Translate this page into:
Ultrastructure alterations of adult male Schistosoma mansoni harbored in albino mice treated with Sidr honey and/or Nigella sativa oil
*Corresponding author at: Biology Department, Faculty of Science, King Khaled University, Abha, P.O. Box 9004, Saudi Arabia. Fax: +966 72289300x1762 osamamostafa@hotmail.com (Osama M.S. Mostafa)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
In this investigation, the effects of Sidr honey, Nigella sativa oil (black-seed oil) and Sidr honey and black-seed oil together on the ultrastructure of tegument, gastrodermis and testes of adult male Schistosoma mansoni harbored in albino mice were studied. The tegument of adult male S. mansoni recovered from mice treated with various types of treatments revealed apparent damages which were severely clear in male worms harbored in mice treated with Sidr honey and black-seed oil together. On the other hand, the gastrodermis and testes of adult male S. mansoni obtained from mice treated with Sidr honey only or black-seed only revealed no apparent damage, while the gastrodermis and testes of worms recovered from mice treated with Sidr honey and black-seed oil together showed severe disorganization. Therefore, these treatments may represent a promising alternative treatment for the control of schistosomiasis mansoni, especially in endemic areas and where drug-resistant strains are found. These natural products may be recommended as useful, pleasant and popular accepted elements of food and drinks in such cases.
Keywords
Schistosoma mansoni
Sidr honey
Black-seed oil
Nigella sativa
Tegument
Testes
Gastrodermis
Ultrastructure
Antischistosomal drugs
1 Introduction
Schistosomiasis continues to occupy the second position in the world among parasites, after malaria, in the term of the extant of endemic areas and number of people infected. Two drugs have been widely used in the treatment of schistosomiasis with good efficacy and low toxicity: oxamniquine and praziquantel (Lescano et al., 2004). Meanwhile, repeated chemotherapy in endemic areas has resulted in the emergence of drug-resistant schistosome strains (Ismail et al., 1999; Kenworthy et al., 2003; Lawn et al., 2003).
The development of such resistance has drawn the attention of many authors to alternative drugs. Many medicinal plants were studied to investigate their antischistosomal potency and found to be effective, like: plant quinghao Artemisia anmia (Abdel Aziz and El-Badawy, 2000), garlic Allium sativum (Zakhary, 1994; Riad et al., 2007, 2008), wild carrots Daucus carota (Shalaby et al., 1999), jungle weed Combretum sp. (McGaw et al., 2001), myrrh Commiphora molmol (Massoud, 1999), ginger Zingiber officinale (Sanderson et al., 2002) and hound’s berry or night shade Solanum nigrum (Ahmed and Rifaat, 2005).
The crude oil of Nigella sativa (black-seed oil) is one of the promising alternative drugs of plant origin that have an antischistosomal effects (EL-Qadri and Emara, 1994; Mostafa, 2001; Mostafa and Soliman, 2002; Mahmoud et al., 2002; Tantawi and Mostafa, 2003; Ahamed and Mostafa, 2003; Mohamed et al., 2005).
The use of honey as a medicine eventually came to exist only in folk medicine but now has been reborn into modern medicine (Al-Waili, 2003). Some researchers began to document the medical properties of honey. The antimicrobial properties of honey may render it beneficial in the treatment of various oral ailments including periodontal disease and mouth ulcers (Molan, 2001; Engeseth et al., 2002; English et al., 2004). In the field of parasitological studies, Zeina et al. (1997) investigated the activities of honey dilutions against three species of Leishmania; their results were compared with the effects of the same concentrations of sugar; honey and sugar both have anti-leishmanial effects in vitro, but the results indicated that honey is superior to sugar. Mostafa (2005) studied the effects of Sidr honey alone, black-seed oil alone and Sidr honey and black-seed oil together on schistosomiasis mansoni in albino mice; his scanning electron microscopical observations revealed that the surface of male worms obtained from mice treated with Sidr honey alone showed extensive loss of spines. Moreover, the worm recovery and the eggs density were lowered in mice treated with Sidr honey.
Alterations in the surface ultrastructure of schistosome worms were used by several investigators for the evaluation of antischistosomal drugs (Mohamed and Fawzi, 1997; El-Sayed and Allam, 1997; Fawzi, 1999; Mostafa and Soliman, 2002; Jiraungkoorskul et al., 2005; Mostafa, 2005). Moreover, the alteration caused by antischistosomal drugs was more pronounced in the male tegument than in that of the female (Shaw and Erasmus, 1987; Shalaby et al., 1991). However, drug related testis alterations of schistosomes and other helminthes have received little attention (Bang and Hairston, 1946; Khayyal, 1964; Molokhia and Smith, 1968; Stammers, 1975; Otubanjo, 1981; Irie et al., 1989; Basch and Clemens, 1989; You et al., 1992; Mohamed, 1999; Fawzi et al., 2001).
On the other hand, the schistosome digestive tract has been more or less ignored in several investigatory efforts (Senft, 1969; Erasmus, 1977); however, there have been a few reports on drug action relative to this system. For instance, Yarinsky et al. (1970), Bogitsh (1975) and Clarkson and Erasmus (1984) have shown that either in vitro or in vivo administration of the drugs astiban, hycanthone, lucanthone and niridizole can precipitate morphological changes in the gastrodermis of schistosomes similar to changes resulting from starvation.
This work was aimed to study the ultrastructure alterations in the tegument, gastrodermis and testes of adult male Schistosoma mansoni harbored in albino mice treated with Sidr honey and/or black-seed oil.
2 Materials and methods
2.1 Infection of mice
S. mansoni cercariae and clean CD1 male albino mice were supplied by the Schistosome Biological Supply Program (SBSP) at Theodor Bilharz Research Institute, Imbaba, Giza, Egypt. Male CD1 albino mice were infected with 100 S. mansoni cercariae via subcutaneous route. The ethical obligations to experimental animals were followed. The experiments were carried out at Zoology Department, Faculty of Science, Ain Shams University, Cairo, Egypt.
2.2 Treatment of mice
Mice were treated orally with pure Sidr honey at a dose of 0.5 ml/mouse and/or N. sativa oil (black-seed oil) at a dose 250 μl/kg body weight, day after day from the first day of infection till the end of the 7th week post-infection. Pure Sidr honey was purchased from Al-Yahya Bees Farms, Abha, Asser, Saudi Arabia. Sidr honey obtained from bees collected nectar of Zizyphus spina-christi flowers. The plant Z. spina-christi is of the Rhamnaceae family, and it grows wild in the mountains of Asser district in southwestern of Saudi Arabia. Black-seed oil is available in drug stores in gelatin capsules under the name of Baraka produced by Pharco Pharmaceuticals, Alexandria, Egypt.
2.3 Experimental design
This study was carried out on 40 mice divided into four groups, 10 mice each: first group was infected with S. mansoni cercariae and non-treated; second group was infected with S. mansoni cercariae and treated with Sidr honey only; third group was infected with S. mansoni cercariae and treated with black-seed oil only and fourth group was infected with S. mansoni cercariae and treated with Sidr honey and black-seed oil together. All mice were sacrificed at the end of the 7th week of the experiment (starting from the day of infection).
2.4 Worm recovery
The recovery of S. mansoni worms from the hepatic portal system and mesenteric veins of sacrificed mice was done by the perfusion technique described by Smithers and Terry (1965).
2.5 Transmission electron microscopical techniques
Worms were fixed in 4% glutaraldehyde in sodium cacodylate buffer for 2 h and washed in the same buffer at a pH of 7.4. Post-fixation was performed with osmium tetraoxide in sodium cacodylate buffer. Specimens were dehydrated in graded ethanol before embedding in Spur resin. Ultrathin sections of the male worms were stained with uranyl acetate and lead citrate and examined with JOEL-1200EX2 electron microscope.
3 Results
3.1 Tegument
Tegumental ultrastructure features of adult male S. mansoni recovered from infected, non-treated mice revealed no apparent damage, showing a superficial syncytial layer forming tubercles with evident surface pits leading to branched intercommunicating channels. The syncytial layer was externally covered by an outer tegumental membrane studded with electron dense tiny discoid bodies. The spines appeared as large densely osmophilic more or less triangular bodies. Underneath the syncytial layer there is an intact layer of longitudinally and transversely cut muscle fibers and tegumental cells (Fig. 1).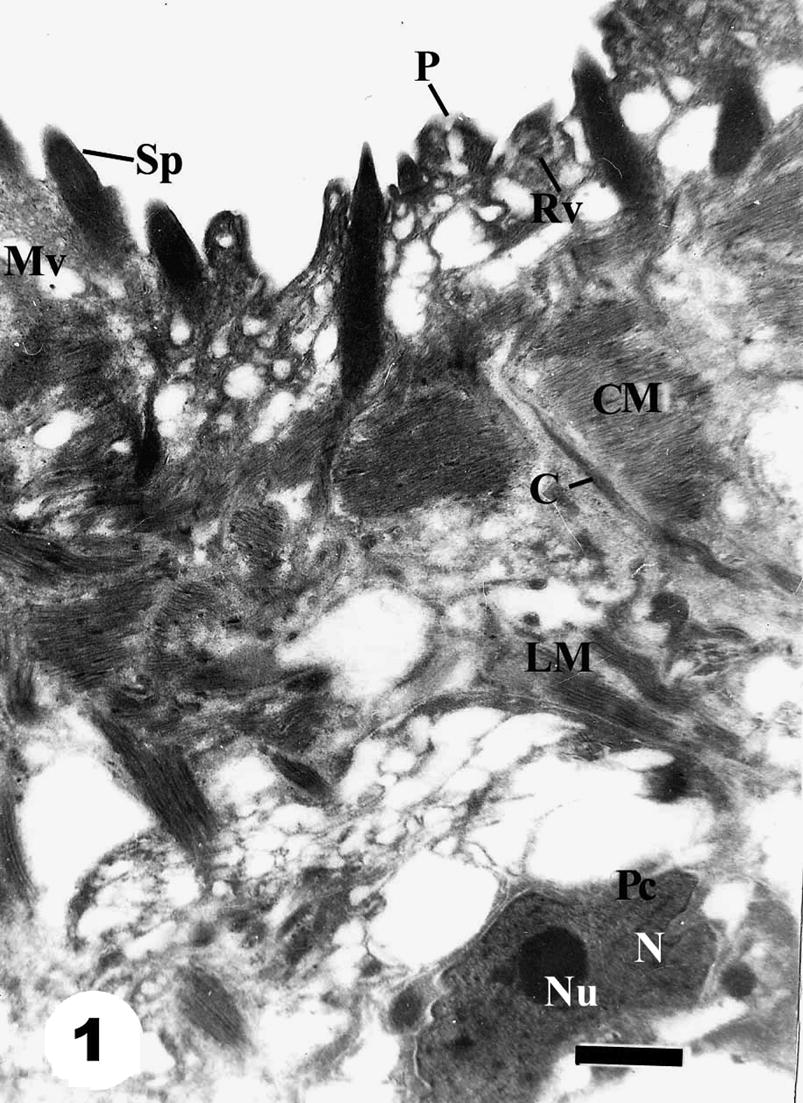
Transmission electron micrograph (TEM) of adult male Schistosoma mansoni tegument recovered from infected, non-treated mice showing numerous spines covering the tubercles (Sp), small papilla with minute pore (P), circular muscle (Cm) and longitudinal muscle (Lm), parenchyma cell (Pc) with large nucleus (N) and nucleolus (Nu). Note also a canal (C) coming from a tegumental cell. Bar = 1 μm.
The tegument of adult male S. mansoni recovered from mice treated with various types of treatment revealed apparent damages which were severely appeared in worms harbored in mice treated with Sidr honey and black-seed oil together. The tegumental features of adult male S. mansoni recovered from infected mice treated with Sidr honey only showed disappearance of most tegumental spines but the muscle layer appeared normal. The cytoplasm of the tegumental cell was vacuolated and the secretory granules were decreased tremendously; in addition the cells showed moderately degeneration in the cytoplasm and nuclear materials (Figs. 2–4).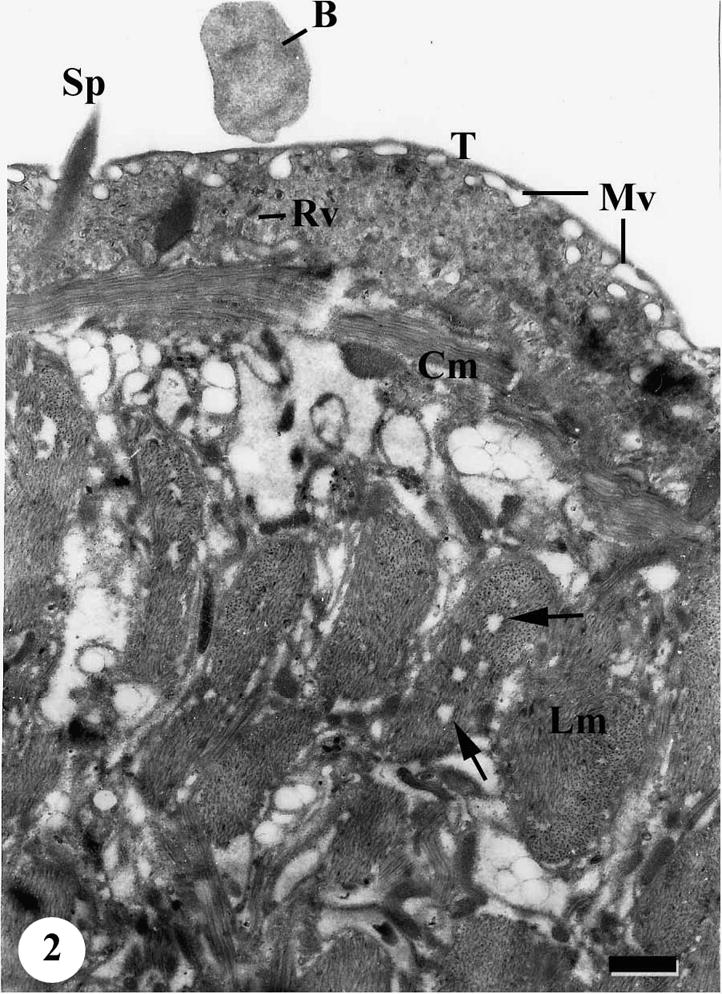
TEM of the tegumental surface of adult male Schistosoma mansoni recovered from infected mice treated with Sidr honey showing blebs (B) on the outer surface of the tegument, numerous membrane bound vesicles (Mv) under outer tegumental surface (T) and rod shaped vesicles (Rv). Note swelling of circular (Cm) and longitudinal (Lm) muscle layers. Some muscle bands riddled with many vacuoles (arrows). Bar = 1 μm.
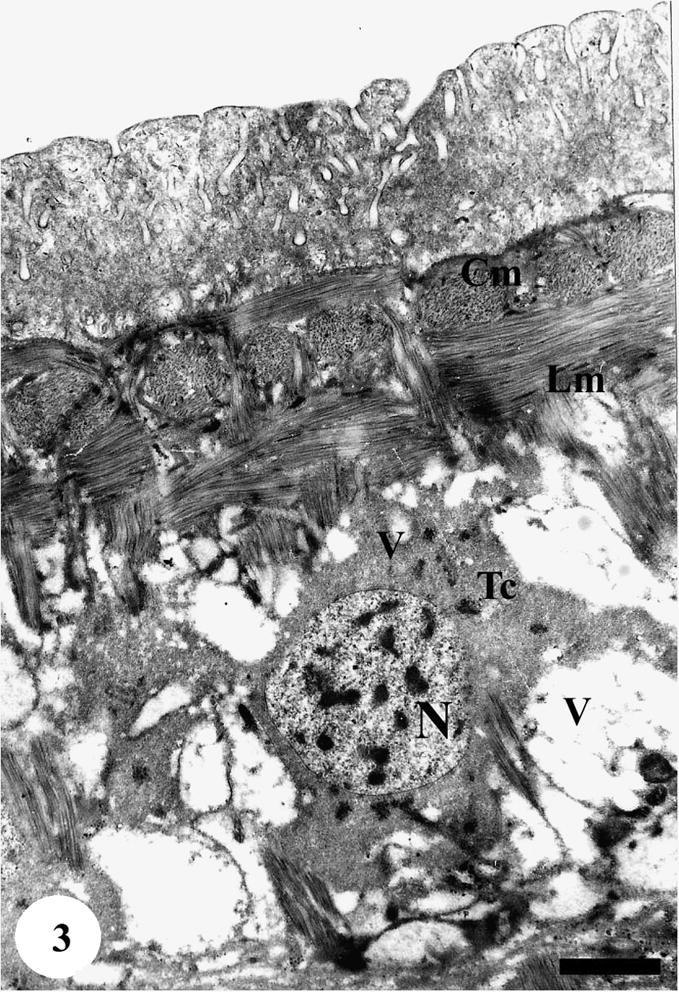
TEM of the ventral side of tegumental surface of adult male Schistosoma mansoni recovered from infected mice treated with Sidr honey. The tegumental cells (Tc) showed tremendous vacuolation (V) in cytoplasm and nuclear material (N). The muscle layers (Cm and Lm) appear normal. Bar = 1 μm.
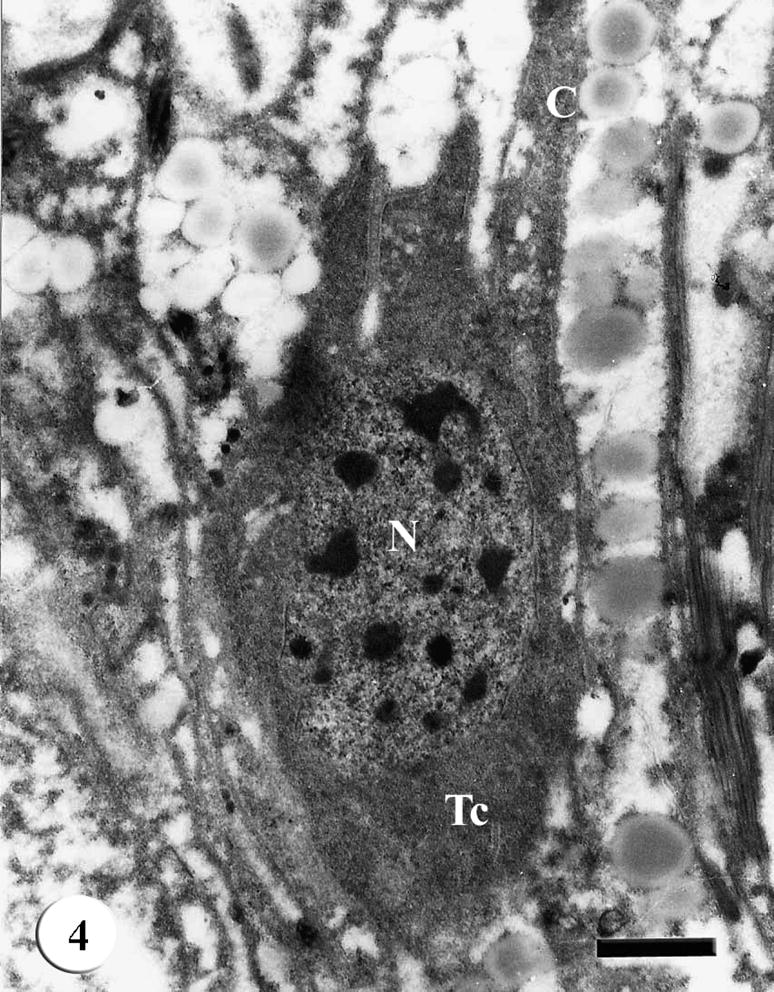
TEM of the tegumental cell (Tc) of adult male Schistosoma mansoni recovered from infected mice treated with Sidr honey showing cytoplasm with decreased secretory granules, nucleus (N) and connecting canal (C). Bar = 1 μm.
The tegumental surface of adult male S. mansoni recovered from infected mice treated with black-seed oil only, showed loss of spines in tegumental tubercles, swelling of the muscle layer and vacuolated loose material appeared beneath the outer tegumental layer. The secretory granules greatly reduced. Some sensory papillae are sunken in the swelling tegument (Fig. 5).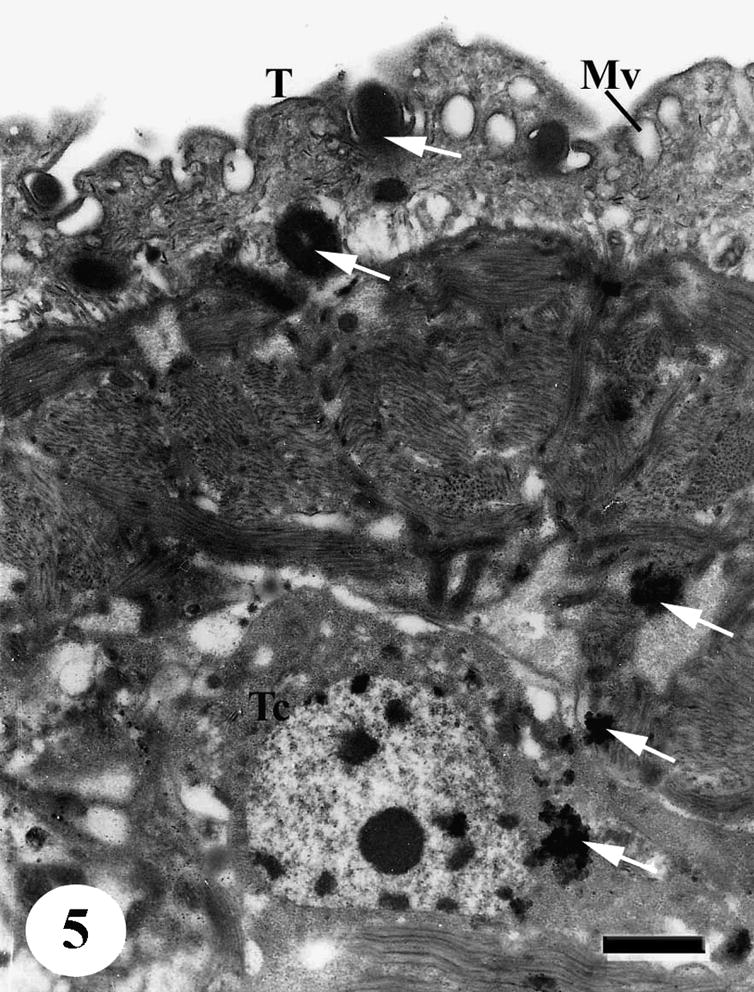
TEM of the ventral tegumental surface of adult male Schistosoma mansoni recovered from infected mice treated with black-seed oil showing distorted tegumental cell with vacuolated cytoplasm while aggregation of dark secretory granules (arrows) appeared in cytoplasm, connecting canal and in outer tegumental layer (T). Note the presence of numerous membrane bound vesicles (Mv). Bar = 1 μm.
The tegumental surface of adult male S. mansoni recovered from infected mice treated with Sidr honey and black-seed oil showed loss of spines and swelling of the muscle layer. Distortion of the tegumental cells was observed, vacuolation was spread everywhere in subtegumental tissue and between muscle bundles. Secretory granules were disappeared completely (Figs. 6–9).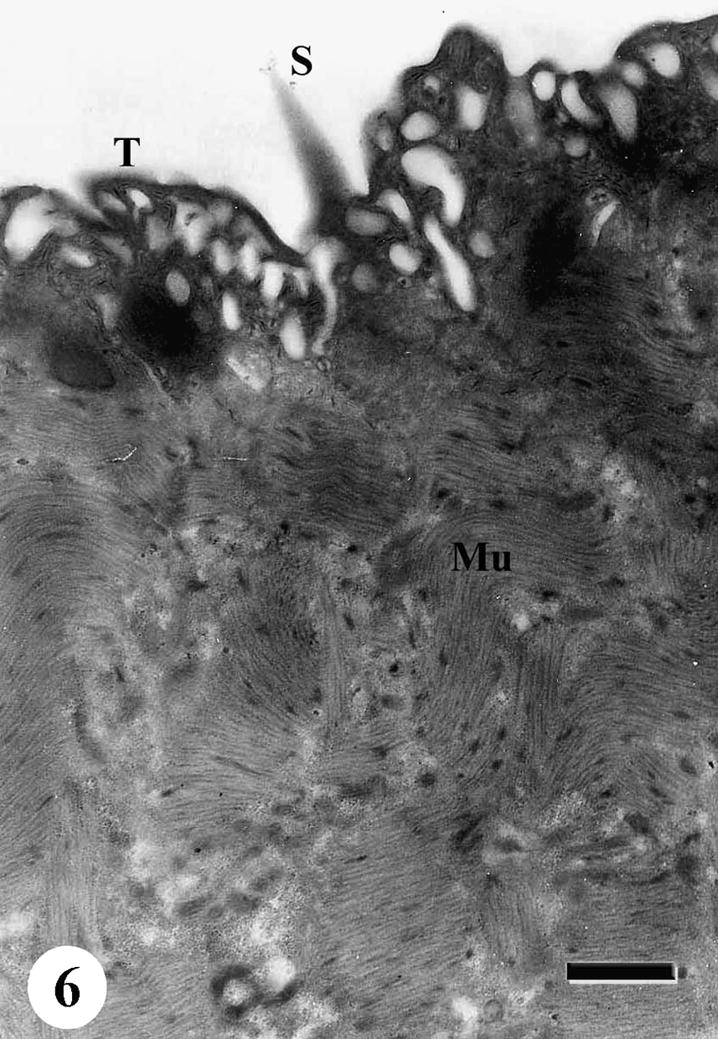
TEM of the tegumental surface of adult male Schistosoma mansoni recovered from infected mice treated with Sidr honey and black-seed oil showing tremendously vacuolated outer tegumental layer (T) with numerous membrane bound vesicles (Mv) and swelling of the muscle layer (Mu). Bar = 1 μm.
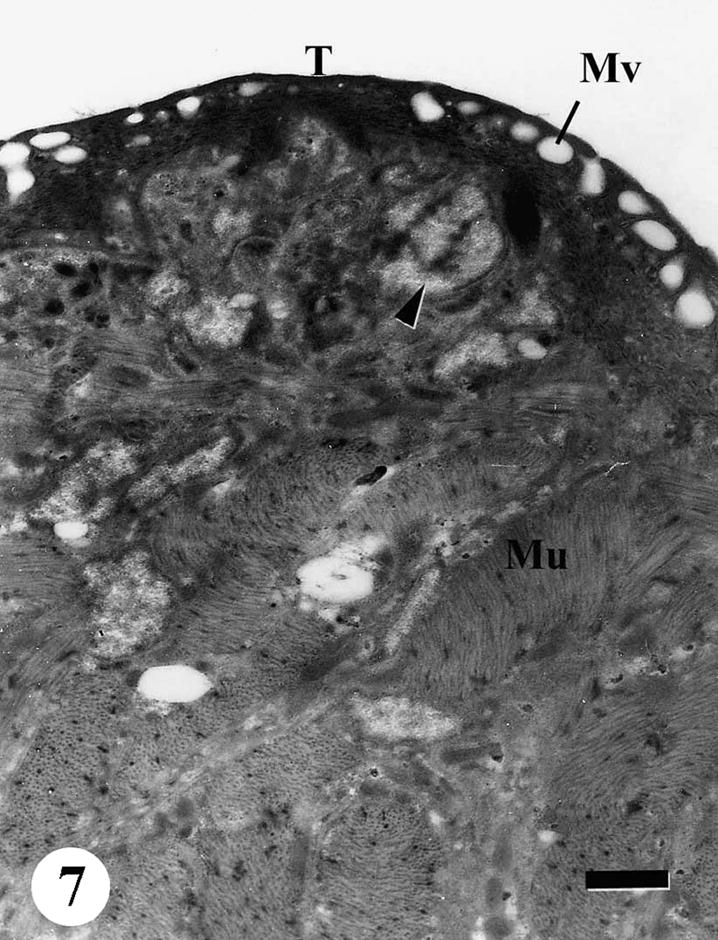
TEM of the tegumental surface of adult male Schistosoma mansoni recovered from infected mice treated with Sidr honey and black-seed oil, spines completely disappeared, membrane bound vesicles (Mv) in the outer tegumental layer (T), abnormal loose material (arrowhead), swelling of the muscle layer (Mu). Bar = 1 μm.
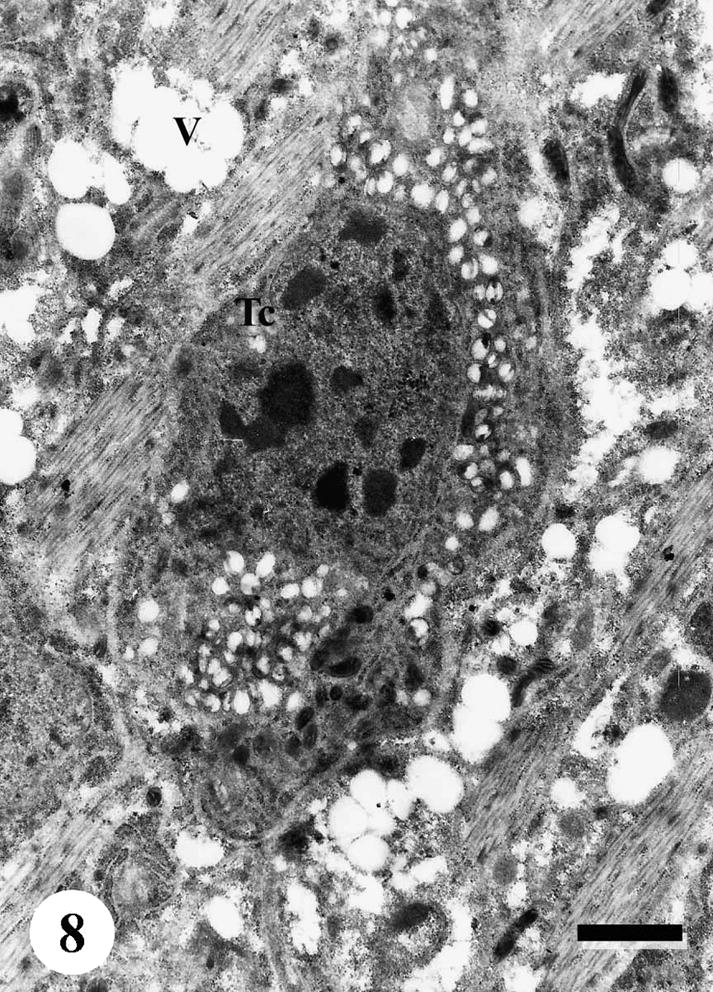
TEM of the subtegumental area of adult male Schistosoma mansoni recovered from infected mice treated with Sidr honey and black-seed oil, showing distortion of the tegumental cells (Tc), with highly vacuolated cytoplasm. Vacuolation spreads everywhere in subtegumental tissue and between muscle bundles (V). Bar = 1 μm.
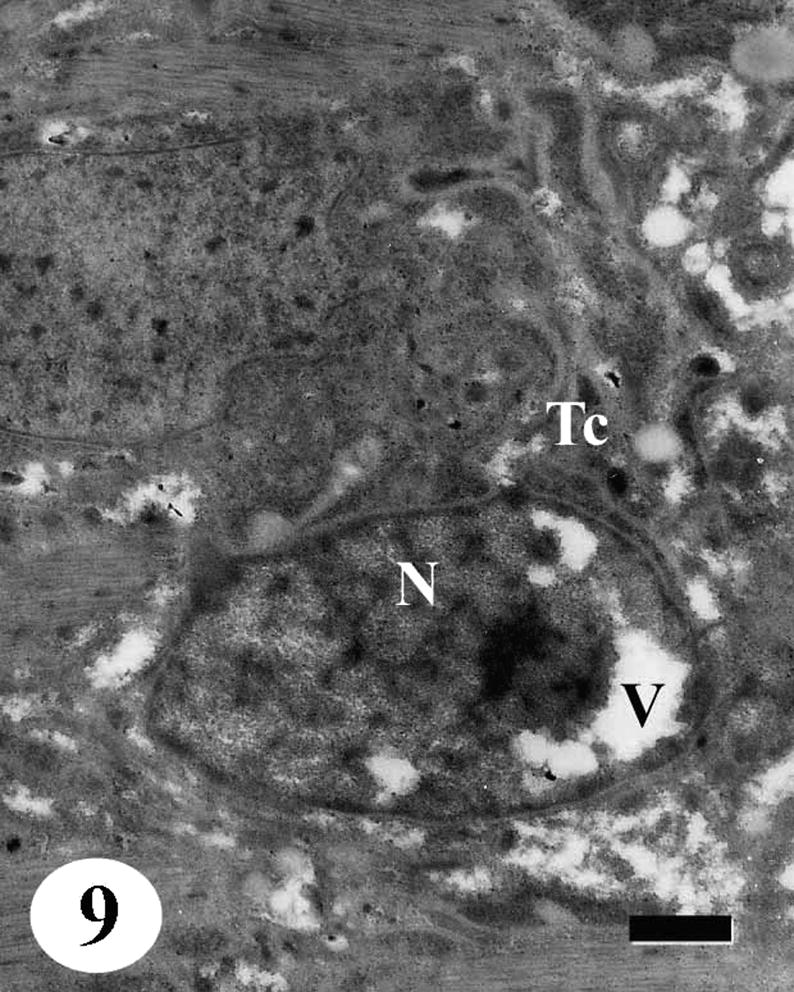
TEM of the tegumental cell of adult male Schistosoma mansoni recovered from infected mice treated with Sidr honey and black-seed oil showing distortion of the tegumental cells (Tc). Nucleus (N) with vacuolated nucleoplasm (V), secretory granules disappeared completely. Bar = 1 μm.
3.2 Gastrodermis
The gastrodermis of adult male S. mansoni recovered from infected, non-treated mice appeared as syncytial with normal nuclei and provided with numerous cytoplasmic extensions (Fig. 10). The gastrodermis of worms recovered from mice treated with Sidr honey only or black-seed only revealed no apparent damage, however the gastrodermis of worms recovered from mice treated with both Sidr honey and black-seed oil showing several disorganization. The first signs of anomaly appeared as blebbing of small components from the apical surface of the gastrodermis into the lumen and a dramatic decrease in the number of surface cytoplasmic extensions as compared to control. Moreover, flattening of the gastrodermis, degeneration of endoplasmic reticulum and darkening of the cytoplasm were noticed (Fig. 11).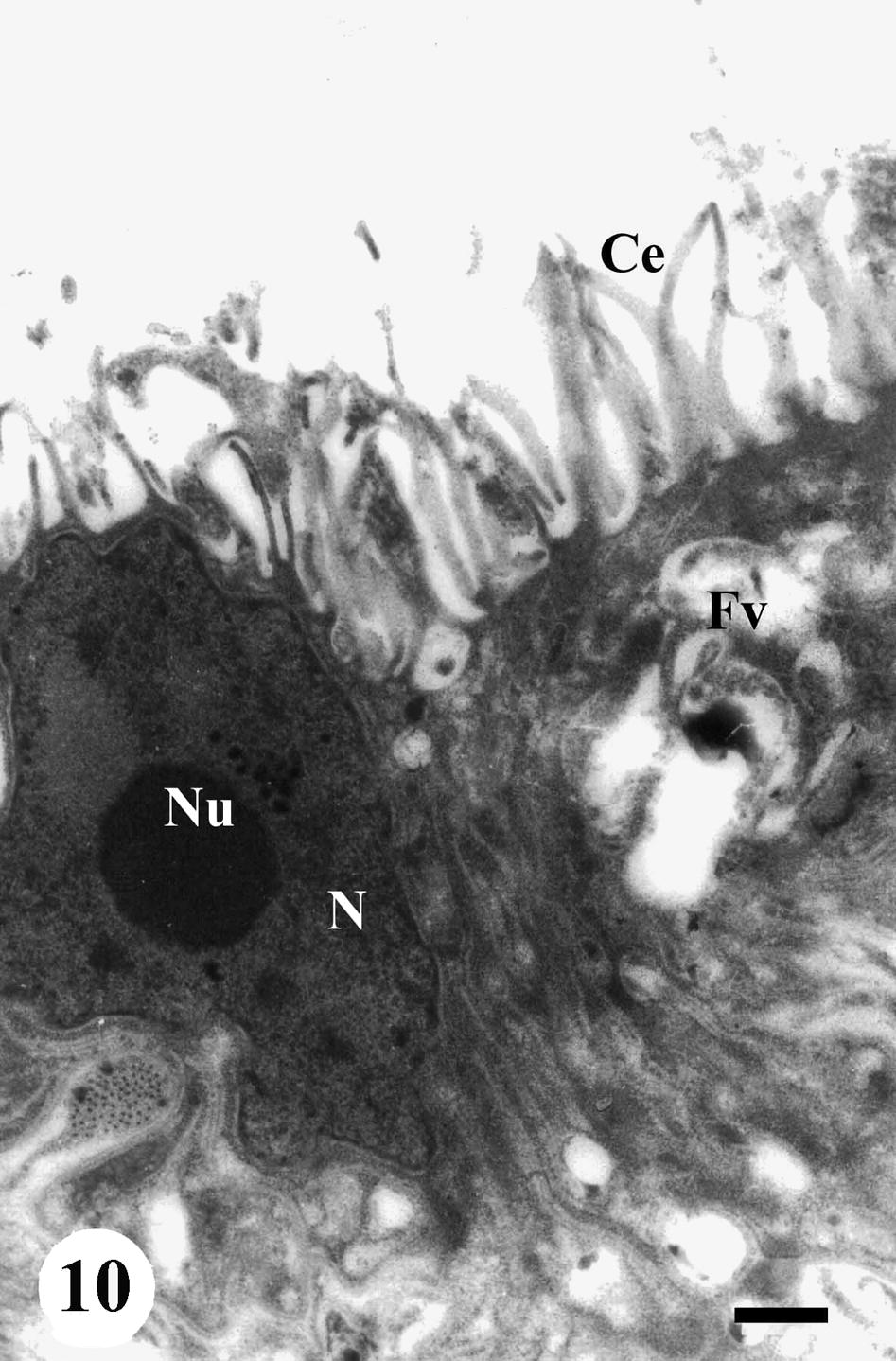
TEM of gastrodermis of adult male Schistosoma mansoni recovered from infected, non-treated mice showing syncytial gastrodermal cells with nucleus (N), nucleolus (Nu), numerous cytoplasmic extensions (Ce) and vacuolated cytoplasm (Fv). Bar = 2 μm.
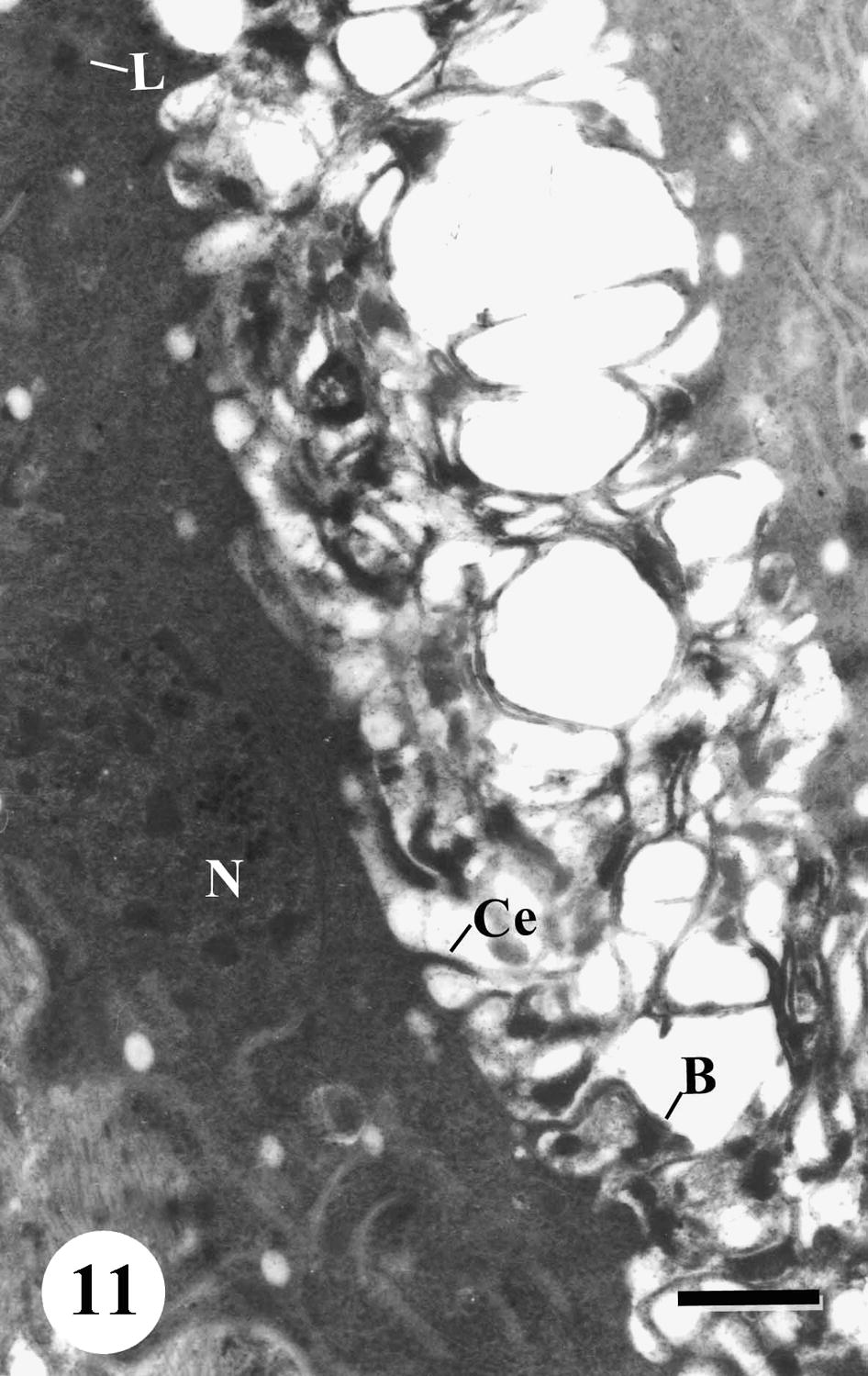
TEM of gastrodermis of adult male Schistosoma mansoni recovered from mice treated with Sidr honey and black-seed oil showing disorganized gastrodermis containing lipid droplets (L) and blebbing (B) of small components from the apical surface. Note extensively decreased cytoplasmic extensions (Ce). Bar = 1 μm.
3.3 Testis
Ultrastructural examination of the testis of S. mansoni males recovered from infected, non-treated mice revealed that the testicular follicles of the worm are formed of germinal and non-germinal cells bounded by a basal lamina enclosed by a thick coat of circularly arranged muscle fibers. The germinal and non-germinal cells are randomly arranged in the testicular follicle. The developing germ cells appeared electron-lucent while the non-germ cells were electron dense. The spermatogonia, which are located peripherally near the basal lamina, possessed oval or rounded nuclei with one large spherical nucleolus and patches of chromatin bodies; these cells were considerably larger than the other germ cells (Fig. 12).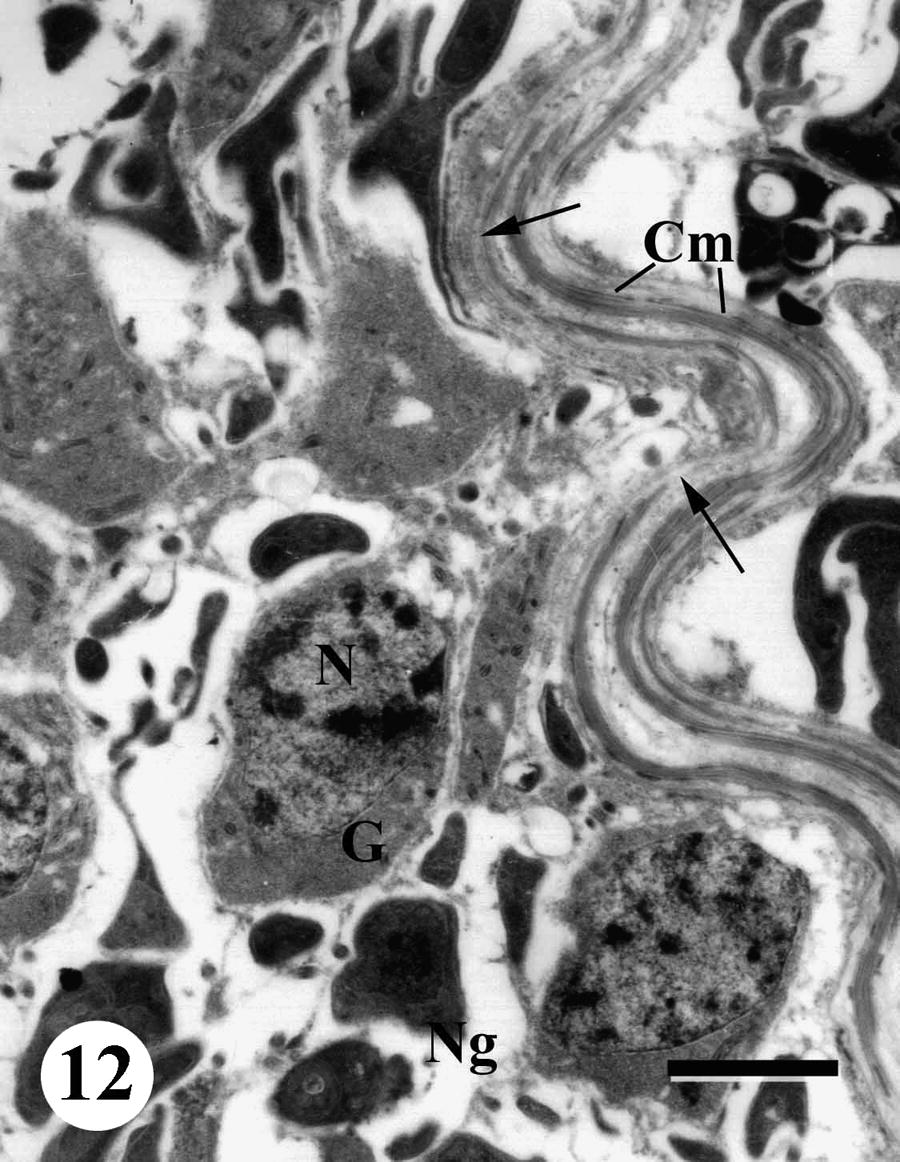
TEM of a testis of adult male Schistosoma mansoni recovered from infected, non-treated mice showing germinal cells (G), non-germinal cells (Ng), nucleus (N), nucleolus (Nu), basal lamina (arrows) and circular muscle (Cm). Bar = 2 μm.
The testis of adult male S. mansoni recovered from mice treated with Sidr honey only or black-seed only revealed no apparent damage. But the testis of worms recovered from mice treated with Sidr honey and black-seed oil revealed several changes. The non-germinal cells exhibited different damage and various degree of degeneration. Their cytoplasm became dark and their nucleus appeared vacuolated while mitochondria appeared as dark bodies with indistinct cristae. Large intercellular spaces were noticed containing debris of degenerating cells. Membranous bodies and aggregation of lipid granules were also observed. Disruption of follicular wall and degeneration of circular muscles were observed (Fig. 13).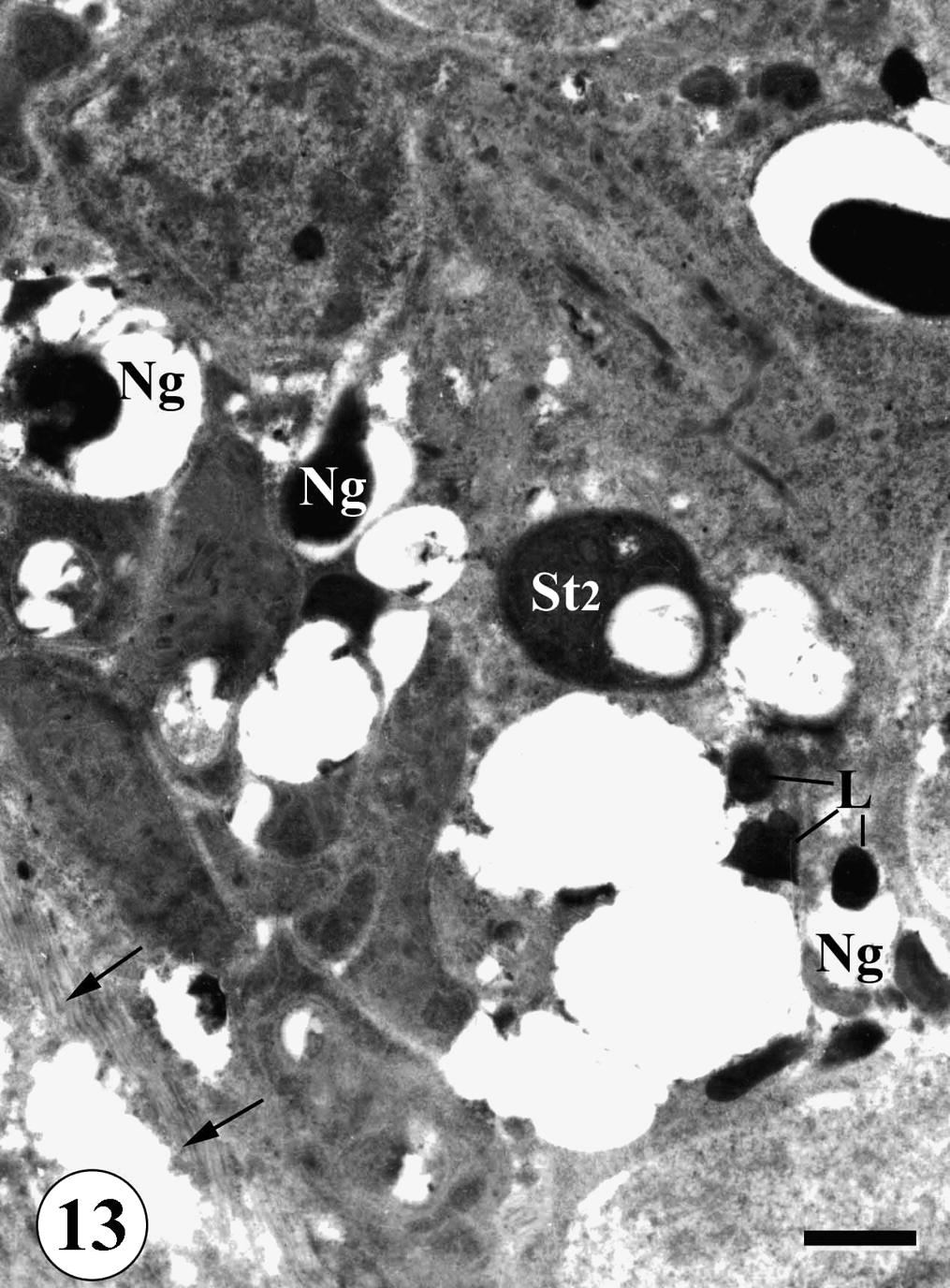
TEM of testis of adult male Schistosoma mansoni recovered from mice treated with Sidr honey and black-seed oil showing degeneration of non-germinal cells (Ng), aggregation of lipid granules (L). Note also disruption of follicular wall (arrows). Bar = 2 μm.
The spermatogonia appeared swelling developing large vacuoles. Chromatin patches disappeared and the nucleolus became condensed. Lipid granules, swelling mitochondria and whorled bodies were observed in the cytoplasm. Degenerating spermatogonia sometimes contained membrane whorls which lay directly below the cell surface and around other cytoplasmic organelles (Fig. 14).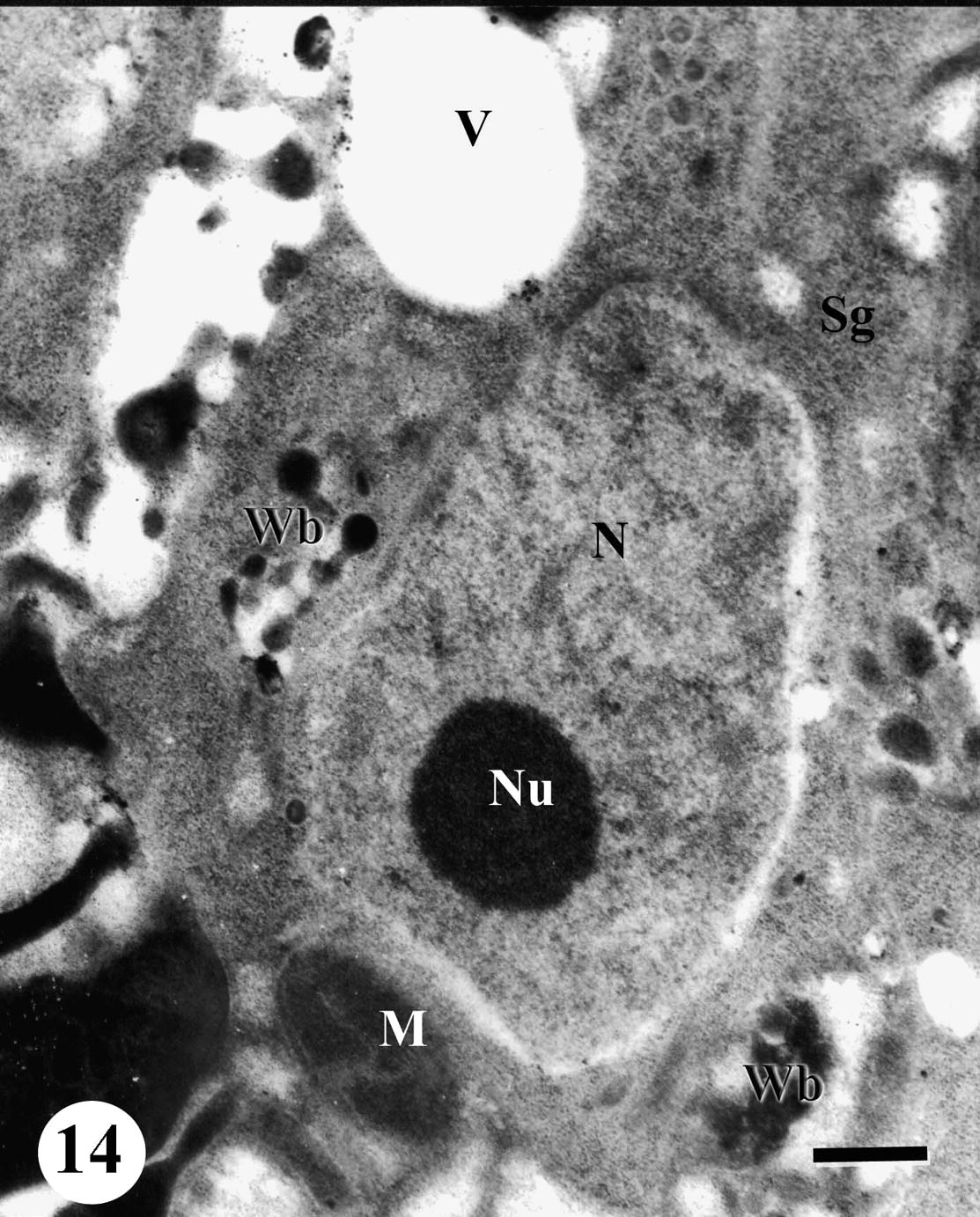
TEM of testis of adult male Schistosoma mansoni recovered from mice treated with Sidr honey and black-seed oil showing disorganized testicular tissue. Note swollen spermatogonia (Sg) develop pronounced vacuolation (V), whorled bodies (Wb) swollen mitochondria (M), nucleus (N) and nucleolus (Nu). Bar = 500 nm.
Degenerative changes occurred in spermatocytes, a prolonged swelling and vacuolation was observed in cytoplasm and nucleus. The swollen nuclei lost their characteristic chromatin patches. Mitochondria exhibited varying morphological changes: some lost their cristae and appeared distended. In addition, some spermatocytes were completely disintegrated (Figs. 15 and 16).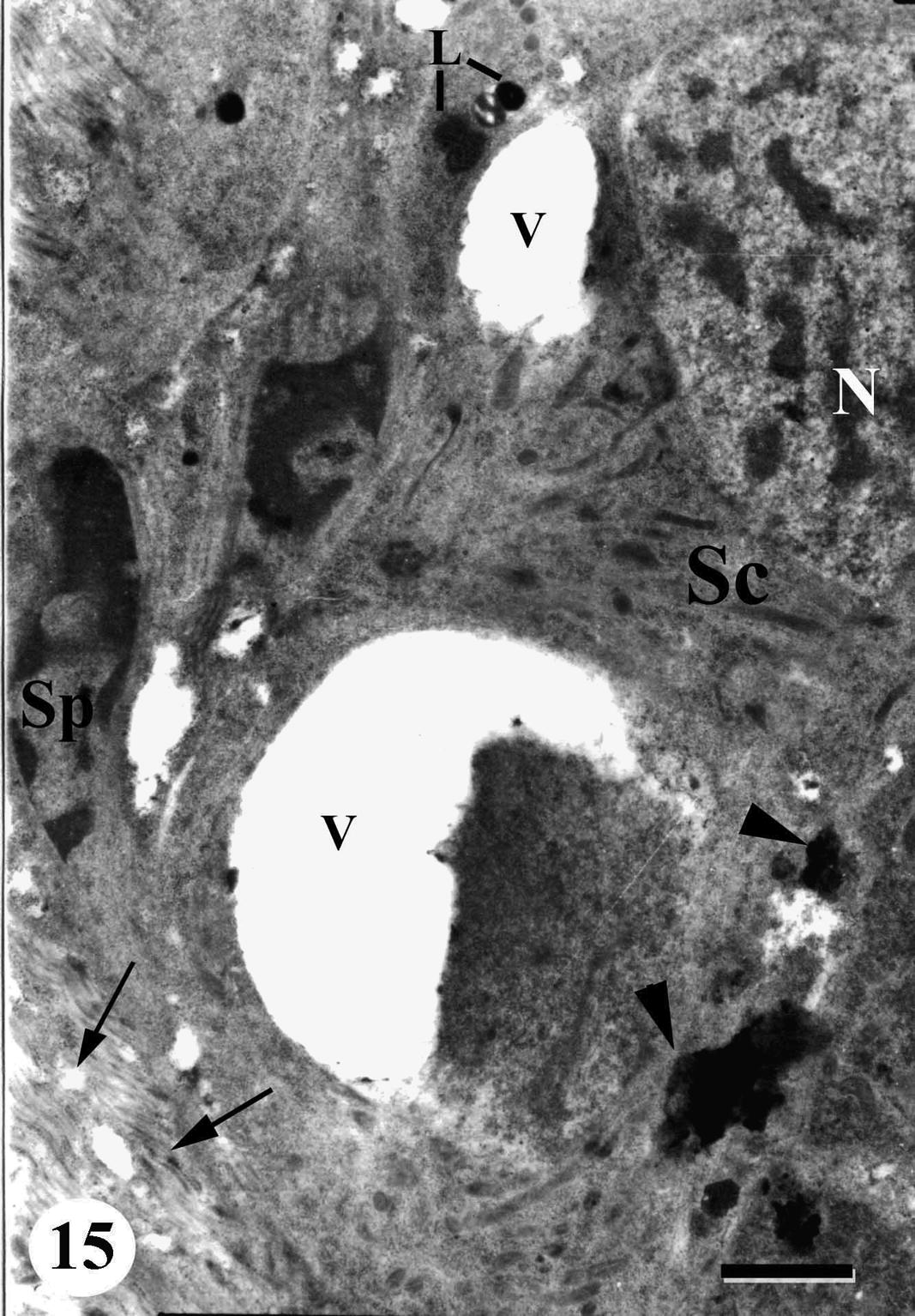
TEM of testis of adult male Schistosoma mansoni recovered from mice treated with Sidr honey and black-seed oil showing degenerated spermatocyte (Sc) with abnormally vacuolated cytoplasm (V), nucleus (N), lipid granules (L), aggregation of dark material in cytoplasm (arrowheads). Note degenerated follicular wall and circular muscles (arrow) and some regressed spermatozoa (Sp). Bar = 1 μm.
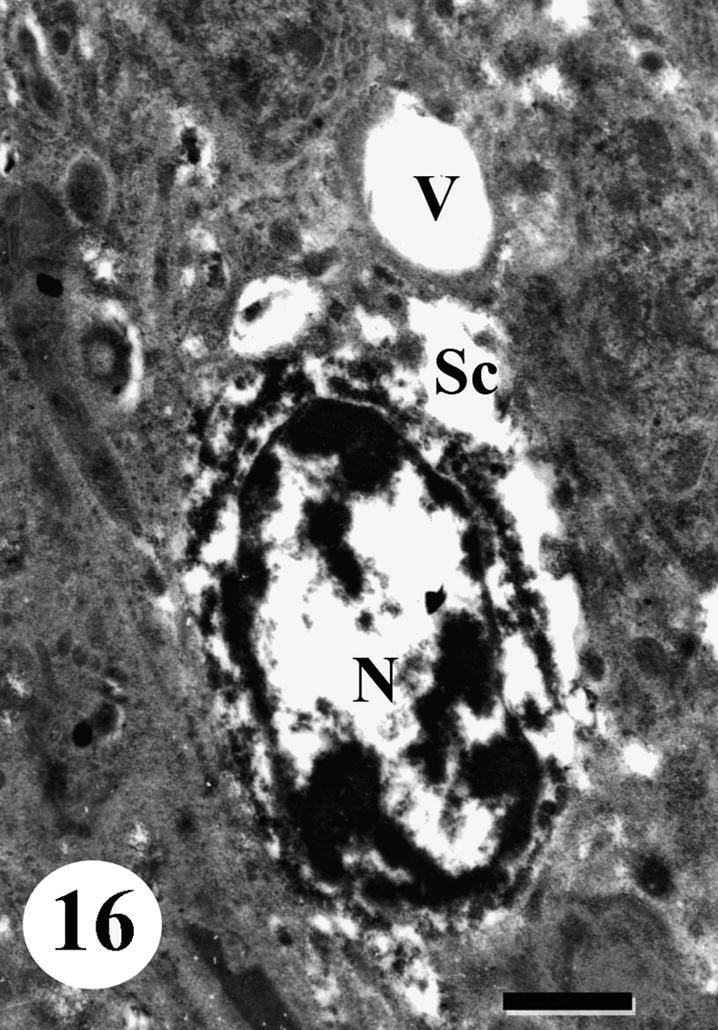
TEM of testis of adult male Schistosoma mansoni recovered from mice treated with Sidr honey and black-seed oil showing another degenerated spermatocyte (Sc) with vacuolated cytoplasm (V). Note seriously degenerated nucleus (N). Bar = 1 μm.
Rosette of early spermatids appeared pale with numerous mitochondria accumulated at nuclear anterior end. Lipid granules were observed in the cytoplasm. Nuclei became very pale with very few chromatin patches while some nuclei degenerated completely. Degeneration and regression of late spermatids were also noted in testicular follicles of treated worms and the cytoplasm develops vacuolation (Figs. 17 and 18). As to spermatozoa, some of them showed abnormal morphology and their nuclei lost characteristic electron-lucent areas (Fig. 19).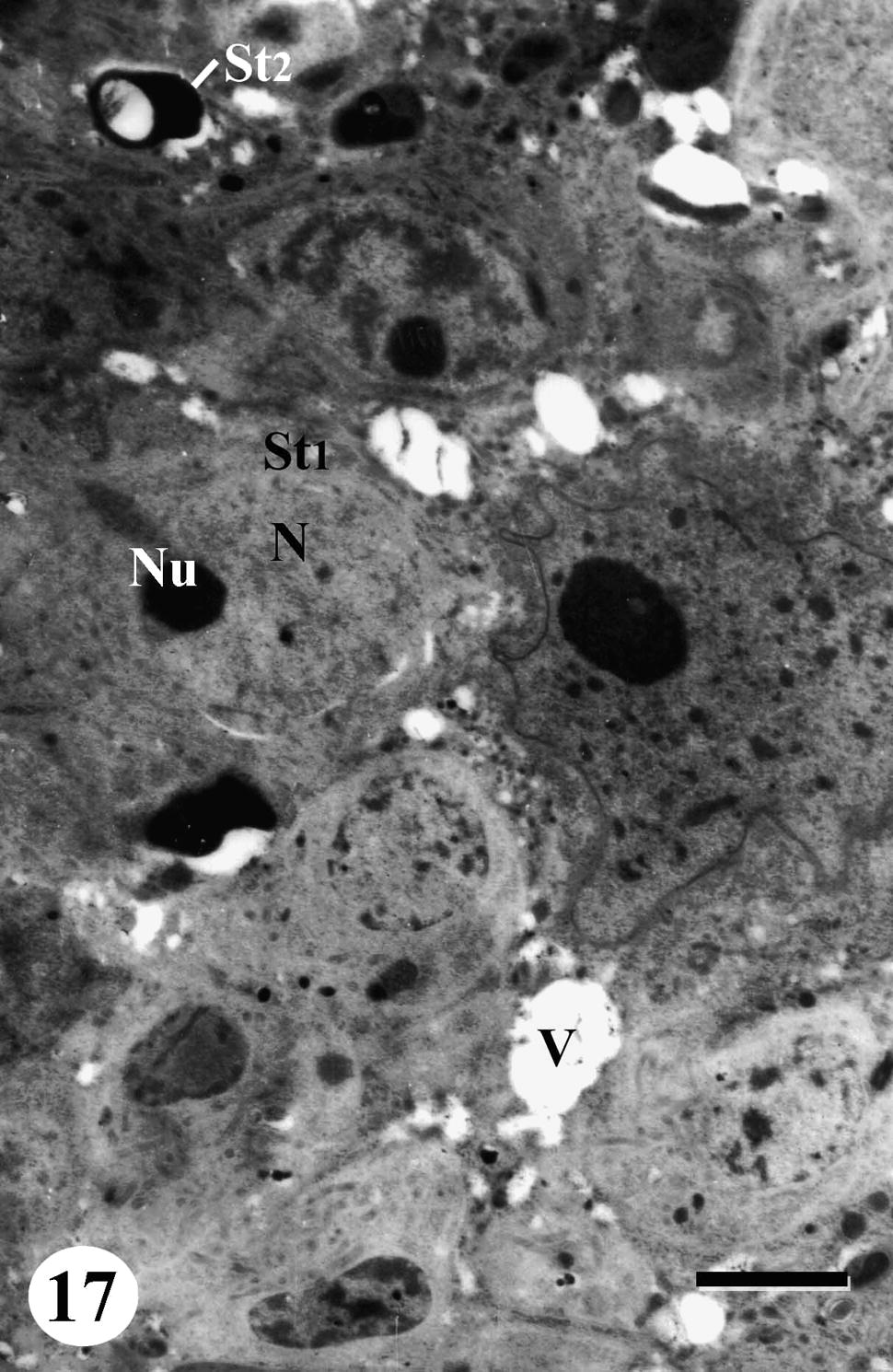
TEM of testis of adult male Schistosoma mansoni recovered from mice treated with Sidr honey and black-seed oil showing rosette of early spermatids (St1), with pale cytoplasm containing numerous mitochondria, pale swollen nucleus (N) with nucleolus (Nu) and few chromatin patches. Note degenerated late spermatid (St2). Bar = 2 μm.
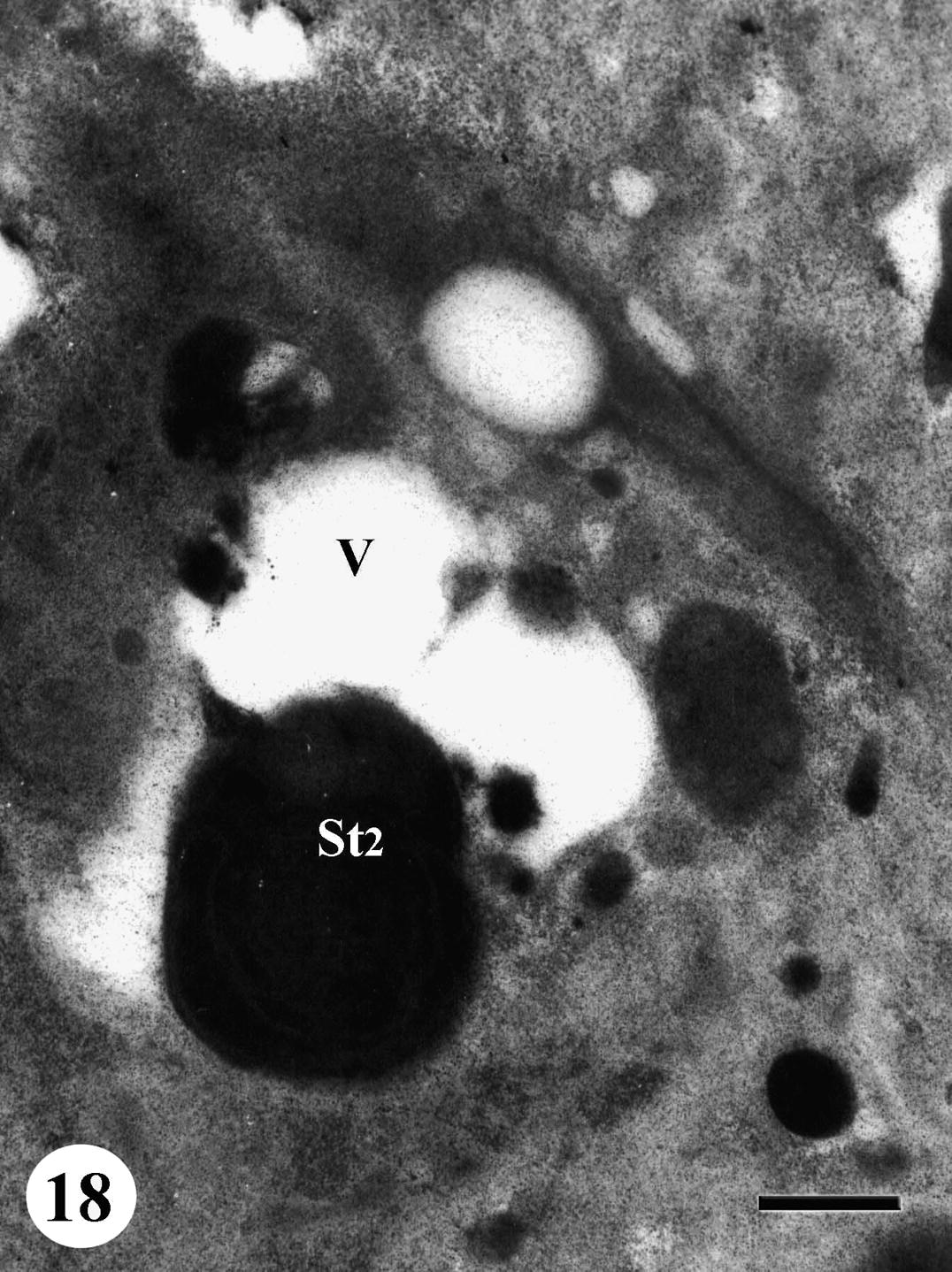
TEM of testis of adult male Schistosoma mansoni recovered from mice treated with Sidr honey and black-seed oil showing regressed late spermatid (St2). Note a pronounced vacuolation (V) of testicular material. Bar = 500 nm.
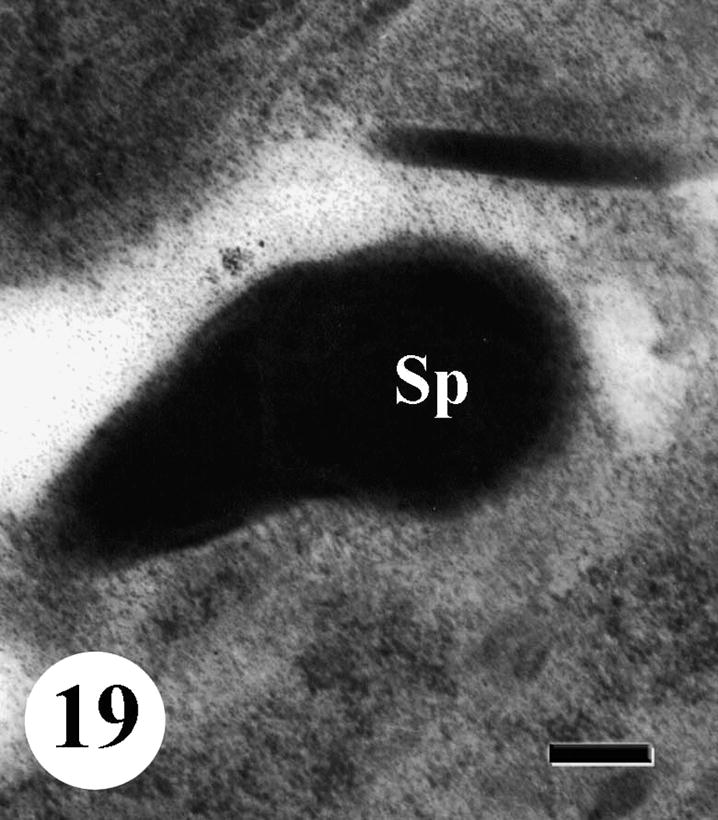
TEM of testis of adult male Schistosoma mansoni recovered from mice treated with Sidr honey and black-seed oil showing spermatozoa (Sp) with abnormal morphology and nucleus lost characteristic electron-lucent area. Bar = 200 nm.
4 Discussion
Tegumental and subtegumental alterations induced by different types of treatment used in the present investigation were characterized by swelling, disappearance of spines, cytoplasm vacuolation, reduction or disappearance of secretory granules, degeneration in the cytoplasm and nuclear materials and sunken of some sensory papilla in the swelling tegument. However, the combined treatment was more sever in action on the tegument compared to single one. More or less similar changes were induced in response to different chemical drugs. Voge and Bueding (1980) presented a detailed study on S. mansoni tegumental surface alterations induced by subcurative doses of the schistosomicide amoscanate. They observed swelling, wrinkling, constriction, collapse of sensory bulbs and erosion of large areas of the surface. Mehlhorn et al. (1981) reported that the primary effect of praziquantel that eventually lead to the death of S. mansoni was the disruption of the tegument of those parasites that are chemotherapeutically susceptible to praziquantel. Tegumental changes induced by oxamniquine treatment of adult S. mansoni worms included marked oedema, wrinkling, distortion, complete disorganization of suckers, destruction of tubercles and collapse of sensory bulbs, as observed by Amin and Mikhail (1989). The tegument of S. mansoni was immediately destroyed after exposure in vitro to triclabendazole leading to the formation of defects in the surface of the worms (El-Sayed and Allam, 1997). Also, Ro15-5458 and R-354 were recorded to cause tegumental damage of S. mansoni in the form of vacuolation (Fawzi, 1999; Taha, 2007).
Kusel et al. (1989) reported some of the functions of glycoproteins in parasite surface: act as receptors for growth substances, as a physical or immunochemical barrier to cells and antibodies of the host immune system and maintain the structure of the surface membrane. Therefore, the tegumental changes induced by Sidr honey and/or black-seed oil in S. mansoni worms could have exerted a profound effect upon the metabolic activities of the parasite. Moreover, the alterations produced in the tegumental surface make the worms vulnerable to the host immune system and attacked by the host’s inflammatory cells. Mehlhorn et al. (1981) reported that after treatment of S. mansoni with praziquantel, leucocytes of the host attacked the damaged surface and penetrated to the interior tissues of the parasite. In addition, it is well known that male worms use tubercles and spines in holding to the wall of the blood capillaries. Since the treatment with Sidr honey and/or black-seed oil causes partial or complete destruction to these structures, the worms can be drifted with the blood stream.
The present finding coincide with the scanning electron microscopical observations that obtained by Mostafa (2005) who found that the surface topography of male worms obtained from mice treated with Sidr honey alone showed extensive loss of spines. The tegument of worms developed in mice treated with black-seed oil showed moderate structural changes, since the tubercles on the dorsal surface of the male showed partial loss of spines. However, the worms developed in mice treated with Sidr honey and black-seed oil together showed the greatest changes; they lost their normal surface architecture, and erosion of the tegument and spines loss were noted. In addition, the obtained results are in harmony with that observed by Mostafa and Soliman (2002) in their study on the surface topography of adult worms of S. mansoni harbored in albino mice treated with black-seed oil; they reported that the tubercles on the dorsal surface of the mature males developed in mice treated with black-seed oil from 0 day of infection showed extensive loss of spines. Spines may be partially or completely disappeared in some worms. Moreover, the size of the tubercles was greatly reduced. The inter-tubercle tegumental regions showed extensive swelling (oedema) while erosion of the surface was observed.
Ultrastructural changes in the gastrodermis following treatment with Sidr honey and black-seed oil may interfere with digestion and, consequently, have a determental effect on nutrient assimilation. The schistosome digestive tract has been more or less ignored in several ultrastructure investigations, although it long has been recognized, because of its role in the physiology and development of the organism (Senft, 1969; Erasmus, 1977). However, there have been a few reports on drug action relative to this organ appeared to be comparable with the present finding. For instance, Leitch and Probert (1990) observed a severe damage to the gastrodermis with the development of autophagic vacuoles containing whorls of myelin and sequestered portions of damaged tissue in S. haematobium recovered from hamsters treated with astiban. Moreover, focal and extensive lysis, decrease in granular endoplasmic reticulum, vacuolation and degeneration of mitochondria were observed in gut epithelium of S. haematobium harbored in mice following amoscanate (Leitch and Probert, 1984), and artemether administration (Xiao et al., 2002, 2006). The increase in number of gastrodermal vacuoles has been documented by Bogitsh (1975) and Clarkson and Erasmus (1984) and is considered to be consistent with changes occurring in the gastrodermis of S. mansoni when subjected to stress conditions, such as starvation or drug treatment, either in vitro or in vivo.
Various alterations in testicular structure of S. mansoni have been recorded in this study as a result of treatment with Sidr honey and black-seed oil. Such alterations included the disintegration of non-germinal cells, cellular swelling, distortion and disorganization of germ cells, vacuolation within the testis and regression of spermatocytes. Non-germinal cells were partially disintegrated in this investigation. Complete disintegration has been reported in the testis of S. mansoni after treatment with astiban (Otubanjo, 1981), Ro15-5458 (Mohamed, 1999) and Ro-354 (Soliman, 2008). Moreover, vacuolation of the non-germinal cells was reported in testis of S. haematobium treated with Ro15-5458 (Fawzi et al., 2001). Non-germinal cells of S. mansoni showed increased phagocytic activity in astiban-induced testicular damage (Otubanjo, 1981).
In this study, the germinal cells show swelling and contained very large vacuoles. Nucleus squeezed by these vacuoles. Chromatin patches disappeared and the nucleus became condensed. Cytoplasm developed lipid granules while mitochondria abnormally increased and appeared as dark bodies with indistinct cristae. Leitch and Probert (1984) similarly reported abnormal increase of mitochondria as evident after treatment of S. haematobium with amoscanate. Mohamed (1999) reported shrinkage of germinal cells leads to separation and intercellular spaces after Ro15-5458 administration in case of S. mansoni. Similarly, Fawzi et al. (2001) found that shrinkage and cell separation in S. haematobium. Jiraungkoorskul et al. (2005) reported swelling of the testicular tissue followed by degeneration leaving several hollow spaces in Eurytrema pancreaticum after using PZQ and triclabendazole.
Vacuolation within the testis and regression of spermatocytes observed in this study are comparable to those recorded by Irie et al. (1989) in their work on S. mansoni treated with PZQ and dextro-PZQ, Mohamed (1999) and Soliman (2008) in their investigation on S. mansoni treated with Ro15-5458 and Ro-354. The anticancer drug procarbazine was found to be profoundly damaging to the primary and secondary spermatocytes and spermatids which were replaced by amorphous granular material (Basch and Clemens, 1989). Degeneration and atrophy of the testis of S. japonicum was also demonstrated by You et al. (1992) after artemether administration. Otubanjo (1981) mentioned that testicular disorganization was prominent initially in spermatozoa and spermatids but became more generalized with drug accumulation. Basch and Clemens (1989) reported disruption of meiotic process, spermatocytes and spermatids were destroyed and replaces by amorphous granular material in S. mansoni by procarbazine treatment.
In conclusion, it is obvious that Sidr honey, black-seed oil and Sidr honey and black-seed oil together had schistosomicidal effects upon the tegument of S. mansoni in albino mice, but treatment with Sidr honey and black-seed oil together was the most effective. On the other hand, Sidr honey alone or black-seed oil alone appeared non-effective against the gastrodermis and testes. The last two organs are affected only by treatment with Sidr honey and black-seed oil together. Therefore, these treatments may represent a promising alternative strategy for control of schistosomiasis mansoni, especially in endemic areas and where drug-resistant strains are found. These natural products may be recommended as useful, pleasant and popular accepted elements of food and drinks in such cases.
References
- Experimental trials of an artemisinin derivative in treatment of Schistosoma mansoni infected mice. Journal of Egyptian Society of Parasitology. 2000;30(1):295-303.
- [Google Scholar]
- Experimental use of black-seed oil against Schistosoma mansoni in albino mice. IV. Oxidative stress markers and some biochemical parameters in the liver and kidney. Journal of Egyptian German Society of Zoology. 2003;41(A):227-253.
- [Google Scholar]
- Effects of Solanum nigrum leaves water extract on the penetration and infectivity of Schistosoma mansoni cercariae. Journal of Egyptian Society of Parasitology. 2005;35(1):33-40.
- [Google Scholar]
- Effects of daily consumption of honey solution on hematological indices and blood levels of minerals and enzymes in normal individuals. Journal of Medicinal Food. 2003;6(2):135-140.
- [Google Scholar]
- Schistosoma mansoni: tegumental surface alterations following oxamniquine treatment of infected mice. Journal of Egyptian Society of Parasitology. 1989;19(Suppl. 2):815-826.
- [Google Scholar]
- Studies on Schistosomiasis japonicum. IV. Chemotherapy of experimental schistosomiasis japonica. American Journal of Hygiene. 1946;44:348-366.
- [Google Scholar]
- Schistosoma mansoni: reversible destruction of testes by procarbazine. Comparative Biochemistry and Physiology. 1989;93:397-401.
- [Google Scholar]
- Cytochemistry of gastrodermal autophagy following starvation in Schistosoma mansoni. Journal of Parasitology. 1975;61:237-248.
- [Google Scholar]
- Schistosoma mansoni: an in vivo study of drug-induced autophagy in the gastrodermis. Journal of Helminthology. 1984;58:59-68.
- [Google Scholar]
- Effect of Nigella sativa crude oil on skin penetration and viability of Schistosoma mansoni cercariae. The New Egyptian Journal of Medicine. 1994;11(1):431-436.
- [Google Scholar]
- Effect of triclabendazole on the tegument of Schistosoma mansoni: a scanning electron microscope study. Journal of Egyptian Society of Parasitology. 1997;27(1):143-152.
- [Google Scholar]
- Antioxidant and antimicrobial activity of honeys against oral pathogens. Journal of Dental Research. 2002;80:349.
- [Google Scholar]
- The effects of manuka honey on plaque and gingivitis: a pilot study. Journal of the International Academy of Periodontology. 2004;6(2):63-67.
- [Google Scholar]
- The host–parasite interface of trematodes. Advances in Parasitology. 1977;15:201-242.
- [Google Scholar]
- Ultrastructural studies on the effect of antischistosomal drug Ro 15-5458 on the tegument of male Schistosoma mansoni. Egyptian Journal of Zoology. 1999;33:21-31.
- [Google Scholar]
- Schistosoma haematobium: testicular damage and repair after in vivo treatment of infected hamsters with the antischistosomal drug Ro15-5458. Egyptian Journal of Zoology. 2001;37:1-14.
- [Google Scholar]
- Schistosoma japonicum and S. mansoni ultrastructural damage in the tegument and reproductive organs after treatment with levo- and dextro-praziquantel. American Journal of Tropical Medicine and Hygiene. 1989;41:204-211.
- [Google Scholar]
- Resistance to praziquantel direct evidence from Schistosoma mansoni isolated from Egyptian villagers. American Journal of Tropical Medicine and Hygiene. 1999;60:932-935.
- [Google Scholar]
- Effects of praziquantel and artesunate on the tegument of adult Schistosoma mekongi harbored in mice. Parasitology International. 2005;54(3):177-183.
- [Google Scholar]
- Field evaluation of a test for praziquantel resistance in Schistosoma sp. Veterinary Parasitology. 2003;113(1):83-87.
- [Google Scholar]
- The effects of antimony uptake on the location and pairing of Schistosoma mansoni. British Journal of Pharmacology. 1964;22:342-348.
- [Google Scholar]
- Effect of irradiation and tunicamycin on the surface glycoproteins of Schistosoma mansoni. Memórias do Instituto Oswaldo Cruz. 1989;84:199-204.
- [Google Scholar]
- Case report: Schistosoma mansoni infection: failure of standard treatment with praziquantel in a returned traveler. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2003;97(1):100-101.
- [Google Scholar]
- Schistosoma haematobium: the effect of Astiban on the cell composition and ultrastructure of the vitelline gland and the ultrastructure of the tegument and gastrodermis. Journal of Helminthology. 1990;64(1):65-69.
- [Google Scholar]
- Schistosoma haematobium: amoscanate and adult worm ultrastructure. Experimental Parasitology. 1984;58:278-289.
- [Google Scholar]
- Antischistosomal activity of artemether in experimental schistosomiasis mansoni. Revista de Saúde Pública. 2004;38(1):1-6.
- [Google Scholar]
- The effect of Nigella sativa oil against the liver damage induced by Schistosoma mansoni infection in mice. Journal of Ethnopharmacology. 2002;70(1):1-11.
- [Google Scholar]
- Myrrh; a schistosomicide for human schistosomiasis mansoni. Ain Shams Medical Journal. 1999;50:1401-1417.
- [Google Scholar]
- An investigation on the biological activity of Combretum species. Journal of Ethnopharmacology. 2001;75(1):45-50.
- [Google Scholar]
- In vivo and in vitro experiments on the effect of praziquantel on Schistosoma mansoni. Arzneim-Forsh Drug Research. 1981;31:544-554.
- [Google Scholar]
- Sativa seeds against Schistosoma mansoni different stages. Memórias do Instituto Oswaldo Cruz. 2005;100(2):205-211.
- [Google Scholar]
- Scanning electron microscopy on adults of Schistosoma mansoni treated in vivo with praziquantel and Ro-15(5458) Qatar University Scientific Journal. 1997;17:439-458.
- [Google Scholar]
- Schistosoma mansoni: testis alterations induced by antischistosomal drug Ro15-5458. Journal of Egyptian Society of Parasitology. 1999;29:325-334.
- [Google Scholar]
- The potential of honey to promote oral wellness. General Dentistry. 2001;49(6):584-589.
- [Google Scholar]
- Antimony uptake by Schistosomes. Annals of Tropical Medicine and Parasitology. 1968;62:158-163.
- [Google Scholar]
- Experimental use of black-seed oil against Schistosoma mansoni in albino mice. II. Surface topography of adult worms. Egyptian Journal of Medical Laboratory Science. 2002;11(1):79-85.
- [Google Scholar]
- Experimental use of black-seed oil against Schistosoma mansoni in albino mice. I. Some parasitological and biochemical parameters. Egyptian Journal of Medical Laboratory Science. 2001;10(2):99-113.
- [Google Scholar]
- Effects of sedr honey and/or black-seed oil on Schistosoma mansoni in albino mice: parasitological, biochemical and scanning electron microscopical studies. Egyptian Journal of Zoology. 2005;45:449-469.
- [Google Scholar]
- Schistosoma mansoni: the sustentacular cells of the testis. Parasitology. 1981;82:125-130.
- [Google Scholar]
- The effect of garlic on some parasitological and on hepatic tissue reactions in experimental schistosomiasis mansoni. Journal of Applied Science Research. 2007;3(10):949-960.
- [Google Scholar]
- The effect of garlic on murine schistosomiasis mansoni: a histological and ultrastructural study on the ileum. Research Journal of Medicine and Medical Sciences. 2008;3(2):188-201.
- [Google Scholar]
- In vitro and in vivo studies on the bioactivity of ginger (Zingiber officinale) extract towards adult schistosomes and their egg production. Journal of Helminthology. 2002;76(3):241-247.
- [Google Scholar]
- Considerations of schistosome physiology in the search for anti-bulharziasis drugs. Annals of the New York Academy of Sciences. 1969;160:571-592.
- [Google Scholar]
- Scanning electron microscopy of the tegumental surface of in vivo treated Schistosoma mansoni (Saudi Arabian geographical strain) with oxamniquine and praziquantel. Journal of Egyptian Society of Parasitology. 1991;21(3):797-810.
- [Google Scholar]
- Effect of Daucus carota var. boissieri extracts on immune response of Schistosoma mansoni infected mice. Folia Microbiologica. 1999;44(4):441-448.
- [Google Scholar]
- Schistosoma mansoni: structural damage and tegumental repair after in vivo treatment with praziquantel. Parasitology. 1987;94:243-254.
- [Google Scholar]
- The infection of laboratory hosts with cercariae of Schistosoma mansoni and the recovery of the adult worms. Parasitology. 1965;55:695-700.
- [Google Scholar]
- Ultrastructural alterations in testis and gastrodermis of Schistosoma mansoni due to treatment of infected mice with the new rhodamine derivative Ro-354. Journal of Biological Science. 2008;8(4):738-745.
- [Google Scholar]
- Disruption of spermiogenesis in Fasciola hepatica by some anthelmintics. Zeitschrift für Parasitenkunde. 1975;47:145-150.
- [Google Scholar]
- Ultrastructural alterations in the tegument of male Schistosoma mansoni caused by a new rhodanine derivative (Ro-354) Egyptian Journal of Zoology. 2007;48:371-384.
- [Google Scholar]
- Experimental use of black-seed oil against Schistosoma mansoni in albino mice. III. Ultrastructural studies of the liver. Egyptian Journal of Zoology. 2003;40:27-45.
- [Google Scholar]
- Schistosoma mansoni: tegumental surface alteration induced by subcurative doses of the schistosomicide amoscanate. Experimental Parasitology. 1980;50:251-259.
- [Google Scholar]
- Ultrastructural alterations of adult Schistosoma haematobium harbored in mice following artemether administration. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi. 2006;24:321-328.
- [Google Scholar]
- Transmission electron microscopic observations on the ultrastructural damage in juvenile Schistosoma mansoni caused by artemether. Acta Tropica. 2002;81:53-61.
- [Google Scholar]
- The uptake of tritiated hycanthone by male and female Schistosoma mansoni worms and distribution of the drug in plasma and whole blood of mice following a single intramuscular injection. Bulletin of the World Health Organization. 1970;42:445-449.
- [Google Scholar]
- Effect of artemether against Schistosoma japonicum. Chung Kuo Yao Li Hsueh Pao. 1992;13:280-284.
- [Google Scholar]
- Biochemical studies on the effect of garlic on liver and intestine of schistosomal mice. Egyptian Journal of Bilharzia. 1994;16:107-127.
- [Google Scholar]
- The effects of honey on Leishmania parasites: an in vitro study. Tropical Doctor. 1997;27(Suppl. 1):36-38.
- [Google Scholar]







