Translate this page into:
Preservation of human spermatozoa in a simple medium
*Corresponding author. Address: Zoology Department, College of Science, King Saud University, Saudi Arabia mohameddkhil@yahoo.com (Mohamed A. Dkhil)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
The objective of this study was to estimate the best conditions for sperm preservation in a simple medium and to examine the changes in sperm morphology and vitality due to preservation.
Swim-up technique was carried out using KSOMaa medium (potassium simplex optimized medium with amino acid) then, such sperms were preserved without/with supplementation of BSA to the medium in two different osmolarities (271 and 800 mOsmal). Sperms were preserved for 2 weeks in three different temperatures (37 °C, 4 °C and −20 °C). Our results demonstrated that: (1) KSOMaa medium is a good medium to obtain progressive motile sperms with a good morphology. (2) The best conditions for preserving human spermatozoa were 800 mOsmol KSOM-BSA and a holding temperature of −20 °C. (3) Light and electron microscopy showed that cryodamage has been induced in some human spermatozoa due to preservation. Collectively, our data indicate that this new simple procedure could be the method of choice for selecting motile and morphologically normal spermatozoa. This new preservation method may help in vitro fertilization centers but should be tested to check the embryonic development after intracytoplasmic sperm injection.
Keywords
Human spermatozoa
Sperm preservation method
KSOM
1 Introduction
Preservation of human spermatozoa is one of the most important issues for the possibility of pregnancy following intrauterine insemination (Bunge and Sherman, 1953). Since about half a century and a lot of methods concerned with freezing of human sperms have been improved (Bunge and Sherman, 1953; Royere et al., 1996).
Recently, the cryopreservation of mammalian spermatozoa is widely spread in many laboratories where it can help the sperm genetic material unaffected so that increases the success rate of the assisted reproduction (Hori et al., 2004; Suppawiwat et al., 2006).
Spermatozoa from some animals have been successfully cryopreserved (Gabriel et al., 2000; Eriksson et al., 2002; Cormier and Bailey, 2003) but, the cryopreservation process affected sperm activity and morphology (Yoshida et al., 1990; Hammadeh et al., 2001).
Preservation of human spermatozoa in liquid nitrogen is the most successful method. Since liquid nitrogen can cause some problems and it is not available in all laboratories so, investigators tried to freeze sperms without liquid nitrogen but this procedure have been succeeded only in mice (An et al., 1999; Nguyen et al., 2005; Kishikawa et al., 1999) but not in human.
The study aimed to apply such a method reported in mice (Nguyen et al., 2005) using human spermatozoa, and to investigate the effects of the preservation method on human spermatozoa in a simple medium with/without supplementation of BSA in different osmolarities and different temperature. This new method is rapid, simple, and inexpensive and recovers most of motile sperms in the specimen.
2 Materials and methods
2.1 Collection and evaluation of semen samples
Twenty healthy donors were used in our study. The semen samples were obtained by masturbation directly into sterile plastic containers after at least 2 days of sexual abstinence. Samples were allowed to liquefy for 30 min at 37 °C (WHO, 1999). Each specimen was evaluated according to standard procedures recommended by the World Health Organization (WHO) manual with a phase-contrast microscope (WHO, 1999). Semen parameters assessed included sperm volume, count, motility, morphology, vitality and viability. Donors specimen were included if they had sperm parameters within the normal range defined by the WHO (WHO, 1999). All studies were approved by the Human Investigation Committee of Libyan health organization.
2.2 Testing of the KSOMaa medium efficiency
One milliliter of each seminal sample was diluted 1:2 with KSOMaa media prepared as previously mentioned (Suppawiwat et al., 2006). Samples were centrifuged for 10 min at 300g, then the supernatant was removed and an aliquot of 0.5 ml of KSOMaa media was added. Specimens were incubated for 18 h in 5% CO2 incubator (RS Biotech Laboratory, UK) (Suppawiwat et al., 2006). Sperm motility was observed and compared to that in fresh semen.
2.3 Usage of KSOMaa medium in swim-up procedure
The used medium in the separation process was KSOMaa medium. Progressively motile sperms have been separated by swim-up technique as mentioned previously (Younglai et al., 2001). After swim-up sperm assessed parameters have been evaluated. To check the efficiency of the KSOMaa medium, the same procedure have been done using the commonly used medium in laboratories FertiCult™ IVF medium–0.4% human serum albumin (FetriCult, Beemen, Belgium) then the results were compared.
2.4 Preservation of spermatozoa
Sperms were collected from swim-up were stored in KSOMaa media with or without supplementation of 4 mg/ml bovine serum albumin (BSA) at different osmolarities (271 and 800 mOsmal) and different temperatures (−20 °C, 4 °C, 37 °C) for 2 weeks. Osmolarity was adjusted as mentioned previously (Nguyen et al., 2005) using Micro-Osmometer 3320 (Advanced® Instruments, USA). Spermatozoa were stored in 1.5 ml sampling tubes at a concentration of 5–8 × 106 spermatozoa/ml.
2.5 Thawing and analysis of the preserved spermatozoa
After 2 weeks storage in KSOMaa medium at −20 °C specimen were thawed in a water bath at 37 °C for 10–15 min. Estimation of sperm count, motility, viability staining, hypoosmotic swelling (HOS) testing have been carried out according to WHO (1999).
2.6 Sperm function testing
Sperm motility was assessed before and after swim-up according to the methods described by the WHO (1999). In brief, a motile sperm was defined as a cell having a progressive or nonprogressive motion, with nonprogressive sperms showing clear flagellar movement but no change in position. Immotile sperms included all nonmoving cells without flagellar motion and sperm heads without a flagellum.
Viability of spermatozoa was determined by mixing 10 μl of an aliquot of spermatozoa with one drop of a supravital stain (0.5% eosin Y in aqueous solution of 0.9% NaCl) (WHO, 1999). Counting of living sperms (unstained) and dead ones (stained) were observed at ×400.
To assess the sperm membrane function, HOS test were carried out as mentioned previously (Jeyendran et al., 1984).
2.7 Evaluation of sperm morphology
Sperm morphology was assessed before and after swim-up and also after preservation and thawing process. The sample (5 μl) was spread along the length of a microscope slide. The resulting thin smear was allowed to air dry for 20 min before staining with Giemsa stain (WHO, 1999). Sperm morphology has been estimated at ×1000 magnification under oil emersion and at least 100 spermatozoa were counted on each slide according to Kruger et al. (1987).
2.8 Transmission electron microscopy
After swim-up, the preserved sperms in KSOM-BSA (of osmolarites 271 and 800 mOsml at −20 °C for 2 weeks) were fixed for 2 h 2.5% glutaraldehyde. The fixed sperms were centrifuged at 1200g for 15 min. The pellet was washed in phosphate buffer then postfixed in 1% buffered osmium tetroxide, dehydrated in ethanol and then, embedded in Epon resin. Semi-thin sections were stained with methylene-blue and examined under the light microscope, and then ultra-thin sections were collected in copper grids contrasted with uranyl acetate and lead citrate and observed with electron microscope (Joel 1200 EXII).
2.9 Statistical analysis
Statistical analyses were performed using an unpaired Student’s t-test. The data were analyzed by using Excel 2000 (Microsoft, USA), and Sigma Plot 2001 (SPSS, USA).
3 Results
3.1 Improvement of sperm assessment parameters in KSOMaa medium
All the chosen specimens were normospermic according to WHO (1999) standards. Swim-up technique by KSOMaa medium improved the quality of sperm count, motility, morphology, and vitality. KSOMaa medium has the same quality as FertiCult™ IVF medium (Table 1). Significant improvements noted in all parameters assessed in comparison to fresh semen (P ⩽ 0.05).
Parameters assessed
Fresh semena
Swim-up using KSOMaa medium
Swim-up using FertiCult™ IVF medium
Count × 106/ml
48.40 ± 10.02
25.20 ± 04.79
24.90 ± 03.40
Motility (%)
Progressive
67.20 ± 04.79
82.60 ± 02.24
81.00 ± 04.20
Nonprogressive
17.20 ± 03.54
10.40 ± 02.15
12.60 ± 03.00
Immotile
15.60 ± 40.04
7.00 ± 02.60
06.40 ± 02.41
Normal morphology (%)
Normal
62.40 ± 05.16
81.40 ± 06.56
83.36 ± 03.17
Head defect
19.40 ± 04.22
12.20 ± 04.01
11.74 ± 04.01
Tail and neck defects
18.20 ± 01.32
06.40 ± 03.87
05.90 ± 03.20
Vitality (eosin test; %)
75.60 ± 01.94
86.00 ± 03.70
85.60 ± 05.59
HOS test (%)
72.70 ± 02.61
89.20 ± 04.70
88.00 ± 02.73
Progressive motile sperms increased significantly (P < 0.5) after swim-up. Both nonprogressive and immotile sperms were significantly reduced after swim-up (Table 1).
Light microscopic examinations showed significant improvements (P ⩽ 0.5) in sperm morphology in specimen after swim-up. Normal spermatozoa were 62.4% while it reached 81.4% in swimed up specimens (Table 1). Sperms head defects were reduced after swim-up (12.2%) in comparison to 19.4% in fresh semen. Tail and neck defects have been reduced in swimed up specimen (Table 1).
In addition, the viability of sperms and the percentage of sperms with intact membrane was increased (86% and 89.2%), respectively (Table 1).
Improvement of the membrane function after swim-up using KSOMaa medium has been quantified using HOS test. Membrane function was increased by about 17% when compared to the fresh semen with a significant of P < 0.5 (Table 1 and Fig. 1).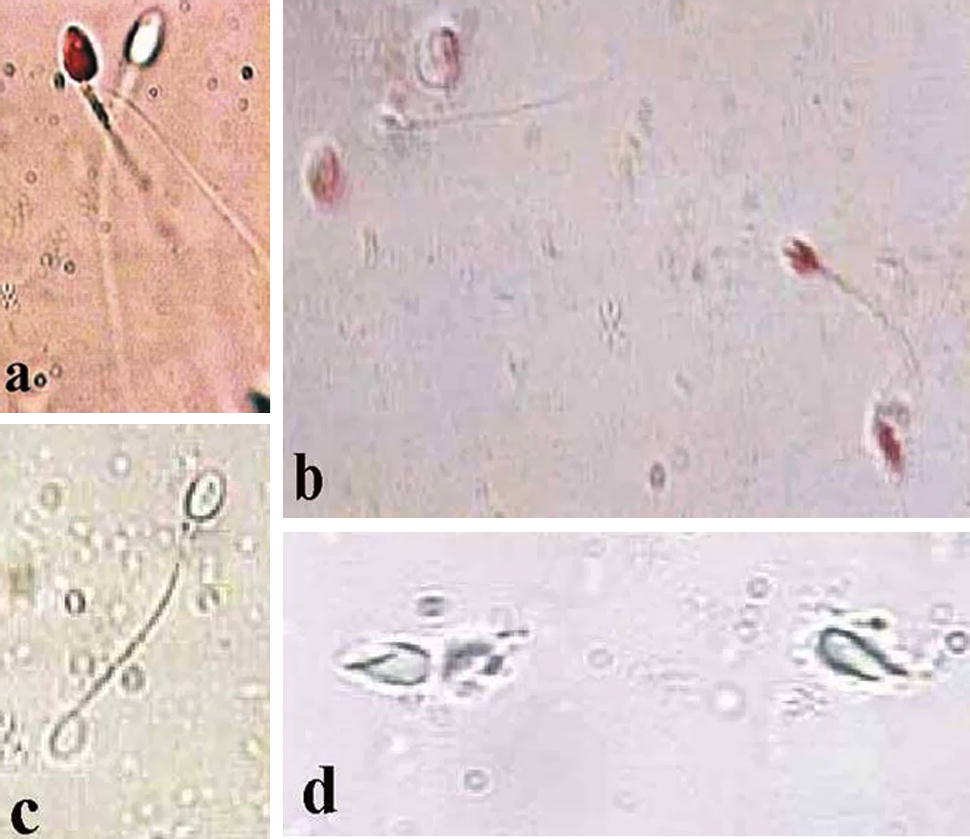
Photomicrograph of human spermatozoa (a) live (unstained) and dead (stained) spermatozoa in fresh semen. (b) Sperms subjected to freezing and stained with eosin Y. (c) Swelled sperm subjected to HOS test. (d) Dead sperms subjected to freezing for 2 weeks appearing with lysed tails and damaged heads
3.2 Changes in sperm concentration after storage in preservation medium
Samples preserved at 271 mOsml showed a reduction in sperm count by 60%, 50%, and 35% at 37 °C, 4 °C, and −20 °C, respectively (Fig. 2). However, samples preserved in 800 mOsml showed a reduction in sperm concentration by approximately 45%, 20%, and 10% at 37 °C, 4 °C, and −20 °C, respectively.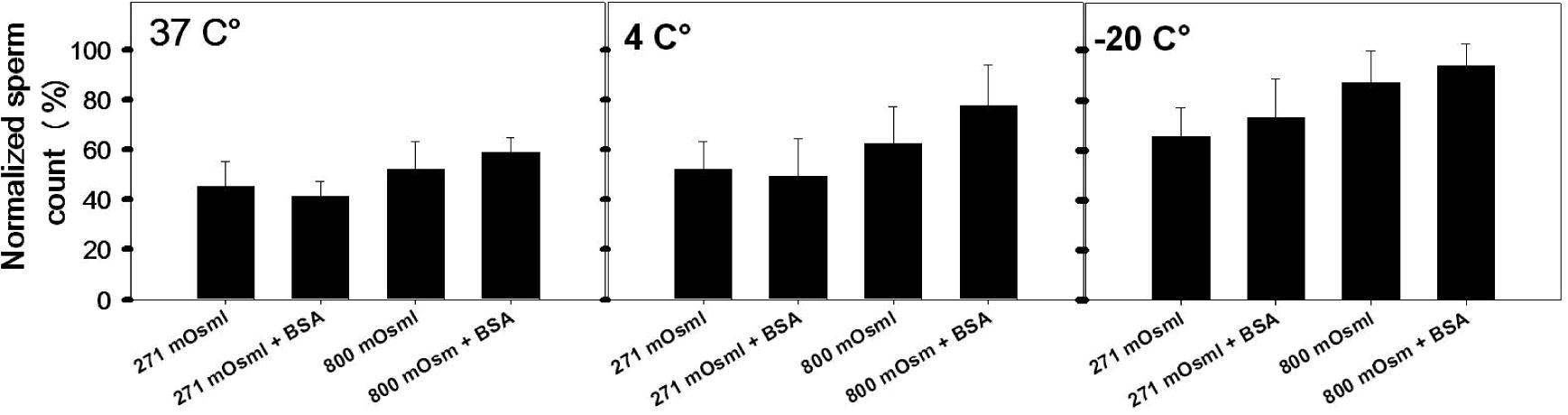
Changes in sperms count (n = 20) after preservation for 2 weeks in KSOMaa medium without/with supplementation of BSA at different temperature and different osmolarities (means ± SD).
Sperm concentration was significantly higher (P < 0.05) when specimen preservation at 800 mOsml than when preservation at 271 mOsml (Fig. 2). Sperm count after preservation at −20 °C was greater than that at 4 °C and 37 °C (Fig. 2). Preserved samples with or without supplementation of BSA to the KSOMaa showed no significant difference in sperm count.
3.3 Effects of storage temperature and osmolarity on sperm morphology
The results of this study showed that, there is no significant difference in sperm morphology between samples preserved with or without the supplementation of BSA to the KSOMaa medium.
Fig. 2 shows that the percentage of normal sperm morphology was more significantly reduced (P < 0.05) when preserved at 37 °C (42.8%) and 4 °C (47.2%) either at 271 or 800 mOsml compared with sperms stored at −20 °C (54.6%).
It is clear that the best preservation condition for sperm morphology is at −20 °C under osmolarity of 800 mOsml for 2 weeks. In general sperm damage was observed after preservation of sperms for 2 weeks. This damage was observed in tails and heads (Fig. 1d).
3.4 Structural changes in spermatozoa preserved at −20 °C
Human spermatozoa were affected by freezing for 2 weeks at −20 °C. Light microscopic examinations showed several types of sperm injury. These injuries were damaged and fragmented heads, isolated or fragmented tails.
Using transmission electron microscopy, human spermatozoa following freezing and thawing were examined. Similar patterns of the structural alterations were observed when spermatozoa were frozen at −20 °C in either 271 mOsmol or 800 mOsmol KSOMaa medium. The plasma membrane of normal spermatozoa appeared intact (Fig. 3a) but the damaged spermatozoa showed structural changes of plasma and acrosomal membranes in addition to the changes induced in the middle piece (Fig. 3a–d). The damaged spermatozoa showed distension, disruption (Fig. 3a and b) and complete loss of the plasma membranes (Fig. 3c). Structural damages to acrosome included swelling (Fig. 3a and b), disruption (Fig. 3a–d) or complete loss of the acrosome membranes (Fig. 3c). The vesiculation of the plasma and outer acrosomal membranes with leakage of acrosome content was also observed (Fig. 3b). A defect in the tail (middle piece) consists of spermatozoa with swelling, disruption or absence of mitochondria (Fig. 3d). Also, destruction or absence of the middle piece plasma membrane was observed (Fig. 3d). Absence of the central (Fig. 3d) or some of the peripheral (Fig. 3d) fibres in the middle piece were observed. In some spermatozoa, the chromatin appeared condensed (Fig. 3c). In other sperms, the chromatin showed an incomplete condensation (Fig. 3a and b).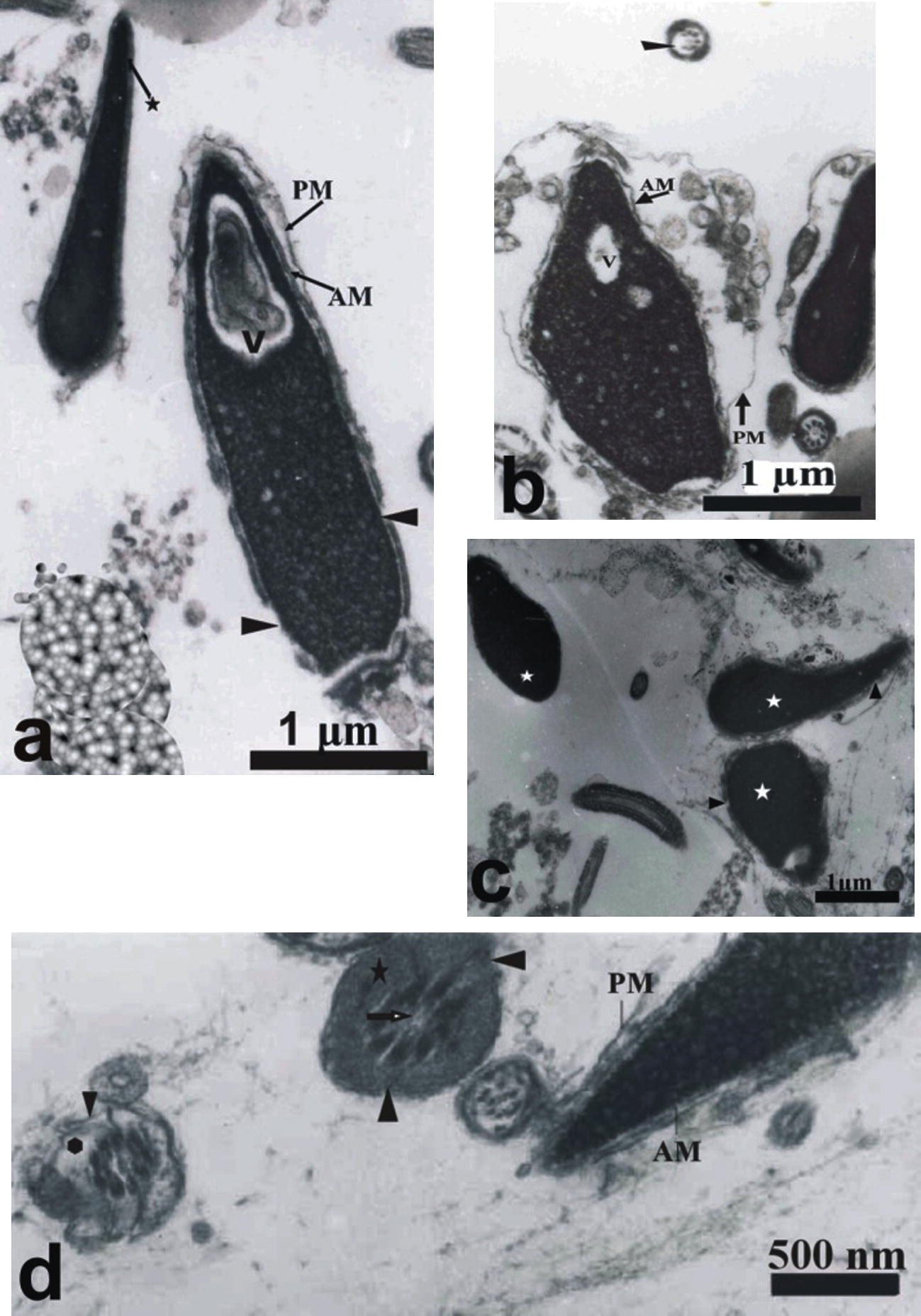
Transmission electron micrographs of frozen–thawed human spermatozoa stored at −20 °C for 2 weeks in 271 or 800 mOsml KSOMaa medium. (a) Normal sperm with intact plasma membranes (star) and sperm with vacuolated nucleus (V) with swelled plasma membrane (PL) and disrupted acrosomal membrane (AM) (head arrow). (b) Spermatozoa with disrupted plasma membrane and swollen acrosome. (c) Sperm with condensed chromatin (white star), disrupted membrane (head arrow), complete loss of plasma membrane and outer acrosomal membrane. (d) Sperm with damaged swelled mitochondria (star), loss of mitochondria (polygon) with the absence of central fibres in the middle piece.
4 Discussion
There are many current protocols are used to preserve human spermatozoa and most of them are complicated and expensive protocols. To overcome these problems, a new simple method has been developed for human sperm preservation.
The qualitative characteristics of fresh spermatozoa subjected to swim-up via KSOMaa medium have been significantly improved (P ⩽ 0.05). This finding supports the fact that spermatozoa separated via various methods have better quality and a greater fertilizing capacity than spermatozoa in the whole ejaculate (Tanphaichitr et al., 1988).
The importance of sperm morphology in the assessment of male fertility is evident in a large number of studies (Hammadeh et al., 2001; Aitken et al., 1995). Our results demonstrated that semen sample subjected to swim-up via KSOMaa medium separate spermatozoa that have improved morphology (81.4%) compared to that in fresh semen (62.4%).
Since the sperm motility is of considerable importance in evaluation and prediction of fertility potential of semen (Zavos and Centola, 1991) so, the used KSOMaa medium improved the sperm motility after swim-up by about 15%.
Osmolarity has great effects on the morphology of spermatozoa where they swell or shrink in response to the change in dilution or concentration of the intracellular components (Drevius, 1972). This will lead to changes in sperm function (Lang et al., 1998) where the flagellar motility is changed due to changes in osmolarity (Gilmore et al., 1988). But, it is known that many cell types including spermatozoa, can maintain the cell volume to overcome the change in osmolarity (Petrunkina et al., 2004). An important finding in this study was the relationship between the osmolarity of the sperm preservation medium and the morphology of stored sperms. In fact, this study showed that sperms stored at −20 °C in 800 mOsml for 2 weeks had much better morphology than that stored at 271 mOsml and more than that stored at different temperature. This result agreed with that had been carried out on mice sperms (Nguyen et al., 2005). At the same time, the reduction in sperms concentration was less when stored at −20 °C for 2 weeks (about 10%).
The present study showed that the procedures of freezing and thawing caused ultrastructural alterations of human spermatozoa. The organelles most frequently damaged after freezing and thawing are the plasma membrane, the acrosome and the mitochondria. This finding agreed with that have been proved by others (Watson, 1995; He and Woods, 2004) that used DMSO medium for the preservation of elephant spermatozoa.
Various types of damage to acrosome of post-thaw spermatozoa were observed in this study including acrosomal swelling, acrosomal membrane disruption, leakage of the acrosomal contents and complete loss of acrosome. Similar defects in acrosome have been described in frozen spermatozoa from different animal species (Krogenaes et al., 1994; Alvarenga et al., 2000). Since the mitochondria of the sperm tail generate energy to support motility, changes in mitochondrial membrane potential could be a good indicator to predict sperm motility (Garner et al., 1997). This study proved that cryopreservation had induced mitochondrial swelling or injury (Fig. 3) which may be a potential cause of low sperm motility. After preservation, the sperm motility were reduced to reach about 1% after preservation in either 271 mOsmol or 800 mOsml KSOMaa medium at different temperature for 2 weeks (data not shown).
In conclusion, this new simple procedure could be the method of choice for selecting motile and morphologically normal spermatozoa. This new preservation method may help in vitro fertilization centers but should be tested to check the embryonic development after intracytoplasmic sperm injection.
References
- Acrosomal ultrastructure of stallion spermatozoa cryopreserved with ethylene glycol using two packaging systems. Equine Vet. J.. 2000;32(6):541-545.
- [Google Scholar]
- Viable spermatozoa can be recovered from refrigerated mice up to seven days after death. Cryobiology. 1999;38(1):27-34.
- [Google Scholar]
- Differential mechanism is involved during heparin- and cryopreservation-induced capacitation of bovine spermatozoa. Biol. Reprod.. 2003;69(1):177-185.
- [Google Scholar]
- Field fertility with exported boar semen frozen in the new flatpack container. Theriogenology. 2002;58(6):1065-1079.
- [Google Scholar]
- Live rhesus offspring by artificial insemination using fresh sperm and cryopreserved sperm. Biol. Reprod.. 2000;63(4):1092-1097.
- [Google Scholar]
- Fluorometric assessments of mitochondrial function and viability in cryopreserved bovine spermatozoa. Biol. Reprod.. 1997;57(6):1401-1406.
- [Google Scholar]
- Determination of plasma membrane characteristics of boar spermatozoa and their relevance to cryopreservation. Biol. Reprod.. 1988;58(1):28-36.
- [Google Scholar]
- Comparison between human sperm preservation medium and TEST-yolk buffer on protecting chromatin and morphology integrity of human spermatozoa in fertile and subfertile men after freeze–thawing procedure. J. Androl.. 2001;22(6):1112-1118.
- [Google Scholar]
- Effects of dimethyl sulfoxide and glycine on cryopreservation induced damage of plasma membranes and mitochondria to striped bass (Morone saxatilis) sperm. Cryobiology. 2004;48(3):254-262.
- [Google Scholar]
- Artificial insemination of frozen epididymal sperm in beagle dogs. J. Vet. Med. Sci.. 2004;66(1):37-41.
- [Google Scholar]
- Development of an assay to assess functional integrity of the human sperm membrane and its relationship to other sperm characteristics. J. Reprod. Fertil.. 1984;70(1):219-228.
- [Google Scholar]
- Fertility of mouse spermatozoa retrieved from cadavers and maintained at 4 degree. J. Reprod. Fertil.. 1999;116(2):217-222.
- [Google Scholar]
- Membrane alterations in bull spermatozoa after freezing and thawing and after in vitro fertilization. Acta Vet. Scand.. 1994;35:17-26.
- [Google Scholar]
- A quick reliable staining technique for sperm morphology. Arch. Androl.. 1987;18(3):275-277.
- [Google Scholar]
- Functional significance of cell volume regulatory mechanisms. Physiol. Rev.. 1998;78(1):247-306.
- [Google Scholar]
- New preservation method for mouse spermatozoa without freezing. Biol. Reprod.. 2005;72(2):444-450.
- [Google Scholar]
- Role of potassium channels, the sodium–potassium pump and the cytoskeleton in the control of dog sperm volume. Theriogenology. 2004;61(1):35-54.
- [Google Scholar]
- Freezing of epididymal spermatozoa from dogs after cool storage for 2 or 4 days. Theriogenology. 2006;66:1633-1636.
- [Google Scholar]
- Egg penetration ability and structural properties of human sperm prepared by Percollgradient centrifugation. Gamete Res.. 1988;20(1):67-73.
- [Google Scholar]
- Recent developments and concepts in the cryopreservation of spermatozoa and the assessment of their post-thaw function. Reprod. Fertil. Dev.. 1995;7(4):871-891.
- [Google Scholar]
- Laboratory Manual for the Examination of Human Semen and Semen Cervical Mucus Interaction (third ed.). Cambridge University Press; 1999.
- Fertilization of fresh and frozen human spermatozoa. Assist. Reprod. Technol. Androl.. 1990;1:164-172.
- [Google Scholar]
- Selection of sperm from oligozoospermic men for ARTA: comparisons between swim-up and the SpermPrep™ filtration. J. Assist. Reprod. Technol. Androl.. 1991;1:338-345.
- [Google Scholar]







