Translate this page into:
Kinetics and vaporization of anil in nitrogen atmosphere – Non-isothermal condition
*Corresponding author. Tel.: +91 4144 239523; mobile: +91 9488476098 pvta1998@yahoo.co.in (V. Thanikachalam)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.

Available online 29 April 2011
Abstract
The thermal vaporization kinetics of Schiff’s base was studied by thermogravimetry (TG) and differential thermal analysis (DTA) techniques using non-isothermal conditions. The kinetic parameters were calculated under model-free (Friedman’s, Kissinger–Akahira–Sunose (KAS) and Flynn–Wall–Ozawa (FWO) methods) and model-fitting (Coats–Redfern, CR) methods. The results of the Friedman’s isoconversional analysis of the TG data suggest that the investigated vaporization process follows a single-step reaction. Based on the obtained results, the mass loss is caused mainly by vaporizations and not by decomposition. The most probable kinetic model for vaporization of the compound is P2 (power law).
Keywords
Schiff’s base
Thermogravimetric analysis
Kinetic parameters
Non-isothermal
Isoconversional
1 Introduction
Schiff bases are nitrogen donor ligands that form complexes by donating an electron pair to a metal atom in the formation of coordination compounds. Schiff bases have found wide applications in the field of coordination chemistry (Sallam, 2006), biological processes (Snatos et al., 2005), several enzymes (Clarkson and Basolo, 1973), microcalorimetry (Li-Xia et al., 2006), cytotoxic (Tarafder et al., 2002), insulin mimetic agents (McNeill et al., 1992), anticonvulsant (Panchagnula et al., 2004), antiproliterative (Vicini et al., 2003), antifungal activities (Pignatello et al., 1974), transport of oxygen in mammalian and other respiratory systems (Singh et al., 2010). Few Schiff’s bases are prepared, characterized and determined the kinetic parameters of chitosan with salicylaldehyde (Guinesi and Cavalheiro, 2006), trans 1,2-cyclohexadiamine with salicylaldehyde (Aranha et al., 2007) and 4-aminoantipyridine with 2-aminophenol (Mohamed et al., 2009). Literature data show that no work has been reported on thermal decomposition of anil at different heating rates (10, 15 and 20 K min−1) under non-isothermal condition in nitrogen atmosphere.
2 Experimental
2.1 Preparation of anil
Anil was prepared by refluxing equimolar quantities of benzaldehyde and aniline in alcohol for about 1–2 h. The resulting solution was cooled and poured into cold water. The precipitated anil was filtered off, washed with cold ethanol and dried. It was recrystallized from alcohol. The purity of anil was checked by melting point and FT-IR spectrum (m.p. 53 °C, lit. 54 °C), microanalysis; Found %C: 86.10, %H: 6.02, %N: 7.68; Calculated %C: 86.18, %H: 6.07, %N: 7.73.
2.2 Measurements
Elemental analysis was performed on a Carlo Analyzer at Central Drug Research Institute (CDRI), Lucknow, India. FT-IR spectrum was recorded in a KBr-pellet on an Avatar-330 spectrometer (with resolution 2 cm−1). The simultaneous TGA and DTA curves were obtained with the thermal analysis system model Perkin Elmer TAC 7/DX Thermal Analysis Controller TAC-7. The TG and DTA analyses of anil were carried out under static nitrogen atmosphere (100 mL min−1), in an alumina crucible with sample mass around 10 mg with heating rates of 10, 15 and 20 K min−1 from 308 to 973 K. The kinetic parameters Ea and ln A were calculated using Microsoft® Excel 2007® Software. The sample temperature, which is controlled by a thermocouple, did not exhibit any systematic deviation from the preset linear temperature program.
3 Theoretical background
3.1 Model fitting method
The integral method (Horowitz and Metzger, 1963) of Coats and Redfern (1964) has been most successfully used for studying the kinetics of dehydration and vaporization of different solid substances (Wendlandt, 1974). The kinetic parameters can be derived from modified Coats and Redfern Eq. (1),
3.2 Model free methods
Friedman’s method (Friedman, 1963) is a differential method and was one of the first isoconversional methods. The non-isothermal rate law Eq. (2)
A plot of ln (β dα/dT) versus 1/T at each α gives Ea from the slope of the plot.
In the present study to evaluate the values of the activation energies of thermal vaporization of solid materials, Flynn–Wall–Ozawa equation (Flynn and Wall, 1966; Ozawa, 1965) (Eq. (4))
and Kissinger–Akahira–Sunose (KAS) equation (Kissinger, 1957; Akahira and Sunose, 1971) (Eq. (5)) were used.
The plots of ln (β dα/dT) versus 1/T (Eq. (3)), ln β versus 1/T (Eq. (4)) and ln (β/T2) versus 1/T (Eq. (5)) have been shown to give the values of apparent activation energies for the vaporization of anil at different α values. According to these equations, the reaction mechanism and shape of g(α) function do not affect the values of the activation energies of the vaporization stage.
3.3 Thermodynamic parameters
The kinetic parameters, energy of activation and pre-exponential factors are obtained from Kissinger single point (Kissinger, 1957) kinetic method using the Eq. (6).
Based on the values of activation energy and pre-exponential factors for the vaporization stage, the values of ΔS≠, ΔH≠ and ΔG≠ for the formation of activated complex from the reactants were calculated (Cordes, 1968).
4 Results and discussion
4.1 TG and DTA curves of vaporization of anil
The TG and DTA curves of vaporization of anil obtained at three heating rates (10, 15 and 20 K min−1) are shown in Fig. 1. The weight loss observed in TG curves on heating the anil from room temperature to 350 °C is associated with the peak of curves. The weight loss is due to complete vaporization of the anil and curves are asymmetric figures and move to high temperature with increase in heating rates (Fig. 1).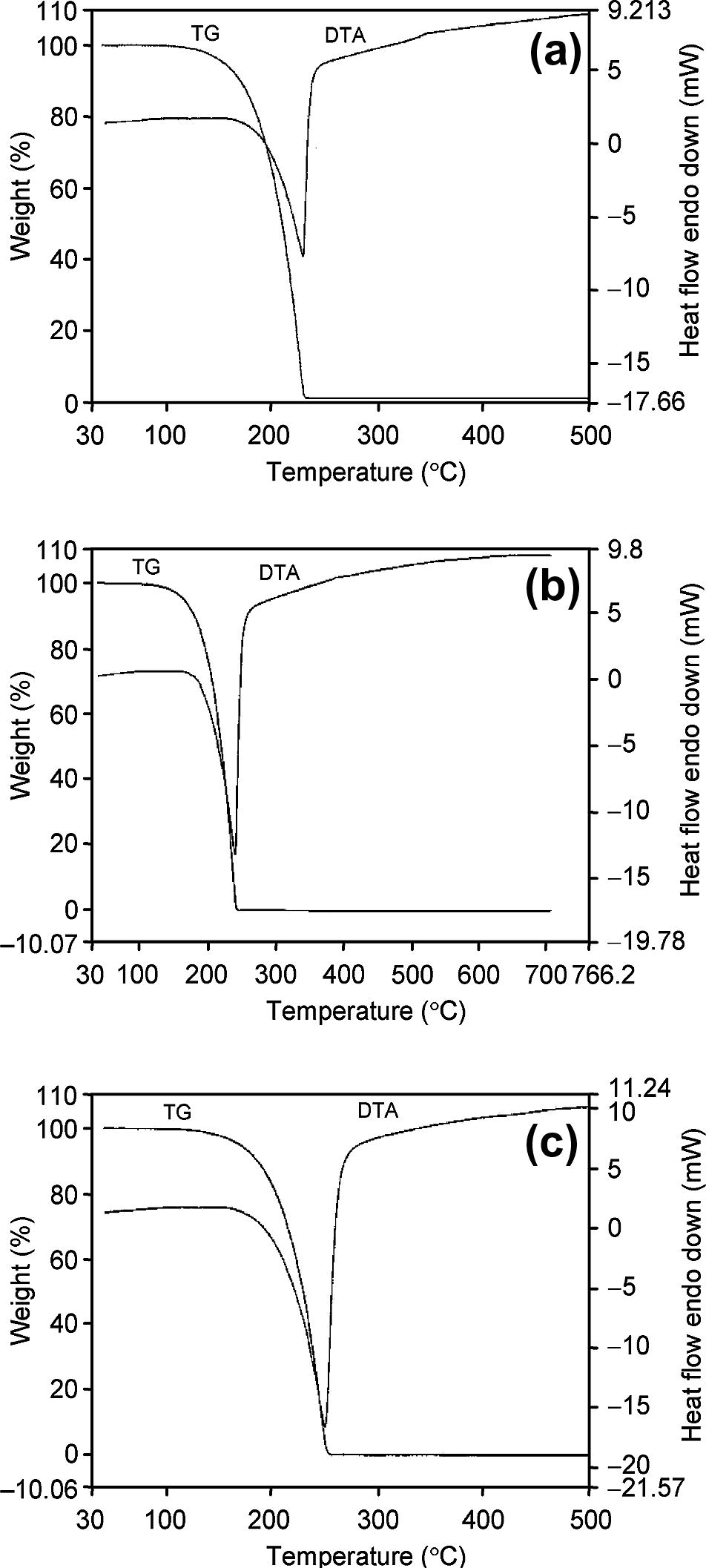
TG and DTA curves of anil at (a) 10 K min−1, (b) 15 K min−1, and (c) 20 K min−1 heating rates in nitrogen atmosphere.
4.2 Model-free analysis
The non-isothermal vaporization kinetics of anil was first analyzed by model-free methods viz., Friedman, Kissinger–Akahira–Sunose and Flynn–Wall–Ozawa. The data show that the variation of apparent activation energy Ea, as a function of extent of conversion α, for vaporization of anil. Ea value increases slightly in the conversion range of 0.20 ⩽ α ⩽ 0.90. It was pointed out (Vyazovkin and Linert, 1995) that when Ea changes with α, the Friedman and KAS isoconversional methods lead to close value of Ea. The applied isoconversional method does not suggest a direct way for evaluating either the pre-exponential factor (A) or the analytical form of the reaction model (f(α)), for the investigated vaporization process of anil. In addition, the obtained data reveal that the dependence of the apparent activation energy (Ea) on the extent of conversion (α) helps not only to disclose the complexity of vaporization process, but also to identify its sublimation or evaporation mechanism. Similar type of effect is observed in the study of dichloroglyoxime (Pourmortazavi et al., 2007). The value of activation energy Ea changes slightly with α which suggests that the vaporization stage is a single step (Sbirrazzuoli et al., 2005). The average values of Ea energy of vaporization are 26.92 ± 1.90 kJ mol−1 (0.20 ⩽ α ⩽ 0.90, Friedman method). From Fig. 2 it is evident that the values of activation energies obtained by the Friedman method is little lower than the values of activation energies obtained by FWO method and closer to KAS method. Regardless of the calculation procedure used, the activation energy remains practically constant in the 0.20 ⩽ α ⩽ 0.90 range, with average values of Ea = 26.92 ± 1.90, 26.43 ± 0.40 and 33.09 ± 0.59 kJ mol−1 calculated by using the Friedman, KAS and FWO methods, respectively. The observed energy of activation error varied from 1% to 5%, in our case KAS method shows least when compared with Friedman method.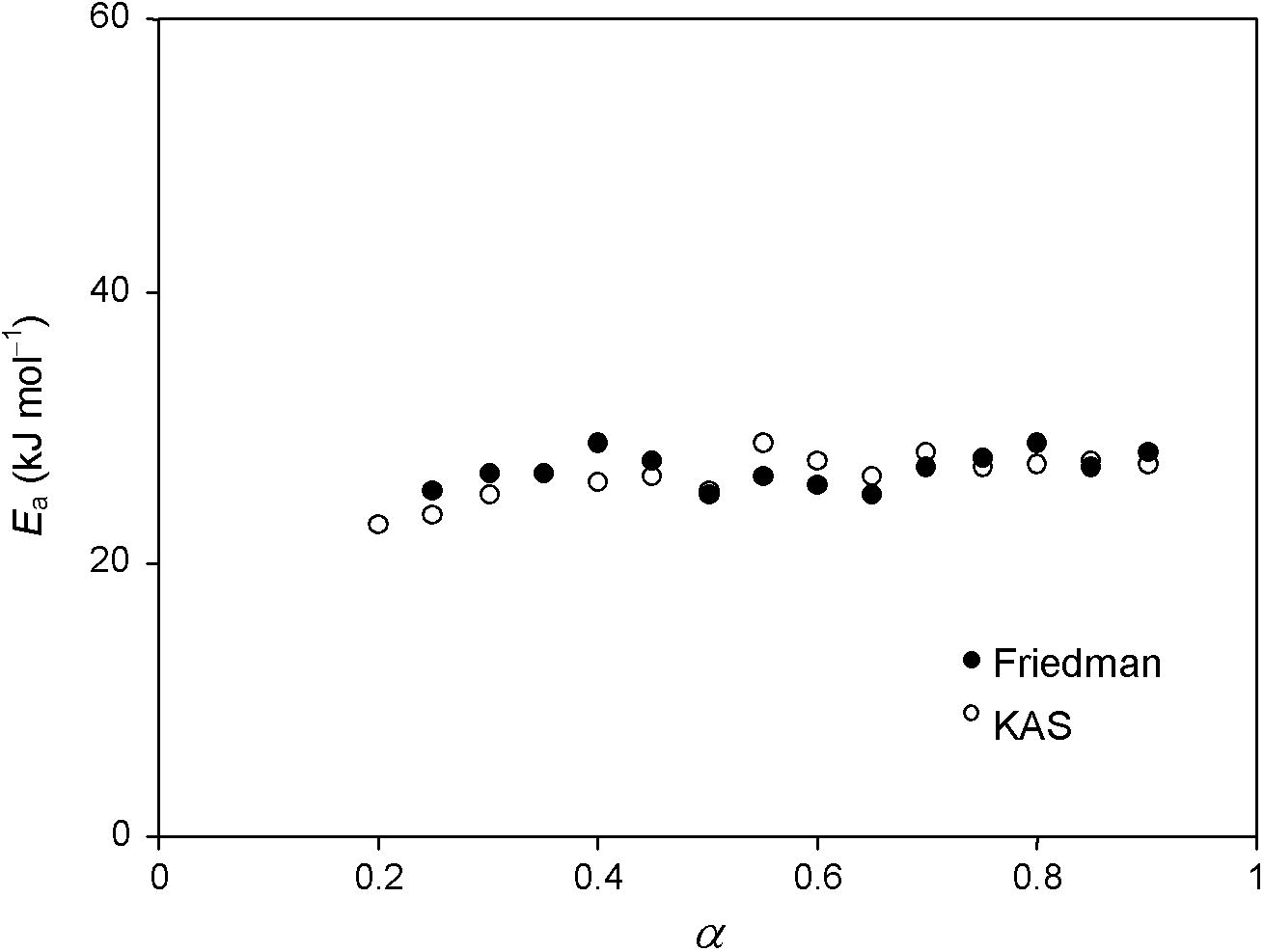
Ea versus α plot for the vaporization of anil under non-isothermal condition.
4.3 Model-fitting analysis
After model-free analysis is performed, model-fitting can be done in the conversion region where apparent activation energy is approximately constant where a single model may fit. The non-isothermal kinetic data of anil at 0.20 ⩽ α ⩽ 0.90 where model-free analysis indicates approximately constant activation energy, were then fitted to each of the 15 models listed in Table 1. As shown in Table 1, for the applied method (Coats–Redfern), Arrhenius parameters (Ea, ln A) for vaporization or decomposition process, exhibit strong dependence on the reaction model chosen. The values of Ea (mean average values are calculated by Friedman method) for anil coincidence with values are calculated by Coats–Redfern method (Ea and ln A). Based on the kinetic data, it is concluded that the vaporization occurred in a single mechanism (i.e., P2) which is also confirmed by invariant kinetic parameters method.
Kinetic model
β = 10 K min−1
β = 15 K min−1
β = 20 K min−1
Ea (kJ mol−1)
ln A (A s−1)
−r
Ea (kJ mol−1)
ln A (A s−1)
−r
Ea (kJ mol−1)
ln A (A s−1)
−r
P2
28.15
4.74
0.999
32.07
5.75
0.999
32.66
5.89
0.999
P3
16.13
1.30
0.999
18.60
2.13
0.999
18.61
2.31
0.999
P4
10.15
−0.59
0.999
11.91
0.16
0.999
12.07
0.35
0.999
F1
85.34
20.12
0.996
97.75
22.47
0.994
99.59
22.43
0.994
F2
111.78
27.41
0.985
130.98
31.13
0.976
133.39
30.99
0.975
F3
143.09
35.96
0.972
171.18
41.51
0.954
174.27
41.23
0.952
D1
146.55
42.13
1.000
164.24
44.99
0.999
167.62
44.89
0.999
D2
148.64
34.91
0.999
167.57
37.97
0.999
170.91
37.78
0.999
D3
163.38
37.41
0.998
185.28
41.03
0.998
188.91
40.78
0.998
D4
169.13
38.65
0.999
193.40
42.73
0.999
197.20
42.46
0.999
A2
38.75
7.88
0.995
44.74
9.28
0.994
45.54
9.39
0.993
A3
22.91
3.47
0.994
26.73
4.55
0.992
27.17
4.71
0.992
A4
15.45
1.24
0.993
18.24
2.19
0.991
18.51
2.36
0.990
R2
74.10
16.29
0.999
84.11
18.18
0.999
85.73
18.19
0.999
R3
70.78
15.63
0.999
80.16
17.41
0.999
81.71
17.43
0.999
4.4 Invariant kinetic parameters (IKP) method
Criado and Morales (1976) reported that almost any α = α(T) or (dα/dt) (T) experimental curve may be correctly described by several conversion functions. The use of an integral or differential model-fitting method leads to different values of the activation parameters. Although obtained with high accuracy, the values change with different heating rates and among conversion functions.
Lesnikovich and Levchik (1983) suggested that correlating these values by the apparent compensation effect, ln A = aβ + bβEa, one obtains the compensation effect parameters, aβ and bβ, which strongly depend on the heating rates (β) as well as on the considered set of conversion functions. The straight lines ln A versus Ea for three constant heating rates should intersect at a point (isoparametric point (Lesnikovich and Levchik, 1985)) which corresponds to the true values of the activation energy and pre-exponential factor. These were named as invariant kinetic parameters. To establish the best combination (r → 1), a better resolution is determining the invariant kinetic parameters and the closest values to the mean isoconversional activation energies (Budrugeac and Segal, 2007; Vyazovkin and Lesnikovich, 1988). For (all kinetics models) AKM – {P4; F1; F2; F3; D1; D2; D3; D4; R2; R3} has the highest correlation coefficient (r = 0.999) and is a true straight line (Table 2). For these six groups, the invariant activation energy is 26.93 kJ mol−1, which is equal to 26.92 ± 1.90 kJ mol−1 obtained by Friedman method and invariant Einv = 26.93 ± 0.3 kJ mol−1 and ln Ainv = 4.48 A s−1. Depending on the chosen group of kinetic models, the compensation effect parameters are obtained with different accuracies, their values and derived invariant activation parameters varying substantially.
Kinetic model
Einv (kJ mol−1)
ln Ainv (A s−1)
−r
AKM
32.71
5.95
1.000
AKM – {D1; D2; D3; D4}
30.15
5.11
0.999
AKM – {F3; D1; D2; D3; D4}
30.74
5.28
0.999
AKM – {P4; F1; F2; F3; D1; D2; D3; D4; R2; R3}
26.93
4.48
0.999
AKM – {P3; P4; D1; D2; D3; D4; R3}
30.24
5.26
0.999
AKM – {P3; P4; F3; D1; D2; D3; D4; R3}
30.96
5.48
0.999
By using Dollimore method (Dollimore et al., 1992), most reliable kinetic model was developed from recreated DTG curves (Fig. S1). The extent of activation energy and pre-exponential factor are calculated from the Arrhenius plot. Table S1 shows the Ea and A values obtained from lines drawn through various ranges of α in order to illustrate the disadvantages of extracting these parameters directly from Arrhenius plot.
Carrying out the vaporization of anil under a dynamic flowing atmosphere of nitrogen, and using a heating rate of 10 K min−1 gave αmax equal to 0.62 and half-width of 59 °C. Referring to Table S2, the data show two possible mechanisms having a αmax range comparable to the experimentally determined value. Thus the P2 equation is possible with magnitude of the half-width making the power law mechanism most suitable. Table S2 shows the reconstructed αmax and half-width values as function of the kinetically related Arrhenius parameters obtained for the vaporization of anil, using the P2 mechanism.
The most probable kinetic model for vaporization process of anil is therefore P2 model. By introducing the derived reaction model g(α) = (α)1/2, the following Eq. (7) is obtained:
The plot of (α)1/2 against (Ea/Rβ)p(x) at the different heating rates is constructed in Fig. 3. By using the above equation, the A value was determined from the slope of the fitted line shown in Fig. 3. For power law model (P2) and Ea = 26.92 ± 0.34 kJ mol−1, the pre-exponential (frequency) factor was found to be A = 1.21 × 102 s−1 (ln A = 4.80). The obtained value of ln A is in good agreement with average value of Friedman isoconversional intercept (ln [Af(α)] = 5.14).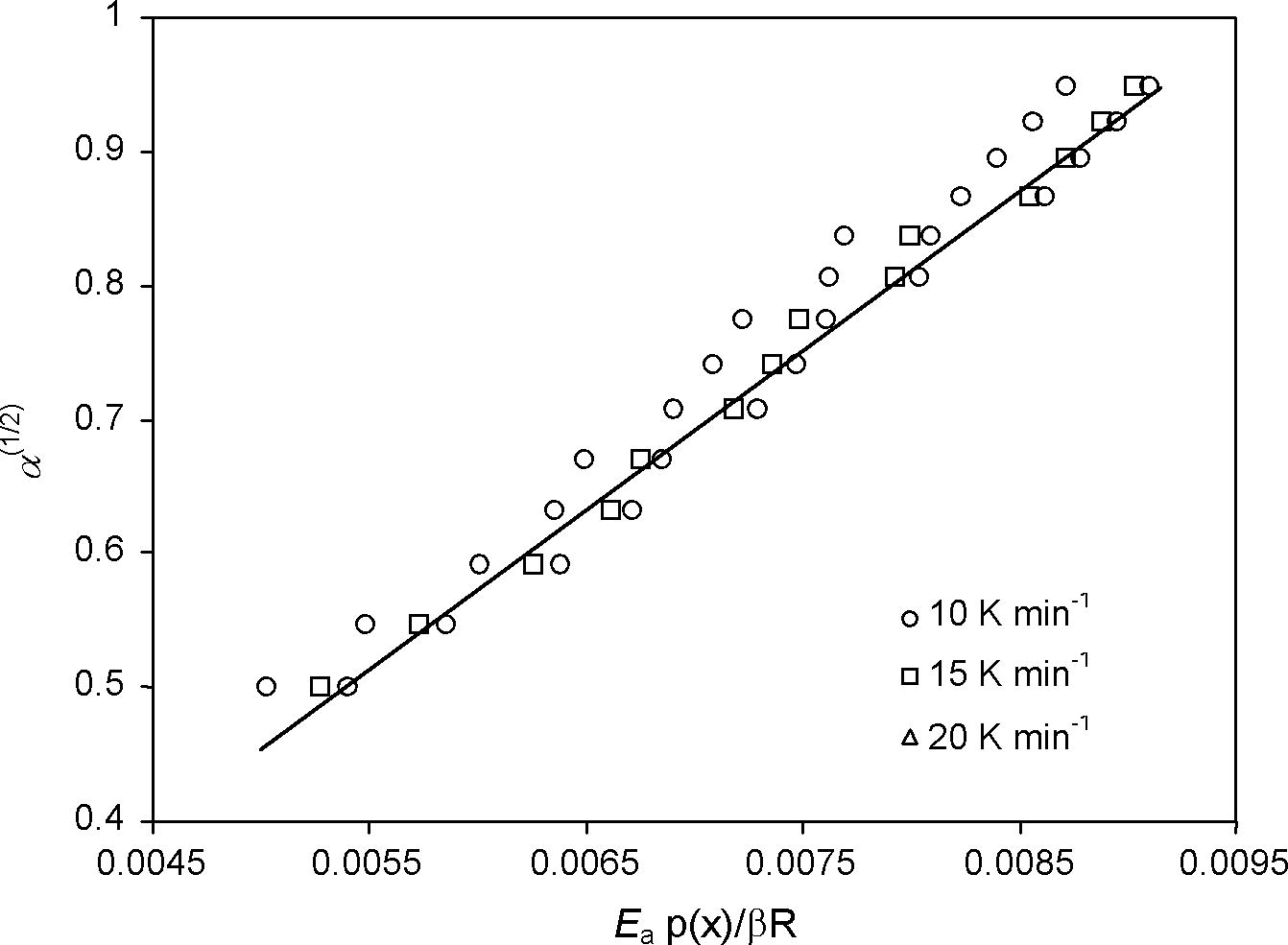
Determination of A value by plotting α(1/2) against Ea p(x)/β R for the vaporization process of anil at the different heating rates (β).
For the non-isothermal decomposition of anil, model P2 is the best for all three heating rates. Model P2 gives raise to an activation energy that varies in the range of 28.15–32.66 kJ mol−1. Fitting of the reaction-order model followed by statistical analysis resulted in the following confidence limits −1.0 ⩽ n < 0.6, 26.92 ⩽ Ea ⩽ 50 kJ mol−1 and 4.8 ⩽ log A/min−1 ⩽ 8. The kinetic triplet corresponding to the minimum (r = −0.999) is n = 0.33, Ea = 26.92 kJ mol−1 and ln |A| min−1 = 4.8. According to the statistical test (Vyazovkin and Wight, 1999) this kinetic triplet is equivalent to the triplet corresponding to model P2.
4.5 Thermodynamic parameters
From the DTA curves, the peak temperatures for anil are 503.04, 514.09 and 522.49 K and used to evaluate single point kinetic parameters (Kissinger, 1957).
The energy of activation for anil is less. Free energy of activation (ΔG#) is more positive, which indicates that vaporization of anil is non spontaneous in nature. The lower value of energy of activation, shows that anil is thermally less stable. The positive values of ΔH≠ and ΔG≠ for anil show that they are connected with absorption of heat and they are non-spontaneous processes (Criado et al., 2005). The obtained Ea value is coincided with invariant kinetic parameter.
Based on the obtained results, the kinetic description of mass loss can be accomplished through one mechanism (P2). The low values of apparent activation energy attributed to phase transformation may occur. So the loss in this stage should be caused mainly by vaporizations and not by decomposition. It is confirmed by TG and DTA curves.
5 Conclusion
The studied compound is vaporized in a single stage with absorption of heat. The model for the vaporization mechanism is P2. The thermal stability of anil is less and the energy of activation also less. Free energy is positive which indicates that the vaporization is non-spontaneous process.
Acknowledgments
The authors thank Prof. L.D. Prabhakar, Chemistry Wing (DDE), Annamalai University, Annamalainagar, India, for his fruitful suggestions and The Head, Indian Institute of Technology, RISC, Chennai for TGA-DTA studies.
References
- Joint convention of four electrical institutes. Res. Report CHIBA Inst. Technol.. 1971;16:22-31.
- [Google Scholar]
- Thermal behaviour of vanadyl complexes with Schiff bases derived from trans-N,N-bis(salicylidene)-1,2-cyclohexadiamine(t-salcn) Thermochim. Acta. 2007;453:9-13.
- [Google Scholar]
- Applicability of the Kissinger equation in thermal analysis revisited. J. Therm. Anal. Calorim.. 2007;88:703-707.
- [Google Scholar]
- Reaction of some cobalt nitrosyl complexes with oxygen. Inorg. Chem.. 1973;12:1528-1534.
- [Google Scholar]
- Preexponential factors for solid-state thermal decomposition. J. Phys. Chem.. 1968;72:2185-2189.
- [Google Scholar]
- Defects of thermogravimetric analysis for discerning between first-order reactions and those taking place through the Avrami-Erofeev’s mechanism. Thermochim. Acta. 1976;16:382-387.
- [Google Scholar]
- Dependence of the preexponential factor on temperature errors in the activation energies calculated by assuming that A is constant. J. Therm. Anal. Calorim.. 2005;82:671-675.
- [Google Scholar]
- Correlation between the shape of a TG/DTG curve and the form of the kinetic mechanism which is applying. Thermochim. Acta. 1992;198:249-257.
- [Google Scholar]
- General treatment of the thermogravimetric of polymers. J. Res. Natl. Bur. Stand. A. 1966;70:487-523.
- [Google Scholar]
- Kinetics of thermal degradation of char-foaming plastics from thermogravimetry-application to a phenolic resin. Polym. Sci. C. 1963;6:183-195.
- [Google Scholar]
- Influence of the degree of substitution in biopolymeric Schiff bases on the kinetic of thermal decomposition by non-isothermal procedure. Thermochim. Acta. 2006;449:1-7.
- [Google Scholar]
- Reaction kinetics in differential thermal analysis. Anal. Chem.. 1957;29:1702-1706.
- [Google Scholar]
- A method of finding invariant values of kinetic parameters. J. Therm. Anal. Calorim.. 1983;27:89-93.
- [Google Scholar]
- Isoparametric kinetic relations for chemical transformations in condensed substances (analytical survey). I. Theoretical fundamentals. J. Therm. Anal. Calorim.. 1985;30:237-262.
- [Google Scholar]
- Inhibitory of some novel Schiff base derivatives on Staphylococcus aureus by microcalorimetry. Thermochim. Acta. 2006;440:51-56.
- [Google Scholar]
- A computer program for kinetic analysis of non-isothermal thermoanalytical data. Thermochim. Acta. 1989;136:337-346.
- [Google Scholar]
- Bis(maltolato)oxovanadium(IV) is a potent insulin mimic. J. Med. Chem.. 1992;35:1489-1491.
- [Google Scholar]
- Biological activity studies on metal complexes of novel tridentate Schiff base ligand. Spectroscopic and thermal characterization. Eur. J. Med. Chem.. 2009;44:4801-4812.
- [Google Scholar]
- A new method of analyzing thermogravimetric data. Bull. Chem. Soc. Jpn.. 1965;38:1881-1886.
- [Google Scholar]
- RP-HPLC method and its validation for the determination of naloxone from a novel transdermal formulation. IL Farmaco. 2004;59:839-842.
- [Google Scholar]
- Schiff bases of N-hydroxy-N′-aminoguanidines as antiviral, antibacterial and anticancer agents. Eur. J. Med. Chem.. 1974;29:781-785.
- [Google Scholar]
- Thermal behaviour kinetic study of dihydroglyoxime and dichloroglyoxime. Mater. Lett.. 2007;61:4670-4673.
- [Google Scholar]
- Binuclear copper(II), nickel(II) and cobalt(II) complexes with N2O2 chromophores of glycylglycine Schiff-bases of acetyl acetone, benzoylacetone and thenoyltrifluoroacetone. Transit. Met. Chem.. 2006;31:46-55.
- [Google Scholar]
- Isoconversional kinetic study of alachlor and metolachlor vaporization by thermal analysis. Int. J. Chem. Kinet.. 2005;37:74-80.
- [Google Scholar]
- Spectroscopic characterization and biological activity of Zn(II), Cd(II), Sn(II) and Pb(II) complexes with Schiff base derived from pyrrole-2-carboxaldehyde and 2-amino phenol. Spectrochim. Acta A. 2010;76:376-383.
- [Google Scholar]
- Thermal behaviour of Schiff bases from chitosan. J. Therm. Anal. Calorim.. 2005;79:243-248.
- [Google Scholar]
- S-Methyldithiocarbazate and its Schiff bases: evaluation of bondings and biological properties. J. Biochem. Mol. Biol. Biophys.. 2002;6:85-91.
- [Google Scholar]
- Synthesis and biological evaluation of benzo[d]isothiazole, benzothiazole and thiazole Schiff bases. Bioorg. Med. Chem.. 2003;11:4785-4789.
- [Google Scholar]
- Estimation of the pre-exponential factor in the isoconversional calculation of effective kinetic parameters. Thermochim. Acta. 1988;128:297-300.
- [Google Scholar]
- False isokinetic relationships found in the nonisothermal decomposition of solids. Chem. Phys.. 1995;193:109-118.
- [Google Scholar]
- Model-free and model-fitting approaches to kinetic analysis of isothermal and non-isothermal data. Thermochim. Acta 1999:53-68.
- [Google Scholar]
- Thermal Methods of Analysis. New York: John Wiley and Sons Inc.; 1974.
Appendix A
Supplementary data
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.jksus.2011.04.002.
Appendix A
Supplementary data
Supplementary data
Supplementary data
Figure and tables.







