologie, Berlin). Due to its small size the peptide stays in the solution. Then 1% FCS was added to the culture media and the culture was incubated for another day at 37 °C in the presence or the absence of 30 μg/ml SWCNT (bundles and raw material). Then cell adhesion was estimated according to the method described above.
2.8 SWCNT adsorbed to the extra-cellular matrix and the integrins
With a series of different proteolytic treatments it was possible to detach proteins adsorbed to extra-cellular matrix (ECM). Epithelial cells exposed to SWCNT bundles and raw material were washed with PBS in order to remove all SWCNT not adsorbed to the extra-cellular matrix and not adsorbed to the substrate (petri dish) respectively. Then SWCNT adsorbed to the extra-cellular membrane were detached by a series of proteolytic treatments by trypsin–EDTA (Cat. No. 25300–054, Invitrogen, Basel, CH); accumax, a mixture of proteolytic and collagenolytic enzymes (Cat. No. A7089, Sigma Buchs, CH) and accutase, a mixture of proteolytic and collagenolytic enzymes (Cat. No. A6964, Sigma Buchs, CH). After each proteolytic treatment the cell suspension was centrifuged and the supernatants with the digested ECM and the detached SWCNT were collected and the cell pellets washed with PBS and again centrifuged. Each incubation step lasted 30 min. Finally, cells were washed 3 times with PBS/0.5% BSA and the supernatants were collected. The collected supernatants were centrifuged at 25,000g for 20 min. to collect the carbon nanotubes. The amount of SWCNT still adsorbed to the ECM of the cells and the amount of SWCNT removed from the ECM by the proteolytic treatments were estimated by measuring the optical density at 405 nm. Cells which were not exposed to SWCNT, and SWCNT adsorbed to the petri dish which were incubated in the absence of cells, were treated in the same manner and used as controls.
2.9 Signalling proteins; FAK and MAPK-Erk1/2
For the analysis of signalling proteins epithelial cell cultures were exposed to 15 and 30 μg/ml SWCNT bundles and raw material for 1, 3 and 5 days. Cell proteins were harvested using a RIPA-buffer and analysed by Western blotting. The following antibodies were used: phosphorylated focal adhesion kinase (p-FAK): mouse anti-p-FAK (Tyr397) (Cat. No. 611807, BD-Transduction Laboratory, CH); total focal adhesion kinase (FAK): rabbit anti-FAK (Cat. No. ab40794, Abcam, UK); phosphorylated Erk1/2 (Thr202/Tyr204): rabbit polyclonal anti-p-Erk1/2 (p44/42, MAPkinase) (Cat. No. 9102, Cell Signaling Technology, USA); total-Erk1/2 (44/42, MAPkinase): rabbit polyclonal anti-Erk1/2 (Cat. No. 9101S, Cell Signaling Technology, USA). As a loading control, GAPDH was analysed with GAPDH antibody HRP-loading control (Cat. No. ab9282, Abcam, UK). Detection of the signalling proteins was performed with secondary antibodies coupled to horseradish peroxidase and detection thereof with the electrochemiluminescence (ECL) Advanced Western Blotting Detection Kit (Cat. No. RPN2135, Amersham Pharmacia Biotech, UK). Protein concentrations were estimated using Biometra-Fluoroimager (Biometra, CH) and the integrated density of the protein signals were analysed with Image J-Software.
2.10 Cell shape and cell spreading
Cell shape and cell spreading were analysed by staining cell components (actin, vinculin and nucleus) with fluorescent dyes and thereafter microscopically analysed. The procedure has been described in detail by Kaiser et al., (Kaiser et al., 2008). Cell area and area of focal adhesion points of cells exposed to SWCNT, as well as cells grown in the absence of SWCNT, were analysed with Image J software.
2.11 Statistical analysis
The experiments were repeated independently three times. Significant effects (∗) were determined using Student’s T-test. Differences were considered significant at p < 0.05. In the figures data are presented as the mean of the three independent experiments and the standard error of the means (SEM) over the mean experimental values of each of the three independent experiments.
3 Results
To avoid the measurement of cell adhesion of dead cells, an acute cytotoxicity test including live/dead staining and cell growth was performed prior to cell adhesion and cell spreading assays to define an optimal SWCNT concentration range to study subtoxic effects. Influence of SWCNT on cell viability has been summarized in Table 1a. Trypan blue was used for staining the dead cells.
Parameter
Control culture
SWCNT bundles 15 μg/ml
SWCNT bundles 30 μg/ml
SWCNT raw material 15 μg/ml
SWCNT raw material 30 μg/ml
Cell viabilitya (cell number) Exposure time: 3 days
100.0%
104.0 ± 1.2%
94.5 ± 6.7%
93.4 ± 7.8%
83.2 ± 3.7%*
Cell viabilitya Exposure time: 5 days
100.0%
100.1 ± 4.8%
94.6 ± 3.2%
95.2 ± 4.8%
79.9 ± 6.7%*
Epithelial cell cultures grown in the presence of up to 30 μm/ml SWCNT bundles and 15 μg/ml SWCNT raw material exceeded the same or slightly reduced cell viability as cells of the control cultures, grown in the absence of SWCNT. Cell viability was only affected when cells were grown in the presence of 30 μg/ml SWCNT raw material.
Apoptosis/necrosis was analysed quantitatively by FACS analysis (Table 1b). In a positive control experiment with “Killer Trail” as an apoptosis inducer, it was proven that these cells could undergo apoptosis. Staurosporine was used as a positive control to induce necrosis. These observations indicate that the presence of SWCNT was not seriously affecting cell viability and by that we can exclude that a change in cell physiology is due to an intoxication of the epithelial lung cells. Cells grown in the presence of SWCNT showed an elevated amount of reactive oxygen species (ROS), compared to the control cultures, which were grown in the absence of SWCNT. The influence of SWCNT on the release of ROS is summarized in Table 1c. Repetitions: n = 3. The release of reactive oxygen species (ROS) was analysed after an exposure time of 1 h. 3-Morpholinosydnonimine hydrochloride (5 μM) was used as positive control.
Parameter
Control culture
SWCNT bundles 15 μg/ml
SWCNT bundles 30 μg/ml
SWCNT raw material 15 μg/ml
SWCNT raw material 30 μg/ml
Cell depth:
Live cells
95.1 ± 1.8%
93.5 ± 2.9%
92.2 ± 3.7%
91.4 ± 5.0%
92.6 ± 2.7%
Apoptotic cells
0.7 ± 0.2%
0.7 ± 2.8%
0.9 ± 0.5%
1.1 ± 0.7%
1.0 ± 0.4%
Necrotic cells
Exposure time: 1 day
4.2 ± 1.9%
5.8 ± 3.0%
6.9 ± 3.5%
7.5 ± 4.8%
6.4 ± 2.4%
Cell depth:
Live cells
95.9 ± 0.3%
94.9 ± 0.3%
95.7 ± 0.8%
93.2 ± 2.5%
93.6 ± 0.3%
Apoptotic cells
0.3 ± 0.1%
0.4 ± 0.1%
0.5 ± 0.1%
0.6 ± 0.2%
0.7 ± 0.2%
Necrotic cells
Exposure time: 3 days
3.8 ± 0.4%
4.6 ± 0.4%
4.5 ± 0.7%
6.3 ± 2.3%
5.9 ± 0.2%
Cell depth:
Live cells
92.4 ± 1.9%
91.7 ± 2.1%
90.4 ± 0.9%
90.1 ± 2.5%
87.3 ± 0.9%
Apoptotic cells
0.2 ± 0.1%
0.4 ± 0.1%
0.4 ± 0.2%
0.4 ± 0.2%
0.7 ± 0.1%
Necrotic cells
Exposure time: 5 days7.4% ± 1.9%
8.0% ± 2.1%
9.2% ± 0.9%
9.5% ± 0.1%
12.1% ± 0.9%*
Positive controls
Cell depth:
Killer trail
Live cells
73.1 ± 2.5%*
Apoptotic cells
12.9 ± 1.1%*
Necrotic cells
14.0 ± 1.3%*
Cell depth:
Staurosporine
Live cells
74.9 ± 1.9%*
Apoptotic cells
3.0 ± 0.6%*
Necrotic cells
22.1 ± 1.6%*
Parameter
Control culture
SWCNT bundles 15 μg/ml
SWCNT bundles 30 μg/ml
SWCNT raw material 15 μg/ml
SWCNT raw material 30 μg/ml
Positive control
Release of reactive oxygen species (ROS)
100%
265 ± 10%*
557 ± 25%*
264 ± 25%*
454 ± 18%*
3131 ± 267%*
In several publications it has been mentioned that parameters, such as cell viability, cell activity, apoptosis/necrosis, cell spreading, cytoskeleton organization and cell adhesion are correlated to each other (Nelson et al., 2005; Nobes and Hall, 1999). Therefore the influence of SWCNT on additional cell parameters affecting cell behaviour was investigated.
Cell adhesion of epithelial cells was estimated by measuring the centrifugal force necessary to detach the cells from the surface. Cells, which were incubated in the presence of SWCNT (bundles and raw material), showed a weaker adherence than the control cells, which were not exposed to SWCNT (Fig. 1a and Fig. 1b). Cells increased the adherence to the underlying surface with increasing incubation time. Also, cells incubated in the presence of SWCNT increased cell adhesion to the underlying surface with prolonged incubation time, however the augmentation in cell adherence was far lower than the one seen in the control cultures. The effects of SWCNT were dependent from the SWCNT purity (bundles or raw material), from the SWCNT concentration and from the incubation period.![Epithelial cells were exposed to SWCNT bundles (15 μg/ml and 30 μg/ml) for 5 days. Cell adherence was estimated with the centrifugal force technique by horizontally rotating the petri dishes with the adherent cells. Acceleration [g] is the maximal acceleration of gravity at the peripheries of the petri dishes with a radius of 45 mm.](/content/185/2013/25/1/img/10.1016_j.jksus.2012.06.003-fig1.png)
Epithelial cells were exposed to SWCNT bundles (15 μg/ml and 30 μg/ml) for 5 days. Cell adherence was estimated with the centrifugal force technique by horizontally rotating the petri dishes with the adherent cells. Acceleration [g] is the maximal acceleration of gravity at the peripheries of the petri dishes with a radius of 45 mm.
![Epithelial cells were exposed to SWCNT raw material (15 μg/ml and 30 μg/ml) for 5 days. Cell adherence was estimated with the centrifugal force technique by horizontally rotating the petri dishes with the adherent cells. Acceleration [g] is the maximal acceleration of gravity at the peripheries of the petri dishes with a radius of 45 mm.](/content/185/2013/25/1/img/10.1016_j.jksus.2012.06.003-fig2.png)
Epithelial cells were exposed to SWCNT raw material (15 μg/ml and 30 μg/ml) for 5 days. Cell adherence was estimated with the centrifugal force technique by horizontally rotating the petri dishes with the adherent cells. Acceleration [g] is the maximal acceleration of gravity at the peripheries of the petri dishes with a radius of 45 mm.
Under in vitro conditions transmembrane-proteins like integrins bind to the RGD-sequence of proteins, such as collagens, which are adsorbed to the substrate. Soluble peptides containing a RGD-sequence will also bind to integrins and probably affect cell adhesion in a similar way to SWCNT. Cell adherence was affected by the SWCNT and the RGD-sequence containing peptide (30 μg/ml) in a synergistic way. SWCNT (30 μg/ml) (bundles or raw material) affected cell adherence in a stronger manner than the same concentration of RGD-sequence containing peptide did (Fig. 2).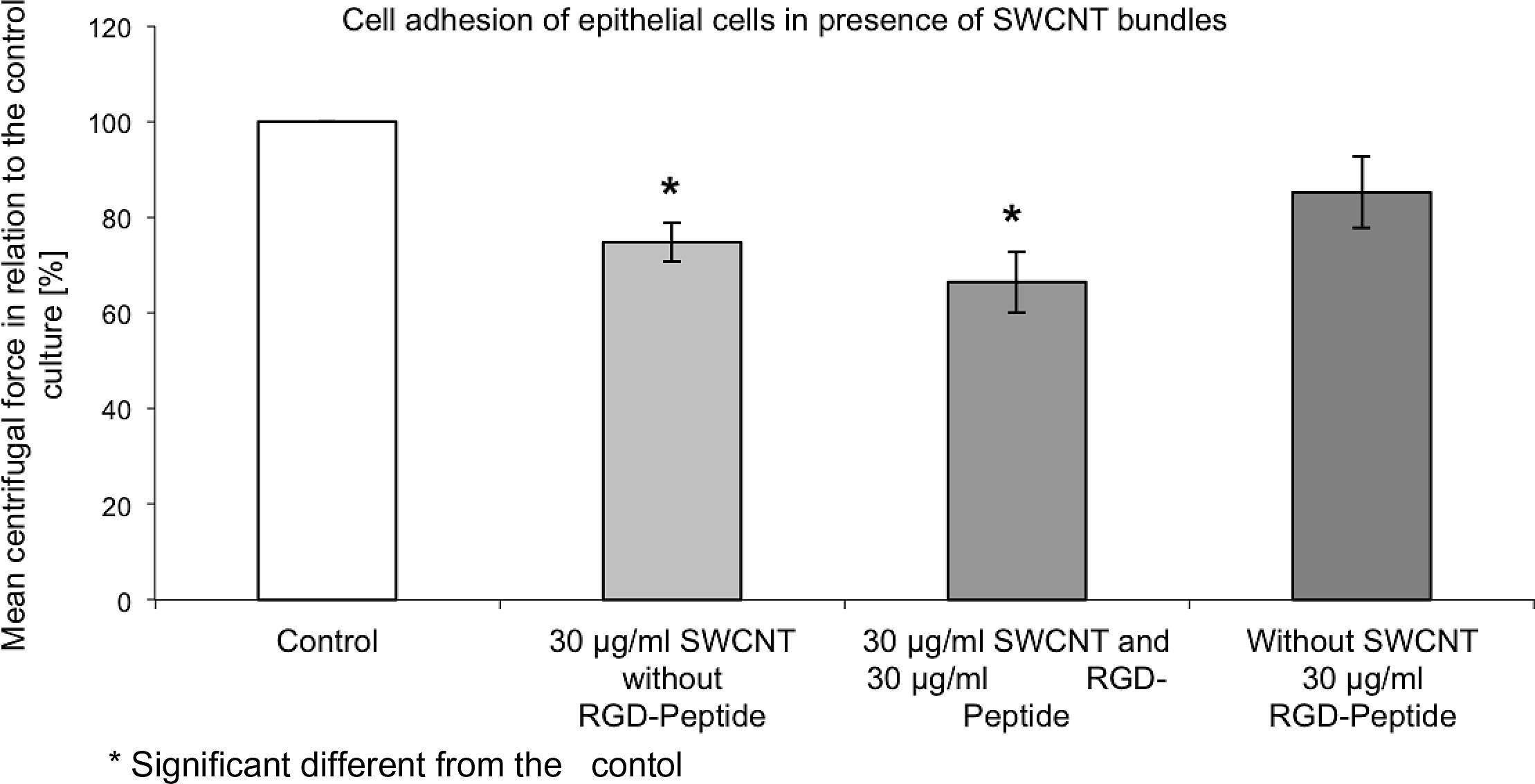
Epithelial cells were exposed to SWCNT bundles (30 μg/ml) with and without an RGD-sequence containing peptide for 1 day. SWCNT affected epithelial cell adhesion in a higher manner than the soluble RGD-sequence containing peptide.
Since SWCNT adhere to proteins it is obvious that they will also adhere to the integrins and other proteins of the extra-cellular matrix. Akiyama et al. (Akiyama and Yamada, 1985) showed that a soluble, labelled fibronectin binds to the surface of the cells and that more than 90% of the labelled fibronectin was released after trypsin treatment. This indicates that the fibronectin was almost exclusively bound on the external surface of the plasma membrane. According to this procedure epithelial cells were incubated in the presence of SWCNT and processed in a similar manner with proteases and collagenases and the released SWCNT estimated by an optical density measurement at 405 nm. The amount of SWCNT still remaining at the extra-cellular matrix was compared with the harvested amount of SWCNT detached from the extra-cellular matrix.
After exposure of epithelial cells for 5 days to 30 μg/ml, for SWCNT bundles and SWCNT raw material 50.3% and 62.1% of the adsorbed SWCNT could be detached from the extra-cellular matrix with this procedure, respectively. Cells exposed to SWCNT (bundles and raw material, 30 μg/ml) for 5 days were darker still than cells grown in the absence of SWCNT (Fig. 3a). This indicates that there were still SWCNT attached to the extra-cellular matrix of the cells after the proteolytic treatments. Fig. 3b shows the released extra-cellular matrix after the proteolytic procedures. The extra-cellular matrix from cells grown in the presence of SWCNT contained adsorbed SWCNT and was therefore much darker than the extra-cellular matrix from cells that were grown in the absence of SWCNT. This clearly indicates that SWCNT were not only internalized into the cells, but also adsorbed to extra-cellular matrix proteins and to integrins. It can be expected that the proteolytic treatments were not disrupting the cell membranes and that there were still SWCNT sticking to the remaining extra-cellular matrix of the cells. Cells were reinoculated after this proteolytic treatment and developed within one day a regular cytoskeleton.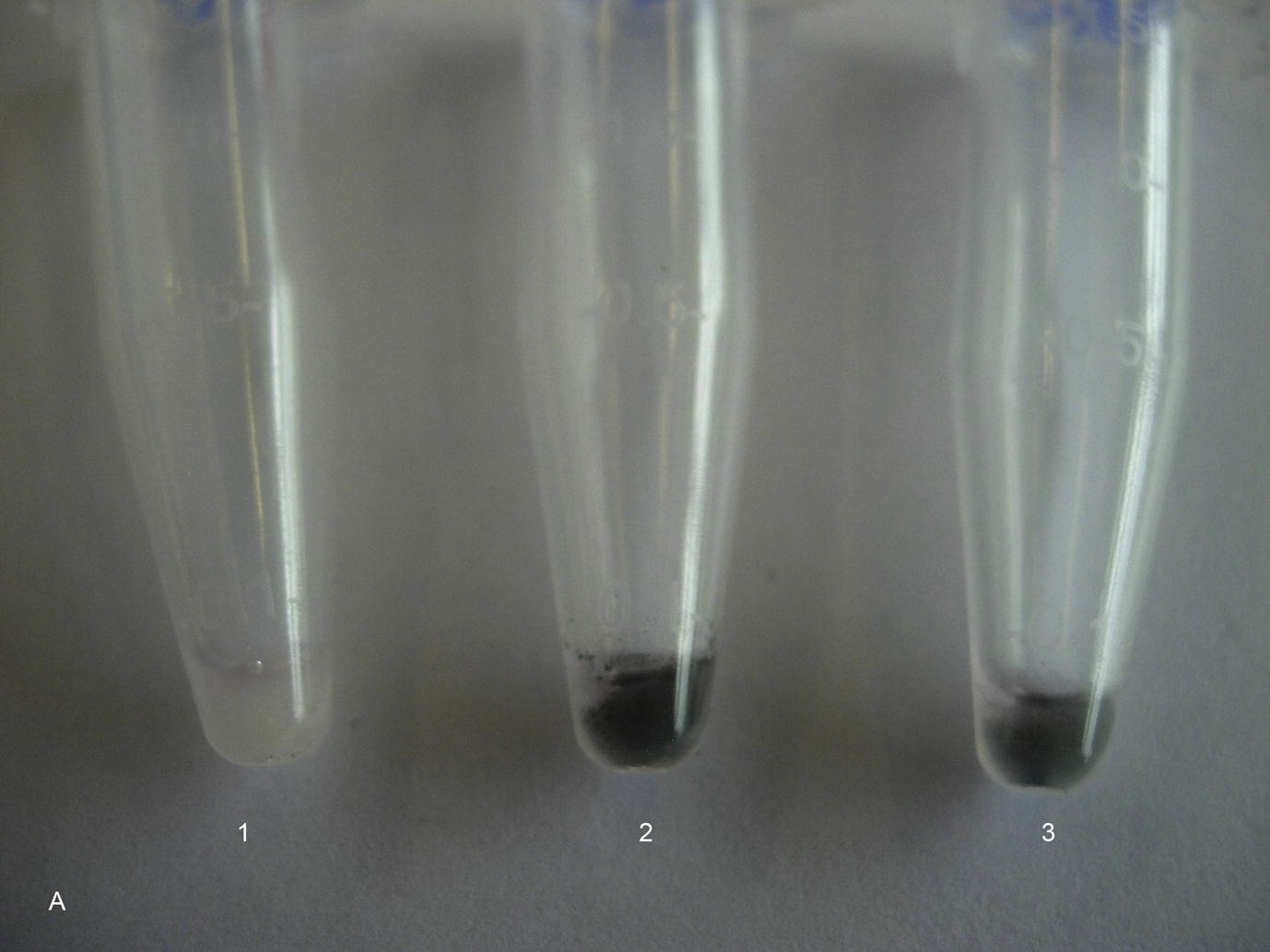
Cells after a series of proteolytic treatments after exposure to SWCNT (bundles and raw material, 30 μg/ml) for 5 days. 1 = Control culture, 2 = Cell culture exposed to SWCNT bundles (30 μg/ml), 3 = Cell culture exposed to SWCNT raw material (30 μg/ml).
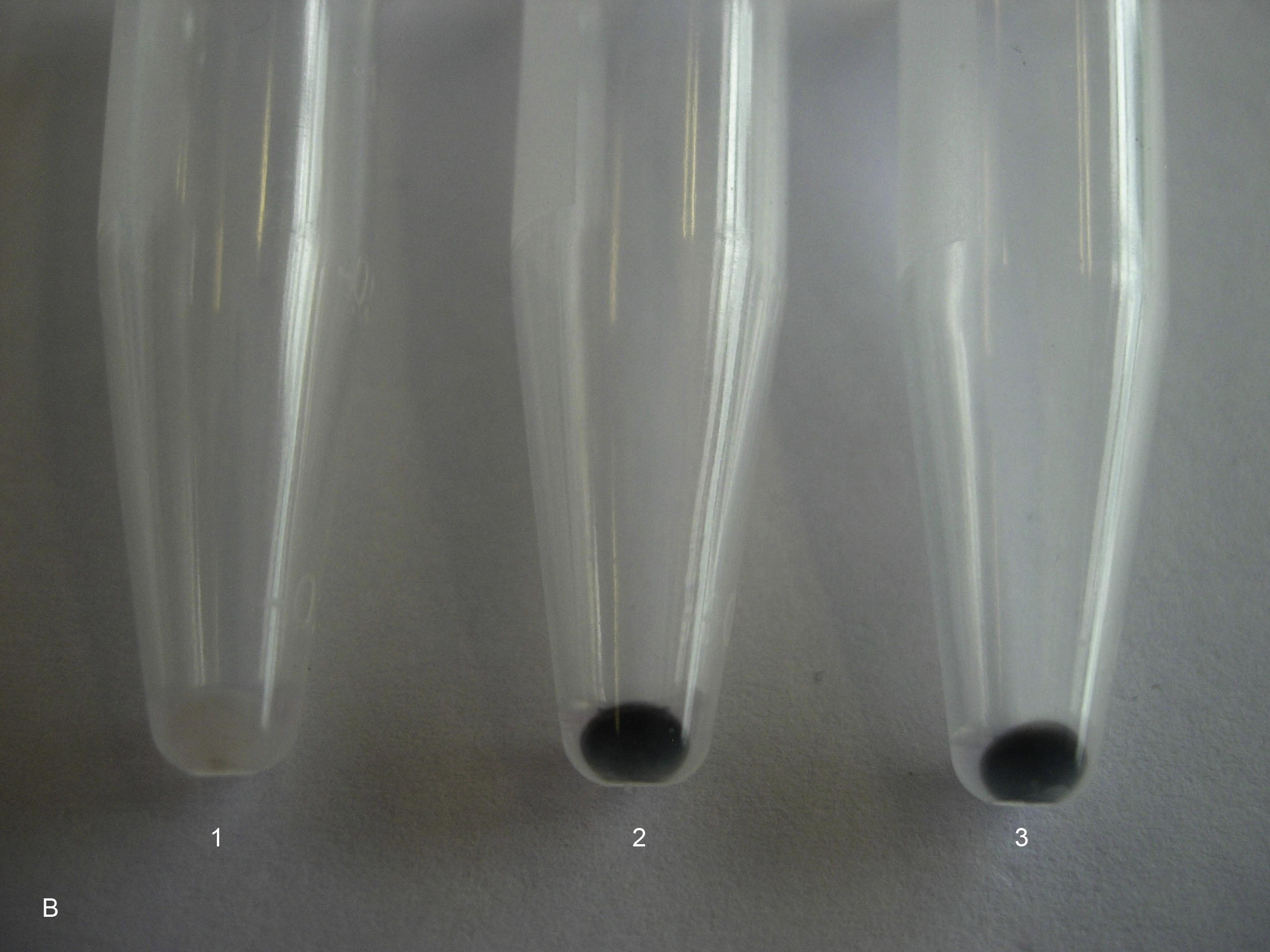
Digested extra-cellular matrix (ECM) with detached SWCNT from the cells after a series of proteolytic treatments. 1 = ECM from a control culture, which has not been exposed to SWCNT, 2 = ECM from a cell culture, which has been exposed to SWCNT bundles (30 μg/ml) for 5 days, 3 = ECM from a cell culture, which has been exposed to SWCNT raw material (30 μg/ml) for 5 days.
To prove that the released SWCNT were not only attached to the unspecific extra-cellular matrix proteins, but also to the integrins, cells were reinoculated after the proteolytic treatments. After an incubation time of 1 day, which allowed the cells to restore their extra-cellular matrix, and to spread and to adhere to the substrate, cell adherence of proteolytic and non-proteolytic treated cell cultures was analysed and compared to analogous treated control cultures. Reincubated cells after proteolytic treatment had a higher adhesion to the underlying surface than cells without proteolytic treatment. These findings were a further indication that SWCNT adhere in a similar way as adsorbed fibronectin to integrins, with the result that the cells with adsorbed SWCNT show a weaker adherence to the underlying substrate than cells grown in the absence of SWCNT.
SWCNT affected not only the cell adhesion, but also the cell spreading and the area of visible focal adhesion points. Cells grown in the presence of 30 μg/ml SWCNT raw material for 5 days showed a reduced spreading area, compared to the control cultures grown in the absence of SWCNT (Fig. 4a). Cells grown in the presence of 30 μg/ml SWCNT raw material showed an average cell spreading which was 14% smaller than the spreading area of cells in the control cultures (Fig. 4b). The areas of visible focal adhesion points of SWCNT exposed cultures were also reduced. Cells grown in the presence of SWCNT raw material (30 μg/ml) for 5 days showed focal adhesion areas which were reduced by 30%, compared to the cells grown in the absence of SWCNT (Fig. 4c). Furthermore less visible focal adhesion points per cell could be observed (Table 2). n = 50.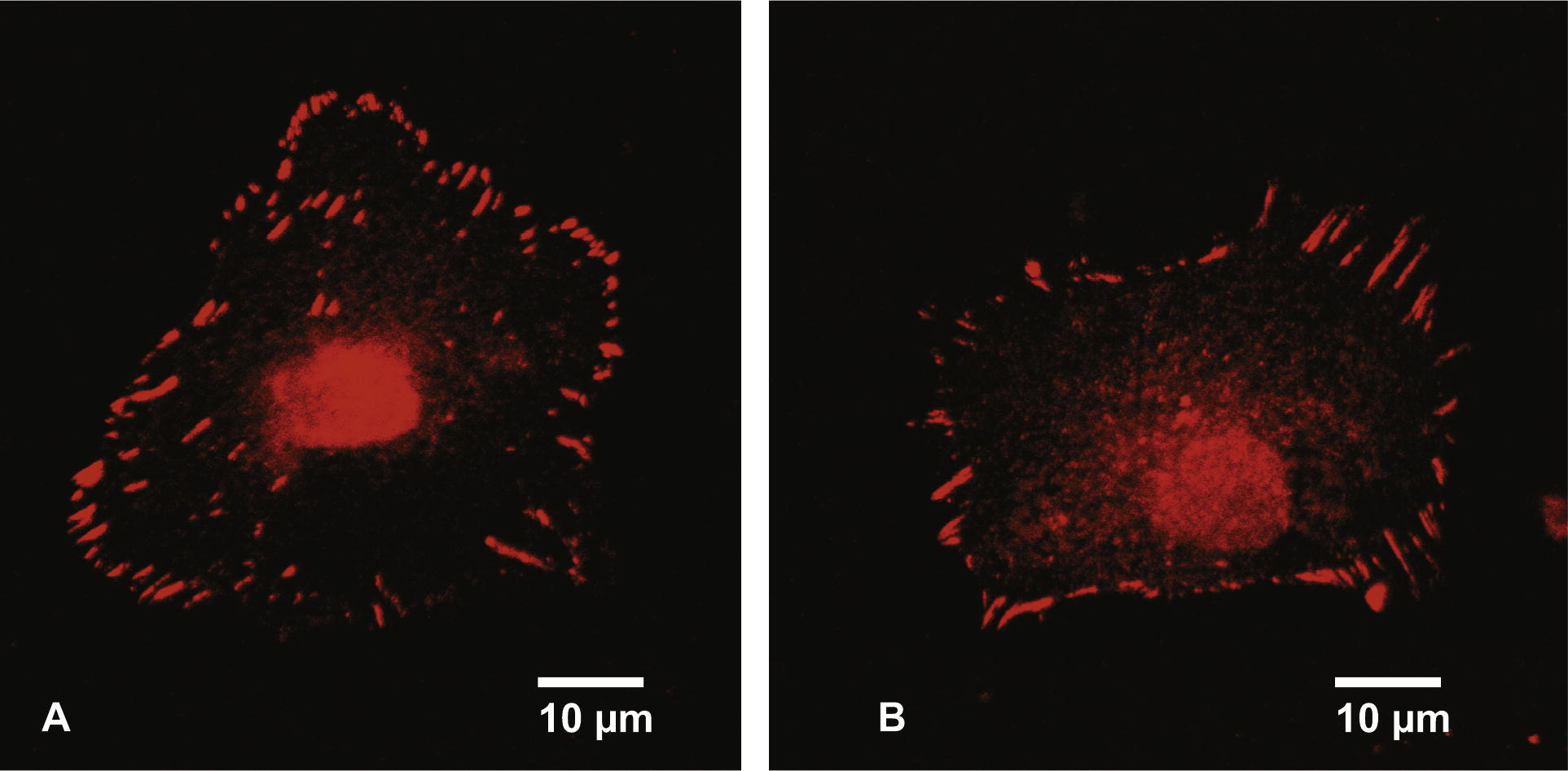
Fluorescence microscope picture of a cell grown in the absence of SWCNT (A). The focal adhesion points were stained with a fluorescent dye conjugated to vinculin. Fluorescent microscope picture of a cell grown for 5 days in the presence of 30 μg/ml SWCNT raw material (B). The focal adhesion points were stained with a fluorescent dye conjugated to vinculin.
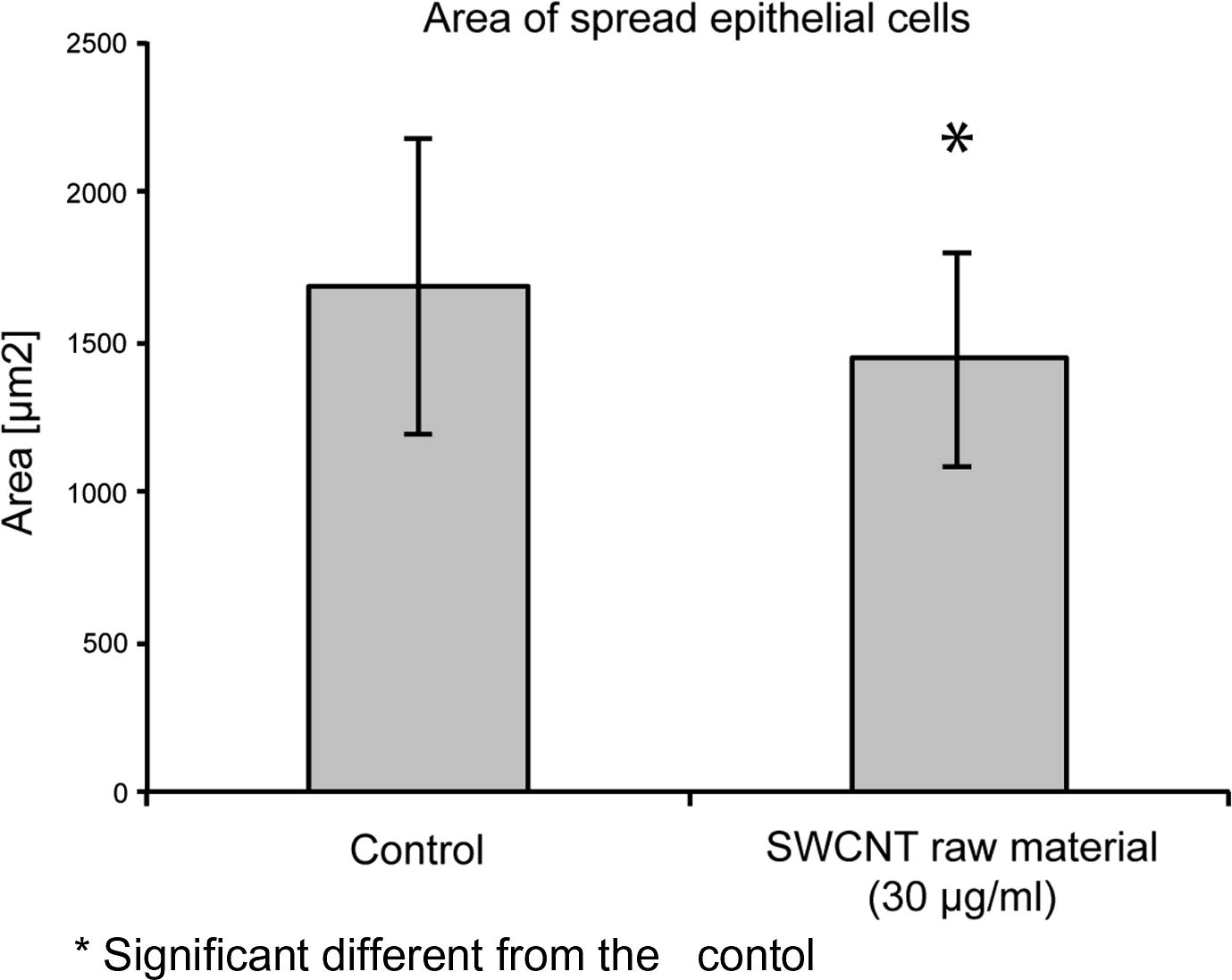
Spreading area of cells grown in the presence of 30 μg/ml SWCNT raw material in comparison to cells grown in the absence of SWCNT.
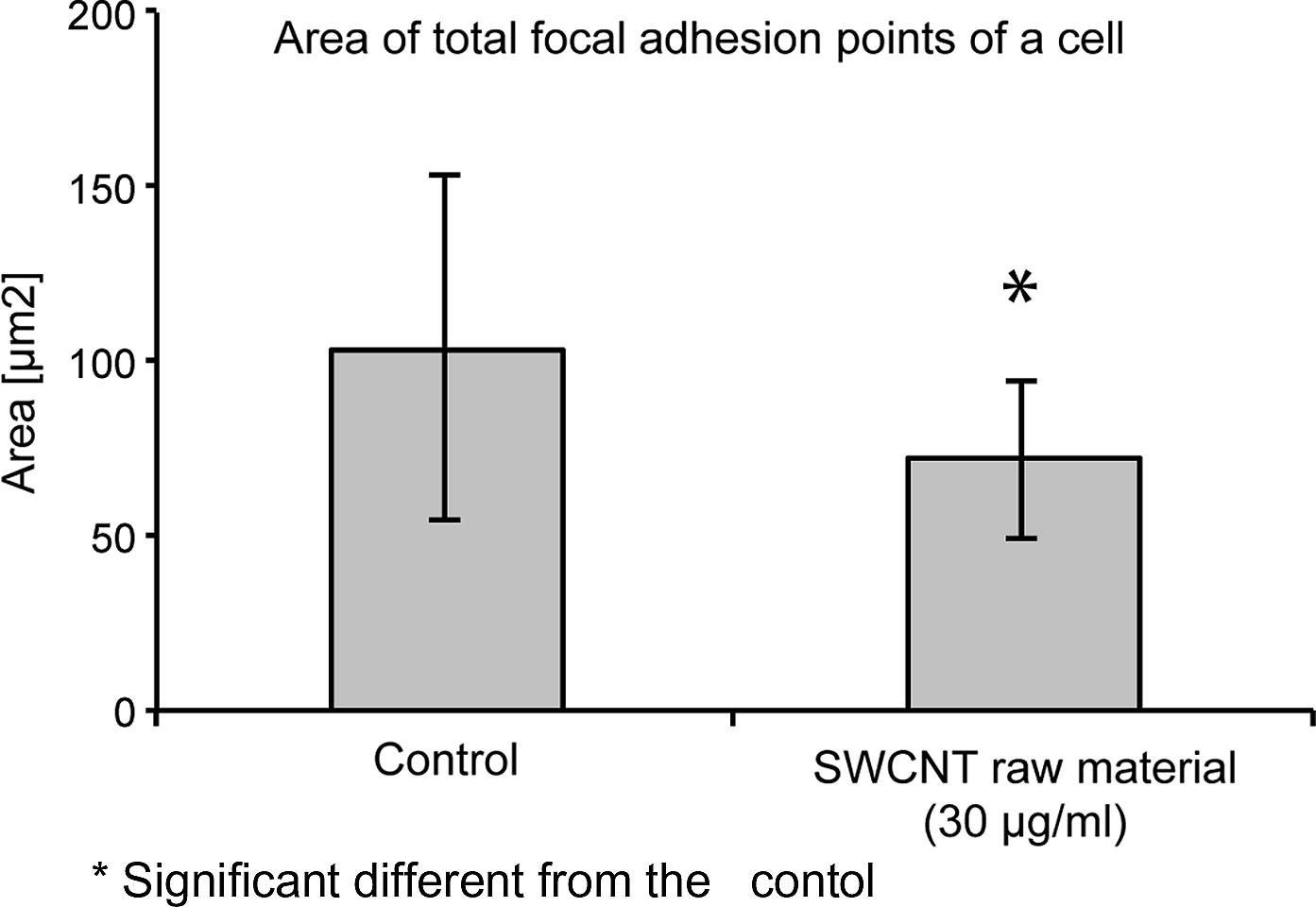
Development of visible focal adhesion points of cells grown in the presence of 30 μg/ml SWCNT raw material in comparison to cells grown in the absence of SWCNT.
Cell area (μm2)
Perimeter (μm)
Area of all visible focal adhesion points (μm2)
Mean area of a visible focal adhesion point (μm2)
Amount of focal adhesion points per cell
Control culture grown in absence of SWCNT
1681.0 ± 488.8
178.6 ± 32.2
103.4 ± 49.5
1.05 ± 0.44
102.9 ± 35.9
Cells grown in presence of SWCNT raw material [30 μg/ml]
1442.9 ± 473.3*
165.2 ± 24.8*
72.13 ± 22.9*
0.90 ± 0.45
87.5 ± 22.3*
No remarkable changes of the actin cytoskeleton organization of the epithelial cells grown in the presence of 30 μg/ml SWCNT bundles were observed after 5 days exposure to SWCNT particles. However, cells exposed to SWCNT raw material were reorganizing their actin cytoskeleton, which resulted in a concentration of actin at the cell peripheries (Fig. 5).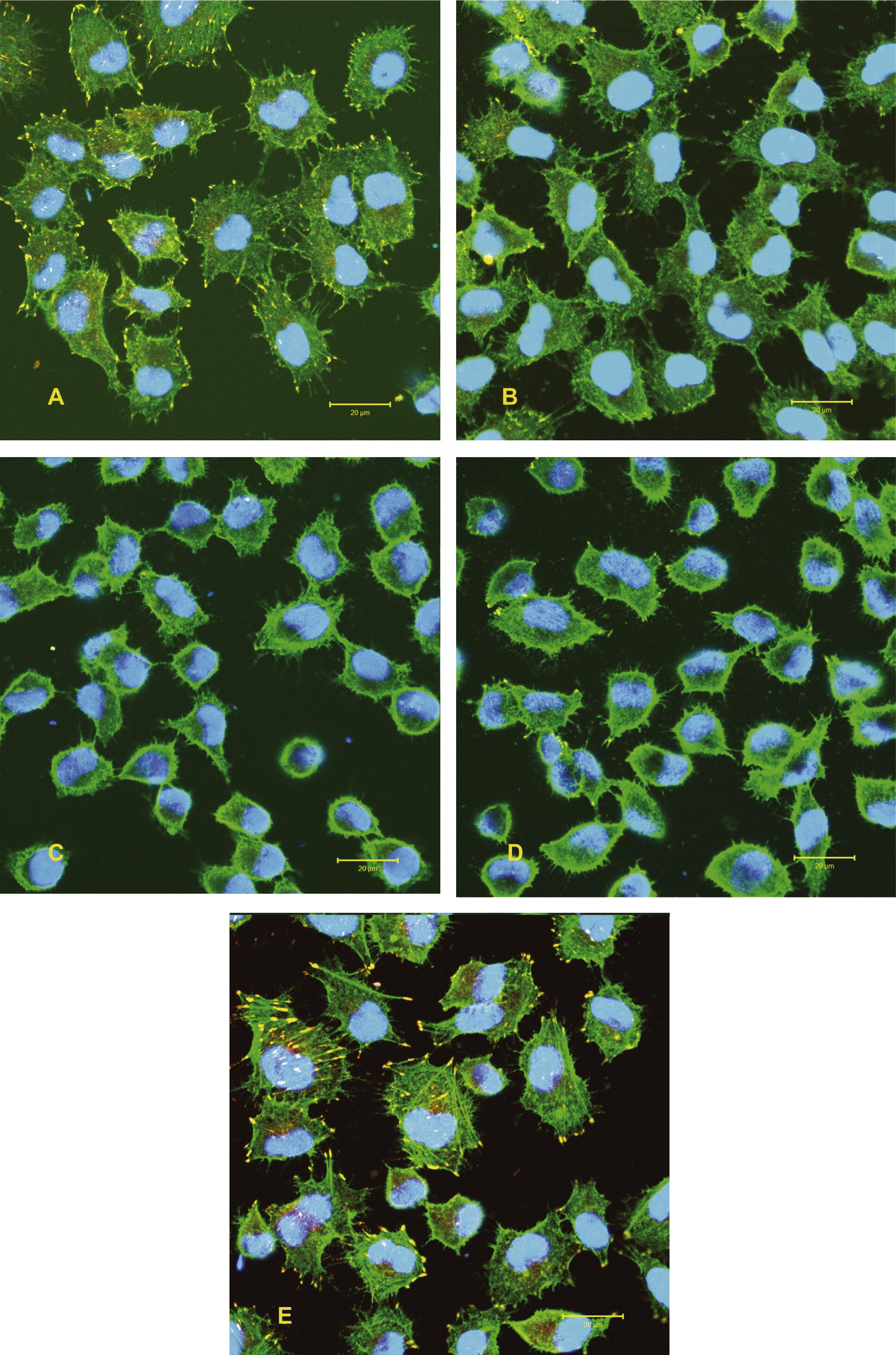
Spreading behaviour (cytoskeleton organization) of epithelial cells exposed to 15 μg/ml and 30 μg/ml SWCNT bundles and SWCNT raw material for 5 days. Staining: actin filaments (green), vinculin (yellow) and nucleus (blue). A = SWCNT bundles (15 μg/ml), B = SWCNT bundles (30 μg/ml), C = SWCNT raw material (15 μg/ml), D = SWCNT raw material (30 μg/ml), E = Control.(For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Integrins mediate signalling from the extra-cellular space into the cell through integrin-associated signalling proteins, such as focal adhesion kinase (FAK). No significant change of the activated FAK (p-FAK) concentration could be observed in the epithelial cell cultures, which were exposed to 15 and 30 μg/ml SWCNT (bundles or raw material). Cultures which were exposed to SWCNT for 1 day showed a slightly lower p-FAK and total-FAK concentration than the control cultures. However, after an exposure period of 5 days the concentration of p-FAK was still insignificantly lower in cultures exposed to SWCNT. The amount of total-FAK in these cultures reached, after an exposure period of 5 days to SWCNT, similar values as the total-FAK concentration in the control cultures (Fig. 6a). Besides FAK, which is localized in the vicinity of the integrins, proteins of the MAPK-Erk1/2 pathway also play an important role in the field of cell adhesion and cell proliferation. The amount of activated Erk1/2 (p-Erk1/2) was estimated in relation to the total-Erk1/2. Cells grown in the presence of SWCNT showed an elevated amount of ROS, however, no significant increase in the ratio p-Erk1/2/total-Erk1/2 was noticed. When cultures were exposed to 15 and 30 μg/ml SWCNT raw material for 1 and 3 days an insignificantly higher ratio of p-Erk1/2/total-Erk1/2 was observed. However, after an incubation period of 5 days to SWCNT all exposed cultures showed a p-Erk1/2/total-Erk1/2 ratio, which was similar to the p-Erk1/2/total-Erk1/2 ratio of the control cultures (Fig. 6b). Connexin-43 and β-catenin are responsible for the gap junctional intercellular communication, and also for cell adhesion. No notable changes in the distribution of these two proteins were observed when cells were grown in the presence of SWCNT, compared to the untreated control cultures. Connexin-43 was randomly spread within the cell body. The same was true for β-catenin, however after an incubation period of 5 days a notable amount of β-catenin was also observed at the cells peripheries. This was true for the control cultures as well as for cultures that were exposed to SWCNT.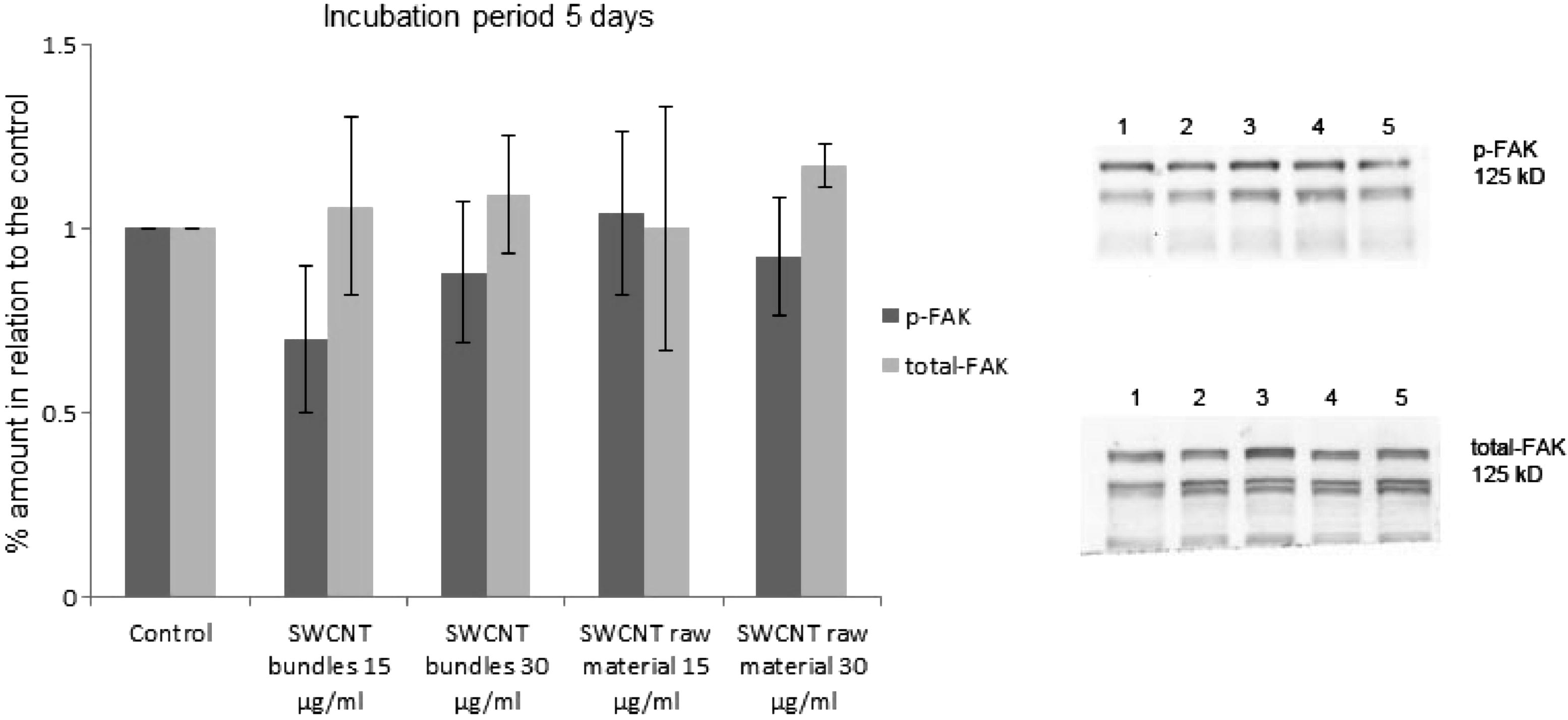
(a) Amount of activated FAK (p-FAK, Tyr 397) as well as total-FAK after exposure of epithelial cell cultures to SWCNT bundles and raw material (15 μg/ml and 30 μg/ml) for 5 days. The amount of activated FAK (p-FAK, Tyr 397) as well as total-FAK has been estimated by Western blotting. 1 = p-FAK, total-FAK from control cultures, 2 = p-FAK, total-FAK from cell cultures, which were exposed to SWCNT bundles (15 μg/ml), 3 = p-FAK, total-FAK from cell cultures, which were exposed to SWCNT bundles (30 μg/ml), 4 = p-FAK, total-FAK from cell cultures, which were exposed to SWCNT raw material (15 μg/ml), 5 = p-FAK, total-FAK from cell cultures, which were exposed to SWCNT raw material (30 μg/ml).
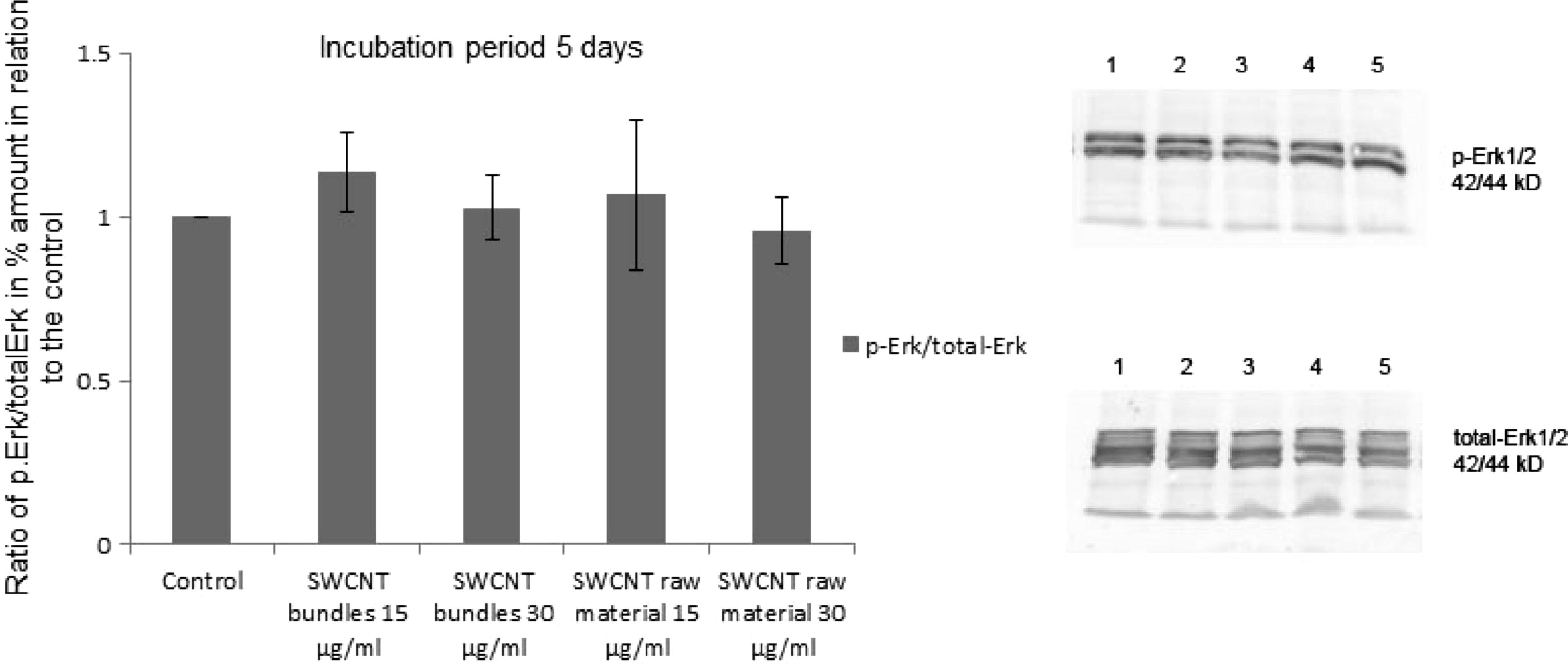
Amount of activated Erk1/2 (p-Erk1/2) as well as total-Erk1/2 after exposure of epithelial cell cultures to SWCNT bundles and raw material (15 μg/ml and 30 μg/ml) for 5 days. The amount of activated p-Erk1/2 as well as total-Erk1/2 has been estimated by Western blotting. 1 = p-Erk1/2, total-Erk1/2 from control cultures, 2 = p-Erk1/2, total-Erk1/2 from cell cultures, which were exposed to SWCNT bundles (15 μg/ml), 3 = p-Erk1/2, total-Erk1/2 from cell cultures, which were exposed to SWCNT bundles (30 μg/ml), 4 = p-Erk1/2, total-Erk1/2 from cell cultures, which were exposed to SWCNT raw material (15 μg/ml), 5 = p-Erk1/2, total-Erk1/2 from cell cultures, which were exposed to SWCNT raw material (30 μg/ml).
4 Discussion
The human cell line epithelial cells (A549) were chosen for these studies, because these cells, found in the human lung, are likely to be exposed to nanoparticles. The exposure of epithelial cells (A549) to short SWCNT did not severely affect cell viability, apoptosis/necrosis, cell–cell communication (distribution of connexin43 and β-catenin), or activation of cell signalling proteins (FAK and Erk) even these SWCNT were taken up by the cells (Muller et al., 2010).
These findings were in agreement with studies where other cell types were exposed to short SWCNT. The effects of well dispersed SWCNT on human mesothelioma cells (MSTO-211H) were far less cytotoxic than rope-like agglomerates or asbestos fibres at the same concentrations (Kaiser et al., 2008; Wick et al., 2007). Primary neurons and glial cells derived from chicken embryonic spinal cord or dorsal root ganglia reacted in a similar way. The toxic effects on the primary chicken cultures were dependent on the agglomeration state of the SWCNT (Belyanskaya et al., 2009). In a study where heart cells were exposed to diesel exhaust particles and engineered nanoparticles such as titanium dioxide and carbon nanotubes, diesel exhaust and titanium dioxide had the most pronounced cytotoxic effects. The mildest effects were observed for SWCNT, for which no clear dose-dependent alterations could be determined (Helfenstein et al., 2008). The same findings could be observed when a triple cell co-culture was exposed to these particles. The triple cell co-culture was far less affected by the purified SWCNT than by the diesel exhaust particles or titanium dioxide. There was no evidence for a synergistic or alleviative effect by the interplay of the different cell types (Muller et al., 2010).
The dissolved SWCNT mimic collagen fibres and fibronectin which are adsorbed to the substrate. Therefore integrin receptors will not only bind to the fibronectin, which is adsorbed to the substrate, but will also bind in a similar way to the dissolved carbon nanotubes. It is known that proteins containing an RGD-sequence bind to integrin proteins, and the exposure to soluble peptides containing a RGD-sequence affected cell adherence. It resulted in a weaker adherence to the underlying substrate compared with cells grown in the absence of SWCNT. Thus, SWCNT adhere in a similar way as RGD-sequences of the dissolved proteins to integrins, which result in a reduced cell spreading, as well as fewer and smaller focal adhesion points. However, it is also possible that extra-cellular matrix proteins are binding to the dissolved SWCNT and the extra-cellular matrix proteins–SWCNT complex are binding to the integrins. This will lead to a reduced attachment to the underlying substrate and will also affect the development of focal adhesion points. Thus, cells grown in the presence of SWCNT show a weaker adherence to the underlying substrate than cells grown in the absence of SWCNT. Since less integrin proteins were able to adhere to the underlying substrate less and smaller focal adhesion points were established. The reduced cell spreading of cells grown in the presence of SWCNT also affected the cytoskeleton organization. Less actin stress fibres could be observed and the cells exposed to SWCNT raw material were reorganizing their actin cytoskeleton, which resulted in a concentration of actin at the cell peripheries. The elevated release of reactive oxygen species (ROS) when cells came in contact with SWCNT was just a spontaneous effect, which had no severe long-lasting effects on other cell parameters.
The effects on cells exposed to subtoxic concentrations of SWCNT have been summarized in a working model (Fig. 7). The dissolved SWCNT, which mimic fibronectin adsorbed to the substrate affected cell spreading, development of focal adhesion points, development of the actin cytoskeleton and cell adhesion, without seriously affecting other important parameters, such as cell viability, apoptosis/necrosis, cell signalling and cell–cell communication. It seems that the important cell functions were not severely affected when a certain number of integrin receptors were attaching to dissolved SWCNT instead of the solid underlying surface, as long as the cells were exposed to a relatively low concentration of SWCNT. It seems that for the cells it makes no difference, if a part of the integrins is binding to dissolved SWCNT, as long as a substantial number of integrins is able to bind via fibronectin to the underlying substrate.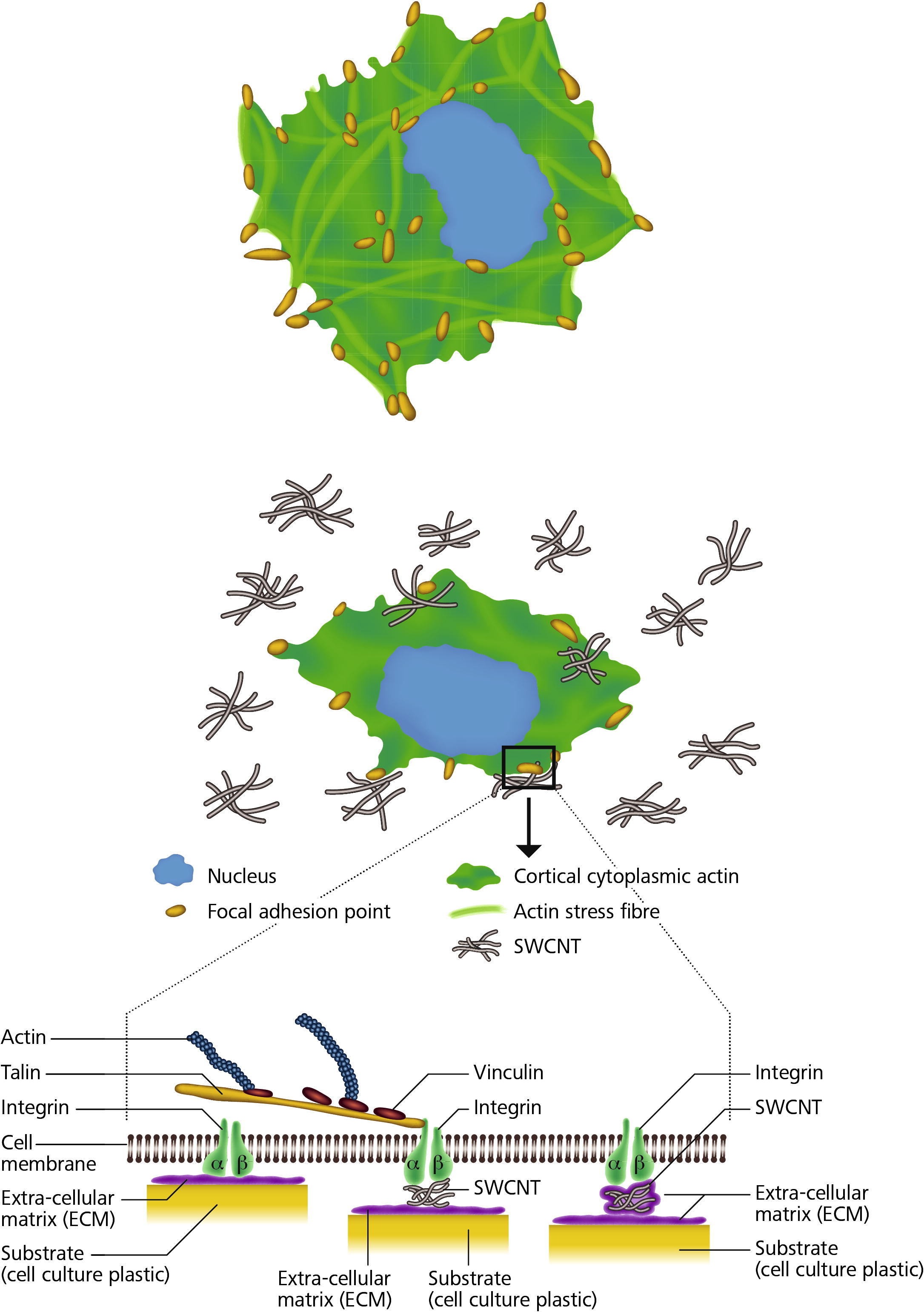
A working model for the adhesion of SWCNT to cell integrins. SWCNT adhere in a similar way as the RGD-sequences of the proteins to integrins, which results in a reduced cell spreading, as well as fewer and smaller focal adhesion points. However, it’s also possible that extra-cellular matrix proteins are binding to the dissolved SWCNT and the extra-cellular matrix proteins-SWCNT complex are binding to the integrins. This will also lead to a reduced attachment to the underlying substrate. Thus cells grown in the presence of SWCNT show a weaker adherence to the underlying substrate than cells grown in the absence of SWCNT.
5 Conclusions
Cells exposed to a subtoxic concentration of SWCNT exhibits no serious dysfunction of important cell behaviours. Since the SWCNT mimic collagen fibres and fibronectin adsorbed to the substrate, cells grown in the presence of SWCNT show no abnormal behaviour. The SWCNT attached to integrin receptors only partially effect cell functions such as adhesion, cell spreading, development of focal adhesion points and cytoskeleton development. These effects were dependent on the concentration of SWCNT and on the exposure time. However, the reduced cell adhesion of cells exposed to SWCNT showed no or only unimportant effects on important cell functions such as cell viability, cell death, cell signalling and cell–cell communication.
6 Authors’ contributions
J.-P. Kaiser participated in the design of the study, carried out the experimental work and wrote the manuscript. T. Buerki made contributions to the analysis and in the interpretation of the data. P. Wick was project leader, he has intellectually accompanied the experimental work; he has been involved in revising the manuscript critically for important intellectual content and has given final approval of the version to be published. All authors read and approved the manuscript.
Acknowledgments
We thank Mr. A. Niederer for drawing the “working model for the adhesion of SWCNT to cell integrins”; Fig. 7. The work was financially supported by Empa, the Seventh Framework Program of the European Commission NanoHouse-Grant (Agreement No. 207816).
References
- The interaction of plasma fibronectin with fibroblastic cells in suspension. J. Biol. Chem.. 1985;260:4492-4500.
- [Google Scholar]
- The reliability and limits of the MTT reduction assay for carbon nanotubes - cell interactions. Carbon. 2007;45:2643-2648.
- [Google Scholar]
- Effects of carbon nanotubes on primary neurons and glial cells. Neurotoxicology. 2009;30:702-711.
- [Google Scholar]
- Involvement of microtubules in the control of adhesion-dependent signal transduction. Curr. Biol.. 1996;6:1279-1289.
- [Google Scholar]
- Large scale CVD synthesis of single walled carbon nanotubes. J. Phys. Chem. B. 1999;103:6484-6492.
- [Google Scholar]
- Carbon nanotubes: a review of their properties in relation to pulmonary toxicology and workplace safety. Toxicol. Sci.. 2006;92:5-22.
- [Google Scholar]
- Exploring the neighborhood: adhesion-coupled cell mechanosensors. Cell. 2002;110:139-142.
- [Google Scholar]
- Catalytic growth of single-walled nanotubes by laser vaporisation. Chem. Phys. Lett.. 1995;243:49-54.
- [Google Scholar]
- Effects of combustion-derived ultrafine particles and manufactured nanoparticles on heart cells in vitro. Toxicology. 2008;253:70-78.
- [Google Scholar]
- Single walled carbon nanotubes (SWCNT) affect cell physiology and cell architecture. J. Mater. Sci. - Mater. Med.. 2008;19:1523-1527.
- [Google Scholar]
- Co-regulation of cell adhesion by nanoscale RGD organization and mechanical stimulus. J. Cell Sci.. 2002;115:1423-1433.
- [Google Scholar]
- Exposure of engineered nanoparticles to human lung epithelial cells: influence of chemical composition and catalytic activity on oxidative stress. Environ. Sci. Technol.. 2007;41:4158-4163.
- [Google Scholar]
- Oxidative stress and inflammation response after nanoparticle exposure: differences between human lung cell monocultures and an advanced three-dimensional model of the human epithelial airways. J. R. Soc. Interface. 2010;7(Suppl 1):S27-40.
- [Google Scholar]
- Emergent patterns of growth controlled by multicellular form and mechanics. Proc. Natl. Acad. Sci. U S A. 2005;102:11594-11599.
- [Google Scholar]
- Rho GTPases control polarity, protrusion, and adhesion during cell movement. J. Cell Biol.. 1999;144:1235-1244.
- [Google Scholar]
- Focal adhesion quantification - a new assay of material biocompatibility? Review. Eur. Cell Mater.. 2005;9:85-96. discussion 85–96
- [Google Scholar]
- Networks and crosstalk: integrin signalling spreads. Nat. Cell Biol.. 2002;4:E65-68.
- [Google Scholar]
- Elastic strain of freely suspended single-wall carbon nanotube ropes. Appl. Phys. Lett.. 1999;74:3803-3805.
- [Google Scholar]
- Relationships among cell attachment, spreading, cytoskeletal organization, and migration rate for anchorage-dependent cells on model surfaces. J. Biomed. Mater. Res.. 2000;49:362-368.
- [Google Scholar]
- The degree and kind of agglomeration affect carbon nanotube cytotoxicity. Toxicol. Lett.. 2007;168:121-131.
- [Google Scholar]
Appendix A
Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.jksus.2012.06.003.
Appendix A
Supplementary data
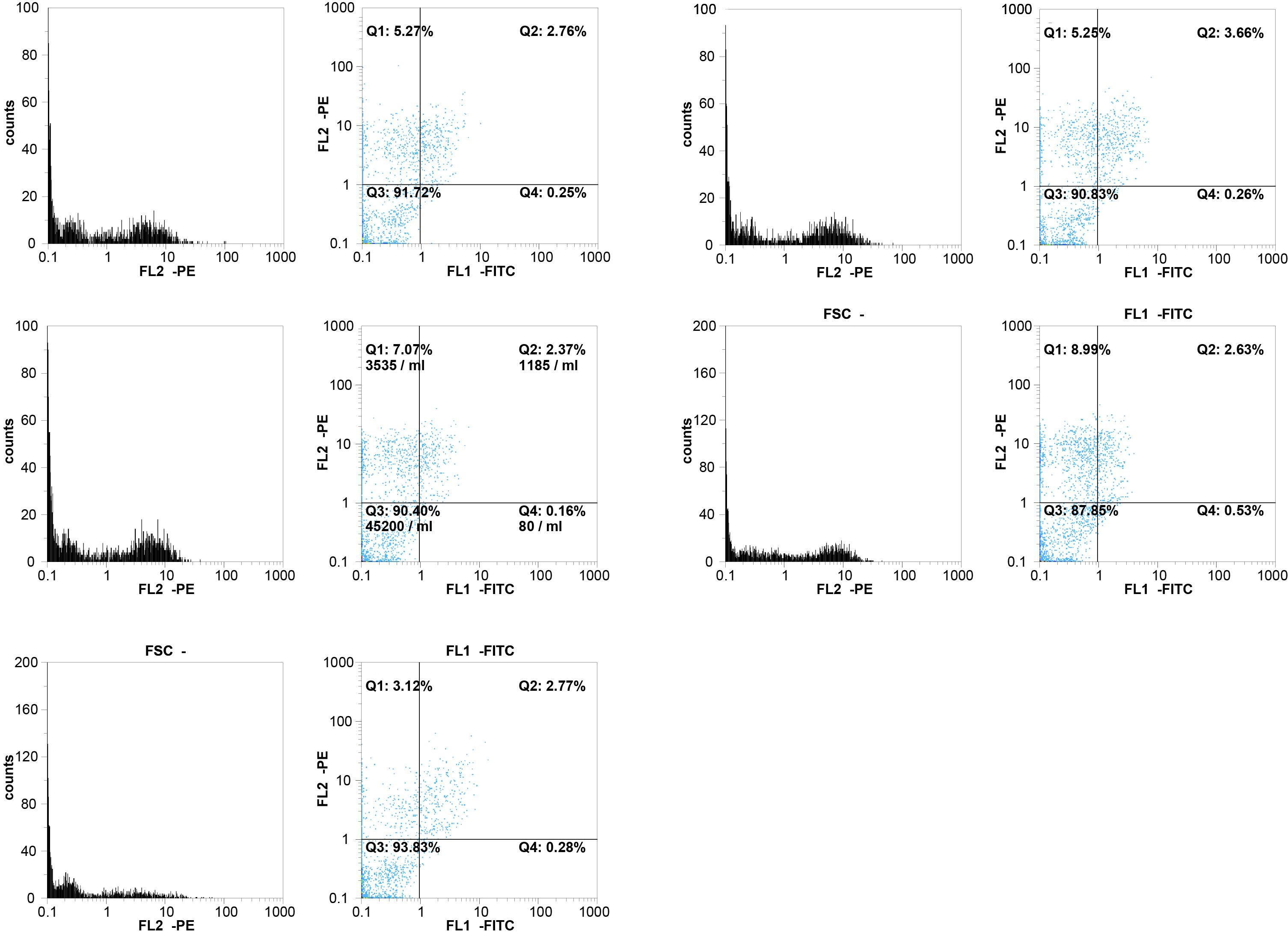
FACS-Analysis: (A) Example of a flow cytometry analysis of an epithelial cell culture grown in the presence of 15 μg/ml SWCNT bundles for 5 days. (B) Example of a flow cytometry analysis of an epithelial cell culture grown in the presence of 30 μg/ml SWCNT bundles for 5 days. (C) Example of a flow cytometry analysis of an epithelial cell culture grown in the presence of 15 μg/ml SWCNT raw material for 5 days. (D) Example of a flow cytometry analysis of an epithelial cell culture grown in the presence of 30 μg/ml SWCNT raw material for 5 days. (E) Example of a flow cytometry analysis of an epithelial cell control culture grown in the absence of SWCNT.







