Translate this page into:
Improvement of the photostabilization of PMMA films in the presence 2N-salicylidene-5-(substituted)-1,3,4-thiadiazole
*Corresponding author. Tel.: +964 7901782816 emad_yousif@hotmail.com (Emad Yousif)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Available online 15 September 2010
Abstract
The photostabilization of poly(methy methacrylate) (PMMA) films by 2N-salicylidene-5-(substituted)-1,3,4-thiadiazole compounds was investigated. The PMMA films containing concentration of complexes 0.5% by weight were produced by the casting method from chloroform solvent. The photostabilization activities of these compounds were determined by monitoring the hydroxyl index with irradiation time. The changes in viscosity average molecular weight of PMMA with irradiation time were also tracked (using benzene as a solvent). The quantum yield of the chain scission (Φcs) of these complexes in PMMA films was evaluated and found to range between 8.74 × 10−5 and 4.65 × 10−5. Results obtained showed that the rate of photostabilization of PMMA in the presence of the additive followed the trend:
SN > SC > SB > SI > SP
According to the experimental results obtained, several mechanisms were suggested depending on the structure of the additive. Among them, UV absorption, peroxide decomposer and radical scavenger for photostabilizer mechanisms were suggested.
Keywords
Photochemistry
PMMA
UV–Vis spectroscopy
Photostabilizer
UV absorber,1,3,4-thiadiazole
Schiff base
1 Introduction
There is a great interest at present in the photo-oxidative degradation of polymeric materials because macromolecules have increasingly widespread commercial applications. Polymeric synthetic, semisynthetic and natural are when exposed to the environment (Grassie and Scott, 1985).
All commercial organic polymers degrade in air when exposed to sunlight as the energy of sunlight is sufficient to cause the breakdown of polymeric C–C bonds as a consequence of degradation. The resulting smaller fragments do not contribute effectively to the mechanical properties and the polymeric article because brittle. Thus the life of thermoplastics for outdoor applications becomes limited due to weathering (Andrady et al., 1988).
Almost all synthetic polymers require stabilization against the adverse effect with the development of synthetic resins it became necessary to look for ways and means to prevent, or at least reduce, the damage caused by the environmental parameters light, air and heat. This can be achieved through addition of special, chemicals, light stabilizers or UV stabilizers, that have to be adjusted to the nature of the resin and the specific application considered.
The photostabilization of polymers may be achieved in many ways. The following stabilizing systems have been developed. Which depend on the action of stabilizer. (1) Light screeners, (2) UV absorbers, (3) excited state quenchers, (4) peroxide decomposers, and (5) Free radical scavengers, of these it is generally believed that types 3, 4 and 5 are the most effective (Yousif et al., 2009).
Most or, indeed all stabilizers are believed to be multifunctional in their mode operation this view is complicated by the fact that mechanism involved in photo-oxidation and these, in turn depend n the polymer structure and other variables, such as manufacturing, operation, processing, conditions, etc. (Harper et al., 1974).
As part of our on-going research on the photostabilization of polymers, the photostabilization of PMMA was studied using 2N-salicylidene-5-(substituted)-1,3,4-thiadiazole compounds.
2 Experimental
2.1 Materials
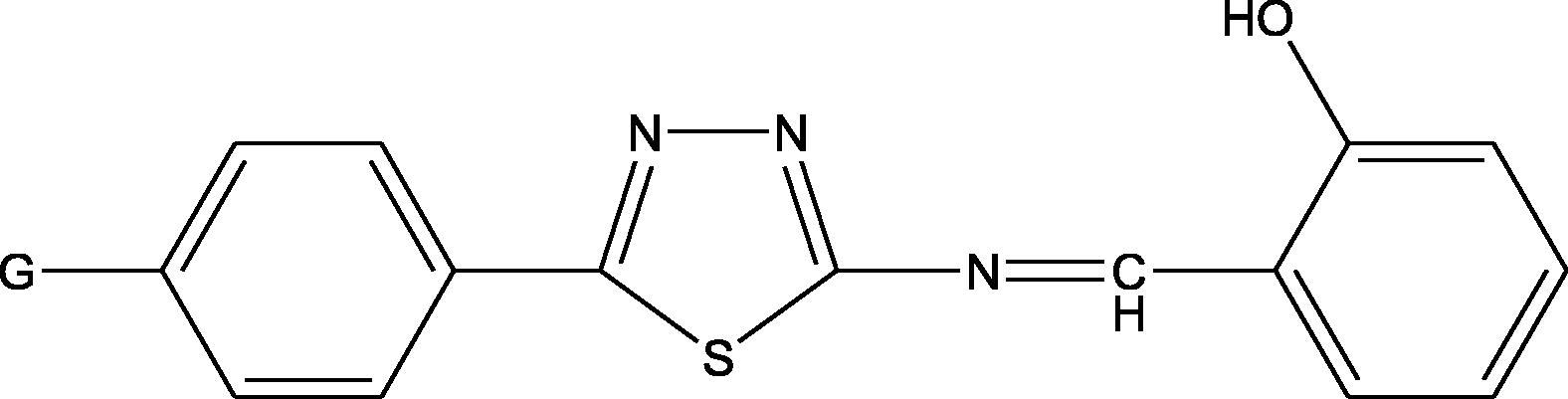
The following 2N-salicylidene-5-(substituted)-1,3,4-thiadiazole compounds were all prepared by the method previously described by Salimon et al. (2010).
2N-salicylidene-5-(P-nitro-phenyl)-1,3,4-thiadiazole
SN
2N-salicylidene-5-(P-chloro-phenyl)-1,3,4-thiadiazole
SC
2N-salicylidene-5-(P-bromo phenyl)-1,3,4-thiadiazole
SB
2N-salicylidene-5-(P-iodo-phenyl)-1,3,4-thiadiazole
SI
2N-salicylidene-5-(phenyl)-1,3,4-thiadiazole
SP
3 Experimental techniques
3.1 Films preparation
Commercial PMMA supplied by Petkim Company (Turkey) was re-precipitated from chloroform solution by alcohol several times and finally dried under vacuum at room temperature for 24 h. Fixed concentrations of PMMA solution (5 g/100 ml) in chloroform were used to prepare polymer films with 40 μm thickness (measured by a micrometer type 2610 A, Germany). The prepared complexes (0.5% concentrations) were added to the films starting at 0 concentrations (blank). It was necessary to control the hygrometry and the rate of evaporation of solvent during casting to maintain good optical quality and very limited turbidity. The film transmission should be greater than 80% in the near-UV range. The films were prepared by evaporation technique at room temperature for 24 h. To remove the possible residual chloroform solvent, film samples were further dried at room temperature for 3 h under reduced pressure. The films were fixed on stands especially used for irradiation. The stand is provided with an aluminum plate (0.6 mm in thickness) supplied by Q-panel company.
3.2 Irradiation experiments
3.2.1 Accelerated testing technique
Accelerated weather-meter Q UV tester (Q panel, company, USA), was used for irradiation of polymers films. The accelerated weathering tester contains stainless steel plate, which has two holes in the front side and a third one behind. Each side contains a lamp (type Fluorescent Ultraviolet Lights) 40 W each. These lamps are of the type UV-B 313 giving spectrum range between 290 and 360 nm with a maximum wavelength 313 nm. The polymer film samples were vertically fixed parallel to the lamps to make sure that the UV incident radiation is perpendicular on the samples. The irradiated samples were rotated from time to time to ensure that the intensity of light incident on all samples is the same.
3.3 Photodegradation measuring methods
3.3.1 Measuring the photodegradation rate of polymer films using infrared spectrophotometery
The degree of photodegradation of polymer film samples was followed by monitoring FTIR spectra in the range 4000–400 cm−1 using FTIR 8300 Shimadzu Spectrophotometer. The position of hydroxyl absorption is specified at 3430 cm−1 (Rabek and Ranby, 1975). The progress of photodegradation during different irradiation times was followed by observing the changes in hydroxyl peak. Then hydroxyl index (IOH) was calculated by comparison of the FTIR absorption peak at 3430 cm−1 with reference peak at 1450 cm−1, respectively. This method is called band index method which includes (Rabek and Ranby, 1975):
Actual absorbance, the difference between the absorbance of top peak and base line (A Top Peak – A Base Line) is calculated using the Base Line method (Rabek and Ranby, 1975).
3.3.2 Determination of average molecular weight ( ) using viscometry method
The viscosity property was used to determine the average molecular weight of polymer, using the Mark–Houwink relation (Mark, 2007).
The intrinsic viscosity of a polymer solution was measured with an Ostwald U-tube viscometer. Solutions were made by dissolving the polymer in a solvent (g/100 ml) and the flow times of polymer solution and pure solvent are t and t0 respectively. Specific viscosity (ηsp) was calculated as follows:
The single–point measurements were converted to intrinsic viscosities by the relation (2).
By applying Eq. (5), the molecular weight of degraded and undergirded polymer can be calculated. Molecular weights of PMMA with and without additives were calculated from intrinsic viscosities measured in benzene solution using Eq. (2).
The quantum yield of main chain scission (ϕcs) (Nakajima et al., 1990) was calculated from viscosity measurement using the following relation:
4 Results and discussion
The 2N-salicylidene-5-(substituted)-1,3,4-thiadiazole compounds were used as additives for the photostabilization of PMMA films. In order to study the photochemical activity of these additives for the photostabilization of PMMA films, the hydroxyl index was monitored with irradiation time using IR spectrophotometry. The irradiation of PMMA films with UV light of wavelength, λ = 313 nm led to a clear change in the FTIR spectrum. Appearance of bands in 3430 cm−1 was attributed to the formation of the hydroxyl group (Andrady and Searle, 1989).
The absorption of the hydroxyl group was used to follow the extent of polymer degradation during irradiation. This absorption was calculated as hydroxyl index. It is reasonable to assume that the growth of hydroxyl index is a measure to the extent of degradation. However, in Fig. 1, the IOH of SP, SI, SB, SC and SN showed lower growth rate with irradiation time with respect to the PMMA control film without additives. Since the growth of hydroxyl index with irradiation time is lower than PMMA blank, as seen in Fig. 1, it is suitable to conclude that these additives might be considered as photostabilizers of PMMA polymer. Efficient photostabilizer shows a longer induction period. Therefore, the SN is consider as the most active photostabilizer, followed by SC, SB, SI and SP which is the least active.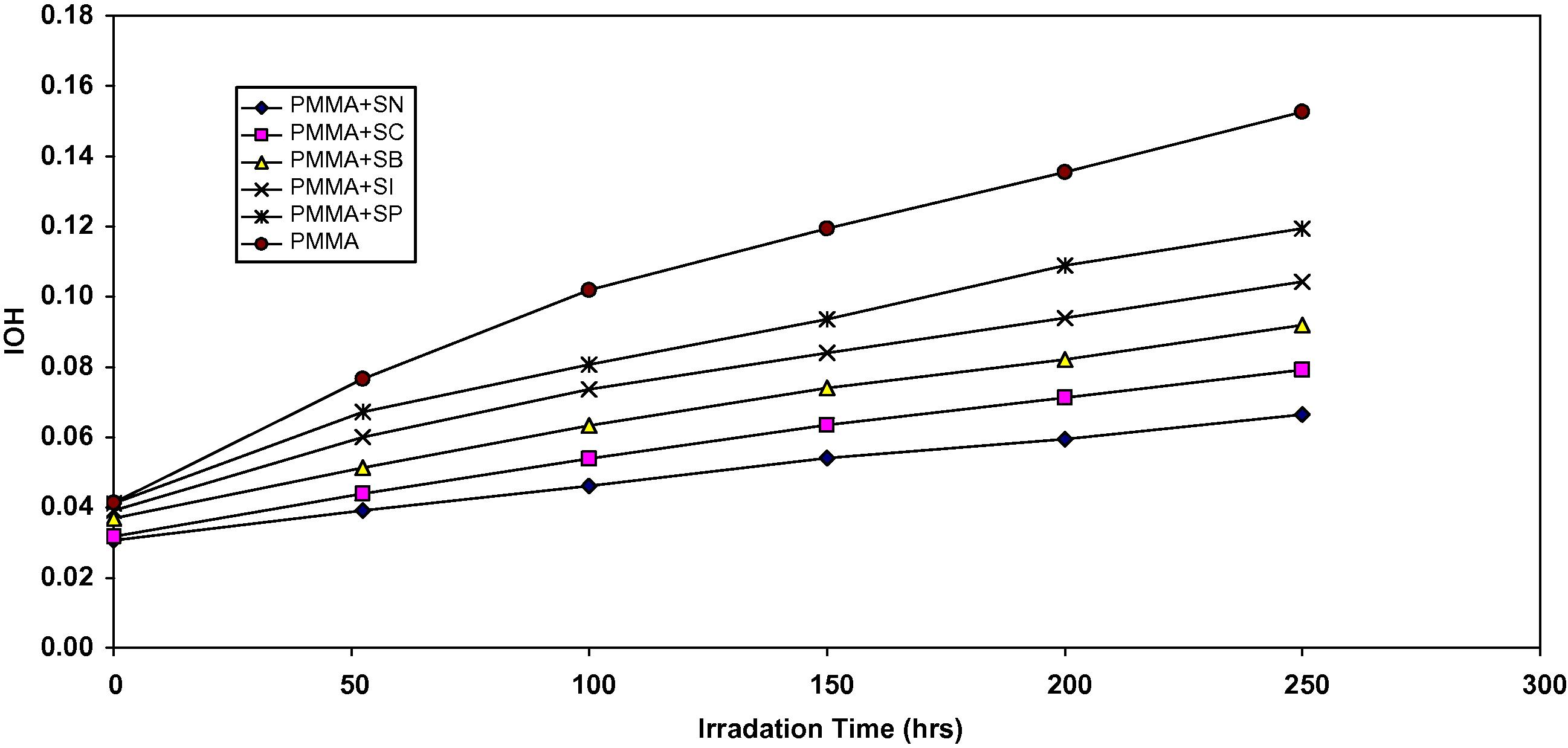
The relationship between the hydroxyl index and irradiation time for PMMA films (40 μm thickness). Containing different additives, concentration of additives are fixed at 0.5% by weight.
4.1 Variation of PMMA molecular weight during photolysis in the presence of by 2N-salicylidene-5-(substituted)-1,3,4-thiadiazole compounds
Analysis of the relative changes in viscosity average molecular weight (
) has been shown to provide a versatile test for random chain scission. Fig. 2 shows the plot of
versus irradiation time for PMMA film with and without 0.5% (wt/wt) of the selected additives, with absorbed light intensity of 1.052 × 10−8 ein dm−3 s−1.
is measured using Eq. (3) with benzene as a solvent at 25 °C.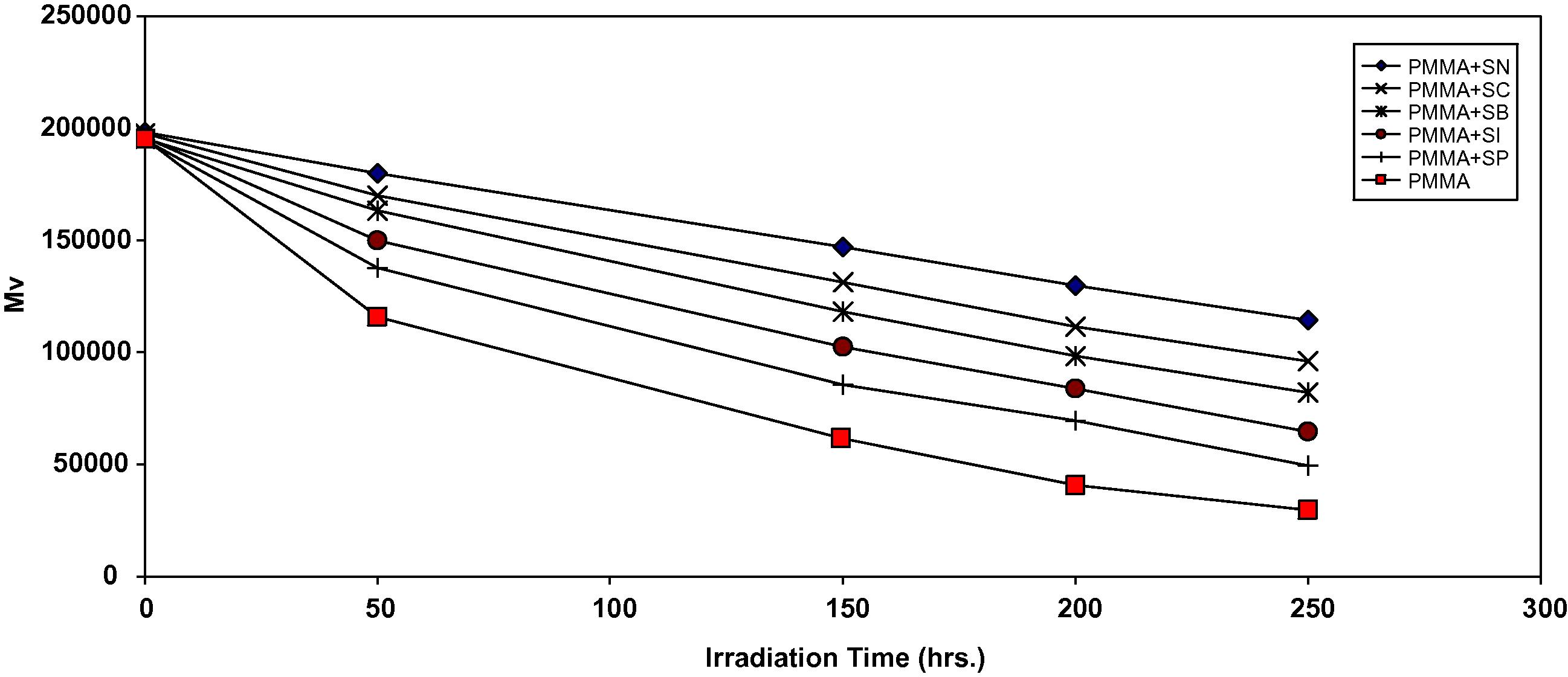
Changes in the viscosity average molecular weight (
) during irradiation of PMMA films (40 μm) (control) and with 0.5 wt% of additives.
It is worth mentioning that traces of the films with additives are not soluble in chloroform indicating that cross-linking or branching in the PMMA chain does occur during the course of photolysis (Mori et al., 1997). For better support of this view, the number of average chain scission (average number cut per single chain) (S) (A. Shyichuk and White, 2000) was calculated using the relation:
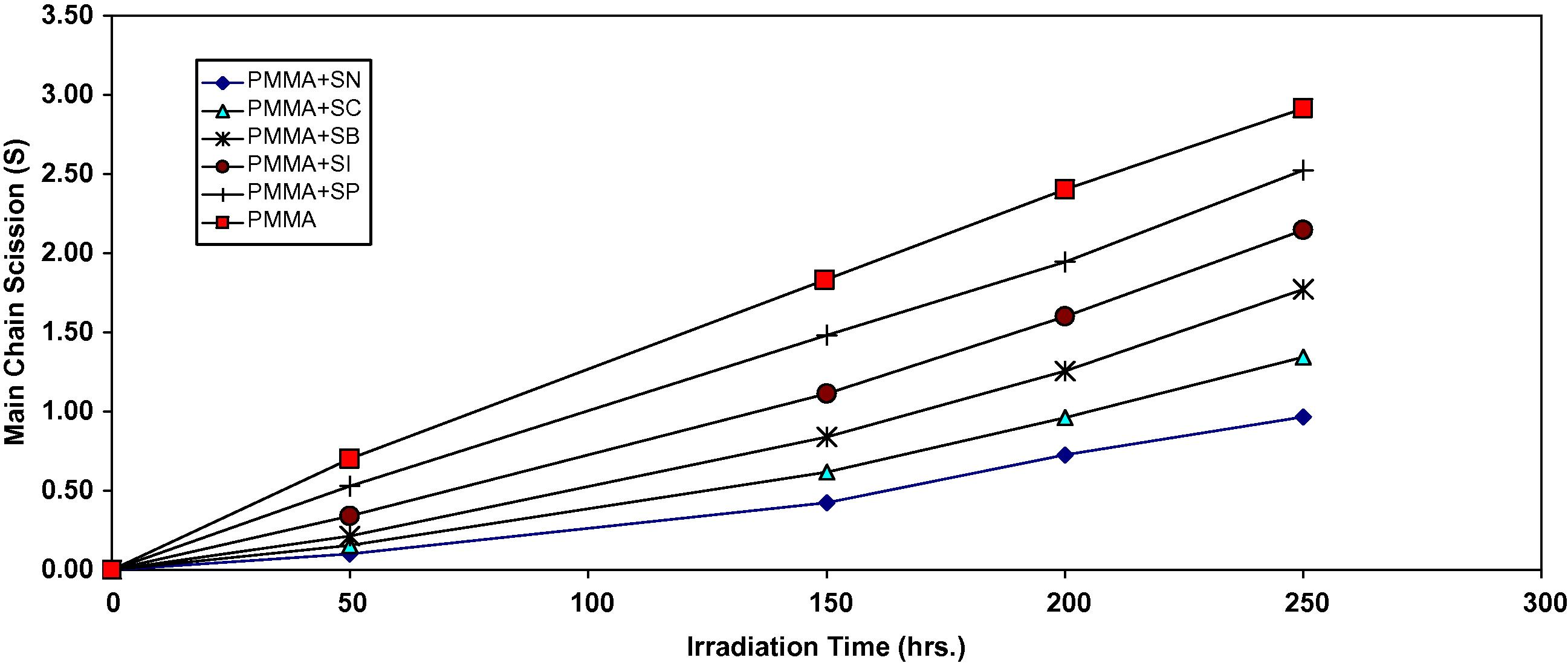
Changes in the main chain scission (S) during irradiation of PMMA films (40 μm) (control) and with 0.5 wt% of additives.
For randomly distributed weak bond links (Gugumus, 1990) which break rapidly in the initial stages of photodegradation, the degree of deterioration α is given as:
The plot of α as a function of irradiation time is shown in Fig. 4.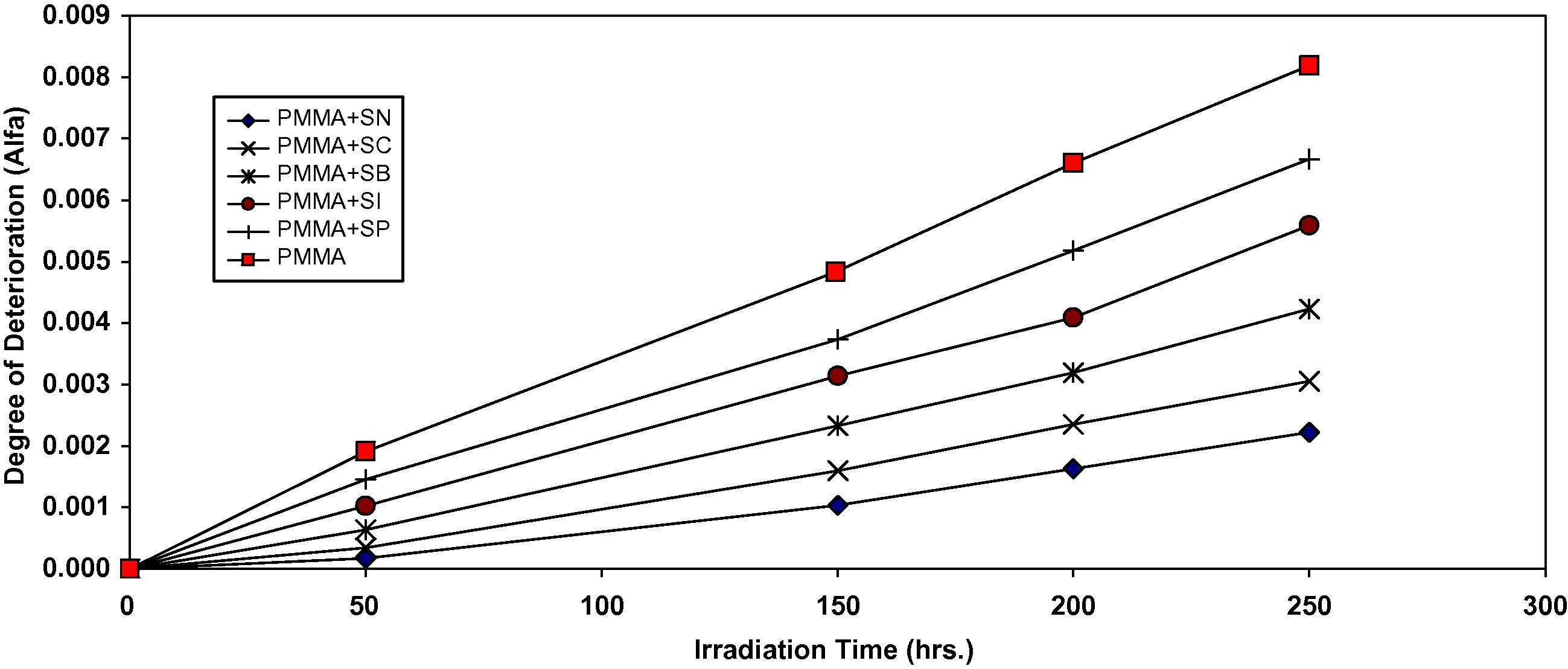
Changes in the degree of deterioration (α) during irradiation of PMMA films (40 μm) (control) and with 0.5 wt% of additives.
The values of α of the irradiated samples are higher when additives are absent and lower in the presence of additives compared to the corresponding values of the additive free PMMA. In the initial stages of photodegradation of PMMA, the value of α increases rapidly with time, these indicators indicates a random breaking of bonds in the polymer chain.
Another way of degradation reaction characterization is the measurement of the quantum yield of the chain scission (Φcs) (Yousif et al., 2010). The quantum yield for chain scission was calculated for PMMA films with and without 0.5% (wt/wt) of additive mentioned above using relation (6). The Φcs values for complexes are tabulated in Table 1.
Additive (0.5 wt.%)
Quantum yield of main chain scission (Φcs)
PMMA +SP
4.65E-05
PMMA +SI
4.36E-05
PMMA +SB
7.21E-05
PMMA +SC
7.56E-05
PMMA +SN
8.74E-05
PMMA (blank)
4.53E-04
The Φcs values for PMMA films in the presence of additive are less than that of additive free PMMA (blank), which increase in the order: SN > SC > SB > SI > SP
4.2 Suggested mechanisms of photostabilization of PMMA by 2N-salicylidene-5-(substituted)-1,3,4-thiadiazole compounds
Through the overall results obtained, the efficiency of thiadiazole derived Schiff base compounds as photostabilizers for PMMA films can be arranged according to the change in the hydroxyl concentration as a reference for the comparison as shown in Fig. 1. SN > SC > SB > SI > SP.
Schiff base stabilize PMMA by different mechanisms such as UV absorber, screener or by radical scavenger.
These stabilizers provide very good long-term stability and are usually referred to these mechanisms.
The most probable mechanisms involved in a photostabilization is the change energy of absorbed photon to the intramolecular proton transfer. This reaction may occur by two proposed cycles Schemes 1 and 2, the first passes by intersystem crossing (ICS) process to the excited triplet state, while the second is referred to internal conversion (IC) process to the ground state.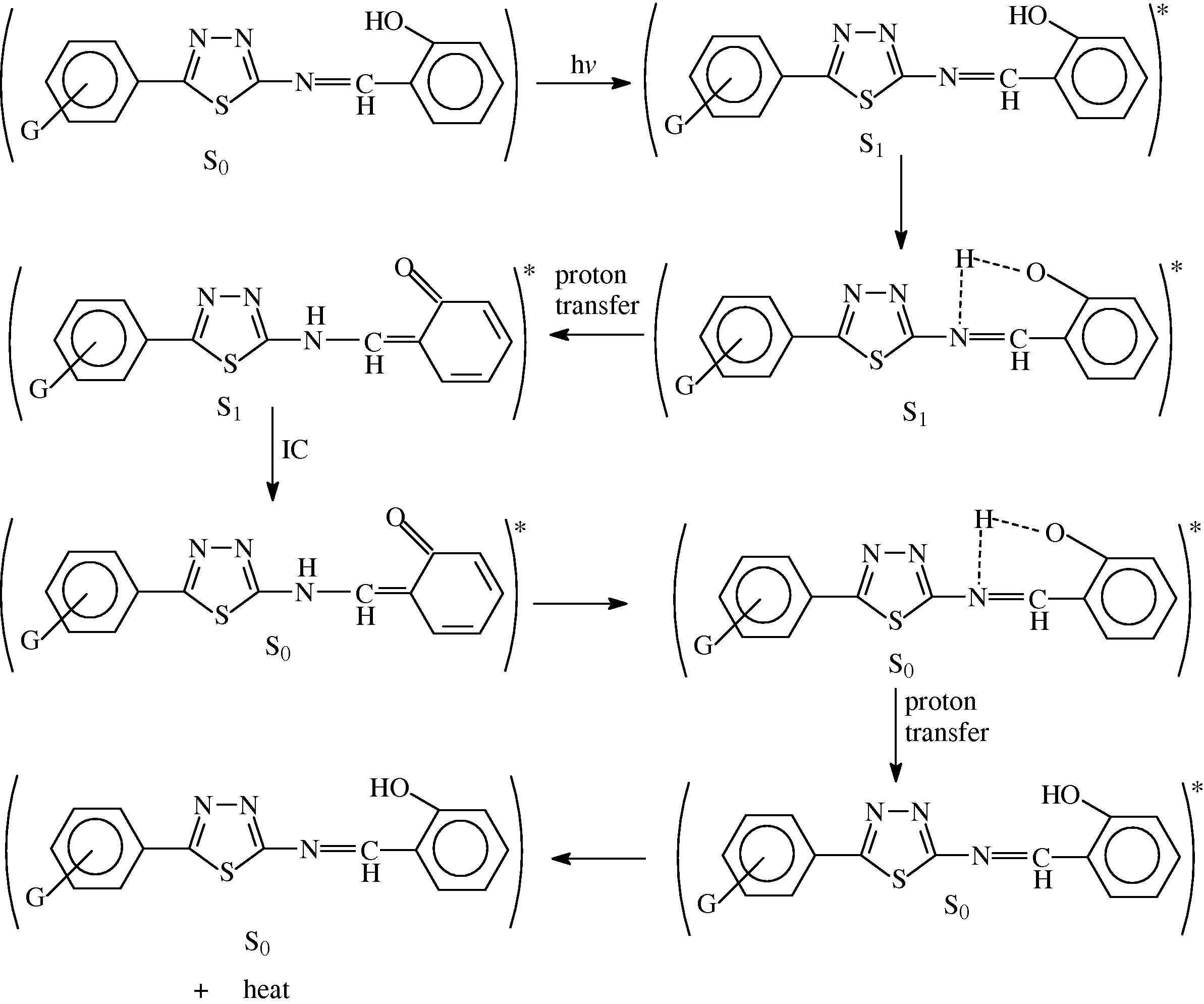
The suggested mechanism of photostabilization of PMMA by SE, SN, SE and SP compounds through absorption of UV light and dissipation light energy as heat.
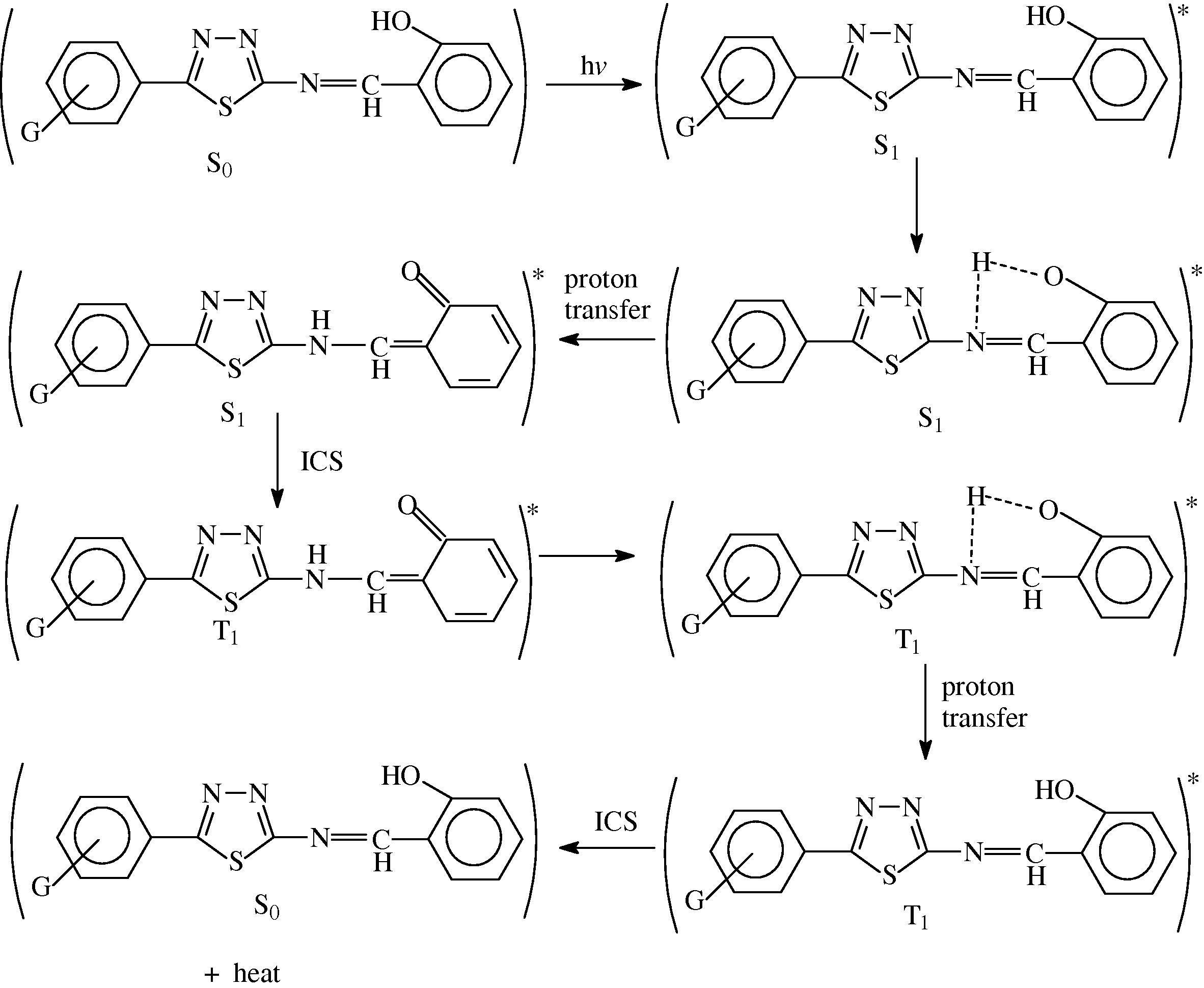
The suggested mechanism of photostabilization of PMMA by SE, SN, SE and SP compounds through absorption of UV light and dissipation light energy as heat.
Other mechanism explains the use of this compound as photostabilizer is by charge separated species which could be form of the excited state such a structure would allow dissipation of energy through rotation on increased vibration about the central bond as shown in Scheme 3.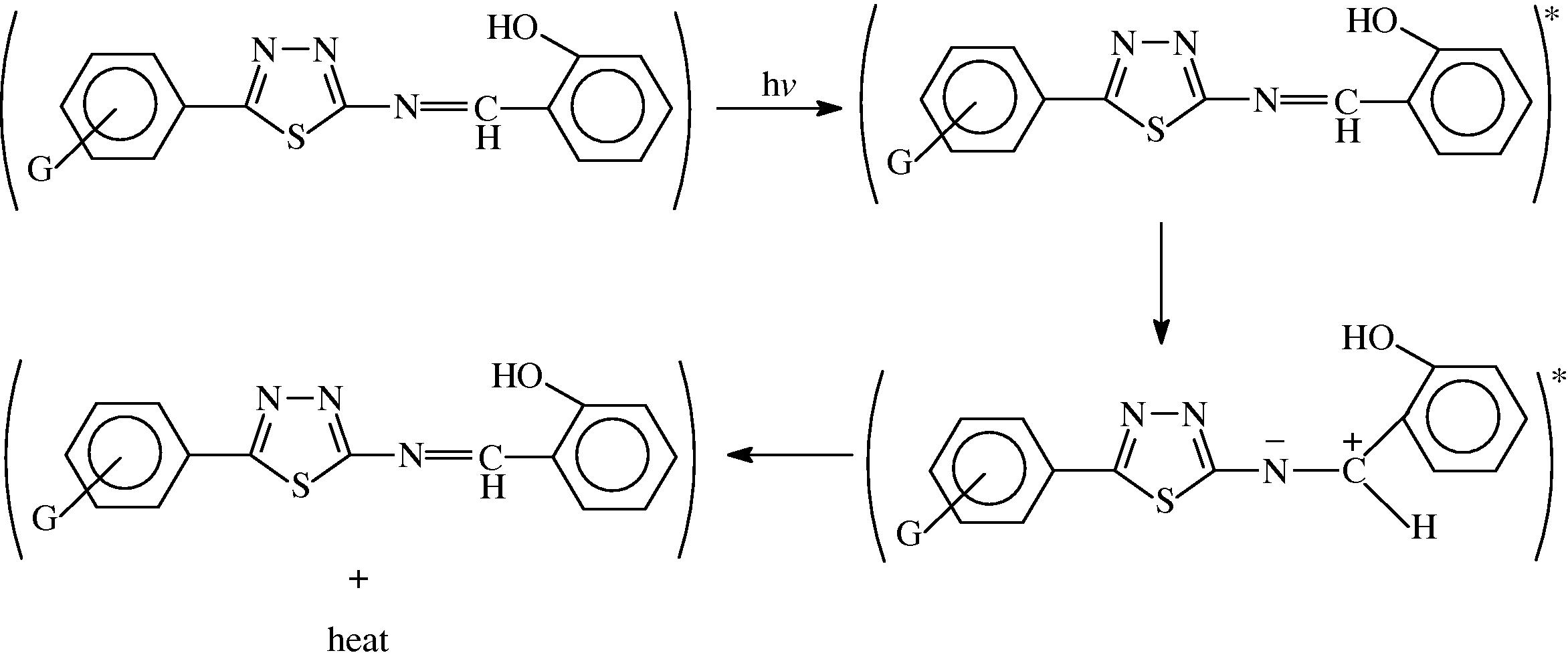
The suggested mechanism of photostabilization of PMMA by SE, SN, SE and SP compounds through absorption of UV light and dissipation light energy as heat.
The hydroxyl group of the additive might acts as radical scavenger for photostabilization process. Therefore this Schiff bases, besides acting as UV absorber they may also act as radical scavenger additives (Rasheed et al., 2009), Scheme 4.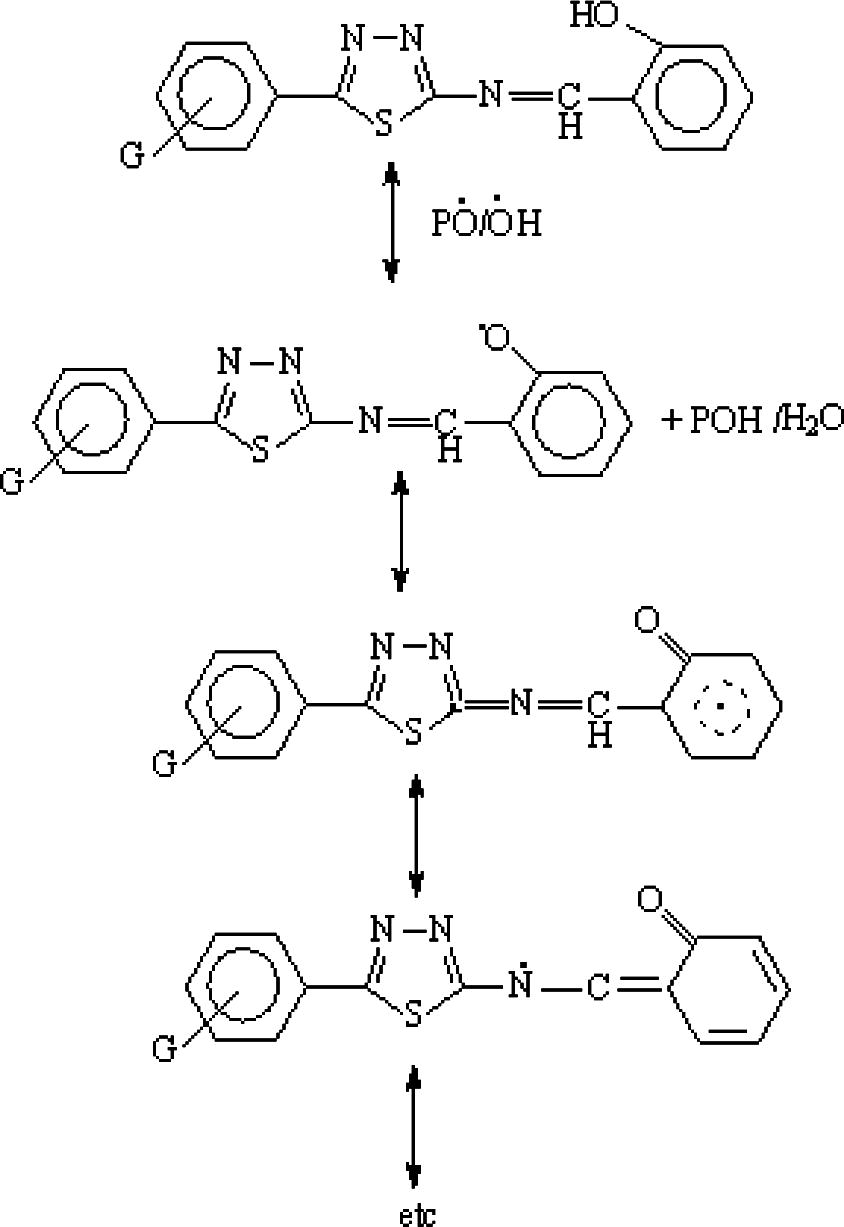
The suggested mechanism of photostabilization of PMMA by SE, SN, SE and SP compounds as radical scavenger.
The rings of 1,3,4-thiadiazole play a role in the mechanism of the stabilizer process by acting as UV absorber. The UV light absorption by these additives containing 1,3,4-thiadiazole dissipates the UV energy to harmless heat energy Scheme 5. Furthermore, this ring plays a role in resonating structures conjugation of radical in peroxide decomposer Scheme 5, which sport it function as a photostabilizer (Yousif et al., 2007).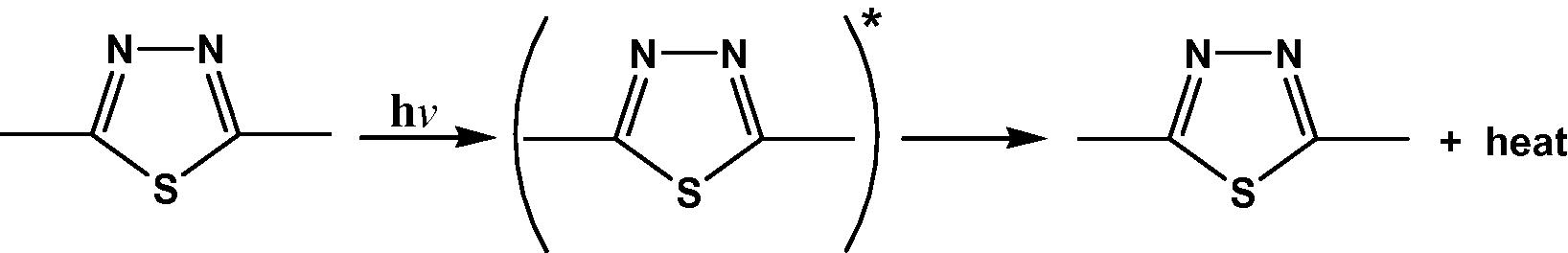
Suggested mechanism of photostabilization of 1,3,4-thiadiazole as UV absorber.
5 Conclusions
In the work described in this paper, the photostabilization of PMMA films using 2N-salicylidene-5-(substituted)-1,3,4-thiadiazole compounds were studied. These additives behave successfully as photostabilizer for PMMA films. The additives take the following order in photostabilization activity according to their decrease in hydroxyl index for PMMA films.
SN > SC > SB > SI > SP
These additives stabilize the PMMA films through UV absorption or screening, peroxide decomposer and radical scavenger mechanisms. The SN compound was found to be the more efficient in photostabilization process according to the photostability and mechanisms mentioned above. These mechanisms support the idea of using 2N-salicylidene-5-(substituted)-1,3,4-thiadiazole compounds as commercial stabilizer for PMMA.
Acknowledgments
The authors acknowledge the IIE, SRF and to the supporting staffs of School of Chemical Sciences and Food Technology, Faculty of Science and Technology, University Kebangsaan Malaysia and Department of Chemistry, College of Science, Al-Nahrain University for their encouragement.
References
- J. Photochem. Photobiol. B Biol.. 1988;46:96-103.
- J. Appl. Poly. Sci.. 1989;37:2789-2802.
- Polymer Degradation and Stabilization. London: Cambridge University Press; 1985.
- Mechanism of Polymer Degradation and Stabilization. Amsterdam: Elsevier; 1990.
- J. Appl. Polym. Sci.. 1974;18:2802-2805.
- Physical Properties of Polymers Handbook. New York: Springer; 2007.
- Die Angewandte Makromolekulare Chemie. 1997;64(1):89-99.
- J. Appl. Poly. Sci.. 1990;41:889-1363.
- Asian J. Chem.. 2010;22:5289-5296.
- J. Appl. Poly. Sci.. 2000;77(13):3015-3023.
- Photodegradation, Photo-oxidation and Photostabilization of Polymers. New York: John Wiley; 1975.
- Eur. J. Sci. Res.. 2009;30:464-477.
- Turk. J. Chem.. 2009;33:339-410.
- J. Al-Nahrain Univ.. 2007;10:7-12.
- Inter. J. Chem.. 2010;2:65-80.







