Translate this page into:
Breeding, embryonic development and salinity tolerance of Skunk clownfish Amphiprion akallopisos
*Corresponding author. Tel.: +91 9786190700; fax: +91 04144 243555 dhanee121@gmail.com (K.V. Dhaneesh)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.

Available online 23 March 2011
Abstract
Breeding and rearing some of the clownfishes most commonly used in the aquarium trade actually represent an economical and ecological tool for broadening development. Culture of clownfish species in low-saline water is still in its infancy. Salinity of the culture environment is one of the more relevant parameters affecting fish physiology, modifying food intake and growth performance in many fish species. The objective of this study was to breed skunk clownfish (Amphiprion akallopisos) in aquarium condition, document the embryonic development, determine the upper and lower lethal salinities of juveniles, tolerance of five different salinities (20, 25, 30, 35, and 40 ppt) and their effect on the survival rate of larvae. Higher (53–55 ppt) and lower (3–6 ppt) salinities produced loss of appetite and movement, finally leading to mortality in juveniles. In a ninety six hour experiment, larvae showed 100% survival at the salinities of 30 (control) and 35 ppt and 88% survival in 40 ppt salinity and 76% survivals in 20 and 25 ppt. In conclusion juveniles of A. akallopisos exhibit satisfactory rates of survival and no signs of stress in high (up to 53 ppt) and low saline (up to 6 ppt) waters. These results demonstrate that using such salinities, which can reduce the incidence of diseases and mortality, does not produce significant physiological alterations in this species. In addition, descriptive studies on embryonic development and mass scale larval rearing were also carried out during the present study.
Keywords
Amphiprion akallopisos
Breeding
Embryonic development
Juvenile
Larva
Salinity tolerance
Survival
1 Introduction
Global ornamental fish trade has increased from US$ 50–US$ 250 million over the past two decades. It has been estimated that 1.5–2 billion aquariums are being kept in households worldwide with more than 600,000 in the US alone (Lewbart et al., 1999; Green, 2003). Global imports on ‘Marine, fresh water fish and invertebrates’ in 2007 have been valued at US$ 327 million. The value of the fish and invertebrates of marine origin in this trade has increased from 9 million US$ in 2003 to reach almost 29 million US$ in 2007 (Tissera, 2010).
The rapid increase in the demand for fish and invertebrates of marine origin within the pet and hobbyist trade poses the threat of increased harvesting effort on natural resources. The increasing demand for marine ornamental fish due to the recent developments in aquarium keeping has resulted in an over exploitation of the natural stock and consequent destruction of reef areas. Captive reproduction of the fish in demand in the aquarium trade stands as the only sustainable means in meeting the increasing demand as well as in replenishing the already depleted natural resources.
Development of early life stage, from fertilization to embryo formation among Teleost fish, generally follows the same pattern (Falk-Petersen, 2005). Spawning and behavioural aspects of many tropical and sub-tropical Pomocentrid species have been well documented. However, description of ova, development of the embryo, and methodology of larval rearing and production of juveniles are available only for a few Pomocentrids and are scarce in the case of species dwelling in Indian waters. Hatching and chronological flow of embryonic development stages differ depending on the species and the specific environmental conditions in which they take place. The free embryo phase begins when the embryo is free of egg membranes. The beginning of the larval period is indicated when the larvae are able to capture feed objects (Chen, 2005).
Salinity of the culture environment is an important abiotic factor that influences marine fish eggs and larval physiology and has a direct effect on their development, growth and survival (Alderdice and Forrester, 1968; Holliday, 1969). By influencing the energy requirement for osmoregulation, salinity directly affects the efficiency of yolk utilization, larval growth and survival (Howell et al., 1998). Therefore, salinity tolerance of a species is an important consideration in the culture of marine and freshwater organisms as it provides information on basic environmental requirements essential for the species to thrive in captivity.
McCormick and Bradshaw (2006) investigated the osmoregulatory functionality of fish that migrate between freshwater and seawater during their life cycle or living in estuaries. Economic considerations on the culture of marine or brackish water species show that low-saline culture could be an attractive alternative to that of freshwater. Studies on captive production and salinity tolerance are not abundant in the clownfish Amphiprion akallopisos. Therefore, the tropical reef dwelling skunk clownfish, belonging to the family Pomacentridae, has been selected for conducting this study. It is expected to provide important information on the development of different life stages of the skunk clownfish that will enhance the captive production technology leading to a sustainable production of this species for the aquarium fish trade.
2 Materials and methods
2.1 Broodstock maintenance and spawning
Skunk clownfish A. akallopisos (TL = 5 ± 0.5 cm, n = 6) and host sea anemones Stichodactyla mertensii (n = 3) were procured from ornamental fish traders in Chennai. These were transported to the hatchery facilities located in the Centre of Advanced Study in Marine Biology of Annamalai University, Tamil Nadu (India). Along with the sea anemones, these fish were acclimated to captive conditions in a cement tank (capacity, 3 ton) for one month. Pair formation took place during this period and pairs were transferred into individual 400 L fibre glass tanks (1.2 × 0.6 × 0.6 M). Interior of these tanks were grey in colour and were initially filled with UV treated estuarine water. A locally made underwater filter was fixed in each of these tanks.
Shrimp, boiled oyster and clam meat were used to feed the fish and sea anemones three times a day at 08:00, 12:00 and 16:00 h. Excretory material and remnant food particles were siphoned out an hour after the feeding. The tanks were illuminated with a 40 W fluorescent tube suspended 45 cm above the water surface. Water quality parameters in the tanks were maintained as temperature 27 ± 1 °C, salinity 25 ± 1 ppt, pH 8 ± 0.2 and dissolved oxygen 6.5 ± 0.3 mg l−1. Light intensity of 800 lux was maintained for 12 h (07:00–19:00 h). Once a week the tanks were given 50% water change. Ceramic tiles and live rocks were provided as spawning substrates. Fish started spawning after 3 months of rearing in the spawning tank. At the time of the first spawning, the male and the female had an average total length of 5.5 and 6.2 cm, respectively. The batch fecundity was estimated by counting the eggs in 1 cm2 and then multiplying with the total area of deposition (Satheesh, 2002).
2.2 Embryonic development
Random samples of 5 eggs were taken at a time with the aid of an ink-filler. Initially egg was taken immediately after fertilization and subsequently, embryonic development stages were observed under a light microscope (Novex, Holland). These were then sequenced based on morphological features and photographed using a digital camera (Cannon, China). The length and width of ova were measured using an ocular micrometer (Erma, Tokyo). The main morphological and functional features of each developmental stage were recorded. The eggs were further monitored in order to study the progress of embryonic development. The onset of cleavage, blastulation, gastrulation, organogenesis, embryo, pre-hatching and larval stage were taken as definitive precincts of development. The time lapsed for 50% of the sampled embryos to attain the above stages were recorded. Milky white eggs, which were considered dead, were removed. These developmental stages were compared with previous studies conducted with other species (Gorshkova et al., 2002; Tachihara and Kawaguchi, 2003; Arezo et al., 2005; Chen, 2005; Dhaneesh et al., 2009).
2.3 Larval rearing
The hatched out larvae were gently collected with the use of a beaker and transferred to white inner coloured oval shaped fibre glass tanks (capacity, 100 L). The stocking density was 3 larvae l−1 of water. Rotifers (Brachionus plicatilis) enriched with algae (Nannochloropsis salina) were added to the larval tank at a density of 6–8 rotifers ml−1 as the initial feed. This was carried out 3 times a day (10:00, 13:00 and 16:00 h) for the first 10 days of initial feeding. The rotifers were substituted with freshly hatched Artemia nauplii at a density of 3–5 nauplii ml−1 starting from the 11th day of feeding. Gentle aeration of the water was provided in order to maintain a satisfactory level of dissolved oxygen and to achieve a homogeneous distribution of the added live feed. The tank bottoms were cleaned and 10% of the tank water was replaced on a daily basis without disturbing the fry needlessly.
Boiled and finely chopped oyster meat was used to feed the growing fry from the 25th day of feeding. Water quality parameters were monitored daily (temperature 27 ± 1 °C, salinity 25 ± 1 ppt, pH 8 ± 0.2 and the dissolved oxygen 6.2 ± 0.2 mg l−1). A twelve hour photoperiod was provided at an intensity of 600 lux (12L–12D).
2.4 Salinity tolerance of larvae
The current study tested the effect of five different levels of salinities on tolerance of the larvae. Two sets of larval rearing tanks were filled with water having salinities of 20, 25, 30, 35, and 40 ppt in each set. Twenty five larvae at the age of 10 days after hatching were introduced into each tank. The set of tanks with a salinity of 30 ppt were taken as the control. Depending on the density of larvae in the rearing tanks, they were fed with freshly hatched nauplii of Artemia at 3–5 nauplii ml−1. The mortality and water quality parameters were recorded daily and a photoperiod of 12 h was maintained.
Temperature was measured using a mercury thermometer with an accuracy of 0.5 °C. A hand-held refractometer (Atago, Japan) was used to measure the salinity and a digital pH pen (Eutech, Singapore) to measure the pH. A Lux meter (Lutron, LX-101, Taiwan) was used to measure the light intensity. The changes in salinity were achieved by mixing different amounts of RO water and concentrated brine solution made by dissolving NaCl in fresh water. Approximately 20% of the rearing water was replenished on a daily basis. Final readings were taken upon reaching total mortality or at the end of the 96 h exposure period. The percentages of larval survival were plotted against the respective values in salinity. The salinity of broodstock and mass scale larval rearing tanks continued to be maintained at 24–26 ppt. But for experiments, open air cement tanks were used for water storage to maintain the larvae at 30 ppt as the control.
2.5 Salinity tolerance of juveniles
The experiments on the salinity tolerance of juveniles were carried out to find both their minimum and maximum tolerance levels of salinity. Twenty juveniles of 30 days of age after hatching having an average total length of 120 mm and an average weight of 60 mg were introduced into two glass tanks in duplicate. Each tank contained 20 L of water at a salinity of 30 ppt. In one set of tanks the salinity was gradually reduced at a rate of 1 ppt every 12 h by adding water from a reverse osmosis (RO) filter. In the other set of tanks the salinity was gradually increased at a rate of 1 ppt every 12 h by adding concentrated brine solution. The salinity changes were started at 07:00 and 19:00 h until 100% mortality occurred in the tanks. Tanks were aerated to assure uniform mixing of water. The readings were taken after the completion to reaffirm that the desired salinities had been attained in the tanks using a calibrated hand-held refractometer (±1 ppt).
Prior to the commencement of the salinity tolerance test, 5 juveniles were randomly taken from the rearing tank and weighed on an electronic balance (Denver, USA) after drying them with water absorbent paper. A metre scale was used to measure their lengths. Fish were fed with boiled oyster meat thrice daily at 09:00, 13:00 and 17:00 h during the experiment. Feeding commenced the day after they were introduced into the experiment tank. The tank bottoms were cleaned by siphoning the faecal matter and remnant feed particles 2 h after each feeding. An exchange of 1/3 of the tank water was given to maintain water quality. Aeration was provided to maintain the dissolved oxygen at satisfactory levels. Dead juveniles, if any, were removed immediately and the mortality recorded on a daily basis. Temperature, dissolved oxygen (DO) levels, pH and light intensity were also monitored and recorded daily. An ambient photoperiod of 12L–12D was maintained during the experiment.
2.6 Statistical analysis
The parameters were subjected to analysis of variance (ANOVA) and correlation by using a statistical software PAST (Ver. 1.89).
3 Results and discussions
3.1 Spawning and embryonic development
The yolk of clownfish is coloured brightly as they are tinted with the parent colour pigments. Wilkerson reported that the eggs of Amphiprion clarkii, A. perideraion and Premnas biaculeatus are yellowish-orange, pinkish and red, respectively (Wilkerson, 2001). However, in the present study, the colour of newly laid eggs of A. akallopisos was found to be pale white in colour (Fig. 1).
A. akallopisos with egg clutch.
The abdomen of the female became swollen prior to spawning. Spawning took place mostly during the morning; between 09:00 and 13:00 h. Females laid capsule-shaped eggs on the cleaned substratum in almost circular or oval patches and subsequently the male fertilized the eggs. The spawning activity lasted from an hour to an hour and forty five minutes. The eggs are adhesive and are covered with a transparent chorion and a narrow perivitalline space. The eggs measured 2.0–2.1 mm in length and 0.9–1.0 mm in width. Approximately 300–400 eggs were spawned at a time. The number of eggs gradually increased in subsequent spawning. One end of the egg capsule identified as the animal pole contained some gelatinous substance to adhere itself to the substratum.
Eggs are white in colour at the time of spawning and may get darker with time. The yolk and comparatively large fat globules are visible. The fertilized eggs hatched within 7–9 days generally between 19:00 and 22:00 h. A major role in parental care was played by the male during incubation. This mainly involves fanning and mouthing the eggs. Fanning was done by flurrying the pectoral fins, which created mild water current over the egg clutch. The unfertilized, dead eggs and dust particles were also removed during the process of mouthing.
The embryonic development of the skunk clownfish, A. akallopisos was classified in to 26 stages (Table 1) (Fig. 2a–z). During the embryonic development, the organism is entirely dependent on the nutrition provided by the mother, mainly by the form of yolk. The period of embryonic development begins at the time of fertilization and can be divided into 2 phases. The first phase is the egg cleavage phase; the interval between the first cell division and the appearance of recognizable precursors of the organ systems, namely, the neural plate. The second or the embryo phase begins when the embryo becomes recognizable as a vertebrate. According to Jobling (2002) and Moyle and Cech (2004), the embryonic period is the phase between fertilization and commencement of organogenesis. The contour of eggs in different species of Pomacentrid fish varies from oval to capsule shape (Moyer and Nakazono, 1978; Pathiyasevee, 1994). Hoff (1996) reported that the length of clownfish eggs ranged from 2.0 to 2.4 mm. The measurements of egg size in some clownfish are given in Table 2.
Stages
Figure no.
Hours after fertilization
Observations
1
2a
0
Cytoplasm is clear with a large fat globule at the centre, un-cleaved cell, animal pole is of semi circle shape and the vegetal pole contains yolk
2
2b
1
During 1st cleavage, blastodisc divides into two blastomeres. The fat globules located at the vegetal pole had become smaller in size
3
2c
1:45 min
Fat globules and four blastomeres of smaller and equal size appeared in second cleavage which took place perpendicular to the first cleavage
4
2d
2:25 min
Eight blastomeres of smaller and equal sizes appeared
5
2e
4
Sixteen blastomeres at animal pole and fat globules at the vegetal pole could be seen
6
2f
4:55 min
Blastomeres that overlap each are other present within the capsule
7
2g
5:15 min
Blastomeres spread laterally and seen as a flat layer
8
2h
6:30 min
Blastomeres were smaller and equal size
9
2i
8
Blastomeres were very small in size and fat globules could be seen at the vegetal pole
10
2j
8:55 min
Blastomeres were still in small size and the embryo had reached the morula stage
11
2k
18:35 min
Blastomeres extended towards the vegetal pole marking the gastrula stage. The number of fat globules had decreased
12
2l
21:50 min
Fat globules had started to disappear. The blastomeres moved further towards the vegetal pole to cover the epibolical part of the yolk
13
2m
27:35 min
Head and neural ectoderm were formed. The outer layer of the embryo formed longitudinal ridges close to the yolk
14
2n
30:35 min
The entire body of the embryo covered with melanophores. Primitive optic buds and longitudinal neural tube had formed
15
2o
34:10 min
The neural tube and head could be clearly seen. The body was transparent due to the absence of the muscular structure
16
2p
42:15 min
Melanophores were increased in the head region. The embryo completely turned itself. The body was attached to the yolk sac with tail movement
17
2q
48:25 min
Pink coloured heart had begun to beat and the eye lens was visible. The freely moved tail detached from the yolk and the body was still attached to the yolk
18
2r
55:05 min
The mouth, eye cup and the lens had become evident. Yolk volume was reduced and the pigments on the head region had increased. The body length had increased distinctly. Blood circulation could be observed
19
2s
64:20 min
The head and tail of the embryo had distinctly separated from the yolk. The large eyes with brown pigments. High pigmentation was in the head but less in the tail region. The caudal and anal fins started to develop
20
2t
75:35 min
The head of the embryo increased in size showing prominent eyes and brown pigmentation. Yolk was covered by the developing body and embryo was growing larger while the yolk was decreasing
21
2u
98:30 min
The melanophores were abundant in the head region. The size of the embryo was increased and shown continuous movement
22
2v
109:25 min
The head of the embryo occupied one third of the capsule space. Fins and eyes were well developed. The yolk sac became quite small
23
2w
119:55 min
The fins have fully developed and clearly visible. The embryo showed vigorous movement inside the egg capsule
24
2x
127:15 min
Capsule fully occupied by the embryo
25
2y
151:05 min
The eyes were seen shining and turning. The embryo tried to hatch out
26
2z
152:10 min
The embryo entered the larval stage. The dorsal, caudal and anal fins were continuous on a longitudinal line. The larva has a total length of about 3.1 mm and 300–400 μm mouth size
![(a–z) Embryonic development and newly hatched larva of A. akallopisos [A: anus, Af: anal fin, Ap: animal pole, B: blastomeres, C: cytoplasm, Cf: caudal fin, E: eye, El: eye lens, Ep: epiboly, G: gastrula, H: head, Ht: heart, M: mouth, Mp: melanin pigments, Mr: morula, Nc: notochord, Nt: neural tube, Ob: optic buds, Og: oil globule, T: tail, Vp: vegital pole and Y: yolk].](/content/185/2012/24/3/img/10.1016_j.jksus.2011.03.005-fig2.png)
(a–z) Embryonic development and newly hatched larva of A. akallopisos [A: anus, Af: anal fin, Ap: animal pole, B: blastomeres, C: cytoplasm, Cf: caudal fin, E: eye, El: eye lens, Ep: epiboly, G: gastrula, H: head, Ht: heart, M: mouth, Mp: melanin pigments, Mr: morula, Nc: notochord, Nt: neural tube, Ob: optic buds, Og: oil globule, T: tail, Vp: vegital pole and Y: yolk].
Species
Measurements (mm)
References
Amphiprion chrysopterus
2.4 × 0.9
Allen (1980)
A. ocellaris
1.5–3.0 × 0.8–1.84
Madhu et al. (2006a)
Premnas biaculeatus
2.8–3.5 × 1.1–1.7
Madhu et al. (2006b)
A. percula
2.0–2.3 × 1.0–1.2
Dhaneesh et al. (2009)
The development rate of fertilized eggs varies depending on ambient temperature and dissolved oxygen content of water (Mariscal, 1970). In the present study, hatching occurred 152 h and 10 min after fertilization. The cleavage pattern of A. akallopisos is as same as of the other anemonefish (Rattanayuvakorn et al., 2005; Dhaneesh et al., 2009). In A. akallopisos, the yolk had different sized fat globules dispersed in the vegetal pole, as reported in A. polymnus (Ross, 1978). There are variations in time and stages of embryonic development among different genus and species of fishes.
3.2 Salinity tolerance of larvae and juveniles
In the present study, survival of 10 days post hatched (10 DPH) A. akallopisos larvae was 100% at the salinities of 30 (control) and 35 ppt throughout the entire 96 h experimental period. At a salinity of 40 ppt, the survival rate by the end of the fourth day was 88%. The larvae reared in 20 and 25 ppt had shown 76% survival (Fig. 3).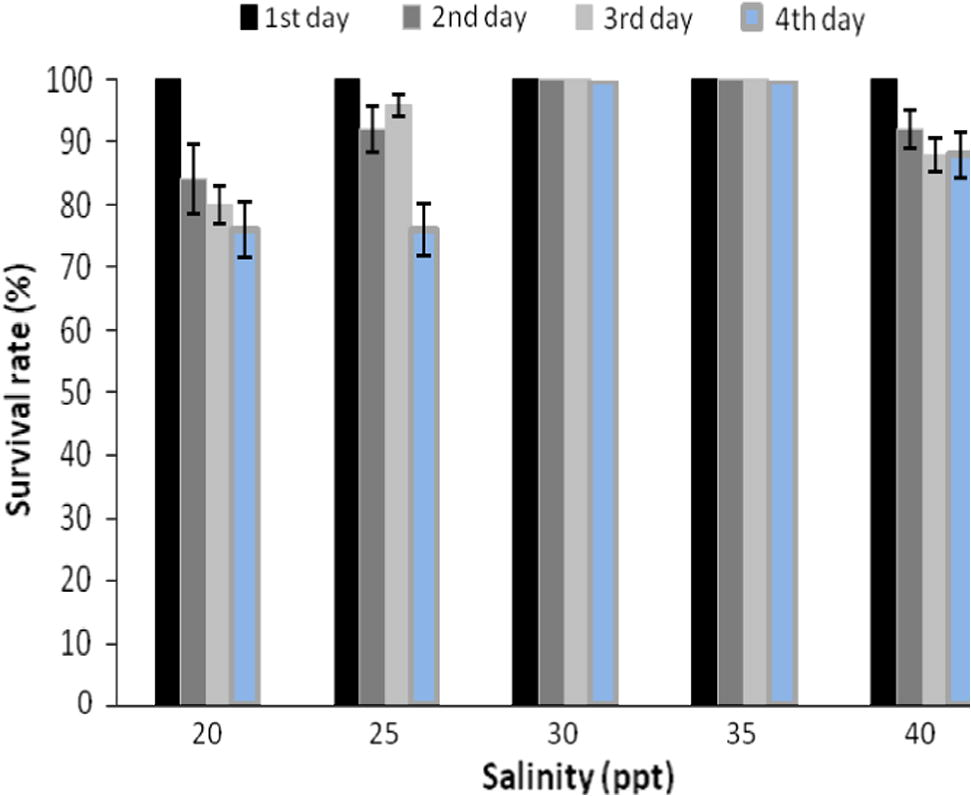
Survival (%) of larvae (10 DPH) at 96 h exposure period to different salinities.
The survival rate of juveniles (30 DPH) reared under different salinities is illustrated in Fig. 4. No mortality was observed when the salinity was brought down to 6 ppt from an initial rearing salinity of 30 ppt. The first mortalities were recorded when the salinity reduced to 5 ppt. Hundred percent mortality was observed when the salinity was brought down to 3 ppt. This showed that the lower tolerable limit of salinity for juveniles of A. akallopisos is at 6 ppt.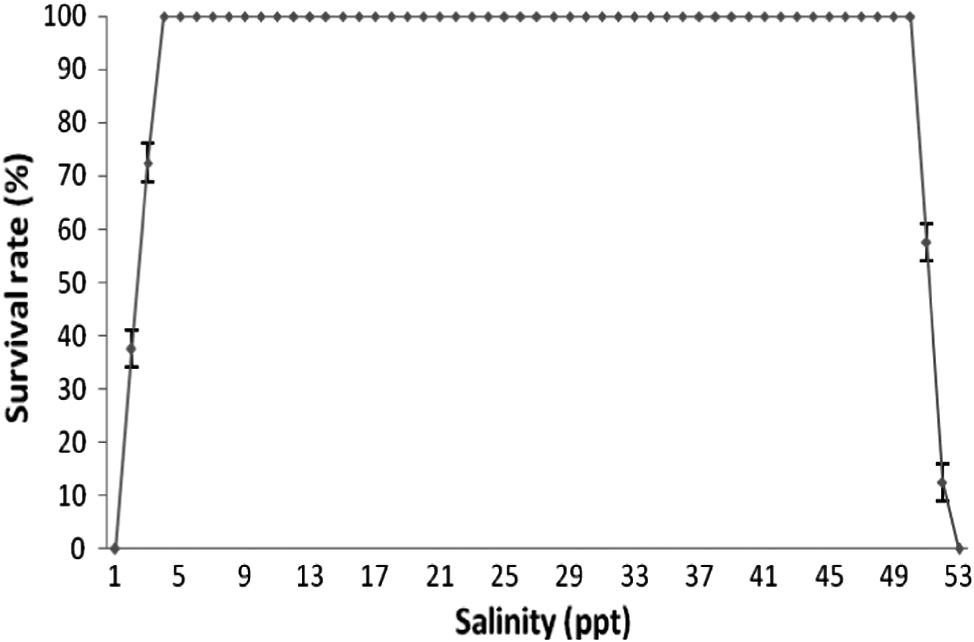
Survival (%) of juveniles (30 DPH) during the salinity tolerance experiment.
In the case of increased salinity, initial mortalities were observed when the salinity increased to 53 ppt. Hundred percent mortality occurred when the salinity reached 55 ppt. Based on 100% mortality as an index for salinity intolerance, it can be safely assumed that the juveniles of A. akallopisos can tolerate a salinity not exceeding 52 ppt. The important symptoms seen in samples taken from the non tolerated hypo and hyper salinity waters were loss of opercular movement and absence of response to external stimuli. Further, the fish showed symptoms of physical weakness, sluggish movements and inappetance.
The experiment for determining the upper lethal level of salinity for the group with an LC-50 of 53 ppt lasted for 13 days. A lethal salinity of 55 ppt was reached within this period resulting in 100% mortality. The experiment for determining the lower lethal level of salinity lasted for 14 days. A lethal saline level of 3 ppt was reached during this period which also resulted in 100% mortality. Biologically significant differences among treatments in the measured water quality parameters were not observed throughout the course of this experiment [Water temperature 27 ± 1 °C, pH 8 ± 0.2, dissolved oxygen 5.8 ± 0.2 mg l−1 and ambient photoperiod (12L–12D) at an intensity of 600 lux]. A positive correlation between the values of the length and weight were observed in each batch taken for the experiment. Differences between the lengths and weights of the treatment groups were significant (F3,16 = 0.023, P < 0.01; F3,16=0.21, P < 0.01 respectively).
The skunk clownfish A. akallopisos is a typical coral reef fish commonly found in Indo-Pacific Ocean, which lives within a salinity range of 33–35. Salinity adaptation studies on clownfish are limited. However, a number of studies on the effect of salinity on growth and survival of a variety of other fish species have been done. Brett mentioned that the higher growth rates of the various species considered clustered around freshwater, 10 ± 2, or 28–35 salinity (Brett, 1979). In the present study, the larvae (10 DPH) showed moderately high survival rate within a wide salinity range of 30–40 when compared with that of 20–25.
Investigations into the salinity tolerance of freshwater species have been predominantly motivated by ecological concerns. The studies made by Bringolf et al. (2005) and Schofield et al. (2006) assessed salinity tolerance as barriers to invasion for the flathead catfish Pylodictis olivaris and Goldfish Carassius auratus, respectively. DiMaggio et al. (2009) studied the evaluation of seminole killifish Fundulus seminolis as a candidate for aquaculture. Killifishes (Cyprinodontiformes) are particularly interesting because of their ability to acclimate to different saline environments and represent useful models for biological research. Osmosensitivity and salinity detection are of high physiological interest. Fish have prolactin (PRL) cells which are directly osmosensitive (Grau et al., 1994). They also possess chemoreceptors, situated in the pseudobranch that are connected to the central nervous system (CNS), providing information on water salinity (Laurent and Dunel-Erb, 1984). These participate in triggering the water drinking mechanism in seawater fish. Many authors have carried out research on the influence of water salinity on fish development (Boeuf and Payan, 2001).
Most information on osmoregulatory physiology of teleost fishes is based on the euryhaline mechanism. Fish are able to regulate the quantity of plasma ions. The relatively low salinity tolerance of African catfish Heterobranchus longifilis has probably prevented its incursion to more saline waters within its natural distribution. The growth and survival potential of H. longifilis in salinity up to 7.5 facilitates the prevention and treatment of most freshwater ectoparasites.
Effects of salinity on osmoregulatory processes and its impact on growth and feed conversion efficiency of fish have been studied in estuarine (euryhaline) species that are well adapted to variations in salinity. Minimum information is available on the tolerance of stenohaline freshwater fish to increased salinity. Research on low salinity aquaculture of marine species is common, but very few studies have been conducted on acclimation of freshwater species to seawater. Experiments examining abrupt transfer of black sea bass Centropristis striata to low salinities have helped to identify a salinity threshold for the successful culture of this species (Young et al., 2006). Similarly, gradual acclimation experiments with Nile tilapia Oreochromis niloticus and blackchin tilapia Sarotherodon melanotheron (Lemarie et al., 2004) and larval salinity tolerance experiments with striped mullet Mugil cephalus, thick-lipped grey mullet Chelon labrosus (Hotos and Vlahos, 1998) and cobia Rachycentron canadum (Faulk and Holt, 2006) have provided valuable information regarding the osmoregulatory ability of a species for use under conventional aquaculture conditions.
Many reports are available on marine and euryhaline species that have shown a faster rate of growth when reared in brackish water (Boeuf and Payan, 2001). It is commonly attributed to the lower energy cost of osmoregulation. Furthermore, it has also been reported that when marine teleosts (Atlantic cod Gadus morhua and turbot Scophthalmus maximus), were raised in environments that are iso-osmotic with plasma, the food conversion efficiency and the growth rate have shown an increase (Imsland et al., 2001). These assertions are also correlated to the findings of the present study. In particular growth performance studies with long-term rearing, from brackish waters up to the upper salinity tolerance levels are wanting.
Boeuf and Payan (2001) concluded in their studies that the marine fish show higher development or growth rates at lower salinities and freshwater fish at higher salinities. This is often, but not systematically, correlated with a lower standard metabolic rate. Future experiments on salinity tolerance of clownfish in combination with feed intake, growth and reproduction would be helpful in optimizing the salinity for aquaculture purposes.
3.3 Mass scale larval rearing
Oval shaped fibre glass tanks (capacity, 100 L) with a white coloured interior were used for mass scale larval rearing. These were filled with 60 L of water and the newly hatched larvae were transferred in to them at a density of 3 larvae per litre of culture water. Continuous aeration was provided using a mini air blower to maintain a DO level between 5.6 and 6.5 mg of O2 l−1. Initially the larvae were fed with rotifers B. plicatilis (6–8 rotifers ml−1) enriched with algae N. salina for a period of 10 days. After this period the larvae accepted freshly hatched Artemia nauplii (3–5 nauplii ml−1). At 25 days post hatch (25 DPH) they were fed with finely chopped boiled Oyster meat. Feeding at larval and juvenile stages was done 3 times a day (10:00, 13:00 and 16:00 h). The eventual survival rates were calculated and shown in Fig. 5. The larvae started attaining the colours of the parents by the 15–16 day after hatching out. First two weeks they were pelagic, but gradually moved to the bottom after metamorphosis. They reached a marketable size of 3 cm within 3 months of rearing (Fig. 6).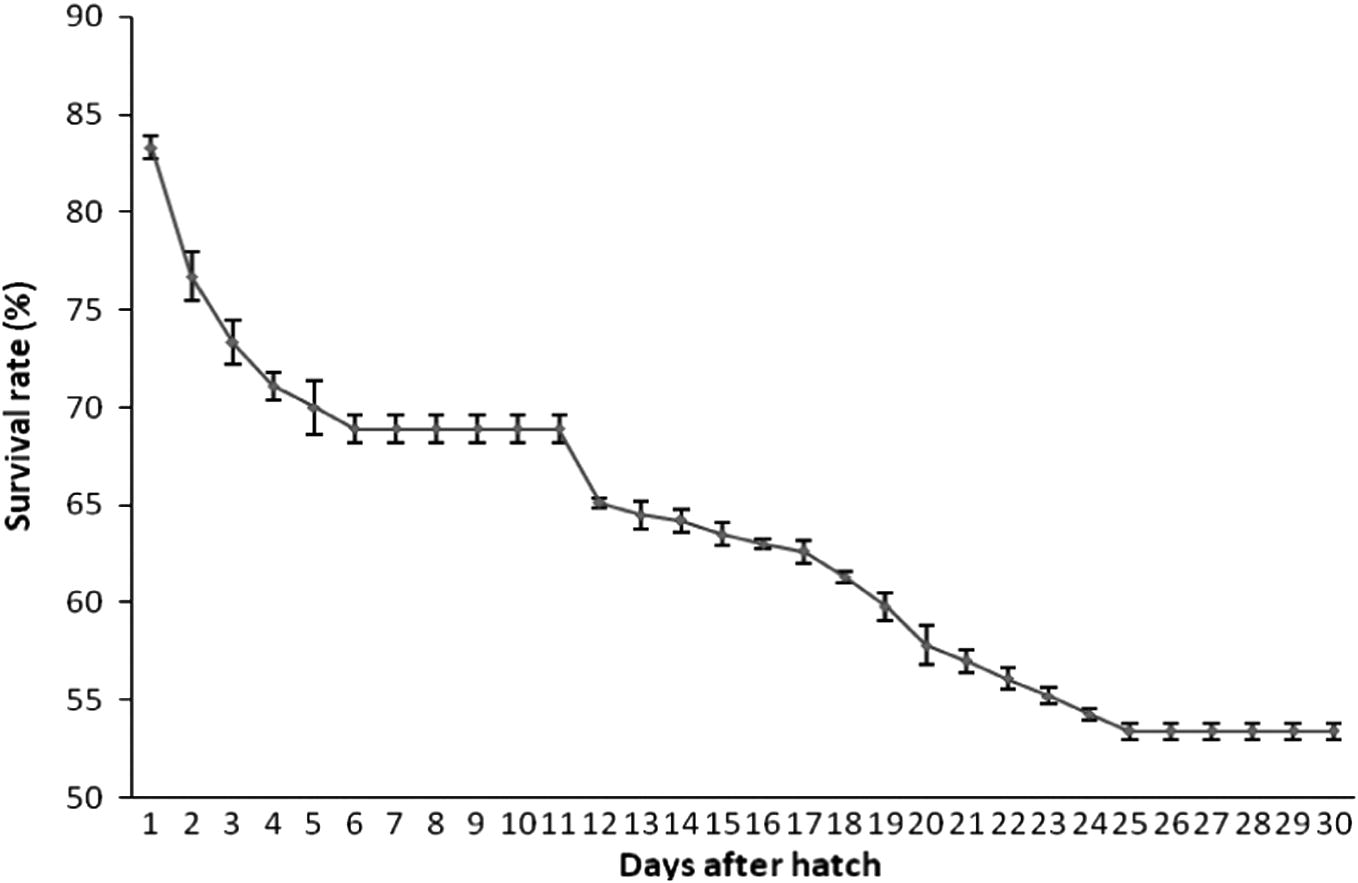
Survival rate of A. akallopisos during scaling up.

Three month old juveniles of A. akallopisos.
Many studies have been carried out with the aim of developing breeding and rearing methodology for marine aquarium ornamentals that are in demand for the trade. The delicate reef ecosystem, from where these species are now being collected, can be preserved by developing sustainable production methodologies for these varieties. Some interesting studies of this nature have been done (Ignatius et al., 2001; Ajith Kumar and Balasubramanian, 2009; Ajith Kumar et al., 2010). However, the present study is one of the first successful attempts on broodstock development, breeding and larval rearing of skunk clownfish A. akallopisos in India. The factors as water quality requirements, nutrition, feeding and appropriate photoperiod that influences the successful hatchery production of this species have been standardized through this study.
Maintaining good water quality was the major challenge in the present study. Exchange of water during larval rearing was imperative and resulted in better survival of larvae. Maintenance of light and dark period is an important factor during larval rearing. Insufficient photoperiod may result in ‘head butting syndrome’ and starvation, which may lead to the death of larvae ultimately. However, 10–20% of larval mortality was observed during 1st and 2nd days after hatching and it was unavoidable. This mortality may be due to the stress and injury caused during larval transfer from the parent tank to the larval rearing tank or due to difficulties in accepting the first feed. Mortalities were also observed during the time when larval feed was changed from rotifers to Artemia. It was also observed that un-hatched Artemia cysts blocked the digestive tract of larvae and interfered with the digestive process. The main infection faced by juveniles during rearing was the marine white spot disease. The treatment given was fresh water dip for 1 min with vigorous aeration.
In the present study, the completion of metamorphosis of A. akallopisos took 15–16 days. This value is within the range of earlier work done with different species of clownfish. Madhu et al. (2006a) recorded the metamorphosis of A. ocellaris at 9–10 days, 12–15 days in A. sebae (Ignatius et al., 2001), 11–12 days in Premnas biaculeatus (Madhu et al., 2000b), 12–15 days in A. chrysogaster (Gopakumar et al., 2001) and 13–15 days in A. percula (Dhaneesh 2009). It is important to note that this study has established the fact that low salinity does not have a major negative effect on the survival or metamorphosis of the species under study. Therefore, the culture technology can be adopted by entrepreneurs based in regions of estuaries, mangroves and back waters.
4 Conclusion
New descriptive investigations into the embryonic development, larval rearing and salinity tolerance should provide valuable information about the ability of the species to handle osmoregulatory stressors and can be extrapolated to many useful ecological applications. In spite of the limitations, the embryonic development protocol, mass scale rearing and salinity tolerance described herein should provide a base for future studies on clownfish and help in achieving conservation and commercial goals.
Acknowledgements
The authors are thankful to the authorities of Annamalai University for the facilities and the Centre for Marine Living Resources and Ecology (Ministry of Earth Sciences), Kochi for financial assistance.
References
- Broodstock development, spawning and larval rearing of the false clownfish, Amphiprion ocellaris in captivity using estuarine water. Curr. Sci.. 2009;97(10):1483-1486.
- [Google Scholar]
- Studies on captive breeding and larval rearing of clownfish Amphiprion sebae (Bleeker 1853) using estuarine water. Indian J. Mar. Sci.. 2010;39(1):114-119.
- [Google Scholar]
- Anemone Fishes of the World: Species, Care and Breeding. Mentor, OH, USA: Aquarium Systems; 1980.
- Some effects of salinity and temperature on early development and survival of the English sole (Parophrys vetulus) J. Fish. Res. Board. Can.. 1968;25:495-521.
- [Google Scholar]
- Early development in the annual fish Cynolebias viarius. J. Fish. Biol.. 2005;66:1357-1370.
- [Google Scholar]
- How should salinity influence fish growth? Comp. Biochem. Phys. C. 2001;130:411-423.
- [Google Scholar]
- Environmental factors and growth. In: Hoar W.S., Randall D.J., Brett J.R., eds. Fish Physiology. Vol vol. 8. New York: Academic Press; 1979. p. :599-675.
- [Google Scholar]
- Salinity tolerance of the Flathead Catfish: implications for dispersal of introduced populations. Trans. Am. Fish. Soc.. 2005;134:927-936.
- [Google Scholar]
- Induced ovulation and embryonic development of ocellated puffer Takigugu ocellatus. J. Appl. Ichthyol.. 2005;21:136-140.
- [Google Scholar]
- Dhaneesh, K.V., 2009. Broodstock management breeding and larval rearing of clownfish Amphiprion percula (Lacepede 1802) in captivity. Dissertation Annamalai University, Tamil Nadu, India.
- Embryonic Development of Percula Clownfish Amphiprion percula (Lacepede 1802) Middle-East J. Sci. Res.. 2009;4(2):84-89.
- [Google Scholar]
- Salinity tolerance of the Seminole killifish Fundulus seminolis a candidate species for marine baitfish aquaculture. Aquaculture. 2009;293:74-80.
- [Google Scholar]
- Comparative organ differentiation during early life stages of marine fish. Fish Shellfish Immun.. 2005;19:397-412.
- [Google Scholar]
- Responses of cobia Rachycentron canadum larvae to abrupt or gradual changes in salinity. Aquaculture. 2006;254:275-283.
- [Google Scholar]
- Hatchery production of the clownfish Amphiprion chrysogaster. In: Menon N.G., Pillai P.P., eds. Perspective in Mariculture. India: The Marine Biological Association of India; 2001. p. :305-310.
- [Google Scholar]
- Cytogenetic examination of early embryonic development in the white grouper Epinephelus aeneus (Pisces, Serranidae) J. Appl. Ichthyol.. 2002;18:29-34.
- [Google Scholar]
- Osmoreception and a simple endocrine reflex of the prolactin cell of the tilapia Oreochromis mossambicus. Perspect. Comp. Endocrinol. 1994:251-256.
- [Google Scholar]
- International trade in marine aquarium species: using the global marine aquarium database. In: Cato J., Brown C., eds. Marine Ornamental Species: Collection Culture and Conservation. Ames, USA: Iowa State Press; 2003. p. :31-48.
- [Google Scholar]
- Hoff, F.H., 1996. Conditioning Spawning and Rearing of Fish with Emphasis on Marine Clown Fish. Aquaculture Consultants.
- Effect of salinity on the eggs and larvae of Teleosts. In: Hoar W.S., Randall D.J., eds. Fish Physiology. Vol vol. 1. New York: Academic Press; 1969. p. :293-311.
- [Google Scholar]
- Salinity tolerance of Mugil cephalus and Chelon labrosus (Pisces: Mugilidae) fry in experimental conditions. Aquaculture. 1998;167:329-338.
- [Google Scholar]
- Early life stages of farmed fish. In: Black K.D., Pickering A.D., eds. Biology of Farmed Fish. UK: Sheffield Academic Press; 1998. p. :27-66.
- [Google Scholar]
- Spawning and larval rearing technique for tropical clownfish Amphiprion sebae under captive conditions. J. Aquacult. Trop.. 2001;16(3):241-249.
- [Google Scholar]
- Interaction of temperature and salinity on growth food conversion in juvenile turbot Scophthalmus maximus. Aquaculture. 2001;198:353-367.
- [Google Scholar]
- Environmental factors and rates of development and growth. In: Hart P.J.B., Reynolds J.D., eds. Handbook of Fish Biology and Fisheries. Vol vol. 1. Fish Biology Blackwell Science Ltd. Blackwell Publishing Company; 2002.
- [Google Scholar]
- The pseudobranch: morphology and function. In: Hoar W.S., ed. Fish Physiology. Vol 10B. New York: Academic Press; 1984. p. :285-320.
- [Google Scholar]
- A simple test to estimate the salinity resistance of fish with specific application to O. Niloticus and S. melanotheron. Aquaculture. 2004;240:575-587.
- [Google Scholar]
- Safety and efficacy of the environmental Products Group Master flow aquarium management system with Aegis Microbe Shield T.M. Aquacult. Eng.. 1999;19:93-98.
- [Google Scholar]
- Spawning and larval rearing of Amphiprion ocellaris under captive conditions. Mar. Fish. Infor. Serv. T. E. Ser.. 2006;188:1-5.
- [Google Scholar]
- Breeding larval rearing and seed production of maroon clown Premnas biaculeatus under captive conditions. Mar. Fish. Infor. Serv. T. E. Ser.. 2006;190:1-5.
- [Google Scholar]
- The nature of symbiosis between Indo-Pacific anemonefishes and sea anemones. Mar. Biol.. 1970;6:58-65.
- [Google Scholar]
- Hormonal control of salt and water balance in vertebrates. Gen. Comp. Endocr.. 2006;147:3-8.
- [Google Scholar]
- Protandrous hermaphroditsm in six species of the anemone fish genus Amphiprion in Japan. Jap. J. Ichthyol.. 1978;25:101-106.
- [Google Scholar]
- Moyle, P.B., Cech, J.J.J.R., 2004. Fishes: an introduction to ichthyology, 5th edn. Pearson Prentice-Hall Inc. Upper Saddle River, NJ.
- Pathiyasevee, U., 1994. Egg-laying behaviour and growth of false clown anemonefish Amphiprion ocellaris (Cuvier 1830). Annual seminar on 1994. National Institute of Coastal Aquaculture Department of Fisheries Bangkok Thailand, pp. 393–412.
- Embryonic Development of Saddleback anemonefish Amphiprion polymnus (Linnaeus 1758) Kasetsart. J. (Nat. Sci).. 2005;39:455-463.
- [Google Scholar]
- Territorial behaviour and ecology of the anemone fish Amphiprion melanopus on Guam. Z. Tierpsychol.. 1978;46:71-83.
- [Google Scholar]
- Satheesh, J.M., 2002. Biology of the clownfish Amphiprion sebae (Bleeker) from Gulf of Mannar (South east coast of India). Dissertation, Annamalai University India.
- Salinity tolerance of goldfish Carassius auratus L. A non-native fish in the United States. Florida Scientist. 2006;69(4):258-268.
- [Google Scholar]
- Morphological development of eggs larvae and juveniles of laboratory-reared Ryukyu-ayu Plecoglossus altivelis ryukyuensis. Fisheries Sci.. 2003;69:323-330.
- [Google Scholar]
- Tissera, K., 2010. Global trade in ornamental fishes. Souvenir Ornamentals Kerala-2010 Dept. of Fisheries Govt. of Kerala, pp. 35–38.
- Wilkerson, D.J., 2001. Clownfishes. A guide to their captive care breeding and natural history. Microcosm Shelburne Vermont.
- Survival and water balance of black sea bass held in a range of salinities and calcium-enhanced environments after abrupt salinity change. Aquaculture. 2006;258:646-649.
- [Google Scholar]







