Translate this page into:
Synthesis, spectroscopic studies and fungicidal activity of some diorganotin(IV) with 2-[(phenylcarbonyl)amino]propanoato
*Tel.: +964 7901782816 emad_yousif@hotmail.com (Emad Yousif)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Available online 17 December 2010
Abstract
New diorganotin(IV) complexes of the type Ph2SnL2, Bu2SnL2 and Me2SnL2 of the ligand 2-[(phenylcarbonyl)amino]propanoic acid (HL) ligand formed by reaction of benzoyl chloride with alanine in presence of sodium hydroxide. The prepared complexes were characterized by infrared, nuclear magnetic resonance (1H, 13C, 119Sn NMR) spectral data and elemental analysis and conductance measurements. From the spectral measurements, monomer structures for the complexes were proposed. Bidentate and Octahedral geometry was proposed for the complexes prepared. Bioassay result in vitro tests for fungicidal activity show that all prepared compounds display good activity to Gibberela, Cercospora arachidicola, Physolospora piricola and Fusarium oxysporum. Moreover, the Ph2SnL2 show a higher inhibition percentage then diorganotin carboxylate.
Keywords
Diorganotin(IV)
Carboxylate
IR
Mulitinnuclear NMR (1H, 13C and 119Sn)
Fungicidal activity
1 Introduction
In the past 3 decades the chemistry of tin compounds has gained considerable importance, both in basic research and in industrial applications. There are many interesting aspects of inorganic and organic tin chemistry discussed in various reviews (Chandrasekhar et al., 2002). Organotin compounds have gained an edge over other organometallics due to their bioavailability in the ecosystem and entrance into the food chain, the fact they are less harmful to the environment and their pharmaceutical applications, including antitumour and anticancer uses. For these reasons, tin and its derivatives are in commercial use more than any other element (Shahid et al., 2003; Hussain et al., 2009).
Organotin carboxylates have attracted considerable attention due to their wide applications in many fields (Tian et al., 2005), such as biological activity and potential antineoplastic and antituberculosis agents (Yousif et al., 2009a; Arks and Balko, 2005) PVC stabilizers (Thoonen et al., 2004; Kuzelova and Vymazal, 1999; Tabassum and Pettinari, 2006) and anti-tumour drugs (Angiolini et al., 2006) as well as polymer catalysts (Katsoulakou et al., 2008). Vast studies have been focused on organotin carboxylates and many of them have been characterized recently either by single crystal structure determination or by spectroscopy (Masood et al., 2004; Pellerito et al., 2010). As a continuation of our series on the synthesis and characterization of organotin carboxylates (Yousif et al., 2009b; Hameed et al. 2009; Farina et al. 2009a; Najeeb et al., 2009; Farina et al., 2009b), we report the diverse fields of applications of organotin complexes, we have synthesized new ligand 2-[(phenylcarbonyl)amino]propanoic acid and its complexes, diphenyltin(IV)bis(2-[(phenylcarbonyl)amino]propanoato) (Ph2SnL2), dibutyltin(IV)bis(2-[(phenylcarbonyl)amino]propanoato) (Bu2SnL2) and dimethyltin(IV)bis(2-[(phenylcarbonyl)amino]propanoato) (Me2SnL2).
2 Experimental
2.1 Synthesis of 2-[(phenylcarbonyl)amino]propanoic acid
One gram of alanine was dissolved in (25 ml) of 5% NaOH solution in a conical flask. To this mixture, benzoyl chloride (2.25 ml) was added in five portions in (0.5 ml increments) and shaken vigorously until all the chloride reacted, acidified with diluted hydrochloric acid and the crude product was washed with cold ether. Finally, the desired product was recrystallized from ethanol.
2.2 Preparation of complexes
Complexes were synthesized by dissolving the free ligand (2 mmol) in hot toluene and adding the diorganotin salts (1 mmol) to the solution. The solution was refluxed for 6 h with a magnetic stirrer and then cooled and filtered. The filtrate was reduced under vacuum to a small volume and solid was precipitated by the addition of petroleum ether, dried at 60 °C and recrystallized from ethanol.
2.3 Instrumentation
Elemental C, H and N analysis were carried out on a Fison EA 1108 analyzer, the FTIR spectra in the range (4000–370) cm−1 cut were recorded as potassium bromide discs using a Perkin–Elmer spectrophotometer GX, molar conductance measurements were made in anhydrous DMF at 25 °C using Inolop-Cond Level 1 WTW, atomic absorption measurements of the prepared complexes were obtained using Shimadzu 680 cc-flame. The 1H, 13C and 119Sn nuclear magnetic resonance spectra were recorded on a jeol 400 MHz spectrometer, relative to the internal standard tetramethylsilane (TMS). Melting points were determined in open capillary tubes using an electrothermal 9300 digital melting point apparatus.
3 Results and discussion
The ligand was prepared by the reaction of benzoyl chloride with alanine in presence of sodium hydroxide. Table 1 shows the physical data for the ligand and the prepared complexes. The purity of the ligand and its complexes were checked by TLC using Silica Gel-G as the adsorbent. The conductance of these complexes has been recorded in DMF at room temperature in the range 7–18 ohm−1 cm2 mol−1, suggesting their non-electrolytic nature. The data of CHNS and Tin analysis were obtained using flame atomic absorption technique. The calculated values were in a good agreement with the experimental values.
Compound
Color
%Yield
M.P. (°C)
Found (calcd.) (%)
C
H
N
Sn
HL
White
85
165–166
62.32(62.17)
5.81(5.74)
7.17(7.25)
–
Ph2SnL2
White
78
142–145
57.65(58.47)
4.99(4.60)
4.12(4.26)
17.71(18.06)
Bu2SnL2
White
81
157–156
53.79(54.48)
6.44(6.20)
3.82(4.54)
19.93(19.23)
Me2SnL2
White
73
171–172
50.06(49.56)
4.06(4.92)
5.53(5.25)
22.41(22.27)
3.1 Infra-red spectroscopy
The FTIR spectrum of the ligand, shows characteristic stretching absorption bands at 3371 cm−1, 3328 cm−1, 1611 cm−1 and 1332 cm−1 assigned to υ(OH), υ(N–H), υ(COO) asym. and υ(COO) sym. groups, respectively.
The COO stretching vibrations are important to predict the bonding mode of the ligand. According to Lebl et al. the values of Δυ [Δυ = υ asym.(COO)−υ sym.(COO)] can be divided into three groups (Reeves and White, 1983); (a) In compounds where Δυ(COO) > 350 cm−1, the carboxylate group binds in a monodentate fashion. However, other very weak intra- and intermolecular interactions cannot be excluded. (b) When Δυ (COO) < 200 cm−1, the carboxylate groups of these compounds can be considered to be bidentate. (c) In compounds where Δυ (COO) > 200 cm−1 and <350 cm−1 an intermediate state between monodentate and bidentate (anisobidentate) occurs. It has also been suggested that the Δυ (COO) value in the chelating mode is less than the Δυ (COO) in a bridging mode (Shahid et al., 2009). From the preceding discussion it is proposed that the investigated compounds have chelating-type carboxylates. The disappearance of hydrogen from the hydroxyl group on complexation indicates that the complexation is through the oxygen atom. The bands for υ(Sn–C) and υ(Sn–O) are assigned in the range of (531–556) and (443–447) cm−1, respectively. The IR data of the complexes are shown in Table 2. The table lists the stretching frequency (υ) for some of the characteristics groups exhibited by the ligand and complexes.
Compound
υ(O–H)
υ(COO) asym.
υ(COO) sym.
υ(Sn–C)
υ(Sn–O)
HL
3771
1611
1332
–
–
Ph2SnL2
–
1540
1323
531
444
Bu2SnL2
–
1539
1318
534
443
Me2SnL2
–
1546
1320
556
447
3.2 Nuclear magnetic resonance
The 1H NMR spectra for all compounds were recorded in [2H6] DMSO using tetramethysilane as the internal standard. The data are compiled in Table 3. The conclusion drawn from 1H NMR studies of a few compounds lend further support to suggested the formation of 2-[(phenylcarbonyl)amino]propanoic acid chelate. Ligand (HL) gives a single resonance near δ 8.71 ppm attributable to the N–H proton. The spectra also exhibit a singlet –OH peak at 9.24 ppm due to hydroxyl group. The hydroxyl resonance is absent in the spectra of the complexes indicting deprotonation and coordination of Tin to the oxygen. There is a small upfield shift of the aromatic protons resonances of the ligand upon chelation with the diorganotin(IV) moiety (Yousif et al., 2009a). The complexes Ph2SnL2, Bu2SnL2 and Me2SnL2 show additional signals. The methyltin (Sn-CH3) accurse at 1.35, 1.33 and 1.31 ppm as on the sharp singlet at integrates for the protons accompanied by satellites due to the 1H–119Sn coupling that corresponds to the hydrogen atom of the methyl protons for the Me2SnL2. In dibutyltin(IV) complex the butyl protons appears as a multiplet and a triplet in the range of 1.55–0.72 ppm due –CH2CH2CH2CH3 group. The aromatic protons in Ph-Sn appears in the 7.06–7.17 ppm.
Compound
O–H
N–H
C–H aromatic
C–(2)H aliphatic
HL
9.24
8.71
7.51–7.81
3.86
Ph2SnL2
–
8.69
7.44–7.77
3.82
Bu2SnL2
–
8.68
7.46–7.81
3.84
Me2SnL2
–
8.67
7.31–7.78
8.81
Table 4 shows the most relevant 13C and 119Sn NMR data. Due to scant solubility of the ligand and its complexes in the CDCL3, their spectra were recorded in [2H6] DMSO. The C⚌O resonance group of the complexes at (160.22–160.31) ppm where shifted downfield compared with the position in the free ligand, which appeared at 165.32 ppm. It is most likely that shift is due to the decrease of electron density at carbon atoms when oxygen is bonded to the metal ion. This observation lends further evidence that the complexation occurred through the oxygen atoms of the carboxylate group. 119Sn NMR spectra for the complexes were recorded in [2H6] DMSO. Diorganotin(IV) complexes gave resonance at −442.86, −436.83 and −431.49 ppm related to Ph2SnL2, Bu2SnL2 and Me2SnL2, respectively which is well within the range for six-coordinated complexes. In Ph2SnL2 the 119Sn resonance appears, as usual, at a lower field region than in Bu2SnL2 and Me2SnL2 in spite of the greater electron withdrawing capability of the phenyl group. The resonance at (−442.86 ppm), probably reflects the greater shielding ability of the phenyl group.
Compound
C⚌O amide
C⚌O acid
C–H aromatic
C–H2 aliphatic
119Sn
HL
165.32
170.43
127.74–131.55
42.65
Ph2SnL2
160.22
165.26
126.86–133.76
41.64
−442.86
Bu2SnL2
160.24
166.14
126.25–131.94
42.13
−436.83
Me2SnL2
160.31
164.19
127.4–131.44
41.38
−431.49
On the basis of the preceding discussion, the structure of the complexes suggested as follows: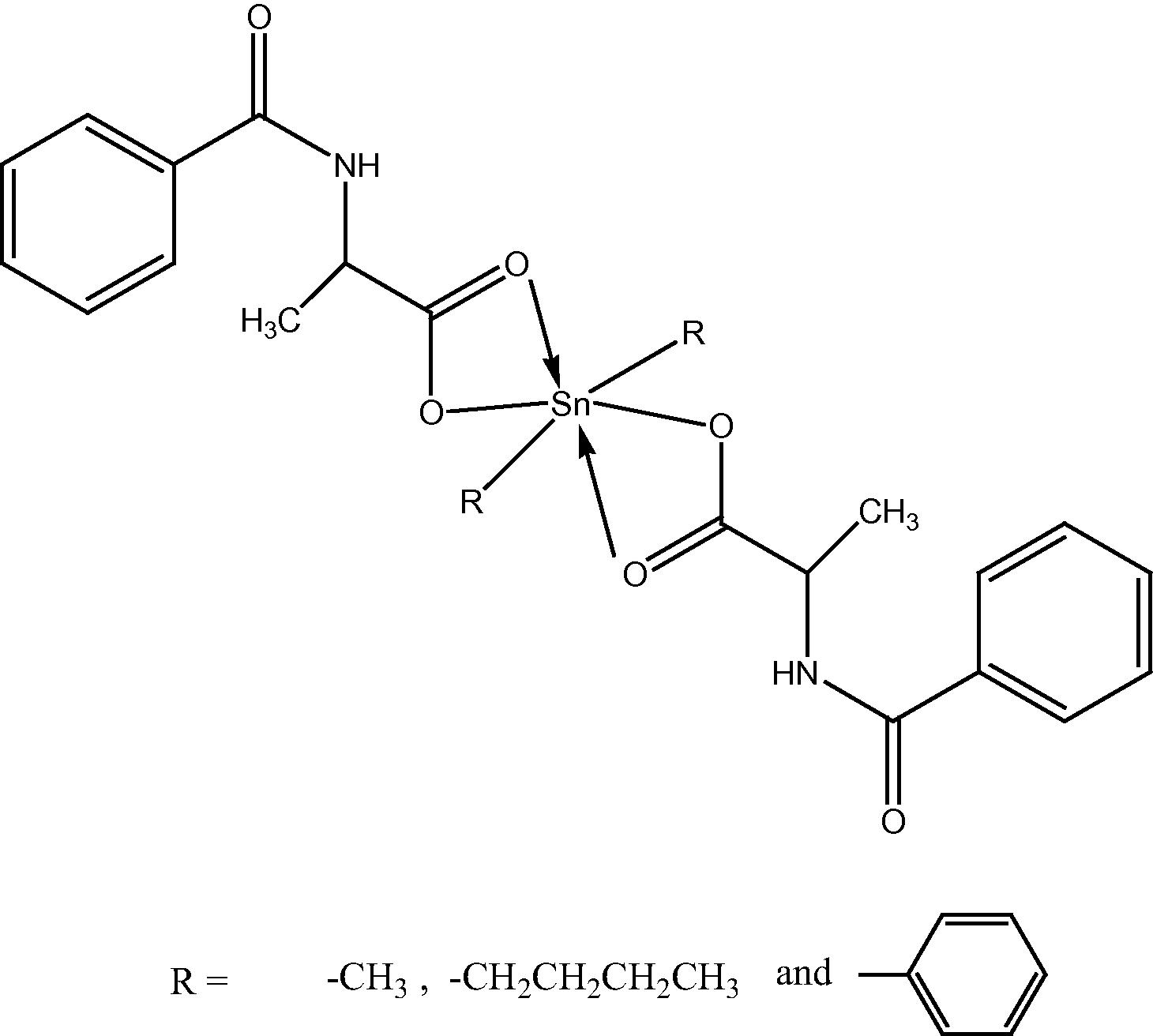
3.3 Biological activity
On the other hand, the newly prepared compounds were screened for their in vitro antifungal activity against Gibberela, Cercospora arachidicola, Physolospora piricola and Fusarium oxysporum in DMF by the serial plate dilution method (Reeves and White, 1983). All the fungal strains were clinical isolates, identified with conventional morphological and biochemical methods. Sabouraud’s agar media was prepared by dissolving peptone (1 g), d-glucose (4 g), and agar (2 g) in distilled water (100 ml) and adjusting the pH to 5.7. Normal saline was used to make a suspension of the spore of fungal strain for lawning. A loopful of a particular fungal strain was transferred to 3 ml saline to get a suspension of the corresponding species. Agar media (20 ml) was poured into each petri dish. Excess suspension was decanted and the plates were dried by placing in an incubator at 37 °C for 1 h. Antifungal activity was determined by measuring the diameter of the inhibition zone. The zone of inhibition observed after respective incubation was measured and percent inhibition of the compounds were calculated. The results are presented in the Table 5 and show that all compounds display certain activity to P. piricola at a low concentration. Moreover, the Ph2SnL2 is more active than the other diorganotin derivatives. In addition, Ph2SnL2 shows the highest inhibition percentage for P. piricola (80.7%) in vitro. (Table 5).
Compound
Inhibition ratio (%) (50 ppm)
Me2SnL2
Bu2SnL2
Ph2SnL2
Gibberela
17.9
20.1
23.3
Cercospora arachidicola
33.8
57.1
36.8
Physolospora piricola
48.4
57.3
80.7
Fusarium oxysporum
11.6
26.2
65.3
4 Conclusion
The ligand 2-[(phenylcarbonyl)amino]propanoic acid was successfully synthesized. The ligand was treated with different diorganotin(IV) salts to afford the corresponding complexes. It may conclude that the ligand coordinated through carboxylate to the Tin atom leading to the formation of a four membered ring chelate. Octahedral geometry was proposed for the prepared complexes. Biological activity data have shown that the reported complexes have a significant biological activity against Gibberela, C. arachidicola, P. piricola and F. oxysporum.
Acknowledgements
The authors acknowledge the University Kebangsaan Malaysia, IIE, SRF, and Department of Chemistry, College of Science, Al-Nahrain University for their encouragement.
References
- Assessment of their catalytic activity in transesterification reactions. J. Organomet. Chem.. 2006;691:3043-3052.
- [Google Scholar]
- Thermal stabilisation of poly(vinyl chloride) by organotin compounds. Poly. Degrad. Stab.. 2005;88:46-51.
- [Google Scholar]
- Synthesis, structural and fungicidal studies of some diorganotin(IV) with benzamidoleucine. Aust. J. Basic Appl. Sci.. 2009;3(3):1670-1673.
- [Google Scholar]
- Synthesis structure and biological activity of some diorganotin (IV) with benzamidomethionine. Mod. Appl. Sci.. 2009;3(4):215-218.
- [Google Scholar]
- Synthesis and characterization and fungicidal activity of triorganotin (IV) with benzamidomethionine. Eur. J. Sci. Res.. 2009;34(2):212-217.
- [Google Scholar]
- Synthesis, structural elucidation and X-ray analysis of triphenyltin(IV) (2-(2,3-dimethylanilino)nicotinate) J. Iran. Chem. Soc.. 2009;6:769-775.
- [Google Scholar]
- Diorganotin(IV) complexes of dipeptides containing the a-aminoisobutyryl residue (Aib): preparation structural characterization antibacterial and antiproliferative activities of (n-Bu)2 Sn(H_1L)(LH = H-Aib-l-Leu-OH, H-Aib-l-Ala-OH) J. Inorg. Biochem.. 2008;102:1397-1405.
- [Google Scholar]
- Contribution to the study of the thermal stabilization of PVC/SAN blends. Eur. Polymer J.. 1999;35:361-364.
- [Google Scholar]
- 1H, 13C, 119Sn NMR, mass, mossbauer and biological studies of tri-, di- and chlorodiorganotin(IV) carboxylates. Turk. J. Chem.. 2004;28:75-85.
- [Google Scholar]
- Synthesis and fungicidal activity of some diorganotin(IV) with benzamidocysteine. J. Al-Nahrain Univ.. 2009;12(1):24-28.
- [Google Scholar]
- Diorganotin(IV) N-acetyl-l-cysteinate complexes: synthesis, solid state solution phase, DFT and biological investigations. J. Inorg. Biochem.. 2010;104:750-758.
- [Google Scholar]
- Principles of Methods of Assaying Antibiotics in Pharmaceutical Microbiology (third ed.). Oxford: Blackwell; 1983.
- Organotin(IV) complexes of aniline derivatives-part-II-synthesis and spectroscopic characterization of organotin(IV) derivatives of 2-[(4-bromoanilino)carboxy]benzoic acid. Turk. J. Chem.. 2003;27:209-215.
- [Google Scholar]
- Synthesis characterization and thermal analysis of organotin(IV) derivatives of 4-(N-maleoyl)butanoate. Turk. J. Chem.. 2009;26:589-597.
- [Google Scholar]
- Chemical and biotechnological developments in organotin chemotherapy. J. Organomet. Chem.. 2006;691:1761-1766.
- [Google Scholar]
- Synthetic aspects of tetraorganotins and organotin(IV) halides. J. Organomet. Chem.. 2004;689:2145-2157.
- [Google Scholar]
- Synthesis, characterization and biological activity of triorganotin 2-phenyl-1,2,3-triazole-4-carboxylates. J. Inorg. Biochem.. 2005;99:1646-1652.
- [Google Scholar]
- Synthesis, characterization and fungicidal activity of some diorganotin(IV) with 2-thioacetic-5-phenyl-1,3,4-oxadiazole. J. Fundam. Sci.. 2009;5(2):94-98.
- [Google Scholar]
- Structure and fungicidal activity of somediorganotin(IV) with benzamidophenylalanine. ARPN. 2009;4(9):39-42.
- [Google Scholar]







