Translate this page into:
Ethanolic extract of Crinum asiaticum attenuates hyperglycemia-mediated oxidative stress and protects hepatocytes in alloxan induced experimental diabetic rats
*Corresponding author. Address: Faculty of Biotechnology, West Campus, PRIST University, Thanjavur 613 403, Tamil Nadu, India. Tel.: +91 9443724611; fax: +91 4362 265140 drravinikesh@yahoo.co.in (S. Ravikumar)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Available online 30 December 2010
Abstract
The present study is to investigate the antioxidant activity in alloxan induced diabetic rats. The experimental rats were randomly divided into three groups: Group I: control; Group II: alloxan induced diabetic rats and Group III: alloxan induced diabetic rats were treated with ethanolic extract of Crinum asiaticum leaves (200 mg/kg/bw). Diabetes mellitus was induced by alloxan in a single dose of 200 mg/kg/bw. A significant decrease in blood sugar level was observed and also an increase in high density lipoprotein (HDL) level and a decrease (P < 0.05) in cholesterol, triglyceride, low density lipoprotein (LDL) and very low density lipoprotein (VLDL) levels were observed after 15 days of treatment with C. asiaticum extract. Besides, the activities of aspartate transaminase (AST), alanine transaminase (ALT), alkaline phosphatase (ALP), acid phosphatase (ACP) and lactate dehydrogenase (LDH) (P < 0.05) were diminished in the C. asiaticum leaves extract of the supplemented group. In addition, the activity of superoxide dismutase (SOD), catalase (CAT) and reduced glutathione (GSH) (P < 0.05) was increased by the C. asiaticum leaves extract administration and increases in lipid peroxides (LPO) in liver were found to be ameliorated after treatment. The findings of the present investigation demonstrated the hepatocyte protective nature of C. asiaticum by attenuating markers of hyperglycemia-mediated oxidative stress and the antioxidant competence in hepatic tissues of diabetic rats.
Keywords
Antioxidant
Crinum asiaticum
Diabetes mellitus
Oxidative stress
Hepatic tissues
1 Introduction
Diabetes mellitus is a metabolic disorder characterized by hyperglycemia and alteration in carbohydrate, fat and protein metabolism associated with absolute or relative deficiencies in insulin secretion or insulin action (Kumar et al., 2006).
Normally blood sugar levels are tightly controlled by insulin, a hormone produced by the pancreas. Insulin lowers the blood glucose level. When the blood glucose elevates, insulin is released from the pancreas to normalize the glucose level. In patients with diabetes, the absence or insufficient production of insulin causes hyperglycemia. Diabetes is a chronic medical condition, meaning that although it can be controlled, it lasts a lifetime.
Diabetes is a common endocrine disorder affecting over 100 million people worldwide. The World Health Organization predicts that this number may increase fivefold in the near future. In India diabetes affects 1–5% of the population. Diabetes mellitus is considered as one of the five leading causes of death in the world.
The World Health Organization (WHO) estimates that 80% of the people of developing countries rely on traditional medicines, mostly plant-derived drugs, for their primary health needs. Medicinal plants are commonly used in treating and preventing specific ailments and are considered to play a significant role in health care. The use of ethanobotanicals has a long folkloric history for the treatment of blood sugar abnormalities (Kiritikar and Basu, 1999).
India is well-known for its rich traditional systems of medicine, i.e. Ayurvedic, Siddha, Unani and Amchi (Tibetan) besides a vast reservoir of living traditions of ethanomedicine. Many rural households in India, with limited access to organized health services practise home remedies, the recipes and formulae of which have been handed down from generation to generation. In Indian systems of medicine, generally the medicines of plant origin are preferred over the medicines of animal origin due to the presence of abundant natural flora (Davis, 1994).
Liver is one of the chief storage organs for glucose reserve in the body and plays a crucial role in the maintenance of blood glucose homeostasis, because it consents to amass the superfluous blood glucose and to demobilize it in hypoglycemic states. Chronic hyperglycemia impedes normal inhibition of hepatic glucose production persuaded by an acute escalation of blood glucose level and enhances phosphoenolpyruvate carboxykinase gene expression, which is an imperative enzyme for the regulation of gluconeogenesis (Shao et al., 2005). Further, liver is the focal organ of oxidative and detoxifying processes as well as free radical reactions and the biomarkers of oxidative stress are elevated in the liver at an early stage in many diseases, including diabetes mellitus (Stadler et al., 2003). In experimental diabetes, streptozotocin exerts its toxic effects on liver and other organs in addition to pancreatic cells. The insulin insufficiency and hyperglycemia that result from cell necrosis further augment liver damage through reactive free radicals mediated lipid peroxidation of hepatocellular membrane (Kume et al., 2004). This pathophysiological sequence sets the scene for considering antioxidant therapy as an adjunct in the management of diabetes. Several studies have recently dealt with either maintenance of antioxidant defense of diabetic liver or reduction of peroxidative stress induced hepatic damage in experimental models (Baydas et al., 2002; Cho et al., 2002; Hünkar et al., 2002). Hence, it is recommended that a therapy with antioxidants may signify a useful pharmacologic overture to the management of diabetes.
The leaves of Crinum asiaticum are used as traditional healers in North east Solomon Islands to treat inflammation (Wiart, 2000). Leaves are used for the treatment of skin disease and inflammation. It has antibacterial activity (Ilavenil et al., 2010). It is also used to treat ulcer and swelling (Goeltenboth et al., 1991). The seeds are considered as purgative (Duke and Ayensu, 1985). In Trobriands, the stem fibers are used for the treatment of Gonorrhoea. Hence, the present study is aimed to investigate ameliorative potential of C. asiaticum on hyperglycemia-mediated oxidative stress and hepatocyte dysfunction in alloxan induced diabetic rats.
2 Materials and methods
2.1 Plant material
C. asiaticum was collected in November month from Cauvery river basin, Thanjavur, Tamil Nadu, India and then transferred to PRIST University, Thanjavur, Tamil Nadu, India. It was taxonomically identified and authenticated by Rev. Dr. S. John Britto SJ, Director, The Rapinat Herbarium and Centre for Molecular Systematics, St. Joseph College (Autonomous), Tiruchirapalli, Tamil Nadu, India. The voucher specimen was deposited at the Rapinat Herbarium and the voucher number is RHCD BP15. The plant was extracted successfully with ethanol by using soxhlet apparatus (Chopra et al., 1981).
2.2 Preparation of ethanolic extract of C. asiaticum leaves
Fresh leaves of C. asiaticum were air dried in shade and powdered. The extraction was carried out by mixing the powdered (800 g) leaves with 1:2 (w/v) in 70% ethanol (v/v) for 2 days. The resulted extract was filtered and concentrated by rotary evaporator under reduced pressure and low temperature. The yield of extract was 15% (w/w) in terms of dried starting material.
2.3 Animals
Male albino Wistar rats of the same age group and body weight of 140–160 g were selected for all the experiments. The animals were housed in polypropylene cages at an ambient temperature of 25–30 °C and 45–55% of relative humidity with 12 h each of dark and light cycles. Rats were fed with pellet diet and water ad libitum. All the animal experimentations were premeditated and executed in compliance with the ethical norms approved by Ministry of Social Justices and Empowerment, Government of India and Institutional Animal Ethics Committee Guidelines (743/03/abc/CPCSEA dt 3.3.03).
2.4 Induction of diabetes for experimental animals
Albino rats were made diabetic by a single intraperitoneal (IP) injection of alloxan (200 mg/kg). Within 48 h after alloxan administration, blood glucose concentrations were measured via tail clip sampling. Animals with a blood glucose concentration 200 mg/dl were considered to be diabetic (Tanquilut et al., 2009).
2.5 Experimental design
The animals were randomly divided into three groups: Group I: control animals (normal, non-diabetic animals) (n = 6); Group II: alloxan diabetic animals (n = 6); and Group III: alloxan diabetic animals treated with ethanolic extract of C. asiaticum leaves (n = 6). Ethanolic extract of C. asiaticum leaves was given to experimental animals orally at a dosage of 200 mg/kg daily for 15 days. After 15 days of treatment, the animals were euthanized. Blood was collected and liver samples were dissected out and washed immediately with ice cold saline to remove as much blood as possible, and immediately stored at −20 °C until analysis. An extra sample of liver was excised and fixed in 10% formalin solution for histopathology analysis.
2.6 Biochemical assay
Aspartate transaminase, alanine transaminase, lactate dehydrogenase, alkaline phosphatase, acid phosphates, glucose, cholesterol, triglyceride, low density lipoprotein, very low density lipoprotein and high density lipoprotein were estimated by using commercially available kits according to the manufacturer’s instruction (AGGAPPE Diagnostic, Kerala and Ensure Biotech Pvt., Hyderabad, India): VLDL-cholesterol levels were calculated by using the following formula (Prakasam et al., 2003):
The activities of enzymatic antioxidants such as SOD (Kakkar et al., 1984), catalase (Sinha, 1982) and non-enzymatic antioxidants such as GSH (Ellman, 1959) were analysed. Levels of lipid peroxides (Berton et al., 1998) were determined.
2.7 Histopathological studies
A portion of the liver was cut into two to three pieces of approximately 6 mm3 size and fixed in phosphate buffered 10% formaldehyde solution. After embedding in paraffin wax, thin sections of 5 μm thickness of liver tissue were cut and stained with haematoxylin–eosin. The thin sections of liver were made into permanent slides and examined (Kleiner et al., 2005) under high resolution microscope with photographic facility and photomicrographs were taken.
2.8 Statistical analysis
Data were evaluated with SPSS/10 software hypothesis testing methods that included one way analysis of variance (ANOVA) followed by least significant difference (LSD) test. P values of less than 0.05 were considered to show statistical significance. All these results were expressed as mean ± SEM for six animals in each group.
3 Results
3.1 Body and organs weight
The body and organ weight of control and experimental rats were given in Table 1. The total body weight decreased during diabetes, but the individual organ weight like, liver, spleen and kidney increased in diabetic rats when compared with control rats. All the diabetic animals showed a progressive decrease in body weight throughout study period. These changes were alleviated to different extents by the rats administered with ethanolic extract of C. asiaticum. Values are expressed mean ± SEM of six animals.
Groups
Body weight (g)
Liver (g)
Kidney (g)
Spleen (g)
Control
134.5 ± 1.3
4.0 ± 0.01
0.87 ± 0.05
0.44 ± 0.01
Alloxan induced rats
107.0 ± 1.5
5.5 ± 0.04
1.02 ± 0.01
0.59 ± 0.01
Alloxan + C. asiaticum
128.0 ± 1.2*
4.5 ± 0.30*
0.90 ± 0.04*
0.53 ± 0.03*
3.2 Serum glucose level
The effect of ethanolic extract of C. asiaticum leaves on blood glucose levels is shown in Fig. 1. The significant increase in glucose concentration in the diabetic animals than that of the control rats indicates a positive response for diabetes. The oral administration of ethanolic extract of C. asiaticum significantly reduced the glucose level in serum when compared with alloxan induced rats. Blood glucose levels of normal, diabetic rats and treated animals were 117 ± 10.2, 230 ± 12.1 and 130 ± 9.3 mg/dl, respectively (P < 0.05).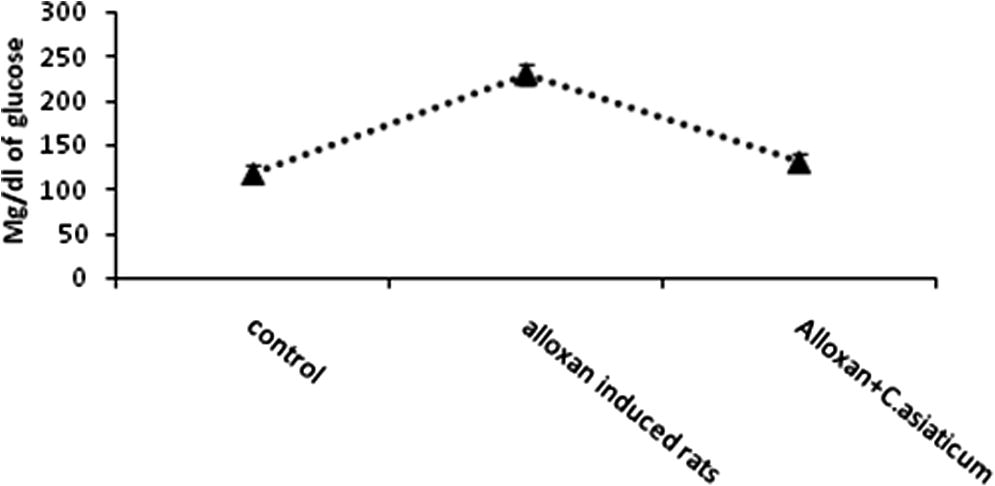
Effect of ethanolic extract of Crinum asiaticum on glucose level in control and experimental animals. Values are expressed mean ± SEM of six animals. ∗P < 0.05, as compared to diabetic induced.
3.3 Serum lipid profile
Variation in the serum cholesterol levels of all groups were observed at the end of the experimental periods (P < 0.05) as presented in Table 2. Serum cholesterol levels were significantly increased in diabetic rats when compared with control group of rats. However, rats administered with ethanolic extract of C. asiaticum leaves had significantly reduced the serum cholesterol levels in diabetic treated rats. In diabetic rats, a significant increase of serum LDL-cholesterol and VLDL-cholesterol was observed as compared with control rats. But decreases in HDL-cholesterol levels were observed. After, rats’ treatment with ethanolic extract of leaves of C. asiaticum was found to reduce LDL-cholesterol (P < 0.05) and VLDL-cholesterol and increase HDL-cholesterol in diabetic treated rats (P < 0.05). Triglyceride levels in serum were increased significantly in diabetic rats as compared with normal rats. However, these effects on rats administered with leaves of C. asiaticum were reversed (P < 0.05). Values are expressed mean ± SEM of six animals.
Groups
Cholesterol (mg/dl)
Triglyceride (mg/dl)
HDL (mg/dl)
LDL (mg/dl)
VLDL (mg/dl)
Control
83.00 ± 0.37
93.00 ± 0.37
26.68 ± 0.06
37.82 ± 0.03
18.60 ± 0.03
Alloxan induced rats
244.44 ± 0.03
177.33 ± 0.49
14.31 ± 0.003
194.44 ± 0.04
35.46 ± 0.29
Alloxan + C. asiaticum
107.00 ± 0.37*
102.00 ± 0.37*
21.43 ± 0.003*
65.17 ± 0.05*
20.40 ± 0.16*
3.4 Serum enzymes profile (AST, ALT, LDH, ALP and ACP)
The study evaluated the activity of AST, ALT, LDH, ALP and ACP in all the groups. A significant elevation of AST, ALT, LDH, ALP and ACP was observed in diabetic induced group of rats. Moreover, treatment of diabetic rats with ethanolic extract of C. asiaticum resulted in significant decreases in the levels of serum AST, ALT, LDH, ALP and ACP when compared with diabetic induced rats. A significant level of reduction in serum-marker enzymes (AST, ALT, LDH, ALP and ACP) were noticed due to the effect of ethanolic extract of C. asiaticum leaves as indicated in Table 3. Values are expressed mean ± SEM of six animals.
Groups
AST (U/l)
ALT (U/l)
LDH (U/l)
ACP (KA units)
ALP(KA units)
Control
69.9 ± 0.04
11.7 ± 0.05
160.8 ± 0.29
6.55 ± 0.01
5.56 ± 0.02
Alloxan induced rats
122.9 ± 0.24
29.1 ± 0.006
320.7 ± 0.03
11.58 ± 0.02
13.50 ± 0.22
Alloxan + C. asiaticum
87.6 ± 0.14*
23.5 ± 0.15*
180.9 ± 0.01*
7.06 ± 0.03*
6.65 ± 0.02*
3.5 Antioxidant and lipid peroxidation level
Table 4 illustrates the enzymic and non-enzymic antioxidant status in control and experimental group of rats. There were significant decreases that were observed in alloxan induced diabetic rates. However, administrations of ethanolic extract of C. asiaticum leaves reversed the decrease level to near normal. There was a significant elevation in serum LPO during diabetes when compared to the control group. Administration of ethanolic extract tends to bring the values to near normal. Values are expressed mean ± SEM of six animals.
Groups
MDA (nm/mg of protein)
Catalase (U/mg of protein)
SOD (U/mg of protein)
GSH (μg/mg of protein)
Control
1.62 ± 0.25
56.50 ± 0.22
2.63 ± 0.01
31.58 ± 0.19
Alloxan induced rats
5.36 ± 0.25
33.00 ± 0.36
1.05 ± 0.07
14.80 ± 0.08
Alloxan + C. asiaticum
2.15 ± 0.48*
48.17 ± 0.09*
1.92 ± 0.02*
30.35 ± 0.09*
3.6 Histological study
Fig. 2A–C illustrates histopathological investigation that also provided an essential evidence for the biochemical analysis. In control rats, liver sections showed normal hepatic cells with well preserved cytoplasm, well-known nucleus, and nucleus and central vein, where as alloxan induced group of rats showed the number of morphological, fatty changes, ballooning of hepatocytes and the loss of boundaries in the liver. However, treatment with C. asiaticum (200 mg/bw) improved the histological architecture when compared to alloxan induced diabetic rats.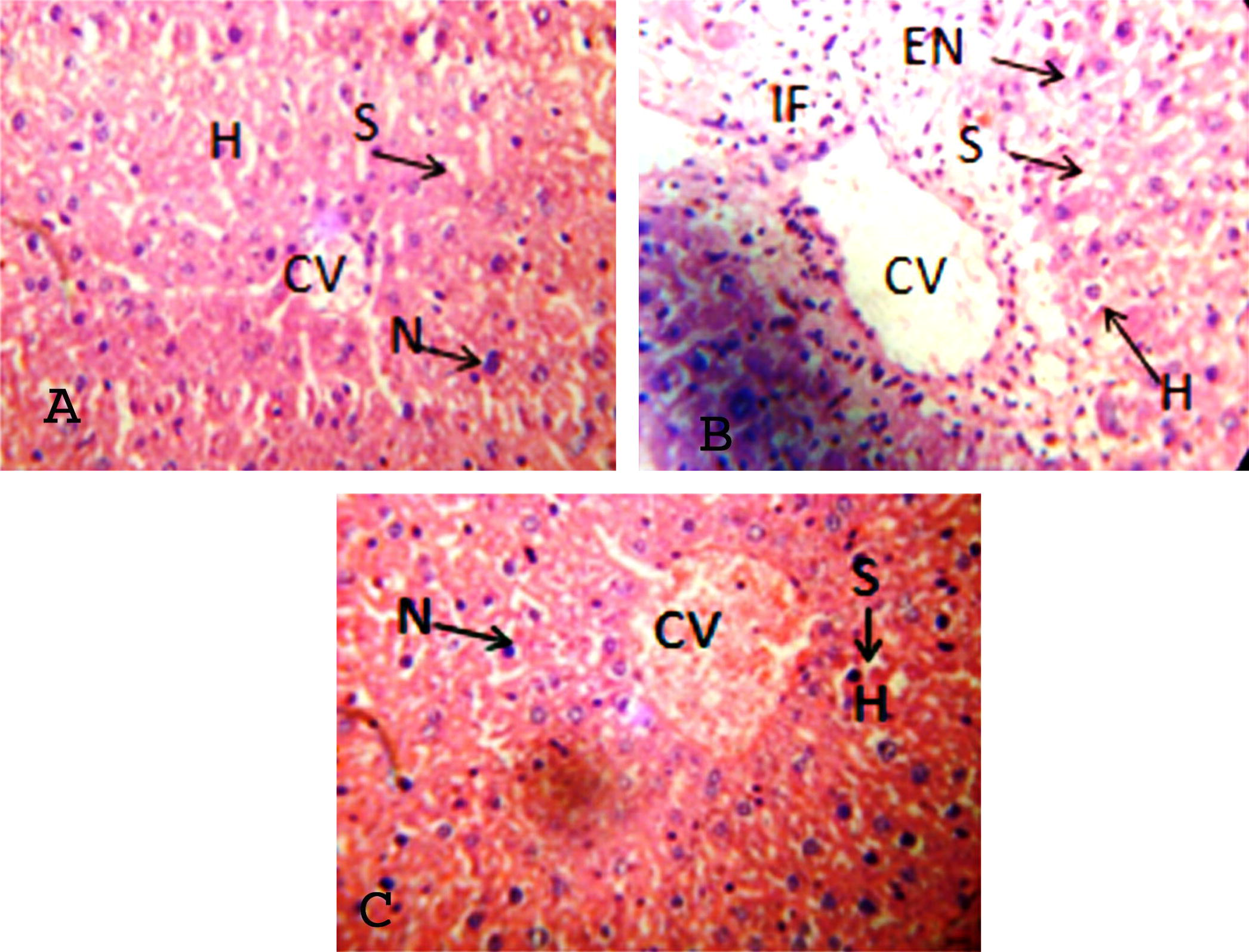
(A) Control histology liver showed intact hepatocytes (H), prominent nucleus (N), sinusoidal space (S) and central vein (CV). (B) Alloxan induced rats showed damage deformation in central vein, enlargement of nucleus (EN), infiltration of cells (IF), degeneration and loss of cell boundaries. Well developed hepatocyte with prominent nucleus and maintained sinusoidal space after Crinum asiaticum (C) treatment.
4 Discussion
Management of diabetes with agents devoid of any side effects is still a challenge to the medical system. This has led to an increase in the demand for natural products with antihyperglycemic activity and fewer side effects. Plants may act on blood glucose through different mechanisms, some of them may have insulin-like substances and some may inhibit insulinase activity (Bopanna et al., 1997. Some plants are involved in the stimulation of cells to produce more insulin (Chang and Johnson, 1980; Collier et al., 1987) and others may increase cells in the pancreas by activating regeneration of pancreatic cells (Chakravarthy et al., 1980).
Alloxan is the most commonly employed agent for the induction of diabetes in experimental animal model. There is increasing evidence that alloxan causes diabetes by rapid depletion of β cells by DNA alkylation and accumulation of cytotoxic free radicals that is suggested to result from initial islet inflammation, followed by infiltration of activated macrophages and lymphocyte in the inflammatory focus. It leads to a reduction in plasma insulin concentration leading to a stable hyperglycemia state (Szkudelski, 2001). In this study significant hyperglycemia was achieved after alloxan injection. Alloxan induced diabetic rats with more than 200 mg/dl of blood glucose level were considered to be diabetic and used for the study.
The present study evaluated the changes in body weight and organs weight in control, diabetic induced and treated animals every week for the entire period of the study, as decrease in body weight is considered as a marker for the development of diabetes. Our results indicated a considerable change in body and organs weight between alloxan induced and treated rats. The treatment with ethanolic extract of C. asiaticum showed a significant increase in body weight in alloxan induced diabetic rats.
The ethanol extract decreased the glucose level in alloxan induced rats. Such an effect may be accounted for, in part, by a decrease in the rats of intestinal glucose absorption, achieved by an extra pancreatic action including the stimulation of peripheral glucose utilization or enhancing glycolytic and glycogenic process with concomitant decrease in glycogenolysis and glyconeogenesis (Luzi et al., 1997).
In uncontrolled diabetes mellitus was observed an increase in total cholesterol, triglyceride, LDL, VLDL cholesterol with decrease in HDL cholesterol, which contribute to coronary artery disease (Arvind et al., 2002). Lipids play an important role in the pathogenesis of diabetes mellitus. Hyperlipidemia is a recognized consequence of diabetes mellitus demonstrated by the elevated levels of tissue cholesterol, phospholipids and free fatty acids (Chakravarthy et al., 1980; Bopanna et al., 1997; Ananthan et al., 2004). Diabetes-induced hyperlipidemia is attributable to the excess mobilization of fat from the adipose tissue due to the under utilization of glucose. The abnormal high concentration of serum lipids in diabetes is mainly due to the increase in the mobilization of free fatty acids from the peripheral depots since insulin inhibits the hormone sensitive lipase. On the other hand, glucagon, catecholamine, and other hormones enhance lipolysis. The level of serum lipids is usually raised in diabetes and such an elevation represents a risk factor for coronary heart disease. However, the levels of serum cholesterol and triglycerides were raised in diabetic rats but were lowered significantly with the treatment of C. asiaticum. It indicates that the ethanol extract of C. asiaticum is more useful in the treatment of diabetes as it has hypolipidemic effect. Moreover, its hypolipidemic effect could represent a protective mechanism against the development of atherosclerosis, which is usually associated with diabetes. Administration of ethanolic extract of C. asiaticum reduced total cholesterol, triglyceride, LDL, VLDL and improved HDL level.
Acid and alkaline phosphatase are a group of enzymes that catalyse the hydrolysis of inorganic phosphate. These enzymes are moderately increased in diabetic rats. Lactate dehydrogenase may be attributed to an increase in glucose utilization through the pentose phosphate pathway (Grover et al., 2000). Supplementation of C. asiaticum showed a significant decrease in enzyme level. The aspartate transaminase and alanine transaminase (AST and ALT) are important liver marker enzymes, which are increased during alloxan treatment. However, after treatment with ethanolic extract of C. asiaticum the level of these enzymes were reduced when compared with diabetic induced group of rats. Similarly, many scientists have reported that alloxan increased the activities of transaminases in both liver and serum of diabetics. This increased level of transaminases, which is active in the absence of insulin because of the availability of amino acid in the blood of diabetics is responsible for the increased gluconeogenisis and ketogenisis metabolism in diabetics (Sumana and Suryawanshi, 2001).
Lipid peroxides are the secondary products of oxidative stress and are unleashed as a result of the toxic effect of reactive oxygen species produced during lipid peroxidation in diabetes (Evans et al., 2002). The elevated levels of lipid peroxides in the liver tissues reveal the degree of lipid peroxidation in hepatic tissues and are considered as the indicator of hepatocyte damage (Albano, 2008). There are several reports in the literature that demonstrated the elevated levels of lipid peroxides in the hepatic tissues of experimental diabetic models (Youssef and McCullough, 2002). The results of the present study also are in line with the previous studies, in which C. asiaticum administration to diabetic group of rats notably declined the levels of liver lipid peroxides. This normalization may be accomplished by the antioxidant and free radical quenching nature of C. asiaticum.
GSH is a major non-protein thiol in living organisms, which plays a central role of co-ordinating the body’s antioxidants defence process. It is implicated in cellular defence against xenobiotics and naturally occurring deleterious compounds such as free radicals (Bandyopadhyay et al., 1999). The decrease in GSH level represents increased utilization for neutralizing free radicals. However, administration of C. asiaticum to alloxan induced rats attained near normal level. It indicates that the plant extract possessed free radical scavenging nature.
Superoxide dismutase and catalase are considered as primary enzymes since they are involved in the direct elimination of oxygen species (Aebi, 1983). Superoxide dismutase is an important defence enzyme, which catalyses the dismutation of superoxide radicals (Kakkar et al., 1984). And catalase is a hemoprotein, which catalyses the reduction of hydrogen peroxides and protects tissues from highly reactive hydroxyl radicals. The reduced activity of superoxide dismutase and catalase in the liver and kidney observed in diabetes may pose deleterious effects as a result of the accumulation of superoxide anion radicals and hydrogen peroxide.
A reduced activity of SOD and catalase in liver has been observed during diabetes and this may result in a number of deleterious effects due to the accumulation of superoxide radicals and hydrogen peroxide. Plants extract treated rats showed decreased lipid peroxidation, which is associated with an increased activity of SOD and CAT. This means that the extract can reduce reactive oxygen free radicals and improve the activities of the hepatic antioxidant enzymes.
The hepatic tissue protective nature of C. asiaticum in control and experimental groups of rats were established by histological study. The diabetogenic potential of alloxan is responsible for a progressive development of the hepatic tissues lesions. The major pathological alteration observed in the diabetic liver was the swelling of the capillaries, loss of cell boundaries and central vein, the thickening of the capillary walls. Most of the diabetic liver sections revealed the augmented fibrosis with plasmacytic infiltrate triggering distortion of typical concentric arrangement of hepatocytes around the central vein. The present studies address the histological findings in hepatocytes and their correlation with liver marker enzymes after C. asiaticum treatment in alloxan induced diabetic rats. There was also congestion of portal vessels and sinusoids and the veins were also dilated (Kleiner et al., 2005).
Therefore, the present investigation imparts novel information on the hepatoprotective activity of C. asiaticum in alloxan induced diabetic rats and provides more evidence that the protective effects are, possibly, due to a decline in oxidants by the hepatic tissues. Further the oral administration of C. asiaticum to alloxan induced diabetic rats exhibited significant ameliorative potential probably by attenuating the hyperglycemia-mediated oxidative stress. Further detailed studies are in progress to elucidate the exact mechanism by which C. asiaticum elicits its modulatory effects.
References
- Oxidative mechanisms in the pathogenesis of alcoholic liver disease. Mol. Aspects Med.. 2008;29:9-16.
- [Google Scholar]
- Modulatory effects of Gymnema montanum leaf extract on alloxan induced oxidative stress in Wistar rats. Nutrition. 2004;20:280-285.
- [Google Scholar]
- Reactive oxygen species: oxidative damage and pathogenesis. Curr. Sci.. 1999;5:658.
- [Google Scholar]
- Comparative analysis of the protective effects of melatonin and vitamin E on streptozocin-induced diabetes mellitus. J. Pineal Res.. 2002;32:225-230.
- [Google Scholar]
- The effect of vitamin E acetate on ultraviolet induced mouse skin carcinogenesis. Mol. Carcinog.. 1998;23:175-184.
- [Google Scholar]
- Antidiabetic and antihyperglycemic effects of Neem seed kernel powder alloxan diabetic rabbits. Indian J. Pharmacol.. 1997;29:162-167.
- [Google Scholar]
- Pancreatic beta cell regeneration a novel antidiabetic mechanism of Petercarpus marsupium. Indian J. Pharmacol.. 1980;12:123-128.
- [Google Scholar]
- Effect of garlic on carbohydrate metabolism and lipid synthesis in rats. J. Nutr.. 1980;110:931-936.
- [Google Scholar]
- Alternation of hepatic antioxidant enzyme activities and lipid profile in streptozotocin-induced diabetic rats by supplementation of dandelion water extract. Clin. Chim. Acta. 2002;317:109-117.
- [Google Scholar]
- Glossary of Indian Medicinal Plants. New Delhi: CSIR Publication; 1981. p. 50
- Partial purification and characterization of insulin like material from spinach and Lemna gibba G3. J. Boil. Chem.. 1987;262:6237-6238.
- [Google Scholar]
- Inactivation of the antibiotics and the dissemination of resistance genes. Science. 1994;264:375-382.
- [Google Scholar]
- Duke, J.A., Ayensu, E.S., 1985. Medicinal Plants of China, vol. 1. Reference Publications Inc.
- Oxidative stress and stress-activated signaling pathways: a unifying hypothesis of type 2 diabetes. Endocr. Rev.. 2002;23:599-622.
- [Google Scholar]
- Goeltenboth, F., Holdworth, D.K., Sakulas, H., Thredgold, H., Woodley, E., Yauieb, A., 1991. In: Woodly, E. (Ed.), Medical Plants of Papua New Guinea. Wau Ecology Institute, F.&T. Mullerbader, Germany.
- Anti-hyperglycemic effect of Eugenia jambolana and Tinospora cordifolia in experimental diabetes and their effect on key metabolic enzymes involved in carbohydrate metabolism. J. Ethnopharmacol.. 2000;73:461-470.
- [Google Scholar]
- Antioxidants in Diabetes-induced Complications (ADIC) Study Group, Effects of cod liver oil on tissue antioxidant pathways in normal and streptozotocin-diabetic rats. Cell Biochem. Funct.. 2002;20:297-302.
- [Google Scholar]
- Evaluation of antibacterial activity and phytochemical analysis of crinum asiaticum. Int. J. Curr. Res.. 2010;1:035-040.
- [Google Scholar]
- A modified spectrophotometric assay of superoxide dismutase. Indian J. Biochem. Biophys.. 1984;21:130-132.
- [Google Scholar]
- Kiritikar, K.R., Basu, B.D., 1999. Indian Medicinal Plants, second ed. International Book Distributors, Dehradun, pp. 205–206.
- Nonalcoholic steatohepatitis clinical research network, design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313-1321.
- [Google Scholar]
- Anti-diabetic activity of fruits of Terminalia chebula on streptozotocin induced diabetic rats. J. Health Sci.. 2006;52:283-291.
- [Google Scholar]
- Hepatic changes in the acute phase of streptozotocin (SZ)-induced diabetes in mice. Exp. Toxicol. Pathol.. 2004;55:467-480.
- [Google Scholar]
- Characterization of the rhesus monkey galactocerebrosidase (GALC) cDNA and gene and identification of the mutation causing globoid cell leukodystrophy (Krabbe disease) in this primate. Genomics. 1997;42:319-324.
- [Google Scholar]
- Hypolipidemic effect of Casearia esculenta root extracts in streptozotocin-induced diabetic rats. Pharmazie. 2003;58:828-832.
- [Google Scholar]
- Chronic hyperglycemia enhances PEPCK gene expression and hepatocellular glucose production via elevated liver activating protein/liver inhibitory protein ratio. Diabetes. 2005;54:976-984.
- [Google Scholar]
- Increased nitric oxide levels as an early sign of premature aging in diabetes. Free Radic. Biol. Med.. 2003;35:1240-1251.
- [Google Scholar]
- Effect of Vinca rosea extracts in treatments of alloxan diabetes in male albino rats. Indian J. Expt. Biol.. 2001;39:748-759.
- [Google Scholar]
- The mechanism of alloxan and streptozotocin action in B-cells of the rat pancreas. Physiol. Res.. 2001;50:536.
- [Google Scholar]
- Hypoglycemic effect of Lagerstroemia speciosa (L.) Pers. on alloxan-induced diabetic mice. J. Med. Plants Res.. 2009;3(12):1066-1071.
- [Google Scholar]
- Wiart, C., 2000. Medicinal Plants of Southeast Asia. Pelanduk Publications, (M) Sdn. Bhd.
- Diabetes mellitus, obesity, and hepatic steatosis. Semin. Gastrointest. Dis.. 2002;13:17-30.
- [Google Scholar]







