Translate this page into:
Production of the lactic acid from mango peel waste – Factorial experiment
*Corresponding author. Tel.: +60 4 653 5203; fax: +60 4 657 3678 abbas@usm.my (Abbas F.M. Alkarkhi)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Available online 16 April 2012
Peer review under responsibility of King Saud University.
Abstract
The production of lactic acid from mango peels using the bio-fermentation method was investigated. The possible relationship between the operational factors, namely process temperature (15 and 35 °C), initial medium pH (4 and 10) and duration of fermentation (3 and 6 days) on lactic acid production as the response was determined using the factorial design. Analysis of the data obtained showed there was a strong significant influence of the operational factors and their interactions on lactic acid production (P < 0.0001) of fermenting microorganisms. The regression model for lactic acid production fitted the data adequately and explained more than 99% of the variation in the response. The results also showed that the maximum production of lactic acid can be achieved at initial medium pH of 10; incubation time of 6 days; and at a temperature of 35 °C. The maximum production of lactic acid was 17.484 g/L. This study highlights the potential of mango peels as a low cost and economically viable fermentation substrate for the production of lactic acid.
Keywords
Mango peel
Fermentation
Lactic acid
Factorial design
Regression model
1 Introduction
The food and agricultural industries produce large volumes of wastes annually worldwide, causing a serious disposal problem. This is especially problematic in countries where the economy is largely based on agriculture and where the farming practice is very intensive. Currently, these agro-wastes are either allowed to decay naturally on the fields or are burnt. However, these wastes are rich in sugars due to their organic nature, are easily assimilated by microorganisms and hence, make them potential substrates for exploitation as raw materials in the production of industrially relevant compounds through microbial conversion. In addition, the reutilization of biological wastes is of great interest since, due to legislation and environmental reasons, the industry is increasingly being forced to find an alternative use for its residual matter (Rodríguez-Couto, 2008). One of the agro-wastes currently causing pollution problems is the peels of the mango (Mangifera indica L.) fruit. Mango is one of the most important fruits marketed in the world with a global production exceeding 26 million tons in 2004 (FAOSTAT, 2004). It is cultivated or grown naturally in over 90 countries worldwide (mainly tropical and subtropical regions) and is known to be the second largest produced tropical fruit crop in the world (Joseph and Abolaji, 1997). The edible tissue makes up 33–85% of the fresh fruit, while the peel and the kernel amount to 7–24% and 9–40%, respectively (Wu et al., 1993). In fact, mango peel as a by-product of mango processing industry could be a rich source of bioactive compounds, and enzymes such as protease, peroxidase, polyphenol oxidase, carotenoids, vitamins C and E, dietary fibers, enzymes and carbohydrate content of 20.80–28.20% in dry weight samples of mango peel (Ajila et al., 2007). While the utilization of mango kernels as a source of fat, natural antioxidants, starch, flour and feed has extensively been investigated (Arogba, 2002; Kaur et al., 2004), studies on peels are scarce. Their use in biogas production (Madhukara et al., 1993; Mahadevaswamy and Venkataraman, 1990) or making of dietary fiber with a high antioxidant activity (Larrauri et al., 1997) has been described in the past. However, mango peels are not currently being utilized commercially in any way, though a large quantity is generated as waste (20–25% of total fruit weight) during mango processing thus, contributing to pollution (Berardini et al., 2005).
Most studies on the exploitation of mango peels have been dealing with their use as a source of pectin, which is considered a high quality dietary fiber (Pedroza-Islas and Aguilar-Esperanza, 1994; Tandon and Garg, 1999). Recently, a screening study of 14 mango cultivars had demonstrated the content and degree of esterification of mango peel pectins to range from 12% to 21% and 56% to 66%, respectively (Berardini et al., 2005). Furthermore, mango peels have been shown to be a rich source of flavonol O- and xanthone C-glycosides (Berardini et al., 2005), gallotannins and benzophenone derivatives (Berardini et al., 2004). However, reports on the use of mango peels for the production of industrially relevant metabolites such as lactic acid through fermentation processes are rare. Thus, cultivation of microorganisms on these wastes may be a value-added process capable of converting these materials, which are otherwise considered to be wastes, into valuable products through processes with techno-economic feasibility.
Lactic acid is used as a preservative and acidulant in foods and one of the main obstacles in the large-scale production of this substance is the cost of the raw material (Reddy et al., 2008). Application of agro-industrial wastes in bioprocesses provides an alternative way to replace the refined and costly raw materials. In addition, the bulk use of such materials helps to solve many environmental hazards. However, the application of microorganisms for the production of lactic acid using cost-effective raw materials is rare. Hence, research efforts are focused on looking for new and effective nutritional sources and new progressive fermentation techniques enabling the achievement of both high substrate conversion and high production (Bulut et al., 2004). Direct conversion of agro waste to lactic acid by bacteria with both amylolytic and lactic acid producing character will eliminate the two step processes of saccharification followed by microbial fermentation which will make it economical.
Therefore, the objective of the present study was to investigate the production of lactic acid from mango peels by microorganisms during fermentation under ambient conditions and to optimize the production of both substances using the factorial design. Experiments were carried out under a variety of operational conditions defined by three independent variables (initial medium pH, process temperature, and fermentation duration). The role of each variable, their interactions and the predicted production of lactic acid during fermentation were determined by applying the factorial design.
2 Materials and methods
2.1 Raw material and chemicals
Mango peels of golden dragon variety were collected by manually peeling off fresh undamaged ripe fruits purchased from a local fruit market in Penang, Malaysia. The underlying pulp on the peels was removed by gently scraping with the blunt edge of a clean knife and the peels were washed with distilled water to remove adhering dust. Crystallized phenol (C6H6O, 99%) was obtained from Panreac Sintesis, while 4-phenylphenol (C6H5C6H4OH, 97%) was obtained from Aldrich Chemical Co. (USA). Trichloroacetic acid (CCl3COOH) and copper (II) sulfate (CuSO4. 5H2O, 98.5–100%) were purchased from Bendosen and R&M Chemicals respectively. All other chemicals and solvents were of analytical grade and obtained from QRëC.
2.2 Preparation and fermentation of mango peel
The washed mango peels were cut into small pieces and blended with ultra pure water in the ratio (1:1) (w/v) using an electrical food processor (Kenwood) at 25 °C for 5 min. The humidity of the washed mango peels to the air dried mango peels before staring the fermentation process was calculated to be 11–13%. While, the humidity of mango peels after mixing with water to be in the fermentation suspension form was found to be 91.40%. The pH of the blended peel was adjusted using 1 M of NaOH or HCl and thereafter, 30 mL was dispensed into conical flasks of 100 mL capacity. The flasks were closed tightly with rubber caps to achieve and maintain microaerophilic fermentation conditions. The spontaneous anaerobic submerged fermentation process was carried out by a consortium of indigenous microorganisms at different temperatures for several days under static incubation conditions. Sterile controls were set up using same conditions after autoclaving flasks containing the blended peel at 121 °C at 15 psi for 15 min. Lactic acid extraction was carried out at the end of incubation. The extraction of lactic acid formed was carried out by diluting 5 mL of withdrawn fermented mango peel (1:5. v/v) with ultra pure water. The diluted paste was shaken vigorously for 5 min. Thereafter, 3 mL of diluted sample was added into a borosilicate screw-capped tube (16 × 150 mm) which already contains 3 mL of 10% trichloroacetic acid in order to stop the fermentation process. This final solution was kept in a fridge at 4 °C for 2 days to obtain a clear solution and to allow for the sedimentation of suspended solids at the bottom of the tube. Subsequently, 0.5 mL of the clear solution was added into 4.5 mL of ultra pure water and stored at 4 °C until used for the estimation of lactic acid level.
2.3 Determination of lactic acid
Lactic acid content of the fermented peel was determined using the colorimetric method according to the method described by Taylor (1996). An aliquot (0.5 mL) of the final clear solution obtained from subsection 2.2 above was placed in a screw-capped tube of 16 × 150 mm borosilicate tubes. Thereafter, 3 mL of concentrated H2SO4 was added and the tubes were heated to boiling for 10 min. On cooling, 50 μL of CuSO4 reagent in addition to 100 μL of para-phenyl phenol (1.5% para-phenyl phenol dissolved in 95% ethanol) were added. The tubes were then incubated at 30 °C for 30 min until all traces of precipitate were dissolved. Finally, the absorbance reading of the resulting solution was taken at the wavelength of 570 nm.
2.4 Experimental design and statistical analysis
Factorial design was employed to study the effect of three factors (independent variables): initial medium pH (X1), process temperature (X2), and incubation time (X1) on lactic acid production being the response. The levels of selected factors were chosen based on preliminary experiments and the levels used include: initial medium pH, 4 and 10; process temperature, 15 and 35 °C, and incubation time, 3 and 6 days. Factorial design of type 23 with five center points was used to study the effect of selected factors within a specified range (Table 1). All experiments were run in random order to minimize the effect of unexpected variability in the observed responses (Montgomery, 2001). The data were analyzed using the analysis of variance (ANOVA) to study the effect of selected factors and their potential interaction effects on lactic acid production. A first-order polynomial with interaction terms was used to fit the data. The regression polynomial model (2FI) is given in the following equation:
pH
Temperature (°C)
Observed
Predicted
Time (day)
Lactic acid (g/l)
Lactic acid (g/l)
4
15
3
2.967
2.967
10
15
3
2.082
2.082
4
35
3
2.175
2.175
10
35
3
9.3
9.3
4
15
6
2.442
2.442
10
15
6
2.517
2.517
4
35
6
3.75
3.75
10
35
6
17.484
17.484
7
25
4.5
5.268
4.9506
7
25
4.5
5.001
4.9506
7
25
4.5
4.626
4.9506
7
25
4.5
5.157
4.9506
7
25
4.5
4.701
4.9506
3 Results and discussion
Lactic acid production was detected in aqueous extraction by the 3rd day of incubation and the amount obtained increased with increase in incubation time up to 6 days. The production of lactic acid from mango peels during the fermentation period could be attributed to the breakdown of peel polysaccharides to glucose and the eventual conversion of the glucose into lactic acid by microorganisms in the fermentation broth. Hydrolysis of starch-like polysaccharides is generally accomplished via liquefaction and saccharification by microbes through the production of amylase and glucosidase (Ye et al., 2008). Thereafter, naturally occurring microbes, especially lactic acid bacteria (LAB), ferment the hexose sugars obtained, such as glucose, by oxidizing NADH generated during glycolysis, with pyruvate serving as the electron acceptor, to form lactic acid as the major product. After substrate loading, there was an extensive growth of microbial cells seen adsorbed onto the mango peel particles followed by dissolution of peel fibres to relatively smooth substrate particles. This observation could be attributed to the structural changes in the composition of the peels taking place during the conversion of peel polysaccharides to glucose, as a result of extra cellular amylopullulanase production, which in turn is converted into lactic acid by amylolytic lactic acid fermentation. No lactic acid production was detected in sterile controls. Vishnu et al. (2006) reported that the amylopullulanase was able to hydrolyze different polymeric substrates such as soluble starch, raw starch, amylopectin, amylose, glycogen and pullulan.
Currently, conventional biotechnological production of lactic acid from starchy materials, for instance, requires pretreatment for gelatinization and liquefaction, which is carried out at high temperatures of 90–130 °C for 15 min followed by enzymatic saccharification to glucose and subsequent conversion of glucose to lactic acid by fermentation (Anuradha et al., 1999). This two step process involving consecutive enzymatic hydrolysis and fermentation makes this process economically unattractive. Moreover, dilute acid hydrolysis used to release the pentose sugars from biomass substrates and make the cellulose amenable to cellulases that often result in the creation of fermentation inhibitors (Dupreez, 1994). Hence, the bioconversion of mango peels in this study to lactic acid by coupling the microbial hydrolysis of the carbohydrate substrate and fermentation of the derived sugar into a single step makes the lactic acid production process effective and economically viable. Similar studies on the successful production of lactic acid from raw starch materials by microorganisms have been reported elsewhere (Naveena et al., 2005; Vishnu et al., 2002). The demand for lactic acid is expected to increase as rated by different surveys due to its use in biodegradable plastics and other large-scale industrial products (Mirasol, 1999). Since, the commercial scale production of lactic acid which involves the addition of glucose is an expensive venture, the use of a cheaper source of carbon, such as the mango peels which can be obtained in large quantities as a waste product from fruit processing industries in combination with amylolytic microorganisms may help to decrease the cost of the overall fermentation process and increase production.
Experiments carried out in order to determine the effect of initial medium pH, process temperature and incubation time on lactic acid production showed that these factors had a significant influence on the production of the fermentation process. There was increase in lactic acid production with increase in initial pH of medium from 4 to 10 and increase in process temperature from 15 to 35 °C. Similarly, increase in production of lactic acid was obtained with increase in incubation time up to the 6th day. Our observation indicates that the production of lactic acid from mango peels depended significantly on these three operational variables and hence, they were used as factors in the subsequent factorial experiment.
3.1 ANOVA and regression analysis
Three factors were thought to be influential on lactic acid production (used as the response) by microorganisms during fermentation of mango peels. The three factors are initial medium pH, process temperature and incubation time. The results of 13-run factorial design including the input factors and their levels, the observed and predicted values are given in Table 1. The results for lactic acid production were analyzed by analysis of variance (ANOVA) and a regression model was developed to describe the relationship between selected factors and the two responses. ANOVA results showed that initial medium pH, process temperature, and incubation time influence lactic acid production. Furthermore, two-factor and three-factor interactions exhibited a significant effect on production of lactic acid (Table 2) which indicates that initial medium pH, process temperature, and incubation time do not work independently and the effect of each factor depends on the level of other factors. The regression model for lactic acid production is given in the following equation:
S.O.V.
Lactic acid
S.S.
D.F.
M.S.
F
P-value
Model
208.32
7
29.76
379.65
<0.0001
pH
50.25
1
50.25
640.99
<0.0001
Temperature
64.42
1
64.42
821.78
<0.0001
Time
11.69
1
11.69
149.08
<0.0003
pH∗Temperature
58.69
1
58.69
748.76
<0.0001
pH∗Time
7.16
1
7.16
91.36
<0.0007
Temperature∗Time
12.13
1
12.13
154.69
<0.0002
pH∗Temperature∗Time
3.99
1
3.99
50.89
<0.0021
Curvature
0.47
1
0.47
5.94
<0.0714
Error
0.31
4
0.078
Total
209.10
12
Three-dimensional response surface plots of lactic acid production as a function of initial medium pH and process temperature showing the behavior of pH and temperature at three different levels of incubation time (3, 4.30 and 6 days) are given in Figs. 1a–c. Higher production of lactic acid from fermenting microorganisms at the three incubation times monitored could be obtained in medium with high initial pH level of 10 and at a temperature of 35 °C. Lower production was obtained with lower initial medium pH and temperature (Figs. 1a–c). Initial medium pH was one of the main factors influencing lactic acid production by the fermenting microbes probably because the catalytic activity of enzymes and metabolic activity of microorganisms depend on extra cellular pH (Silva and Mancilha, 1991). In medium with low initial pH, production of lactic acid during fermentation may have lowered the pH of the fermentation broth rapidly to a point that it becomes inhibitory to the fermenting microorganisms. Fermentation broths with initial pH of 10 may have sustained a pH friendly environment during the duration of the fermentation process due to the buffering effect of complex natural waste materials as shown in Fig. 3, before decline in pH to levels inhibitory to the microbes. This may explain the higher production of lactic acid obtained at higher initial pH of medium. The decrease in pH with time during the fermentation process (Fig. 3) may be attributed to the production of lactic acid from sugars. A similar observation was made during fermentation of wheat flour hydrolysate and brewer’s spent grain for lactic acid production, where the pH decreased with time during the initial fermentation period as a result of lactic acid production by microorganisms (Hofvendahl and Hahn-Hagerdal, 1997; Mussatto et al., 2008). Some authors (Hofvendahl and Hahn-Hagerdal, 1997; Kashket, 1987) had stated that weak acids, e.g., lactic acid inhibit bacterial growth because as the external pH declines, the acid is protonized as soon as it is exported out of the bacteria. Uncharged, it diffuses back into the cell and dissociates due to the higher intracellular pH. The cell then has to use ATP to pump out protons, eventually depleting energy and resulting in growth cessation and cell death. A previous report has also shown that when the pH of the fermentation broth for lactic acid production was at 6 and not controlled, the microorganism metabolism was affected and the glucose consumption and lactic acid production practically ceased after 12 h of process, when the media pH dropped to approx. 4.6 (Mussatto et al., 2008).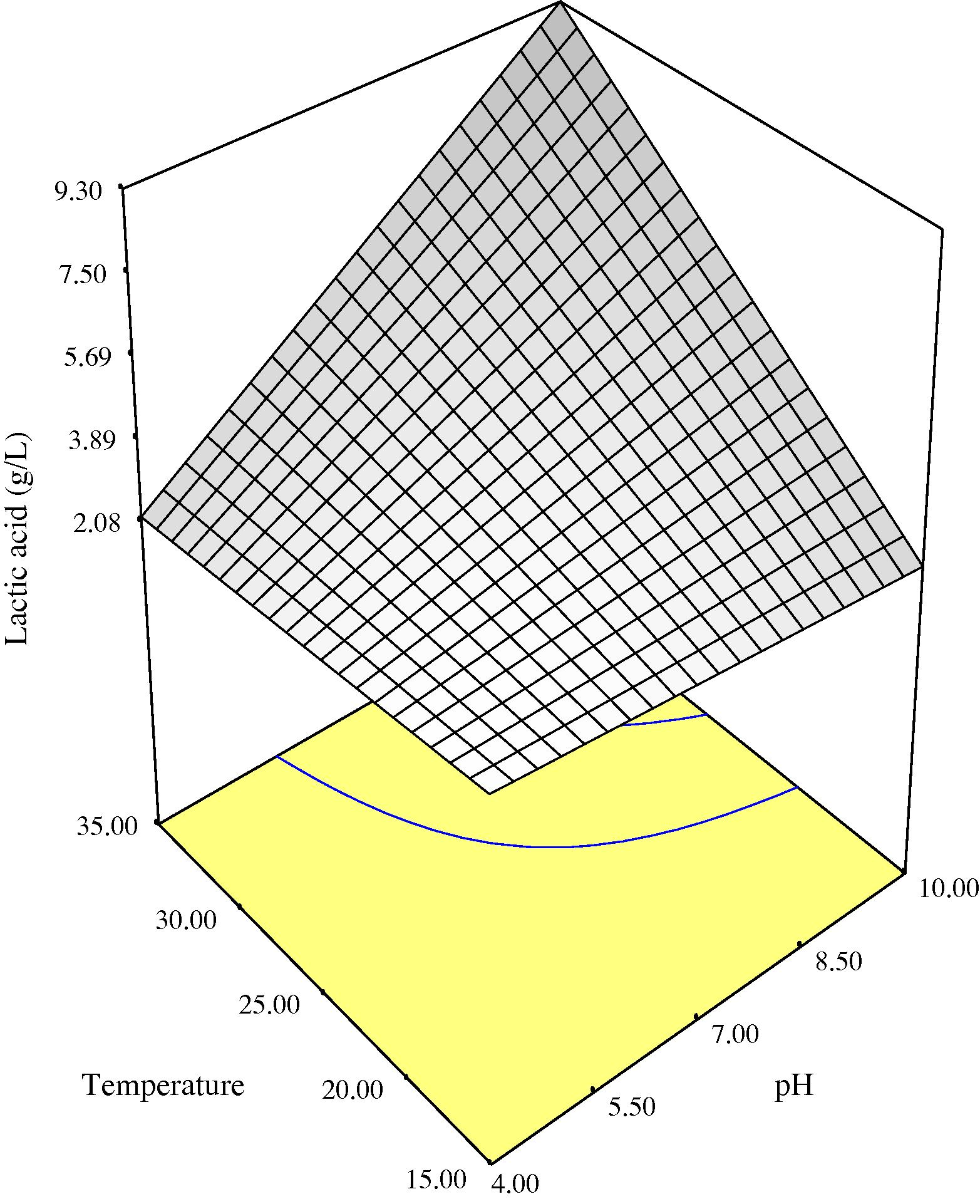
Three-dimensional response surface for lactic acid as a function of pH and temperature at 3 days anaerobic fermentation time.
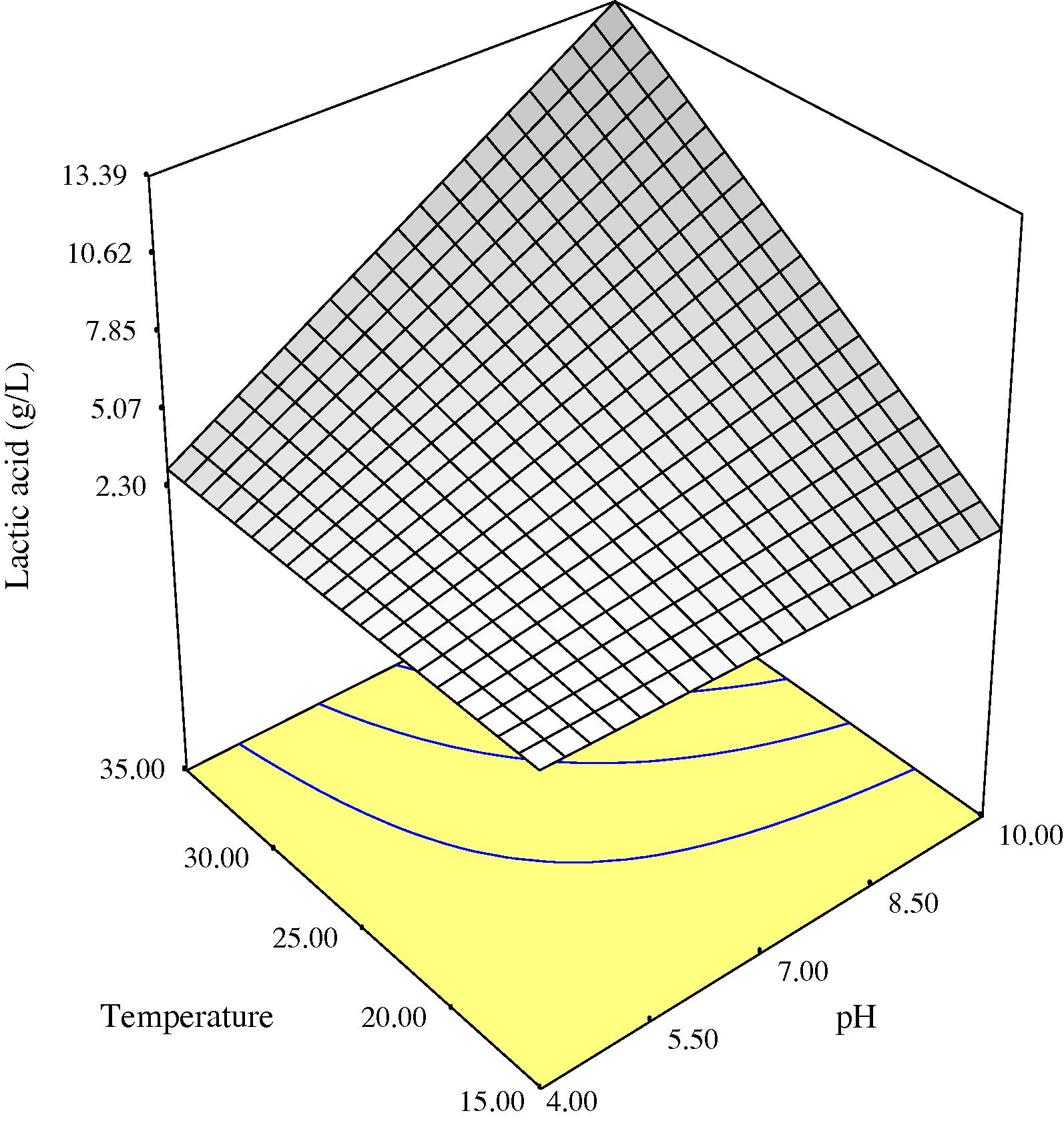
Three-dimensional response surface for lactic acid as a function of pH and temperature at 4.3 days anaerobic fermentation time.
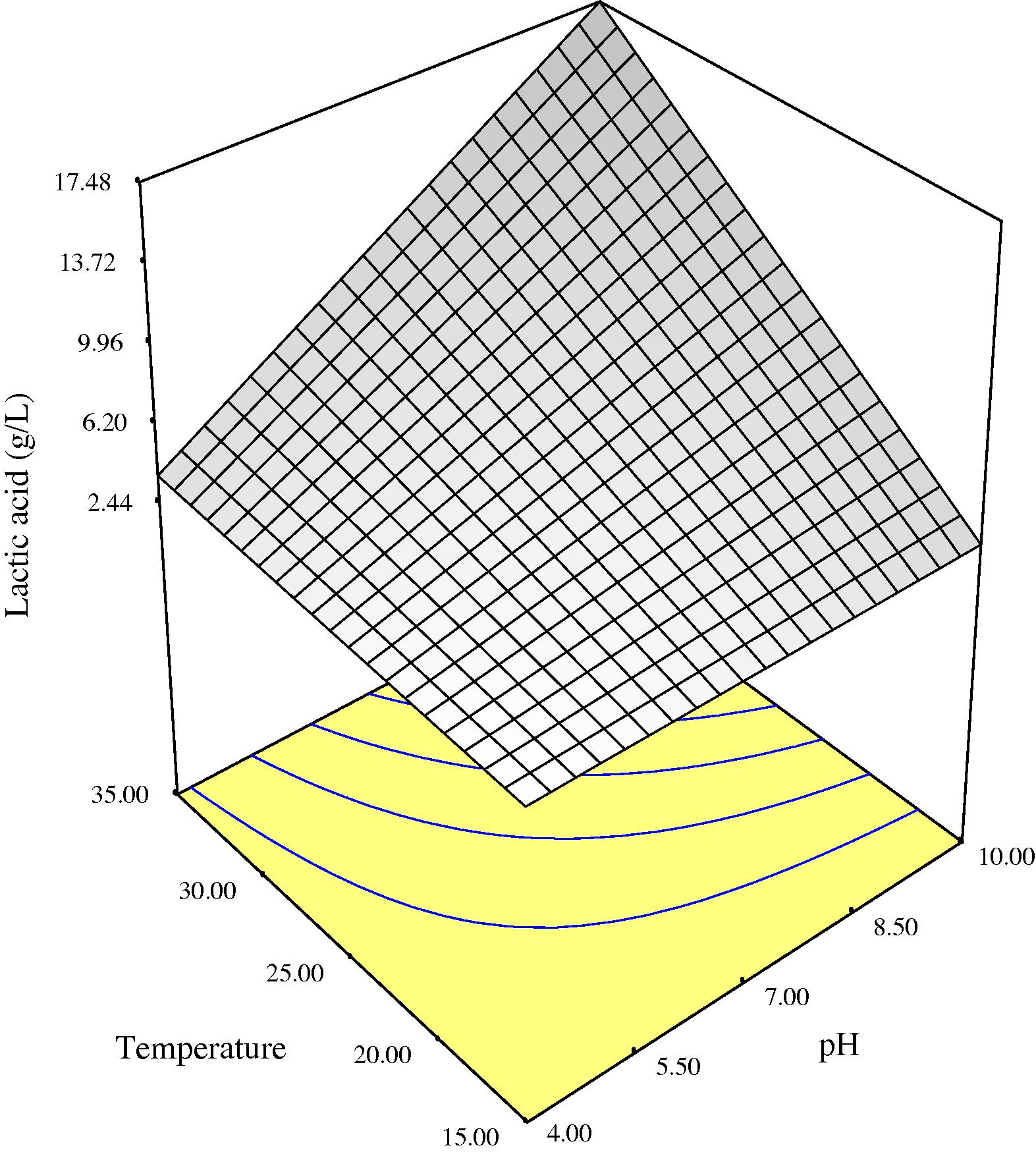
Three-dimensional response surface for lactic acid as a function of pH and temperature at 6 days anaerobic fermentation time
Interaction plots showing the effect of initial medium pH and process temperature on lactic acid production at three different incubation times (low, center and high levels) are presented in Figs. 2a–c. It was evident that the effect of initial medium pH was dependent on the fermentation temperature (Figs. 2a–c) and vice versa. This significant interaction could be due to the obvious influence of both parameters having on microorganisms and their metabolic activities. pH and temperature are known to be some of the important factors that affect the growth of microorganisms. An appropriate pH is beneficial to microbial activity as low and high pH levels could be harmful to microbial cells. On the other hand, most species have a characteristic range of temperature in which they can grow, but they do not grow at the same rate over the whole temperature range (Idris and Suzana, 2006). Most lactic acid bacteria which are responsible for the conversion of sugar to lactic acid are classified as thermophilic or mesophilic bacteria and usually have an optimum growth between 20 and 40 °C. This may explain the low production of lactic acid and reduced interactive effect obtained at 15 °C and the enhanced production and significant interactive effect obtained at 35 °C. Nevertheless, as most chemical hydrolysis and enzymatic hydrolysis are usually operated under high temperatures (Mosier et al., 2005), the mesophilic microbial system of this study seems to be advantageous in practical applications due to its requirement for less energy input.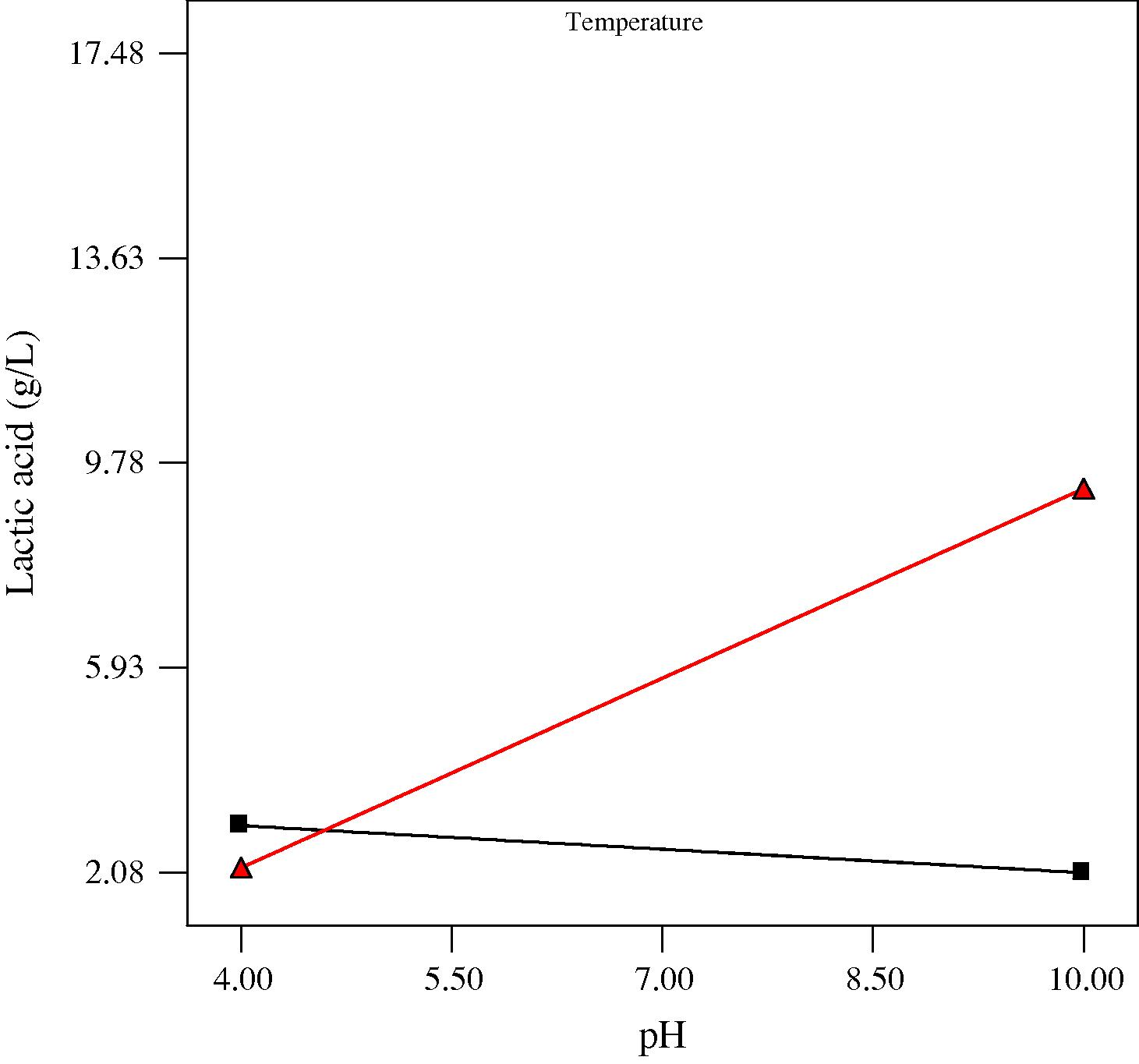
Plot for the interaction between pH and temperature on lactic acid at 3 days anaerobic fermentation time. At temperature ■ 15 °C, ▴ 35 °C.
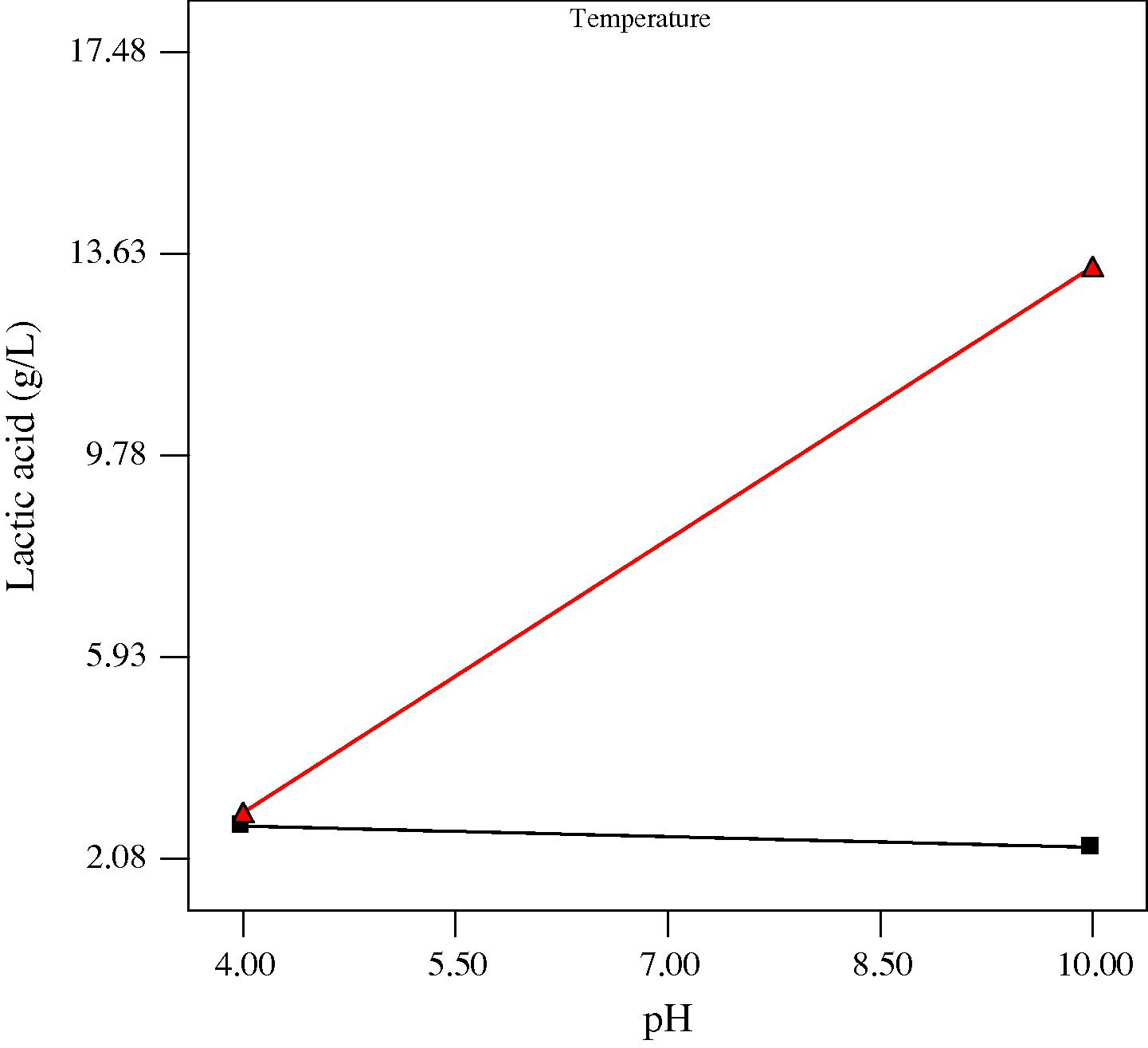
Plot for the interaction between pH and temperature on lactic acid at 4.3 days anaerobic fermentation time. At temperature ■ 15 °C, ▴ 35 °C.
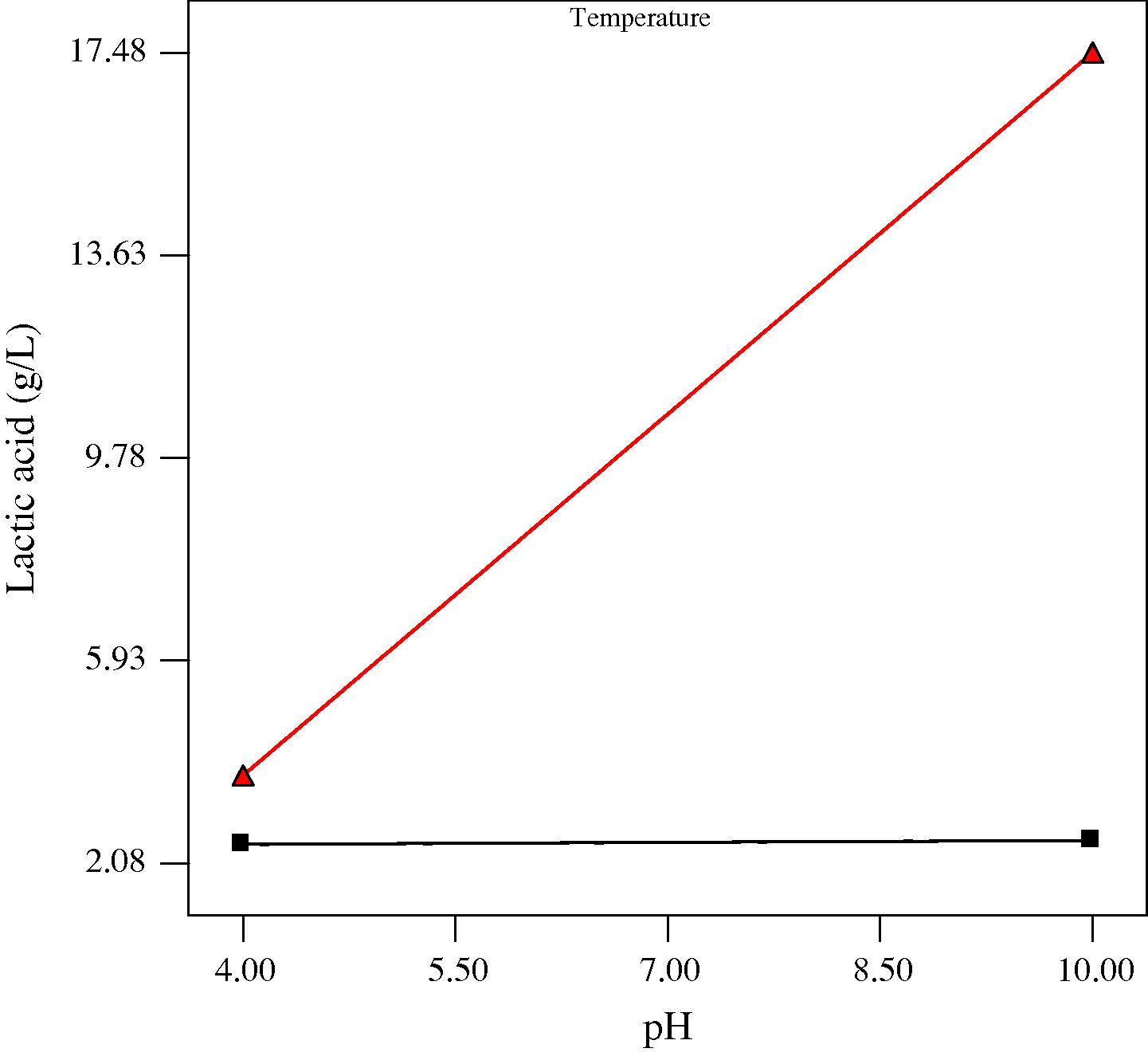
Plot for the Interaction between pH and temperature on lactic acid at 6 days anaerobic fermentation time. At temperature ■ 15 °C, ▴ 35 °C.
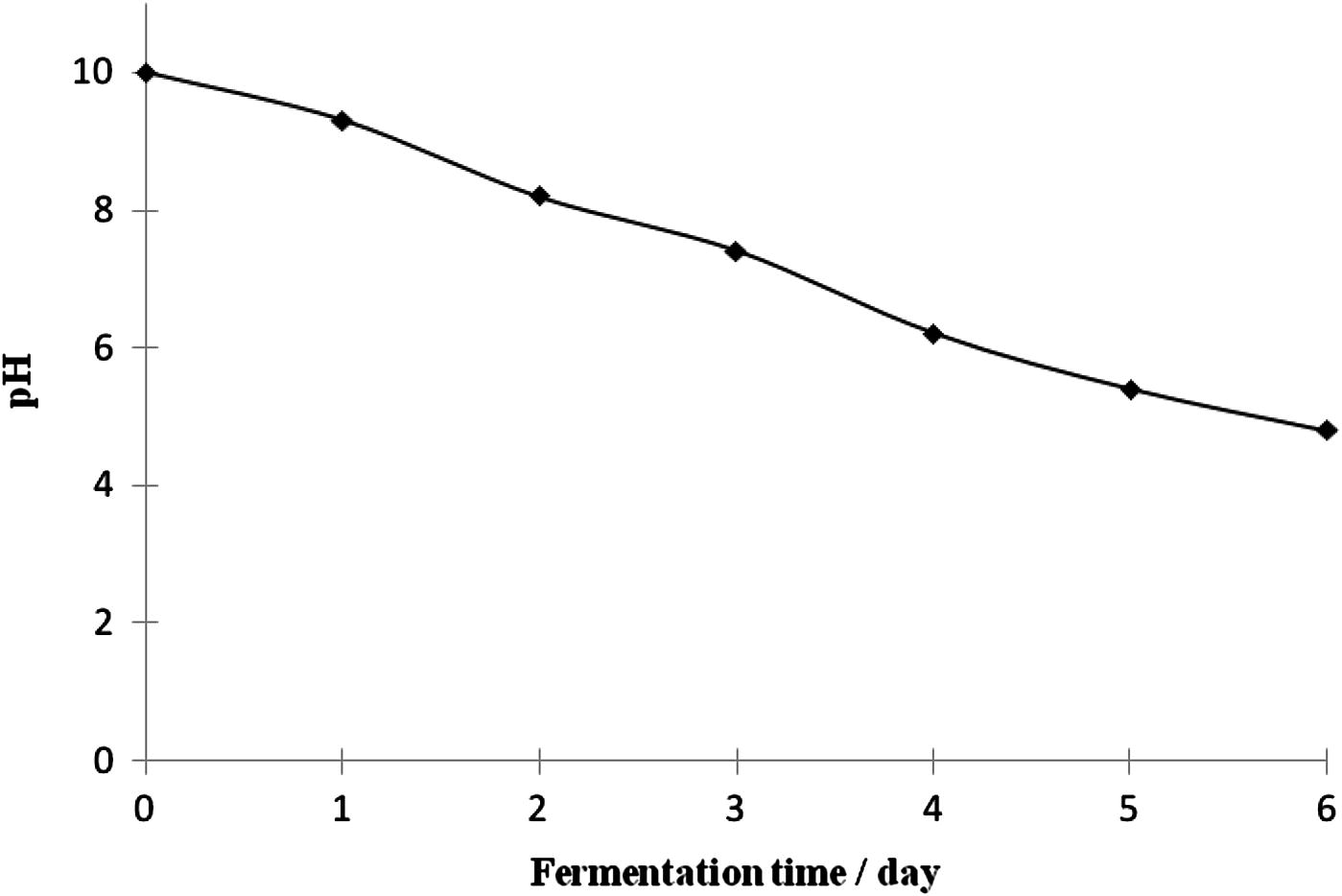
The change in pH during the fermentation time.
In summary, maximum lactic acid production from mango peels using the regression model given in Eq. (1) can be achieved within a fermentation duration of 6 days by setting initial medium pH at high level (10) and process temperature at 35 °C. A confirmatory experiment carried out using these specified conditions resulted in lactic acid production of 17.484 g/l, respectively.
4 Conclusions
In this study, mango peel was investigated for its potential as a novel and potential raw material for lactic acid production. Preliminary experiments showed that lactic acid was produced from mango peels by fermenting microorganisms over a period of six days. The effects of pH, temperature and incubation time were studied using the factorial design and the results obtained showed that these parameters had a significant influence on the production process. Optimization studies on lactic acid production using mango peels showed that maximum production could be obtained in 6 days when initial medium pH of 10 and process temperature of 35 °C are utilized during fermentation of the mango peels. This study has shown that mango peels can be used as a low cost substrate for lactic acid production and that the optimization of the production process will make the fermentation process economically viable and sustainable.
Acknowledgments
The study was funded through the USM short term grant (internal research grant) Grant Number PTEKIND/6310054. The authors acknowledge the Universiti Sains Malaysia (USM) for providing all facilities.
References
- Valuable components of raw and ripe peels from two Indian mango varieties. Food Chem.. 2007;102:1006-1011.
- [Google Scholar]
- Simultaneous saccharification and fermentation of starch to lactic acid. Process Biochem.. 1999;35:367-375.
- [Google Scholar]
- Quality characteristics of a model biscuit containing processed mango (Mangifera indica) kernel flour. Int. J. Food Properties. 2002;5:249-260.
- [Google Scholar]
- Characterization of gallotannins and benzophenone derivatives from mango (Mangifera indica L. cv. FTommy Atkins) peels, pulp and kernels by high-performance liquid chromatography/electrospray ionization mass spectrometry. Rapid Commun. Mass Spectrom.. 2004;18:2208-2216.
- [Google Scholar]
- Screening of mango (Mangifera indica L) cultivars for their contents of flavonol O- and xanthone C-glycosides, anthocyanins and pectin. J. Agric. Food Chem.. 2005;53:1563-1570.
- [Google Scholar]
- Effect of different carbon sources on L(+)-lactic acid production by Rhizopus oryzae. Biochem. Eng. J.. 2004;21:33-37.
- [Google Scholar]
- Process parameters and environmental-factors affecting D-xylose fermentation by yeasts. Enzyme Microb. Technol.. 1994;16:944-956.
- [Google Scholar]
- FAOSTAT, 2004. FAO statistics, food and agriculture organization of the United Nations, Rome, Italy <http://faostat.fao.org/>.
- L-lactic acid production from whole wheat flour hydrolysate using strains of Lactobacilli and Lactococci. Enzyme Microb. Technol.. 1997;20:301-307.
- [Google Scholar]
- Effect of sodium alginate concentration, bead diameter, initial pH and temperature on lactic acid production from pineapple waste using immobilized Lactobacillus delbrueckii. Process Biochem.. 2006;41:1117-1123.
- [Google Scholar]
- Effects of replacing maize with graded levels of cooked Nigerian mango-seed kernels (Mangifera indica) on the performance carcass yield and meat quality of broiler chickens. Bioresour. Technol.. 1997;61:99-102.
- [Google Scholar]
- Physicochemical, morphological, thermal and rheological properties of starches separated from kernels of some Indian mango cultivars (Mangifera indica L) Food Chem.. 2004;85:131-140.
- [Google Scholar]
- Bioenergetics of lactic acid bacteria: cytoplasmic pH and osmotolerance. FEMS Microbial. Rev.. 1987;46:233-244.
- [Google Scholar]
- Mango peels fibres with antioxidant activity. Z. Lebensmitteluntersuch. Forsch. A. 1997;205:39-42.
- [Google Scholar]
- Integrated utilization of fruit-processing wastes for biogas and fish production. Biol. Wastes. 1990;32:243-251.
- [Google Scholar]
- Mirasol, F., 1999. Lactic acid prices falter as competition toughens. Chemical Market Reporter, March.
- Design and Analysis of Experiments (fifth ed). New York: Wiley; 2001.
- Features of promising technologies for pretreatment of lignocellulosic biomass. Bioresour. Technol.. 2005;96:673-686.
- [Google Scholar]
- Effects of medium supplementation and pH control on lactic acid production from brewer’s spent grain. Biochem. Engineer. J.. 2008;40:437-444.
- [Google Scholar]
- Direct fermentation of starch to L(+) lactic acid in SSF by Lactobacillus amylophilus GV6 using wheat bran as support and substrate: medium optimization using RSM. Process Biochem.. 2005;40:681-690.
- [Google Scholar]
- Obtaining pectins from solid wastes derived from mango (Mangifera indica) processing. AIChE Symp. Ser.. 1994;300:36-41.
- [Google Scholar]
- Reddy, G., Altaf, M., Naveena, B.J., Venkateshwar, M., Vijay Kumar, E., 2008. Amylolytic bacterial lactic acid fermentation – a review. Biotechnol. Adv. 26, 22–34.
- Exploitation of biological wastes for the production of value-added products under solid-state fermentation conditions. Biotechnol. J.. 2008;3:859-870.
- [Google Scholar]
- Aproveitamento de reśıduos agro-industriais: ́acido ĺactico, uma alternative. Bol. SBCTA. 1991;25:37-40.
- [Google Scholar]
- Mango waste: a potential source of pectin, fiber, and starch. Indian J. Environ. Prot.. 1999;19:924-927.
- [Google Scholar]
- A simple colorimetric assay for muramic acid and lactic acid. Appl. Biochem. Biotechnol.. 1996;56:49-58.
- [Google Scholar]
- Direct fermentation of various pure and crude starchy substrates to L(+) lactic acid using Lactobacillus amylophilus GV6. World J. Microbiol. Biotechnol.. 2002;18:429-433.
- [Google Scholar]
- Amylopullulanase – a novel enzyme of L. amylophilus GV6 in direct fermentation of starch to L(+) lactic acid. Enzyme Microb. Technol.. 2006;38:545-550.
- [Google Scholar]
- Mango Juice. In: Nagy S., Chen C.S., Shaw P.E., eds. Fruit juice processing technology Auburndale. Florida, USA: Agscience Inc.; 1993. pp. 620–655
- [Google Scholar]
- Lactic acid production from dining-hall food waste by Lactobacillus plantarum using response surface methodology. J. Chem. Technol. Biotechnol.. 2008;83:1541-1550.
- [Google Scholar]







