Translate this page into:
Carbamazepine (CBZ) induced enzymatic stress in gill, liver and muscle of a common carp, Cyprinus carpio
*Corresponding author. Tel.: +91 422 2428493; fax: +91 422 2422387 mathanramesh@yahoo.com (Mathan Ramesh)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Available online 9 January 2011
Abstract
Toxicity and effects of an antiepileptic drug, carbamazepine (CBZ) on transaminases like glutamate oxaloacetate transaminase (GOT) and glutamate pyruvate transaminase (GPT); lactate dehydrogenase (LDH) activities in gill, liver and muscle of a freshwater fish, Cyprinus carpio were investigated. The median lethal concentration (LC50) of CBZ to C. carpio for 24 h was determined (59.70 mg l−l). 1/10th of LC50 value was taken as a sublethal concentration (5.97 mg l−l). Fish were exposed to both acute and sublethal CBZ concentration for 24 h and 35 days (at weekly intervals), respectively. During acute treatment, GOT activity was decreased in all the organs (gill, liver and muscle); GPT and LDH activities were increased in liver and muscle while decreased in gill. During sublethal treatment, GOT activity was decreased in liver and muscle, whereas GPT activity was increased in these two organs. A biphasic trend was noted in GOT and GPT activity in gill and LDH activity in gill, liver and muscle. The present study indicates that CBZ induced alterations in the activities of GOT, GPT and LDH in various organs of fish; these enzymes may be used as logical candidates to monitor the toxic levels of pharmaceuticals in aquatic organisms.
Keywords
Cyprinus carpio
Transaminases
LDH
Carbamazepine
Acute
Sublethal toxicity
1 Introduction
The presence of pharmaceuticals and personal care products (PPCPs) in the aquatic ecosystem has emerged as a serious concern due to their rapid growing and unregulated disposal practices (Richardson and Bowron, 1985; Daughton and Ternes, 1999; Zuccato et al., 2005; Laura Martín-Díaz et al., 2009). These aquatic micropollutants disturb the ecological balance and attracted the public as well as scientific community (Halling-Sørensen et al., 1998; Yamamoto et al., 2009). As a result, a number of investigations have been reported on widespread occurrence of pharmaceuticals in the aquatic environment (Ternes, 1998; Gomez et al., 2007; Letzel et al., 2009).
To date, more than 100 pharmaceutical compounds (anti-inflammatory, beta-blockers, sympathomimetics, antiepileptics, lipid regulators, antibiotics, etc.) have been reported in sewage, rivers and creeks, seawater, surface water, groundwater and drinking water resources throughout the world (Fent et al., 2006; Choi et al., 2008; Rosal et al., 2009). Major cause of the population crash in vultures due to diclofenac provided evidence in India and Indian subcontinent, Pakistan and Nepal (Oaks et al., 2004; Shultz et al., 2004; Muralidharan and Dhananjayan, 2010). Although these emerging pollutants introduced into aquatic environment by discharges from sewage treatment plants, industrial and hospital wastewater, landfill leachates, disposal of unused drugs, effluents from aquaculture, agricultural use and so on (Daughton and Jones-Lepp, 2001; Thomas and Hilton, 2004; Isidori et al., 2005; Kasprzyk-Hordern et al., 2009), the occurrence of drugs in the environment was usually in low concentrations (ng-Ag/L), where in rivers, lakes and seawater ranges from ng/L (Zuccato et al., 2000; Thomas and Hilton, 2004; Gros et al., 2006). Many pharmaceuticals are not completely degraded after application, upon entering to the aquatic ecosystem the metabolites and some unchanged form may interfere with molecules, cells and organs of aquatic organisms due to their lipophilic nature and may leads to biological effect (Halling-Sørensen et al., 1998; Fent et al., 2006). In aquatic environment, the impact of drugs on organisms may occur after a long term exposure of pharmaceuticals at lower concentrations; and this to the non-target organisms is scanty (Thibaut and Porte, 2008).
In the present study we aimed to investigate the toxicity and impact of carbamazepine on certain enzymatic parameters of a freshwater fish Cyprinus carpio to fill up this lacuna. The selection of this group is based on the fact among different groups of pharmaceuticals detected in the aquatic environment. CBZ is a frequently detected pharmaceutical residue and anthropogenic marker in water bodies (Clara et al., 2004). CBZ is consistently found in many cases and proposed as an anthropogenic marker of urban contamination because of its persistence (about 100 days) in effluent and surface waters (Clara et al., 2004; Gagne et al., 2006). It is an antiepileptic drug widely used to control seizures disorders, for relief of neuralgia, and for a variety of mental disorders (Jones et al., 2002; RxList, 2006). It has been frequently found in the aquatic environment and detected in groundwater up to 610 ng l−1 (Drewes et al., 2002). Antiepileptic drugs like CBZ act on the CNS by decreasing the neuronal activity. Based on its toxicity level, CBZ classified as a potentially harmful drug to aquatic organisms. Further, the impact of CBZ on aquatic organisms particularly on freshwater fish and its ecotoxicological data remain largely unknown (Ayscough et al., 2000; Sanderson et al., 2004).
Fish respond to toxicants by altering their enzyme activities and the inhibition or induction of these enzyme activities has been used to indicate tissue damage (Nemcsok and Boross, 1982; Webb et al., 2005). Many enzymatic like carboxyl esterase (CE), lactate dehydrogenase (LDH), alkaline and acid phosphates (ALP, ACP), glutamate oxaloacetate transaminase and glutamate pyruvate transaminase (GOT and GPT) are measured as useful biomarkers to determine cellular impairment and cell rupture. Transaminases such as GOT and GPT play a vital role in protein and carbohydrate metabolism and act as an indicator for tissue damage (Nemcsok et al., 1981; Nemcsok and Boross, 1982). LDH was also used as indicative criteria of exposure due to chemical stress and anaerobic capacity of tissue (Diamantino et al., 2001; Rendon-von Osten et al., 2005).
The present investigation intended to monitor the impact of a pharmaceutical drug, CBZ on a freshwater teleost fish C. carpio using certain enzymatic parameters to assess the possible risk caused by water borne drugs on survival and physiology of aquatic organisms.
2 Material and methods
The Department of Zoology, Bharathiar University, Coimbatore-641046 has been registered with the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Government of India and the experiment was conducted as per the guidelines.
2.1 Test substance
Carbamazepine (CBZ) was chosen to evaluate its toxicity on freshwater fish at acute and sub lethal level. Carbamazepine (CAS No. 298-46-4, purity >99%) was purchased from Sigma–Aldrich Corporation, USA. Carbamazepine (1.0 g) was dissolved in appropriate amount of dimethylsulphoxide (DMSO) and then diluted with double-deionized water to make stock solutions. The DMSO concentration in the exposure solutions, including controls, was 0.01% (v/v) which is non-effective dose and used for further studies.
2.2 Fish and maintenance
C. carpio, a common freshwater fish (average length of 7.5 cm and average weight of 15.0 g) and widely cultivable in many aquaculture farms in India were obtained from Tamil Nadu Fisheries Development Corporation Limited; Aliyar Fish Farm, Tamil Nadu, India and they were safely transported to the laboratory in well packed polythene bags containing oxygenated water. Fish were stocked in a large tank containing dechlorinated tap water and acclimatized to test conditions for 20 days to their use in the experiment. During acclimatization, fish were fed with groundnut oil cake and rice bran in 2:1 ratio at ad libitum. Water (three fourth of the water) was replaced every 24 h to maintain healthy environment. The physico-chemical characteristics of tap water such as temperature, pH, dissolved oxygen, total alkalinity and total hardness were measured according to APHA (1998) and maintained (27.4 ± 1.2 °C; 7.2 ± 0.09; 6.4 ± 0.04 mg l−l; 18.6 ± 8.0 mg l−l; 18.4 ± 0.5 mg l−l) during the study. A static bioassay was performed on fish by the method of APHA (1971).
2.3 Preparation of 24 h LC50 and toxicity stress
The LC50 concentration of C. carpio for 24 h was calculated by the probit analysis method of Finney (1978). Stock solution of CBZ (1000 mg l−l) was prepared using dechlorinated water with nominal concentration (40, 50, 60, 70 and 80 mg l−l) to locate LC50 value for 24 h. For each concentration, 10 fish were randomly selected from the stock and introduced into separate glass tanks (120 cm × 80 cm × 40 cm). For each concentration a control was also maintained (free from toxicant) with three replicates. The concentration at which 50% mortality of fish occurred after 24 h was taken as the median lethal concentration (LC50), which was 59.70 mg l−l. 1/10th value of the LC50 concentration for 24 h of carbamazepine (5.97 mg l−l) was taken for sublethal studies according to Sprague (1971).
2.4 Experimental design and exposure
2.4.1 Acute concentration of CBZ
A static acute toxicity test was employed, using 50 L aerated glass tank (three replicates per treatment) filled with 30 l of water. Then, LC50 (24 h) concentration of CBZ (59.70 mg l−l) was added and 10 healthy fishes from the stock were introduced into each tank after removal of the same quantity of water. A common control was also maintained simultaneously (free from toxicant). After 24 h, fish from control and experiment were taken randomly; tissues (gill, liver and muscle) were removed for the estimation of GOT, GPT and LDH activities.
2.4.2 Sublethal concentration of CBZ
For sublethal studies, 200 healthy fish from the stock were randomly selected and transferred to aquaria of 500 l capacity which was filled with tap water. After introduction of the fish to the aquaria contains toxicant water, they were continuously exposed to CBZ concentration (5.97 mg l−l) under static conditions for 35 days and the toxicant water was renewed every day. A separate control was maintained (toxicant free). During the study period fish were fed with ad libitum with rice bran and groundnut oil cake (2:1 ratio), excess food and faecal matter were removed from the aquaria to reduce water quality deterioration. At the end of 7, 14, 21, 28 and 35 days exposure, samples of tissues (gill, liver and muscle) from control and CBZ treated fish were collected and immediately processed for enzymological (GOT, GPT and LDH) studies.
2.5 Biomarker analysis
After removal of organs (gill, liver and muscle), blotted dry with Whattman filter paper. Then 100 mg of each tissue were homogenates (100 mg/2.5 mL, w/v) with 0.25 M sucrose solution in ice cold condition (Hogeboom et al., 1948). The homogenates were centrifuged for 20 min at 6000 rpm (ice cold condition) and the clear supernatant fluid was removed and used to determine the level of GOT, GPT and LDH activities. GOT and GPT activities were measured according to Reitmen and Franckel (1957) at 505 nm against distilled water. The LDH activity was measured following the methodology described by Tietz (1976) at 340 nm against distilled water. Optical density was measured with the help of a UV-spectrophotometer. Activity of all enzymes was expressed in IU/L.
2.6 Statistical analysis
The statistical analysis was made between control and CBZ treated groups and the mean value of five individual observations was taken for each parameter. The standard error for the sample mean was calculated and the significance of sample means between control and drug treated fish was tested by using student’s t-test.
3 Results
3.1 Acute toxicity – enzymological changes
During acute treatment, GOT activity in gill, liver and muscle of drug treated fish was found to be decreased (Table 1). A maximum decrease was observed in liver (24.22%) followed by gill (49.82%) and muscle (59.87%) of treated fish contrast to control. GPT and LDH activities were observed to be significantly increased in liver and muscle of CBZ exposed fish, while a significant (p < 0.05) decrease in gill of treated fish was noticed compared to control. Values are means ± SE of five individual observations; (−) denotes percent decrease and (+) denotes percent increase over control in the parenthesis.
Enzymological parameters
Control fish
CBZ treated fish
GOT (IU/L)
Gill
57.00 ± 1.183
28.60 ± 0.400* (−49.82)
Liver
64.40 ± 0.509
48.00 ± 2.154* (−24.22)
Muscle
62.80 ± 0.200
25.20 ± 0.374* (−59.87)
GPT (IU/L)
Gill
55.80 ± 0.490
42.20 ± 4.587* (−24.37)
Liver
57.80 ± 0.490
62.20 ± 0.812* (+7.612)
Muscle
42.90 ± 0.872
58.30 ± 5.767* (+35.89)
LDH (IU/L)
Gill
4814.00 ± 9.311
4594.60 ± 55.12* (−4.55)
Liver
1600.60 ± 5.306
4118.00 ± 7.092* (+157.27)
Muscle
2370.00 ± 9.121
4769.00 ± 6.811* (+101.22)
3.2 Sublethal toxicity
3.2.1 Changes in GOT activity
During sublethal treatment GOT activity in gill of drug treated fish was slightly increased (0.69%) at the end of 7th day (Fig. 1). After 7th day a progressive decrease in GOT activity was observed up to 28th day. However at the end of 35th day GOT activity was increased (24.08%) which is not significant (p > 0.05) when compared to that of the control values. GOT activity was observed to be significantly (p < 0.05) decreased throughout the study period in both liver and muscle (Figs. 2 and 3).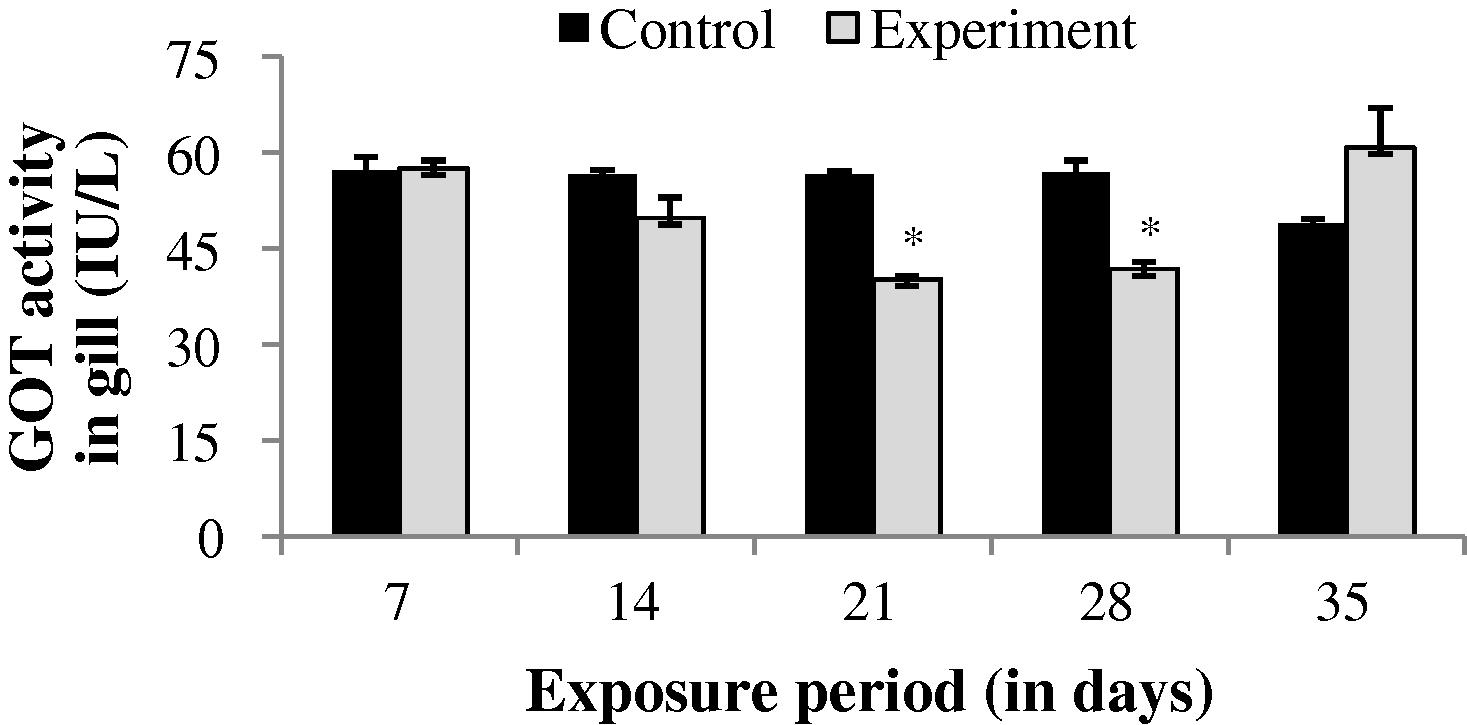
GOT activity in gill of control and carbamazepine treated fish (5.97 ppm; 35 days). Bar represents SE of the mean. Comparisons of means (control and treated fish) were done by Student’s t-test. ∗ Significant at 5% level (p < 0.05).
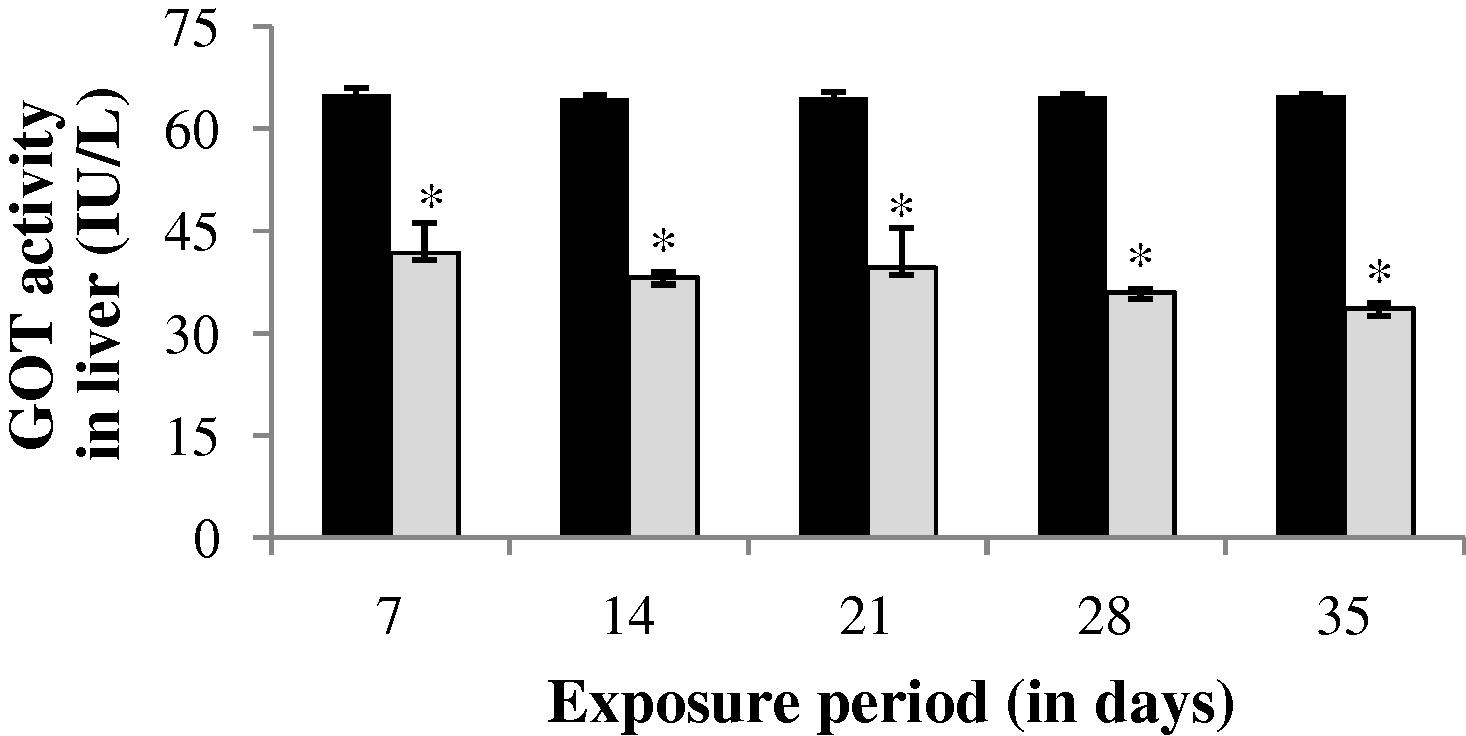
GOT activity in liver of control and carbamazepine treated fish (5.97 ppm; 35 days). Bar represents SE of the mean. Comparisons of means (control and treated fish) were done by Student’s t-test. ∗ Significant at 5% level (p < 0.05).
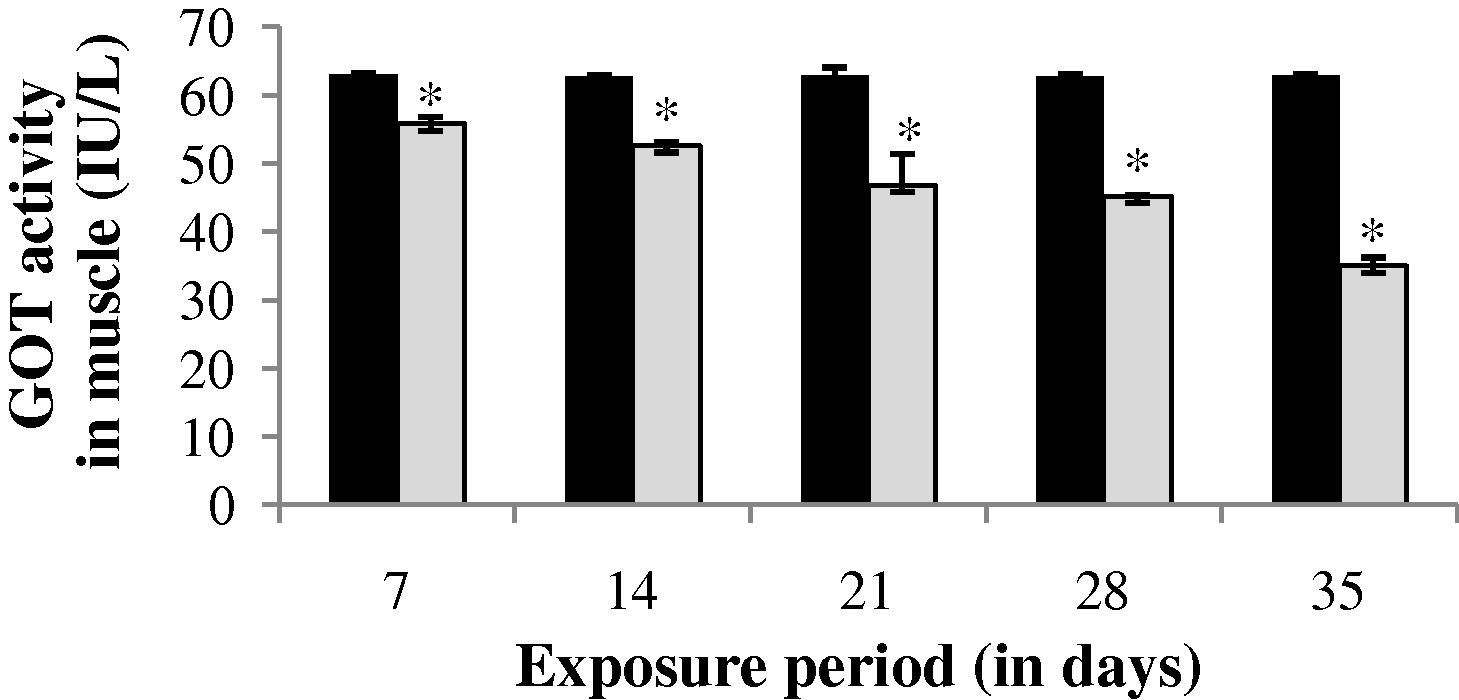
GOT activity in muscle of control and carbamazepine treated fish (5.97 ppm; 35 days). Bar represents SE of the mean. Comparisons of means (control and treated fish) were done by Student’s t-test. ∗ Significant at 5% level (p < 0.05).
3.2.2 Changes in GPT activity
The GPT level in gill, liver and muscle of the experimental fish was found to be increased throughout the study period (35 days) except at the end of 35th day in gill (−14.38%) and at the end of 7th day in liver (−1.04%) (Figs. 4–6). The values of gill during the study period were not significant (p > 0.05). The GPT activity was found to be not significant at the end of 7 and 35th day in liver and 35th day in muscle.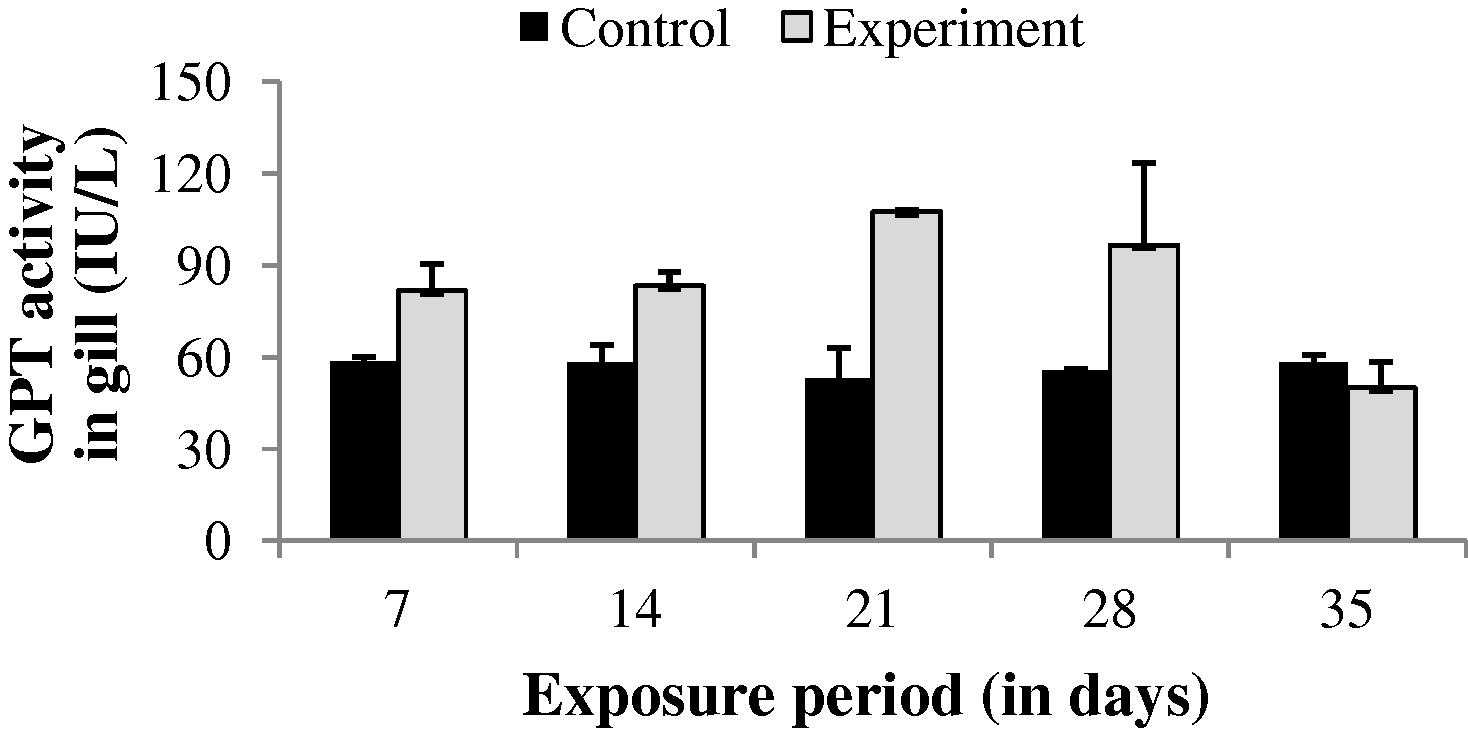
GPT activity in gill of control and carbamazepine treated fish (5.97 ppm; 35 days). Bar represents SE of the mean. Comparisons of means (control and treated fish) were done by Student’s t-test. ∗ Significant at 5% level (p < 0.05).
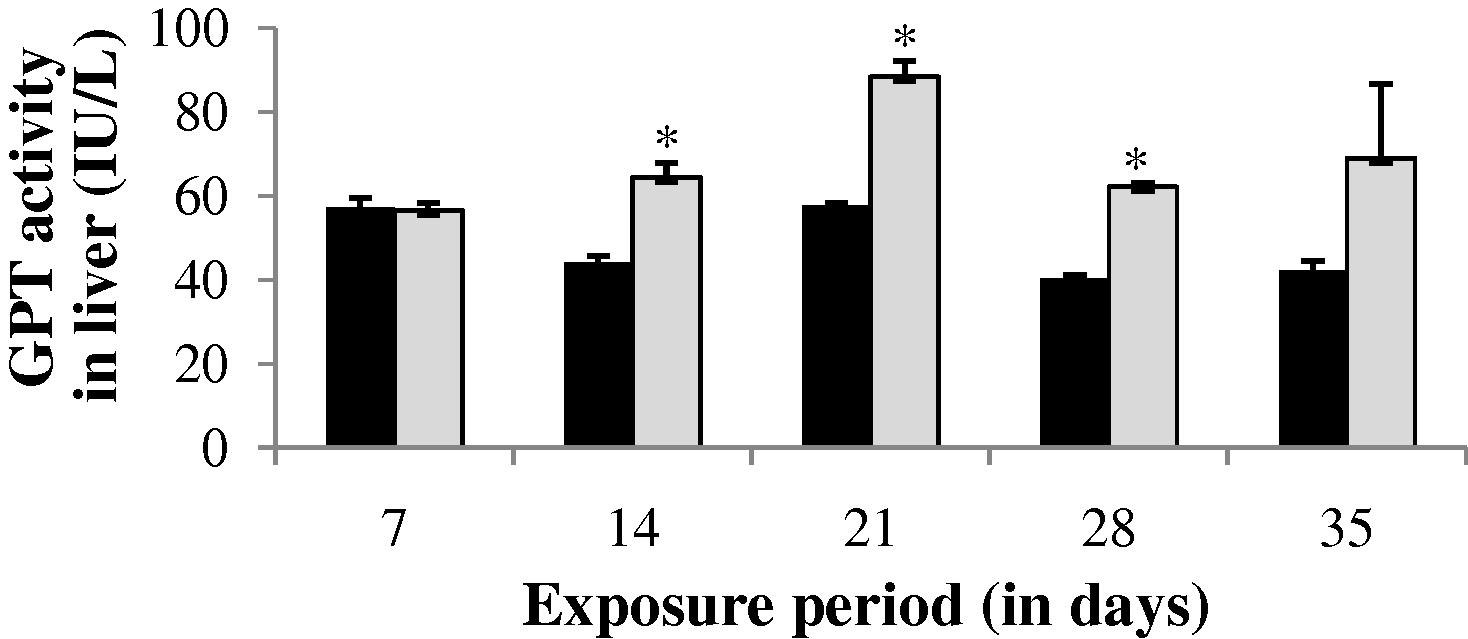
GPT activity in liver of control and carbamazepine treated fish (5.97 ppm; 35 days). Bar represents SE of the mean. Comparisons of means (control and treated fish) were done by Student’s t-test. ∗ Significant at 5% level (p < 0.05).
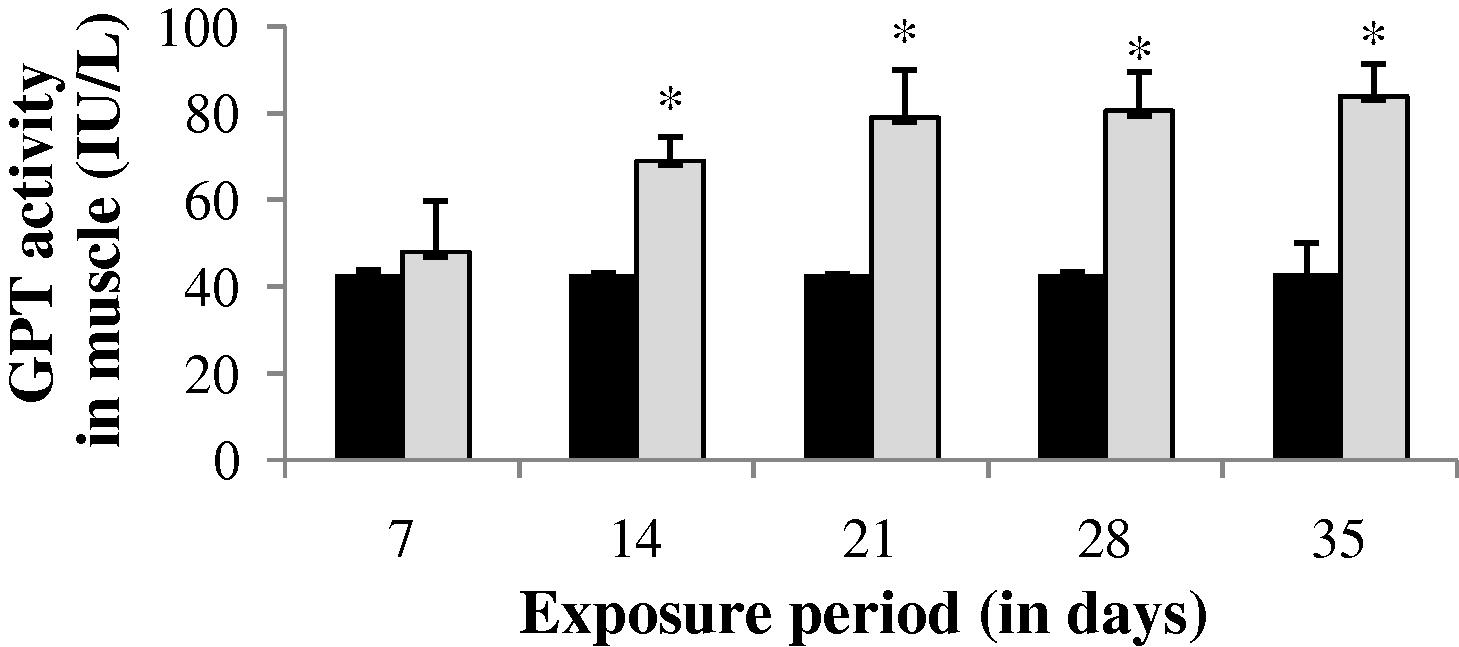
GPT activity in muscle of control and carbamazepine treated fish (5.97 ppm; 35 days). Bar represents SE of the mean. Comparisons of means (control and treated fish) were done by Student’s t-test. ∗ Significant at 5% level (p < 0.05).
3.2.3 Changes in LDH activity
The LDH activity in the gill of experimental fish was found to be significantly (p < 0.05) increased up to 14th day (1.968%). After 14th day, a progressive decrease in LDH level was observed throughout the study period (Fig. 7). An increase in LDH activity was observed throughout the study period except 35th day in liver (−36.57%) and muscle (14.72%). The values obtained during the study period in liver and muscle were statistically significant except the value of 35th day in muscle (p < 0.05) (Figs. 8 and 9).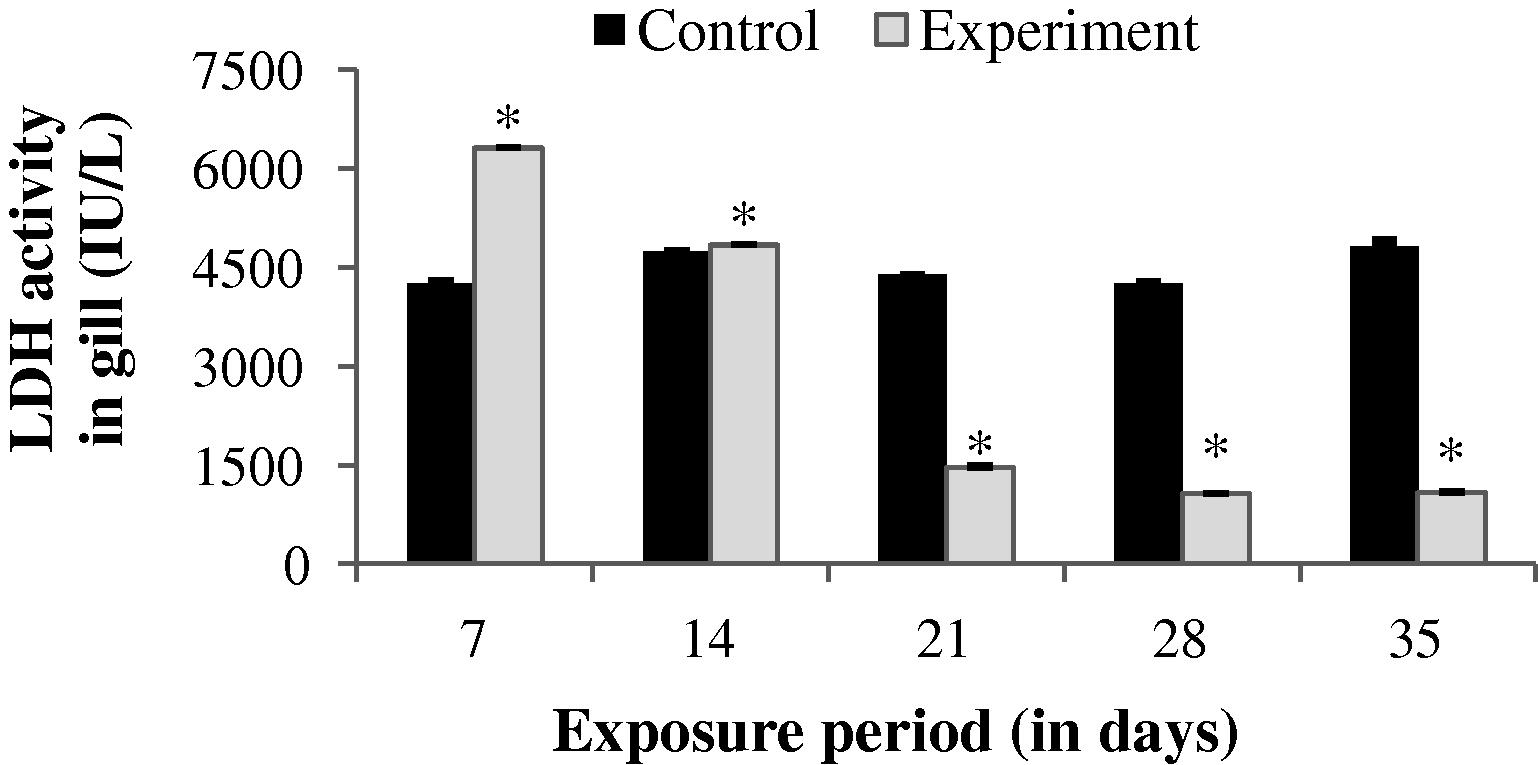
LDH activity in gill of control and carbamazepine treated fish (5.97 ppm; 35 days). Bar represents SE of the mean. Comparisons of means (control and treated fish) were done by Student’s t-test. ∗ Significant at 5% level (p < 0.05).
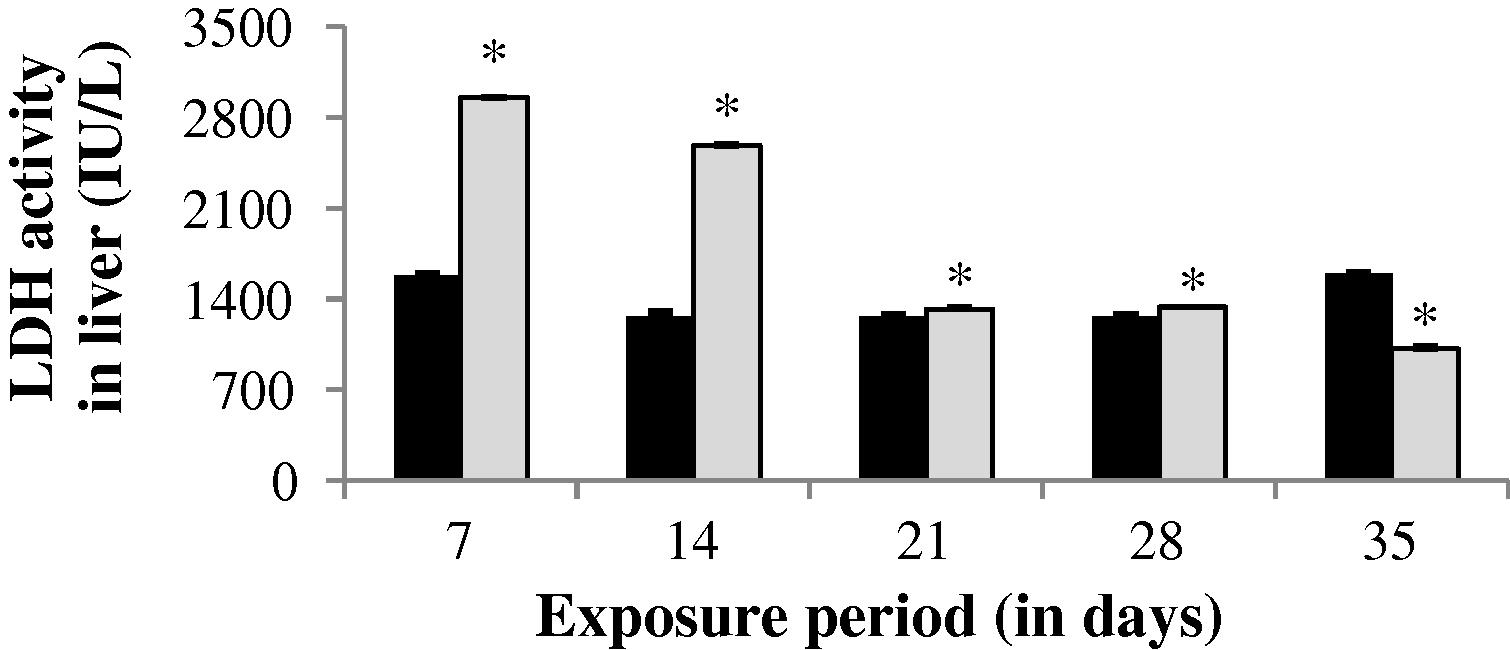
LDH activity in liver of control and carbamazepine treated fish (5.97 ppm; 35 days). Bar represents SE of the mean. Comparisons of means (control and treated fish) were done by Student’s t-test. ∗ Significant at 5% level (p < 0.05).
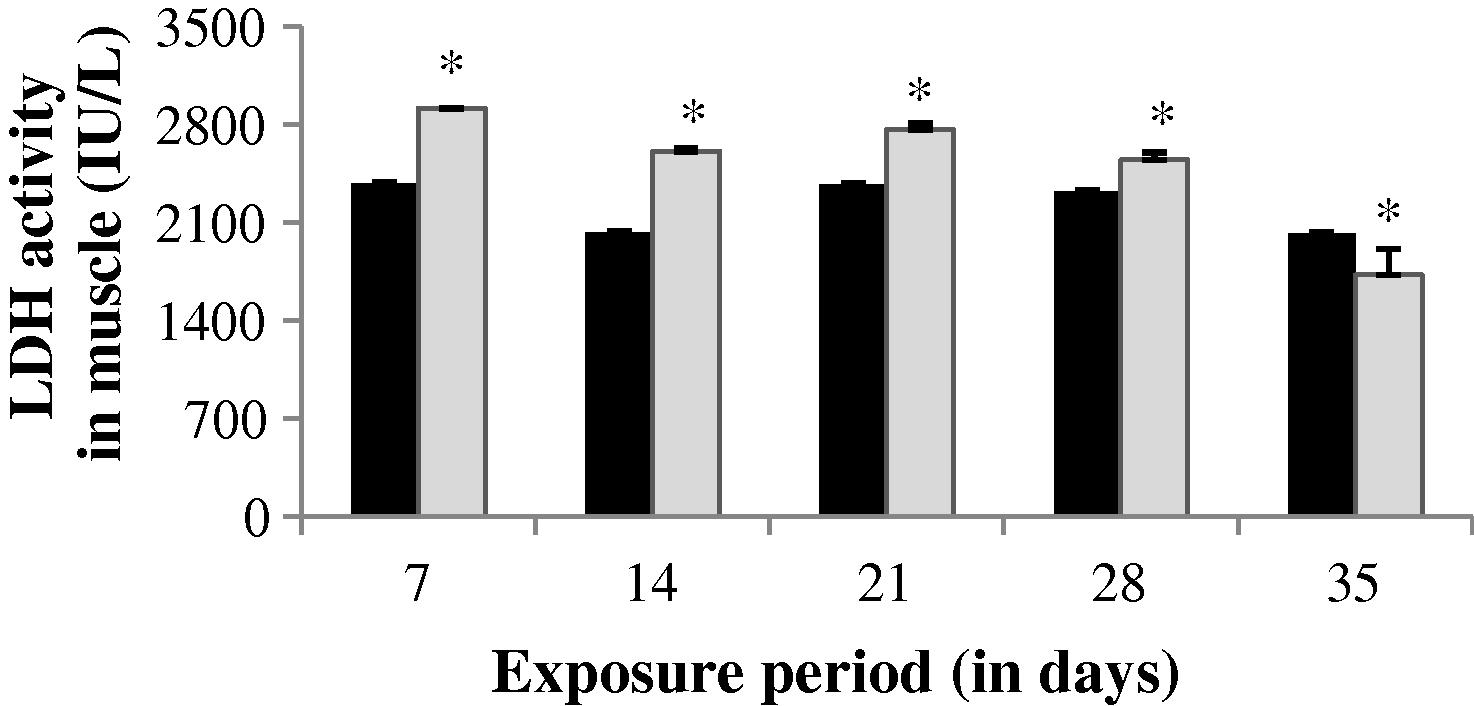
LDH activity in muscle of control and carbamazepine treated fish (5.97 ppm; 35 days). Bar represents SE of the mean. Comparisons of means (control and treated fish) were done by Student’s t-test. ∗ Significant at 5% level (p < 0.05).
4 Discussion
Ecotoxicity procedure for the mode of action of pharmaceuticals on non-target organisms is presently attracted throughout the world (Pomati et al., 2004). The presence of pharmaceuticals in the aquatic environment may act on molecules, cells and organs of organisms through unexpected mode of action (Fent et al., 2006). Hoeger et al. (2008) suggested that although pharmaceutical drugs are usually in low concentration, and are also considered to be non-toxic compounds, they can exert toxic effects on non-target species. Knowledge on the acute toxicity of pharmaceuticals could provide valuable information on the mode of action and toxicity of these compounds. Recently, many studies concentrated on the acute toxicity of pharmaceuticals in organisms such as algae, cnidarians, crustaceans, mussels and fishes (Canesi et al., 2007; Yang et al., 2008; Haap et al., 2008; Choi et al., 2008). However Laura Martín-Díaz et al. (2009) suggested that chronic studies based on specific endpoints should be used in risk assessment. The present study reports the acute and sublethal toxicity of carbamazepine on a freshwater fish C. carpio using certain enzymatic parameters as an end points test.
In the present study, the 24 h median lethal concentration of CBZ to a freshwater teleost fish C. carpio was found to be 59.70 mg l−l. For sublethal studies 1/10th of the above concentration (5.97 mg l−l) was used. Many studies have reported that acute toxicity of CBZ to aquatic organisms was found to be below 100 mg l−1. The acute toxicity of CBZ was found to be 17.2 mg l−1 in Daphnia and 34.4 mg l−l in midges, 43.0 μg l−l in zebra fish (Thaker, 2005), 52.5 mg l−l in Vibrio fischeri and 76.3 mg l−l in Daphnia magna (Jos et al., 2003), 15.0–35.4 mg l−l in medaka fish (Kim et al., 2007); 74.0–138.0 mg l−l to daphnids, zebra fish, and amphibian (Pfluger and Dietrich, 2001). In the present study, CBZ was found to be toxic to the fish C. carpio.
Aquatic vertebrates particularly fish appear to have similar enzyme and receptor systems as in mammalian system (Huggett et al., 2003). Fish react to environmental toxicants by changing and adapting their metabolic functions. Changes in the enzymatic activities of aquatic organisms are widely used to demonstrate tissue damage and also diagnosis of fish diseases (Nemcsok and Boross, 1982; Pacheco and Santos, 2002). GOT and GPT usually present within cell membranes, cytoplasm and mitochondria. The accumulation or binding of toxicants in these cells may lead to damage and disintegration of cells, releasing these enzymes into blood circulation. As a result, a dramatic increase in blood serum transaminases may be expected during stress conditions (Nemcsok et al., 1981; Galina et al., 1992). Elevation in GOT activity may indicate muscle damage especially cardiac muscle, where as higher GPT activity indicates damage in liver cells. Damage in liver, kidney and gills is evident from elevated transaminase activities (Bernet et al., 2001). Changes in protein and carbohydrate metabolism during stress conditions may also affect the activity of GOT and GPT and the elevation of transaminases can be taken as a measure of compensatory mechanism to impaired metabolism (Reddy and Venugopal, 1991). In aquatic monitoring, increased activities of GOT and GPT indicated hepatic tissue damage. Increase in GOT and GPT activity in monocrotophos treated fish Channa punctatus indicates liver damage (Agrahari et al., 2007). Increase in the activities of plasma GOT and GPT can be used as a sensitive indicator to assess even very minute cellular damage (Van der et al., 2003).
In the present study, the significant increase in GOT and GPT activity in gill, liver and muscle during acute and sublethal treatment indicates that the damage of the organs due to drug toxicity or the organism tries to mitigate the drug induced stress by increased rate of metabolism. However the observed decrease in GOT and GPT activity in gill, liver and muscle during acute and sublethal treatment signifies that detoxification mechanism may not be sufficiently effective to prevent the action of the drug on the system. The decreased activities of GOT indicate disturbance in the structure and integrity of cell organelles.
LDH involved in carbohydrate metabolism and the determination of this enzyme activity can be used as a good indicator of the anaerobic capacity of a tissue, chemical exposure and stress in fish (Gagnon, 2002; Rendon-von Osten et al., 2005). LDH is present in all tissues and normally associated with cellular metabolic action; it is used as potential marker for assessing the toxicity of a chemical (Agrahari et al., 2007). Any changes in protein and carbohydrate metabolism may cause change in LDH activity (Abston and Yarbrough, 1976). Normal LDH activity patterns were found to be altered in situations of chemical stress (Diamantino et al., 2001). Elevated LDH activity in gills suggests that the aerobic catabolism of glycogen and glucose has shifted towards the formation of lactate, which may have adverse long-term effects on the organisms (Szegletes et al., 1995). Increased release of LDH into the medium may indicate damage in the integrity of cell membranes or heart muscle (Nemcsok et al., 1984). In the present study, the elevation of LDH activity in gill, liver and muscle has occurred may be due to the metabolic changes caused by the drug CBZ. Further disruption of respiratory epithelium might have caused tissue hypoxia resulting in a decrease in oxidative metabolism which may be responsible for increase in LDH activity in toxicant stressed animals (Gill et al., 1990).
Jos et al. (2003) reported that leakage of LDH is a marker of membrane permeability and cell death. They also suggested that a slight increase in LDH activity may be due to stabilization of cytoplasmic membrane and good viability of the cultures exposed to CBZ. Elevation of LDH activity in mosquito fish, Gambusia holbrooki after acute exposure to clofibric acid may be due to stress response caused by clofibric acid (Nunes et al., 2004). Similarly in CBZ treated rainbow trout Oncorhynchus mykiss, plasma enzymes like LDH and GPT levels were increased during chronic exposure (Li et al., 2009). The significant increase of these enzymes may indicate the changes in the histological structure of the hepatic and extra hepatic tissues (Svoboda et al., 2001; El-Sayed et al., 2007). In C. carpio, alterations in cellular responses such as increased number of macrophages in liver and increase in the membrane material in the cytoplasm were noticed after exposure to CBZ (Triebskorn et al., 2007). Similarly, gill shows epithelial lifting, hypertrophy and hyperplasia of mucus cells.
Inhibition in the activity of the LDH may be either due to the change in mitochondrial membrane junction or it may be due to impaired glycolysis (Sastry and Siddiqui, 1984). Yadav et al. (2007) reported that fertilizer industry effluent caused marked reduction in tissue LDH activity in Channa striatus. Decreased LDH activity may be due to lower metabolic rate under toxic conditions (Agrahari et al., 2007). Gravel and Vijayan (2007) reported that salicylate depressed liver GR (glucocorticoid receptor) protein content and also modified the stressor-mediated liver metabolic capacity in rainbow trout O. mykiss. Severe histopathological alterations in gill and liver and inhibition of cyclooxygenase activity were observed in diclofenac treated fish Salmo trutta (Hoeger et al., 2005). Similar histopathological alteration was also reported in rainbow trout O. mykiss (Schwaiger et al., 2004) and cytological effects (Triebskorn et al., 2004). Probably, the inhibition of LDH in the present study during acute and sublethal treatment may be due to impaired carbohydrate metabolism. Thus, the measurement of alteration in the LDH activity in gill, liver and kidney can be used as a biomarker indicating stress.
Li et al. (2010) reported a slight increase in SOD, CAT, and GPx activity in CBZ-treated fish O. mykiss during the first period (7 days). However, activities of antioxidant enzymes were significantly inhibited after 42 days. They also suggested that prolonged exposure to CBZ resulted in excess reactive oxygen species formation, finally resulting in oxidative damage to lipids, proteins and inhibited antioxidant capacities in fish brain. In rainbow trout O. mykiss after prolonged exposure to CBZ, the levels of LPO and CP in fish gill were increased whereas significant inhibition of the antioxidant enzymes such as SOD, CAT, GR, GPx, glutathione level and Na+, K+-ATPase activity was noticed (Li et al., 2009). Inhibition of AchE activity was noted in muscle and gills of black tiger shrimp Penaeus monodon exposed to antibiotics (Tu et al., 2009).
In the present study it was concluded that CBZ induced alterations in the activities of the enzymes such as GOT, GPT and LDH in a freshwater fish C. carpio and these enzymes may be used to assess the effects of carbamazepine in test organisms.
5 Conclusion
The present study indicates that CBZ induced alterations in the enzymatic activities of the freshwater fish both at acute and sublethal concentrations. These alterations can be considered as a tool for biomonitoring of pharmaceutical drug substances in the aquatic environment. However, further studies are needed to understand the risk of these compounds by using different end points.
6 Conflict of interest statement
None.
Acknowledgements
This work was supported by the University Grants Commission-Major Research Project “Environmental Risk Assessment of Selected Pharmaceuticals to Aquatic Species: Using Physiological and Nonspecific Biomarkers” [Ref. F. No: 33-352/2007 (SR)] financed by the University Grants Commission, New Delhi, India.
References
- The in vivo effect of mirex on soluble hepatic enzymes in the rat. Pestic. Biochem. Physiol.. 1976;6:192-199.
- [Google Scholar]
- Biochemical alteration induced by monocrotophos in the blood plasma of fish, Channa punctatus (Bloch) Pestic. Biochem. Physiol.. 2007;88:268-272.
- [Google Scholar]
- Standard Method for the Examination of Water and Wastewater (13th ed.). New York, USA: American Public Health Association; 1971. p. 874
- Standard Methods for the Examination of Water and Wastewater (20th ed.). Washington, DC: American Public Health Association; 1998.
- Ayscough, N.J., Fawell, J., Franklin, G., Young, W., 2000. Review of Human Pharmaceuticals in the Environment. Technical Report, Environment Agency, Bristol, p. 390.
- Effluent from a sewage treatment works causes changes in serum biochemistry of brown trout (Salmo trutta L.) Ecotoxicol. Environ. Saf.. 2001;48:140-147.
- [Google Scholar]
- Effects of Triclosan on Mytilus galloprovincialis hemocyte function and digestive gland enzyme activities: possible modes of action on non target organisms. Comp. Biochem. Physiol. Part C. 2007;145:464-472.
- [Google Scholar]
- Occurrences and ecological risks of roxithromycin, trimethoprim, and chloramphenicol in the Han River, Korea. Environ. Toxicol. Chem.. 2008;27:711-719.
- [Google Scholar]
- Carbamazepine as a possible anthropogenic marker in the aquatic environment: investigations on the behaviour of carbamazepine in wastewater treatment and during groundwater infiltration. Water Res.. 2004;38:947-954.
- [Google Scholar]
- Daughton, C.G., Jones-Lepp, T., 2001. Pharmaceuticals and personal care products in the environment- scientific and regulatory issues. ACS Symposium Series, vol. 791. American Chemical Society, Washington, DC.
- Pharmaceuticals and personal care products in the environment: agents of subtle change? Environ. Health Perspect.. 1999;107(6):907-938.
- [Google Scholar]
- Lactate dehydrogenase activity as an effect criterion in toxicity tests with Daphnia magna straus. Chemosphere. 2001;45:553-560.
- [Google Scholar]
- Fate of pharmaceuticals during indirect potable reuse. Water Sci. Technol.. 2002;46:73-80.
- [Google Scholar]
- Acute intoxication of deltamethrin in monosex Nile tilapia, Oreochromis niloticus with special reference to the clinical, biochemical and haematological effects. Environ. Toxicol. Pharmacol.. 2007;24:212-217.
- [Google Scholar]
- Statistical Methods in Biological Assay (third ed.). Berlin: Springer; 1978. p. 508
- Occurrence of pharmaceutical products in a municipal effluent and toxicity to rainbow trout (Oncorhynchus mykiss) hepatocytes. Ecotoxicol. Environ. Saf.. 2006;64(3):329-336.
- [Google Scholar]
- Metabolic enzymes as biochemical markers of effect following exposure of fish to sodium pentachlorophenate (Na-PCP) Bull. Environ. Contam. Toxicol.. 2002;69:570-575.
- [Google Scholar]
- Acute effect of sublethal ammonia concentration on common carp, Cyprinus carpio L. II. Effect of ammonia on blood plasma transaminases (GOT, GPT), GIDH enzyme activity, and ATP value. Aquaculture. 1992;104:149-156.
- [Google Scholar]
- Use of the fish enzyme system in monitoring water quality. Effects of mercury on tissue enzymes. Comp. Biochem. Physiol. Part C. 1990;97:287-292.
- [Google Scholar]
- Pilot survey monitoring pharmaceuticals and related compounds in a sewage treatment plant located on the Mediterranean coast. Chemosphere. 2007;66(6):993-1002.
- [Google Scholar]
- Salicylate impacts the physiological responses to an acute handling disturbance in rainbow trout. Aquat. Toxicol.. 2007;85:87-95.
- [Google Scholar]
- Development of a multi-residue analytical methodology based on liquid chromatography–tandem mass spectrometry (LC–MS/MS) for screening and trace level determination of pharmaceuticals in surface and wastewaters. Talanta. 2006;70(4):678-690.
- [Google Scholar]
- Acute effects of diclofenac and DMSO to Daphnia magna: immobilization and hsp70-induction. Chemosphere. 2008;73:353-359.
- [Google Scholar]
- Occurrence, fate and effects of pharmaceutical substances in the environment – a review. Chemosphere. 1998;36:357-393.
- [Google Scholar]
- Water-borne diclofenac affects kidney and gill integrity and selected immune parameters in brown trout (Salmo trutta f. fario) Aquat. Toxicol.. 2005;75(1):53-64.
- [Google Scholar]
- Distribution of intraperitoneally injected diclofenac in brown trout (Salmo trutta f. Fario) Ecotoxicol. Environ. Saf.. 2008;71:412-418.
- [Google Scholar]
- Cytochemical studies of mammalian tissues: 1. Isolation of intact mitochondria from rat liver; some biochemical properties of mitochondria and submicroscopic particulate material. J. Biol. Chem.. 1948;172:619-635.
- [Google Scholar]
- A theoretical model for utilizing mammalian pharmacology and safety data to prioritize potential impacts of human pharmaceuticals to fish. Hum. Ecol. Risk Assess.. 2003;9:1789-1799.
- [Google Scholar]
- Toxic and genotoxic evaluation of six antibiotics on non-target organisms. Sci. Total Environ.. 2005;346:87-98.
- [Google Scholar]
- Aquatic environmental assessment of the top 25 English prescription pharmaceuticals. Water Res.. 2002;36:5013-5022.
- [Google Scholar]
- Ecotoxicological evaluation of carbamazepine using six different model systems with eighteen endpoints. Toxicol. In Vitro. 2003;17:525-532.
- [Google Scholar]
- Illicit drugs and pharmaceuticals in the environment-forensic applications of environmental data, Part 2: pharmaceuticals as chemical markers of faecal water contamination. Environ. Pollut.. 2009;157:1778-1786.
- [Google Scholar]
- Aquatic toxicity of acetaminophen, carbamazepine, cimetidine, diltiazem and six major sulfonamides, and their potential ecological risks in Korea. Environ. Int.. 2007;33:370-375.
- [Google Scholar]
- The use of biochemical responses to assess ecotoxicological effects of pharmaceutical and personal care products (PPCPs) after injection in the mussel Elliptio complanata. Environ. Toxicol. Pharmacol.. 2009;28(2):237-242.
- [Google Scholar]
- Exposure assessment of the pharmaceutical diclofenac based on long-term measurements of the aquatic input. Environ. Int.. 2009;35:363-368.
- [Google Scholar]
- Responses of antioxidant status and Na+/K+-ATPase activity in gill of rainbow trout, Oncorhynchus mykiss, chronically treated with carbamazepine. Chemosphere. 2009;77(11):1476-1481.
- [Google Scholar]
- Modulation of antioxidant defence system in brain of rainbow trout (Oncorhynchus mykiss) after chronic carbamazepine treatment. Comp. Biochem. Physiol. Part C: Toxicol. Pharmacol.. 2010;151(1):137-141.
- [Google Scholar]
- Diclofenac residues in blood plasma and tissues of vultures collected from Ahmedabad, India. Bull. Environ. Contam. Toxicol.. 2010;85:377-380.
- [Google Scholar]
- Comparative studies on the sensitivity of different fish species to metal pollution. Acta Biol. Hung.. 1982;33:23-27.
- [Google Scholar]
- Sub cellular localization of transaminase enzyme in fishes and their significance in the detection of water pollution. Acta Biol. Szeged.. 1981;27:9-15.
- [Google Scholar]
- Effect of copper, zinc and paraquat on acetylcholinesterase activity in carp, Cyprinus carpio (L) Aquat. Toxicol.. 1984;5:23-31.
- [Google Scholar]
- Acute and chronic effects of clofibrate and clofibric acid on the enzymes acetylcholinesterase, lactate dehydrogenase and catalase of the mosquitofish, Gambusia holbrooki. Chemosphere. 2004;57:1581-1589.
- [Google Scholar]
- Diclofenac residues as the cause of population decline of vultures in Pakistan. Nature. 2004;427:630-633.
- [Google Scholar]
- Biotransformation, ecotoxic and histopathological effects of environmental contaminants in European eel, Anguilla anguilla (L) Ecotoxicol. Environ. Saf.. 2002;53:331-347.
- [Google Scholar]
- Effects on pharmaceuticals in the environment-an overview and principle considerations. In: Kummerer K., ed. Pharmaceuticals in the Environment-Sources, Fate, Effects and Risks. Berlin: Springer; 2001. p. :11-17.
- [Google Scholar]
- Effects of erythromycin, tetracycline and ibuprofen on the growth of Synechocystis sp. and Lemna minor. Aquat. Toxicol.. 2004;67:387-396.
- [Google Scholar]
- In vivo effects of cadmium chloride on certain aspects of protein metabolism in tissues of a freshwater field crab, Barytelphusa guerini. Bull. Environ. Contam. Toxicol.. 1991;46:583-590.
- [Google Scholar]
- A colorimetric method for the determination of serum glutamic oxalo acetic and glutamic pyruvic transaminase. Am. J. Clin. Pathol. 1957:28-56.
- [Google Scholar]
- In vivo evaluation of three biomarkers in the mosquito fish, Gambusia yucatana exposed to pesticides. Chemosphere. 2005;58:627-636.
- [Google Scholar]
- The fate of pharmaceutical chemicals in the aquatic environment. J. Pharm. Pharmacol.. 1985;37:1-12.
- [Google Scholar]
- Identification of intermediates and assessment of ecotoxicity in the oxidation products generated during the ozonation of clofibric acid. J. Hazard. Mater.. 2009;172(2–3):1061-1068.
- [Google Scholar]
- RxList, 2006. The Internet Drug Index. <http://www.rxlist.com>.
- Ranking and prioritization of environmental risks of pharmaceuticals in surface waters. Regul. Toxicol. Pharmacol.. 2004;39:158-183.
- [Google Scholar]
- Some haematological, biochemical and enzymological parameters of a freshwater teleost fish, Channa punctatus, exposed to sublethal concentration of quinalphos. Pestic. Biochem. Physiol.. 1984;22:8-13.
- [Google Scholar]
- Toxic effects of the non-steroidal anti-inflammatory drug diclofenac. Part I: histopathological alterations and bioaccumulation in rainbow trout. Aquat. Toxicol.. 2004;68:141-150.
- [Google Scholar]
- Diclofenac poisoning is widespread in decline vulture populations across the Indian subcontinent. Proc. R. Soc. London B. 2004;271(6):S458-S460. doi: 10.1098/rsbl.2004.0223
- [Google Scholar]
- Measurement of pollutant toxicity to fish. III. Sublethal effects and ‘safe’ concentrations. Water Res.. 1971;5:245-266.
- [Google Scholar]
- The effect of diazinon on haematological indices of common carp (Cyprinus carpio L.) Acta Vet. BRNO.. 2001;70:457-465.
- [Google Scholar]
- In vivo effects of deltamethrin on some biochemical parameters of carp, Cyprinus carpio (L.) Environ. Monit. Assess.. 1995;35:97-111.
- [Google Scholar]
- Occurrence of drugs in German sewage treatment, plants and rivers. Water Res.. 1998;32(11):3245-3260.
- [Google Scholar]
- Pharmaceutical data elude researchers. Environ. Sci. Technol.. 2005;139(9):193A-194A.
- [Google Scholar]
- Effects of fibrates, anti-inflammatory drugs and antidepressants in the fish hepatoma cell line PLHC-1: cytotoxicity and interactions with cytochrome P450 1A. Toxicol. In Vitro. 2008;22:1128-1135.
- [Google Scholar]
- The occurrence of selected human pharmaceutical compounds in UK estuaries. Mar. Pollut. Bull.. 2004;49:436-444.
- [Google Scholar]
- Fundamentals of Clinical Chemistry. Philadelphia, PA: W.B. Saunders Co.; 1976. p. 652
- Toxic effects of the nonsterodial anti-inflammatory drug diclofenac: Part II. Cytological effects in liver, kidney, gills and intestine of rainbow trout (Oncorhynchus mykiss) Aquat. Toxicol.. 2004;68:151-166.
- [Google Scholar]
- Ultrastructural effects of pharmaceuticals (carbamazepine, clofibric acid, metoprolol, diclofenac) in rainbow trout (Oncorhynchus mykiss) and common carp (Cyprinus carpio) Anal. Bioanal. Chem.. 2007;387:1405-1416.
- [Google Scholar]
- Acetylcholinesterase activity as a biomarker of exposure to antibiotics and pesticides in the black tiger shrimp (Penaeus monodon) Ecotoxicol. Environ. Saf.. 2009;72(5):1463-1470.
- [Google Scholar]
- Fish bioaccumulation and biomarkers in environmental risk assessment, a review. Environ. Toxicol. Pharmacol.. 2003;13:57-149.
- [Google Scholar]
- Metabolic enzyme activities in black bream, Acanthopagrus butcheri from the swan canning estuary, Western Australia. Comp. Biochem. Physiol. Part C. 2005;141:356-365.
- [Google Scholar]
- Fertilizer Industry Effluent Induced Biochemical Changes in Fresh water Teleost, Channa striatus (Bloch) Bull. Environ. Contam. Toxicol.. 2007;79:588-595.
- [Google Scholar]
- Persistence and partitioning of eight selected pharmaceuticals in the aquatic environment: laboratory photolysis, biodegradation, and sorption experiments. Water Res.. 2009;43:351-362.
- [Google Scholar]
- Growth inhibiting effects of 12 antibacterial agents and their mixtures on the freshwater micro alga Pseudokirchneriella subcapitata. Environ. Toxicol. Chem.. 2008;27(5):1201-1208.
- [Google Scholar]
- Identification of the pharmaceuticals for human use contaminating the Italian aquatic environment. J. Hazard. Mater.. 2005;122:205-209.
- [Google Scholar]







