Translate this page into:
Kinetics of the oxidative decolorization of some organic dyes utilizing Fenton-like reaction in water
*Corresponding author hmadian@hotmail.com (Hesham A.A. Medien)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
The decolorization kinetics of some organic pollutants in water such as commercial dyes, namely, Malachite green (MG), Rhodamine B (RB), Methylene blue (MB) and Crystal violet (CV) were studied using a Fenton-like reagent. The effects of different parameters like the initial dye concentration, Fe3+, concentration of H2O2, pH of the solution, reaction temperature, and added electrolytes on the kinetics were determined. The results indicate that the optimum pH for the decolorization of all dyes examined is in the range between 3 and 4. The rate of decolorization showed remarkable dependence on the initial Fe3+ concentration. Using dilute dye solutions in the range of (0.30–1.50 × 10−5 mol dm−3) the rate increased as the Fe3+ concentration was increased from 1.5 × 10−4 to 6.0 × 10−4 mol dm−3, and becomes practically constant when it exceeds 6.0 × 10−4 mol dm−3. Below 1.5 × 10−4 mol dm−3 of Fe3+, the decolorization reaction is too sluggish to account for any practical significance. But the decolorization rate of all examined dyes decreased at high Fe3+ concentrations (⩾1.0 × 10−3 mol dm−3). There is an optimum H2O2 concentration in the range of 0.01–0.04 mol dm−3 that is effective for decolorization of the dyes. It was observed that the presence of halide salts at the same concentration level substantially decreased the rate and the extent of decolorization, whereas for nitrate salt, there is an increase in the extent of dye decolorization. An increase in the extent of decolorization of the dye was observed when the reaction temperature was raised. The results will be useful for designing the treatment systems of various dye-containing wastewaters. It was also found that the decolorization of the dyes undergoes a fast reaction than the mineralization.
Keywords
Kinetic
Oxidation
Decolorization
Organic dyes
1 Introduction
Synthetic dyes are visible pollutants, which is undesirable even at trace level due to its chemistry and appearance. Many dyes are made water-soluble to meet the color requirements of most fabrics. Textile dyes and other commercial colorants have become a major concern with regard to environmental pollution. A total of 15% of the total world production of dyes is lost during the dyeing process and is released in textile effluents (Zollinger, 1991). Some frequent users of these chemicals include paper and pulp manufacturing, dyeing of cloth, leather treatment, printing etc. Most of the used solutions containing such dyes are discharged as effluents (Bukallah et al., 2007). The discharge of colored wastes into receiving streams not only affects the aesthetic nature but also interferes with the transmission of sunlight into streams and therefore reduces photosynthetic activity (Namasivayam et al., 2001). Wastewaters offer considerable resistance for their biodegradation due to the presence of these heat and light stable dyes, thus upsetting aquatic life (Mall et al., 2006). Thus, pollution caused by industrial wastewaters has become a common problem for many countries (Hamadaoui, 2006).
Several methods have been tested for color removal from the industrial effluents to decrease their impact on the environment. These methods include adsorption onto inorganic or organic matrices (Lata et al., 2008; Gong et al., 2008; Rauf et al., 2008; Cheng et al., 2008; Augustine, 2008; Xiaofei and Khalil, 2008; Hameed and El-Khaiary, 2008a,b), chemical precipitation, coagulation, electrocoagulation (Daneshvar et al., 2003).
Recent developments of chemical treatment of wastewater resulted in a considerable improvement in the oxidative decolorization of organic compounds dissolved in aqueous media. Among these methods called advanced oxidation processes (AOPs), homogenous chemical oxidation using ultraviolet radiation in the presence of H2O2 (photooxidative decolorization) (Modirshahla and Behnajady, 2006; Jiaqing et al., 2006; Andreozzi et al., 2006), Fenton’s reaction (Ntampegliotis et al., 2006) and electro-Fenton processes (Oturan et al., 2008).
In this work, Fenton-like reactions were used for the decolorization of four commercial dyes, namely, Malachite green (MG), Rhodamine B (RB), Methylene blue (MB) and Crystal violet (CV) in dilute solutions. The aim of the study is to examine in detail the effect of the major system parameters on the decolorization kinetics of these dyes. The parameters, which are examined separately, are the solution pH, the concentration of H2O2, the concentration of the Fe3+ ions and the initial dye concentration. This method seems to decolorize dyes with a variety of molecular structures. The rate of decolorization seems to be affected very little by structure, making it an attractive option for the treatment or pre-treatment of textile effluents containing a wide range of dyes.
2 Materials and methods
2.1 Reagents
Ferric nitrate [(Fe(NO3)3·9H2O)], and 35% hydrogen peroxide (H2O2) were purchased from Panreac Quimica Comp., Spain. All dyes were purchased from Acros Organics, New Jersey, USA, and were used without further purification. Other chemicals used in the experiment were all of analytical grade. A known dye concentration was prepared in deionized water and used as the stock solution for all studies.
The structures of all dyes used in the study are shown in Fig. 1.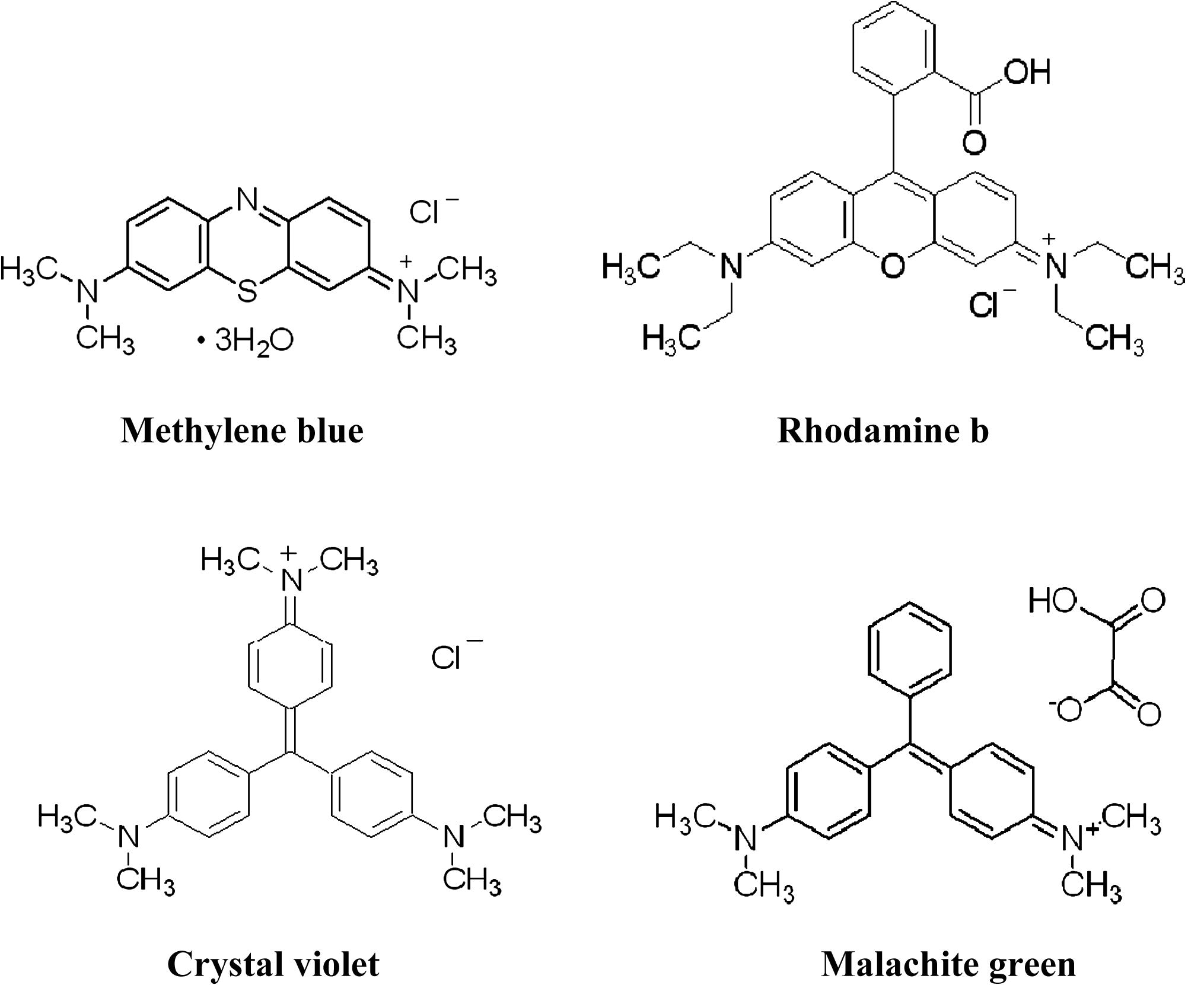
Structures of the used dyes.
3 Experimental procedures
The UV–VIS spectra of the dyes were recorded from 200–800 nm using a UV–VIS–NIR spectrophotometer (JASCO, V-570).The maximum absorbance wavelength (λmax) of Malachite green (MG), Rhodamine B (RB), Methylene blue (MB) and Crystal violet (CV) are 618, 553, 665 and 590 nm respectively from the spectra.
All experiments were carried out at room temperature, 25 ± 1 °C. Solution pH was adjusted by using nitric acid or sodium hydroxide using a pH meter (OAKTON PC 510). The aqueous dye solutions were placed in measuring flasks (10 ml), the required amount of Fe3+ and H2O2 were added into the dye solution, and subsequently the intensity (absorbance) of the dye were measured using a Selecta UV-2005 spectrophotometer at λmax, the absorption maximum for each dye, at different time in order to study the course of the decolorization reactions. Most of the experiments were conducted twice in identical conditions. The difference in results in tow consecutive experiments did not exceed more than 3%. Total organic carbons (TOC) was analyzed with a Shimadzu 5000 TOC analyzer.
4 Results and discussion
In the literature, Fenton and Fenton-like reactions for the treatment of textile effluents are very efficient in a pH range between 2 and 5 (Lunar et al., 2000a,b). Furthermore, in most papers, it is mentioned that the optimum pH value is around 3 (Lunar et al., 2000b; Kitis et al., 1999; Kang and Hwang, 2000; Schafer and Buettner, 2000).
The general mechanism using Fenton reagents, via which the hydroxyl radicals are produced, is a number of cyclic reactions, which utilize the Fe2+ or Fe3+ ions as a catalyst to decompose the H2O2. These ions are regenerated in their original state at the end of the cyclic reactions according to the following scheme of reactions (Kitis et al., 1999; Gallard and De Laat, 2000; Ri et al., 2001):
More than 25 reactions occur, several involving the dye molecule, but the above seven are the most significant involving ions and radicals. When a Fenton-like reagent is used, the sequence of reactions begins with reaction (3). There are many bibliographic sources which report that Fenton reagent is more effective than Fenton-like reagent (Gallard and De Laat, 2000; Solozhenko et al., 1995; Li, 1999; Kwon et al., 1999). However, in most cases it seems that it does not matter whether Fe2+ or Fe3+ is used. The above sequence of reactions begins very quickly if there is an abundant supply of H2O2 and of dye.
4.1 Determination of initial rates
As found from the concentration versus time plots, the concentration of the dye changes very rapidly in the first few minutes of the decolorization and subsequently this rate slows remarkably. In the present work, the reactions have been monitored by noting the change of the dye only; no attempts have been made to determine the hydrogen peroxide or ferric concentrations in the reaction medium at different time intervals.
An attempt has been made to represent the rate of the decolorization reaction in the form of a power-law equation for the initial period of reaction. The initial rates were calculated by drawing a tangent at time t = 0 on the concentration versus time profile. The kinetic analysis was done for those experiments in which the dye concentration, ferric ion concentration and hydrogen peroxide concentration were varied in the studied ranges.
4.2 Effect of pH
Fig. 2 illustrates the effect of pH on the rate of decolorization of the examined dyes (MG, RB, MB and CV) in water. The following can be observed from Fig. 2: (1) the dependence of the decolorization rate on the pH is similar for all dyes examined; (2) the decolorization rate reaches a maximum as the pH increases from 3.0–4.0; (3) in highly acidic solutions (=or below 2), the decolorization rates become significantly slower with decreasing pH while a similar behavior is also found in highly alkaline solutions, where above pH 10 the decolorization rate becomes very small (not measurable in the present study, and so, not shown in Fig. 2). Therefore, it seems that the optimum pH for the decolorization of all dyes examined is in the range between 3 and 4. The findings of this study are consistent with other results reported in the literature (Lunar et al., 2000a,b; Kitis et al., 1999; Kang and Hwang, 2000; Schafer and Buettner, 2000; Solozhenko et al., 1995; Li, 1999; Andreozzi et al., 1999).![Effect of pH on the rates of decolorization of different dyes. [dye] = 1.5 × 10−5 mol dm-3, [Fe3+] = 6.0 × 10−4 mol dm−3, [H2O2] = 0.04 mol dm−3, at 25 °C, (×) CV, (♦) MG, (□) MB and (▴) RB.](/content/185/2010/22/3/img/10.1016_j.jksus.2010.04.002-fig2.png)
Effect of pH on the rates of decolorization of different dyes. [dye] = 1.5 × 10−5 mol dm-3, [Fe3+] = 6.0 × 10−4 mol dm−3, [H2O2] = 0.04 mol dm−3, at 25 °C, (×) CV, (♦) MG, (□) MB and (▴) RB.
The drastic decrease of the decolorization rate in highly acidic solutions is due to the drastic reduction of hydroxyl radicals produced in the sequence of reactions (1)–(7). High concentrations of hydrogen ions result in the reversal of Eq. (3).
Eq. (3) occurs via the following mechanism:
In highly acidic conditions, H2O2 is stabilized and this inhibits the production of the intermediate ion , which reacts with Fe3+ to produce Fe–OOH2+. The Fe–OOH2+ ion decomposes to produce Fe2+, which is necessary in reaction (1) in order to generate hydroxyl radicals (Lunar et al., 2000b; Ahn et al., 1999). Electron transfer through the solution to Fe3+, which occurs by means of , is therefore hindered.
At highly alkaline pH, decolorization efficiency is reduced because of the reduction in solubility of Fe2+ and Fe3+ (Schafer and Buettner, 2000) and the formation of Fe(OH)3 and Fe2O3·nH2O, resulting in the decrease in Fe3+ ions in the solution and subsequently in a reduction in the concentration of Fe2+ ions, which are more efficient than Fe3+ ions because they directly produce OH• (Lunar et al., 2000b).
Also, if the pH is too high, the iron precipitates as Fe(OH)3 and catalytically decomposes the H2O2 to oxygen, which reduces its concentration in the solution, potentially creating a hazardous situation (Lunar et al., 2000a).
4.3 Effect of initial dye concentration
In order to investigate the effect of initial dye concentration on the rate of decolorization, dye solutions having 0.30 × 10−5, 0.75 × 10−5, 1.20 × 10−5 and 1.50 × 10−5 mol dm−3 were separately treated with 3.0 × 10−4 mol dm−3 and 0.02 mol dm−3 solutions. All runs were conducted at the optimum pH. In all cases, the reaction took place at a fast rate initially and subsequently the rate of oxidation dropped.
Generally, the methods of waste treatment are strongly dependent on the initial concentration of the organic substrate (Li, 1999; Centi et al., 2000). This is also consistent with the modeling of the reaction as a first-order kinetic model (−d(Cdye/dt) = kobs Cdye), for the very early stages of the reaction. This also explains the initial rapid drop in the absorbance versus time and subsequently the near leveling off of the absorbance. This implies that Fenton-like reagent is more efficient for a pre-treatment of textile dye effluents. It is observed that, the fractional conversion after 30 min for (MG) was 95% for 0.3 × 10−5 mol dm−3, 92% for 0.75 × 10−5 mol dm−3, 84% for 1.2 × 10−5 mol dm−3 and 68.5% for 1.5 × 10−5 mol dm−3; for (RB) was 90% for 0.3 × 10−5 mol dm−3, 84.4% for 0.75 × 10−5 mol dm−3, 60.8% for 1.2 × 10−5 mol dm−3 and 55.3% for 1.5 × 10−5 mol dm−3; for (CV) was 67% for 0.75 × 10−5 mol dm−3, 63% for 1.2 × 10−5 mol dm−3, 53% for 1.5 × 10−5 mol dm−3 and 38% for 3 × 10−5 mol dm−3; for (MB) was 100% for 0.3 × 10−5 mol dm−3, 100% for 0.75 × 10−5 mol dm−3, 83% for 1.2 × 10−5 mol dm−3 and 77% for 1.5 × 10−5 mol dm−3.
4.4 Effect of [Fe3+] on dye decolorization
Baseline experiments were performed to determine the effect of Fe3+ only in the absence of H2O2 on the decolorization of the dye when practically no decolorization was achieved. With 1.5 × 10−5 mol dm−3 of dye solutions, the rate of decolorization showed remarkable dependence on the initial Fe3+ concentration used. Subsequently, experiments were performed at different Fe3+ concentrations (1.5 × 10−4 to 10.0 × 10−4 mol dm−3) for fixed initial concentration of H2O2 and dye of 0.02 and 1.5 × 10−5 mol dm−3, respectively. By comparing the results of the dye after 30 min, it was found that the rate increased as the Fe3+ concentration was increased from 1.5 × 10−4 to 6.0 × 10−4 mol dm−3, and becomes practically constant when it exceeds 6.0 × 10−4 mol dm−3. Below 1.5 × 10−4 mol dm−3 of Fe3+, the decolorization reaction is too sluggish to account for any practical significance. But the decolorization rate of all examined dyes decreased at high Fe3+ concentrations (⩾1.0 × 10−3 mol dm−3). Thus from the extent of decolorization of the dye after 1 h, we find that there is an optimum Fe3+ concentration for the most effective decolorization of 1.5 × 10−5 mol dm−3 dye solution. Keeping the dye and H2O2 fixed at above concentrations, we studied the effect of variation of Fe3+ concentration for complete destruction of the dye.
The observed decrease in decolorization reaction rate at high Fe3+ concentrations is due to the fact that Fe2+ concentrations increase rapidly through the reactions (3) and (5) and scavenge hydroxyl radicals (Lunar et al., 2000a; Tang and Chen, 1996) through reaction (2). At low Fe3+concentrations (below 1.5 × 10−4 mol dm−3), the decolorization rate decreases even more rapidly. This is due to the lack of Fe3+ and subsequently of Fe2+, which is necessary for the formation of hydroxyl radicals (Tang and Chen, 1996). Therefore, at low Fe3+ concentrations the reaction is starved in OH•, while at high Fe3+ concentrations the OH• concentration is reduced because of the scavenging effect by Fe2+, therefore giving an optimum range between 1.5 × 10−4 and 6.0 × 10−4 mol dm−3.
4.5 Effect of the H2O2 concentration
Fig. 3 shows the he dependence of the decolorization rate of the examined dyes on the H2O2 at constant dye initial concentration, pH and Fe3+ concentration was examined. Experiments were conducted at different H2O2 concentrations with 1.5 × 10−5 mol dm−3 dyes solutions and 3 × 10−4 mol dm−3 Fe3+ solution. In all experiments, the decolorization of the dye increased as H2O2 was added. The H2O2 concentration was varied from 0.01 to 0.15 mol dm−3. The H2O2 concentration of 0.04 mol dm−3 was affecting the decolorization by about 81% of (CV) dye in 40 min; 94% of (MG) dye in 40 min; 98% 0f (RB) dye in 40 min and 100% of (MB) dye in 20 min at a temperature of 298 K. The fractional conversion after 1 h remains almost same for H2O2 concentration of 0.04 to 0.09 mol dm−3. But the decolorization rate of all examined dyes decreased at high H2O2 concentrations (⩾0.1 mol dm−3). Therefore, there is an optimum H2O2 concentration that is effective for decolorization of the dye.![Effect of the initial H2O2 concentration on the rate of dye decolrization at pH = 3.5 and 25 °C; [dye] = 1.5 × 10−5 mol dm−3, [Fe3+] = 6.0 × 10−4 mol dm−3; (▪) MB, (○) RB, (♦) MG and (▴) CV.](/content/185/2010/22/3/img/10.1016_j.jksus.2010.04.002-fig3.png)
Effect of the initial H2O2 concentration on the rate of dye decolrization at pH = 3.5 and 25 °C; [dye] = 1.5 × 10−5 mol dm−3, [Fe3+] = 6.0 × 10−4 mol dm−3; (▪) MB, (○) RB, (♦) MG and (▴) CV.
The occurrence of an optimum H2O2 concentration for the decolorization of the examined dyes could be explained as follows. At low H2O2 concentrations and as the H2O2 concentration is decreased, the rate rapidly decreases. This occurs because the concentration of Fe3+ ions produced through reaction (3) and (4) decrease rapidly with decreasing H2O2 concentration. As a result, the production of hydroxyl radicals through Eq. (3) is drastically reduced. At high H2O2 concentrations (⩾0.1 mol dm−3), the rate tends to decreasing with increasing H2O2 concentration. This is most probably due to the scavenging of the hydroxyl radicals by the H2O2 through Eq. (7) (Kitis et al., 1999; Solozhenko et al., 1995). It was also observed from the experiments that at high H2O2 concentrations (⩾0.1 mol dm−3), H2O2 decomposes into O2 and H2O2, and this results in a rapid increase in solution temperature, decreasing in effect the oxidative power of the Fenton-like reagent because of large decrease in the concentration of H2O2 (Centi et al., 2000).
4.6 Effect of electrolytes on conversion
Industrial wastewater might contain a number of salts, more generally electrolytes, dissolved in them. It was therefore thought worthwhile to investigate the effect of dissolved electrolytes such as sodium chloride, potassium chloride and potassium nitrate on the rate and the extent of the oxidative decolorization of the dye. The effect of dissolved electrolytes was studied only at the optimum concentrations of the dye, H2O2, and Fe3+. Fig. 4 shows the effects of these salts on the rate of decolorization, and it was observed that the presence of halide salts at the same concentration level substantially decreased the rate and the extent of decolorization, whereas for nitrate salt, there is an increase in the extent of dye decolorization. Halide salts reduced the rate of conversion of the dye due to the interaction of the electronegative chloride anion with the hydroxyl radical that inhibited the rate of the decolorization reaction (Ming-Chun et al., 2005). While in the presence of nitrate salts, It is not clear why the rates of decolorization were increased; it might be due to the availability of soluble Fe3+ ions (From ferric nitrate) concentration for catalyzing was increased.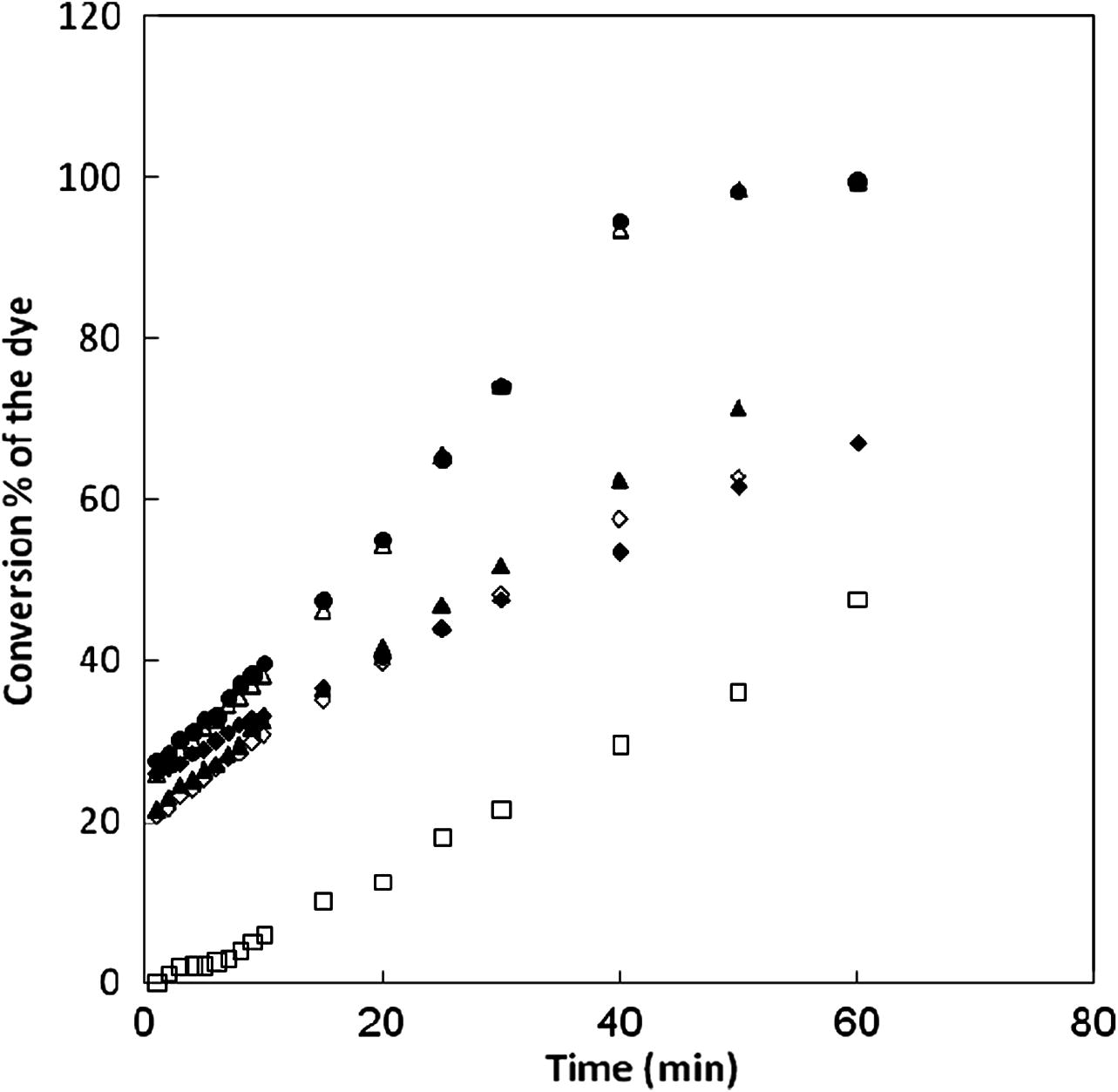
Effect of added electrolytes on the conversion of 1.5 × 10−5 mol dm−3 Malachite green in water with 0.02 mol dm−3 H2O2 and 3.0 × 10−4 mol dm−3 Fe3+: (▴) 0.5% NaCl, (◊) 0.5% KCl, (♦) 1% NaCl, (□) 1% KCl, (•) 0.5% KNO2, and (△) 1% KNO3.
4.7 Effect of temperature on the decolorization of the examined dyes
Experiments were conducted at four different temperatures between 298 and 328 K to investigate the effect of temperature on the decolorization kinetics of aqueous dye solutions. The results are shown in Table 1. An increase in the extent of decolorization of the dye was observed when the reaction temperature was raised. There was practically no difference in the rate and extent of decolorization of the reaction in the temperature between 323 and 328 K. At higher reaction temperature more than 328 K, the rates of decolorization were decreased. This might be due to the thermal decomposition of the hydrogen peroxide resulted in the reduction of its effective concentration towards making hydroxyl radicals, and this was possibly the reason behind lower conversion of the dye at higher reaction temperatures.
Dye
104 k (S−1)
ΔE#
ΔH#
ΔG#
ΔS# e.u
298 K
303 K
313 K
323 K
KJ mol−1
MG
4.01
6.14
20.1
44.1
79.52 ± 1.2
76.99 ± 1.1
93.425 ± 1.0
−54.22 ± 2.0
RB
4.83
8.83
26.5
56.81
79.716 ± 1.3
77.187 ± 1.2
93.425 ± 1.0
−53.59 ± 1.0
MB
5.6
11.5
30.0
70.0
79.75 ± 1.3
77.22 ± 1.2
93.425 ± 1.0
−53.45 ± 1.1
CV
2.50
5.10
15.0
30.1
79.73 ± 1.1
77.20 ± 1.1
93.425 ± 1.0
−53.53 ± 1.0
The activation parameters were calculated for all dyes at 303 K and are shown in Table 1. The values of free energy of activation ΔG# indicate the independence of ΔG# on the structural of the dyes used.
4.8 Determination of the order of decolorization
The kinetic analysis was done for those experiments in which the dye concentration, ferric ion concentration and hydrogen peroxide concentration were varied in the optimum ranges.
The initial rate of decolorization of the dyes is given by:
4.9 Mineralization of all studied dyes
It is known that reaction intermediates can form during the oxidation of studied dyes and some of them could be long-lived and even more toxic than the parent compounds. Therefore, it is necessary to realize the mineralization of these dyes simultaneously although we focus our study on the decolorization. To quantitatively characterize the mineralization of the studied dyes in the solution, the TOC removal ratio is used in the study, which is defined as follows:
Figs. 5 and 6 present the concentration degradation and TOC removal ratio of the studied dyes as a function of time at pH 3.5. As we can see in Fig. 5, dye decolorization significantly increased with increasing reaction time for all dyes. After 30 min of reaction time, dye decolorization was 53%, 55%, 64% and 77% for CV, RB, MG and MB respectively. However, Fig. 6 shows almost no TOC removal efficiency for the first 30 min. It demonstrates that intermediates form by oxidation of dyes during the first 30 min reaction. After then, TOC removal efficiency increases slowly even though a complete dye decolorization is achieved. Meanwhile, at 200 min reaction time, all of the TOC removal ratios reach to about 27%. Compared with the results of decolorization and TOC removal ratio, it can be seen that the decolorization of the studied dyes undergoes a fast reaction rate than the mineralization under the same conditions.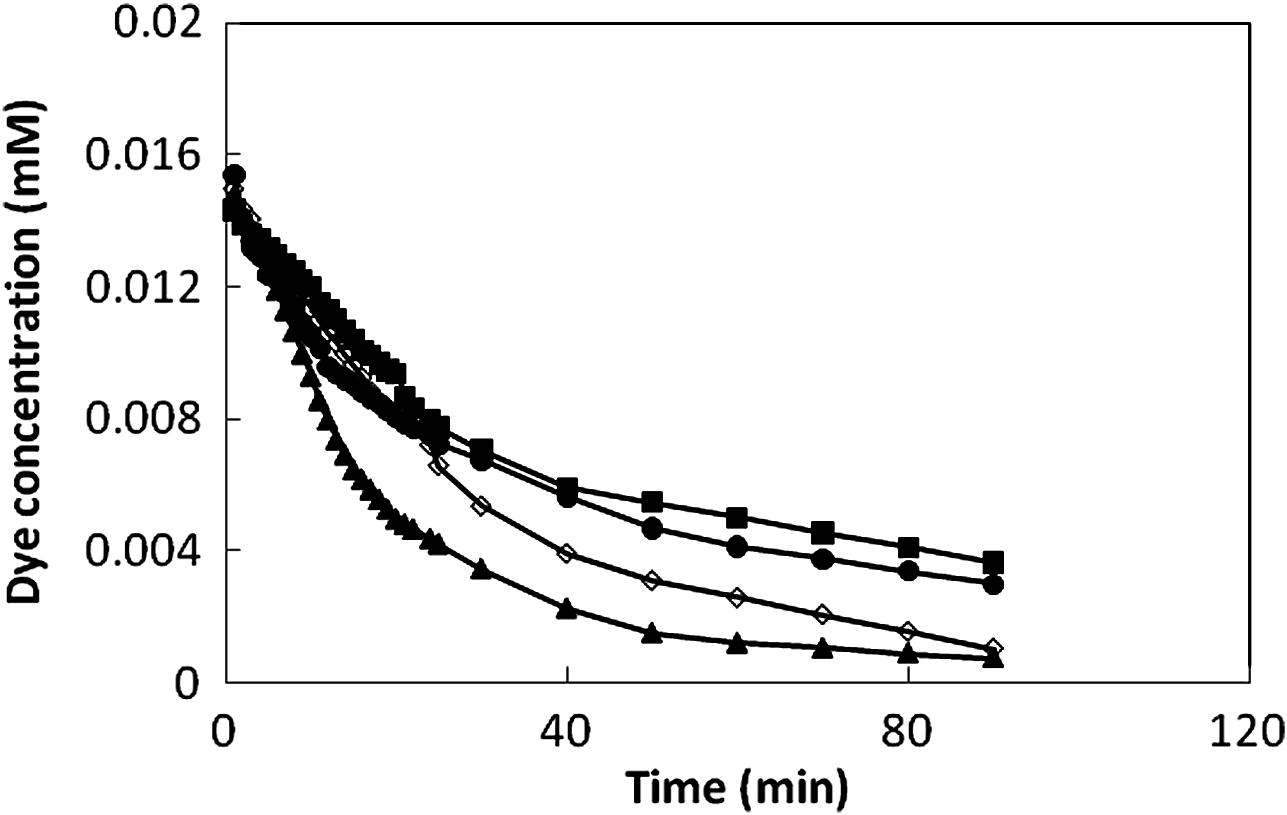
The change of concentration of the dyes as a function of reaction time: (▪) CV, (•) Rb, (◊) MG and (▴) MB.
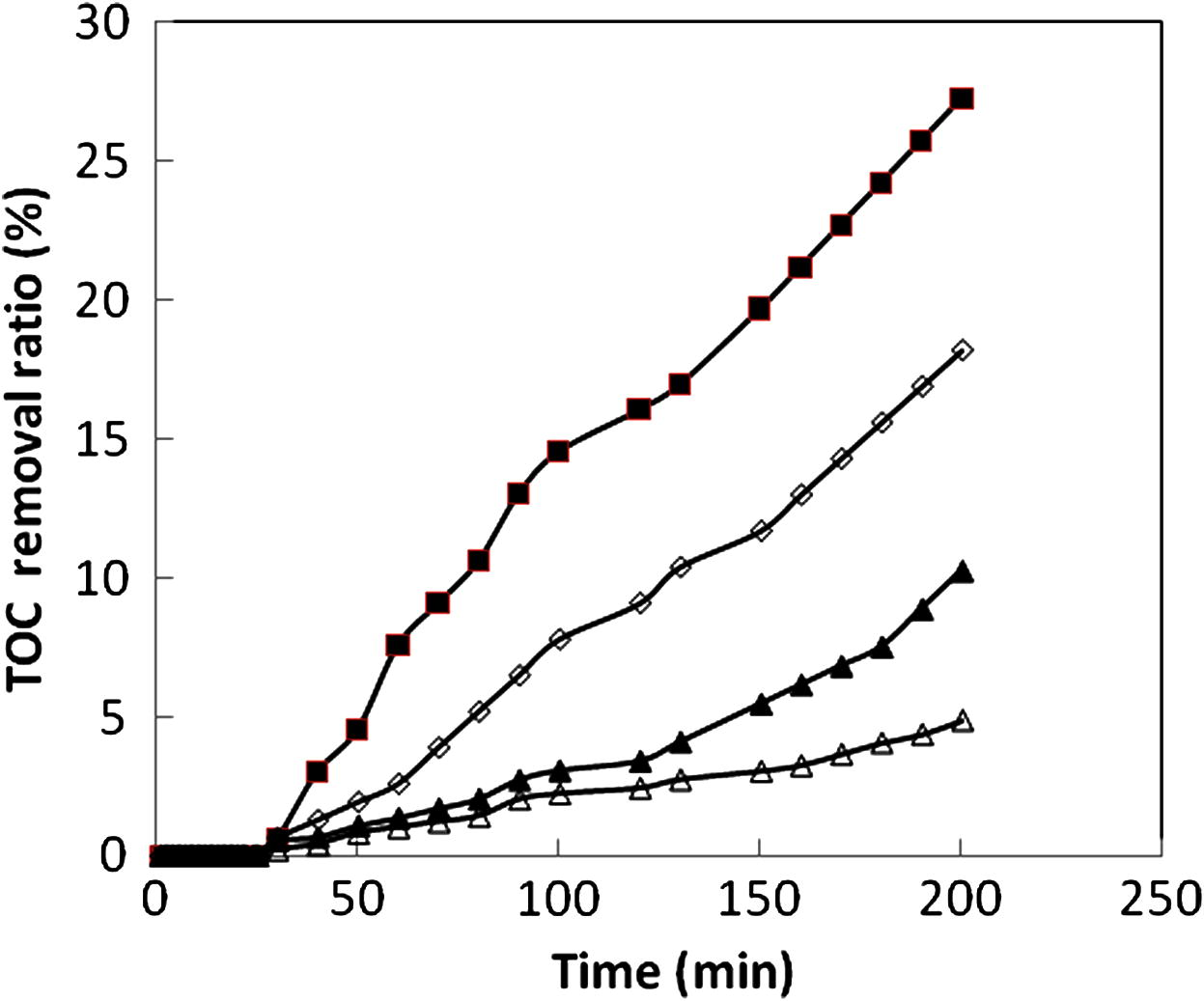
The change of TOC removal ratio of the dyes as a function time: (♦) CV, (◊) Rb, (▴) MG and (△) MB.
5 Conclusions
The kinetics of oxidative decolorization of four commercial dyes, namely, Malachite green (MG), Rhodamine B (RB), Methylene blue (MB) and Crystal violet (CV) were studied using a Fenton-like reagent. The optimum concentrations of Fe3+and H2O2 were, respectively, 6.0 × 10−4 mol dm−3 and 0.04 mol dm−3 for the decolorization of a 1.5 × 10−5 mol dm−3 dye solution at 298 K. The rate of decolorization of the dye was found to be very rapid in the initial period of reaction. The solution pH strongly influences the decolorization reaction rate. The optimum pH range is between 3 and 4. Dissolved electrolytes such as halides suppressed the rate of decolorization, possibly due to the interaction of the anions with the hydroxyl radical. The rate of decolorization was found to be practically dependent on temperature in the range of 298–323 K. The initial rate of decolorization could be expressed in terms of a power-low equation as follows: , where, m, n and p are the obtained orders with respect to dye, Fe3+ and H2O2 respectively. It was also found that the decolorization of the dyes undergoes a fast reaction than the mineralization.
Acknowledgement
The authors are highly indebted to Taibah university for finical support of this work through the funding project no. (117/28).
References
- Process Biochem.. 1999;34:429-439.
- Catal. Today. 1999;53:51-59.
- Water Res.. 2006;40:3785-3792.
- Biochem. Eng. J.. 2008;40:8-18.
- Dyes Pigments. 2007;74:85-87.
- Catal. Today. 2000;55:61-67.
- Biochem. Eng. J.. 2008;39:538-546.
- Sep. Purif. Technol.. 2003;31:153-162.
- Water Resour.. 2000;34:3107-3116.
- Bioresour. Technol.. 2008;99:4510-4514.
- J. Hazard. Mater.. 2006;135:264-273.
- J. Hazard. Mater.. 2008;155:601-609.
- J. Hazard. Mater.. 2008;159:574-579.
- Electrochim. Acta. 2006;51:4942-4949.
- Water Resour.. 2000;34:2786-2790.
- Water Resour.. 1999;33:2561-2568.
- Water Resour.. 1999;33:2110-2118.
- Desalination. 2008;219:250-261.
- Waste Manage.. 1999;19:495-502.
- Water Resour.. 2000;34:1791-1802.
- Water Resour.. 2000;34:3400-3412.
- Dyes Pigments. 2006;69:210-223.
- J. Environ. Mange.. 2005;75:177-182.
- Dyes Pigments. 2006;70:54-59.
- Waste Manage.. 2001;21:381-387.
- J. Hazard. Mater.. 2006;136:75-84.
- Appl. Catal. B: Environ.. 2008;82:244-254.
- Chem. Eng. J.. 2008;137:238-243.
- Water Resour.. 2001;35:387-396.
- Free Radical Biol. Med.. 2000;28:1175-1181.
- Water Resour.. 1995;29:2206-2210.
- Chemosphere. 1996;32:947-958.
- J. Hazard. Mater.. 2008;166:407-414.
- Color Chemistry Synthesis, Properties and Applications of Chemistry and Pigments (second ed.). Weinheim: VCH; 1991.







