Translate this page into:
Comparative evaluation of phytochemical screening, in vitro antioxidant & α-Glucosidase inhibitory properties of Ceiba pentandra & Basella rubra leaf extracts: Identification of active principles by Q-TOFLCMS, ADMET prediction & molecular docking approach
*Corresponding author: E-mail address: quahmed@iium.edu.my (Q.U. Ahmed)
-
Received: ,
Accepted: ,
Abstract
Ceiba pentandra and Basella rubra leaves are traditionally used in Indonesia to treat ailments like diabetes. This study aimed to validate their use by assessing their antioxidant and α-glucosidase inhibitory properties. Initially, maceration of the leaves of both plants yielded dichloromethane, methanol (MeOH), and aqueous extracts. These were phytochemically profiled, and the most active extracts underwent quadrupole time of flight- liquid chromatography mass spectrometry (Q-TOF LCMS) analysis to identify potentially active principles, followed by molecular docking to ascertain their mechanism of action. The results revealed that the aqueous and methanolic extracts of C. pentandra exhibited potent antioxidant activities, with IC50 values of 17.66 ± 0.7 and 53.58 ± 0.25 µg/mg ascorbic acid equivalent for ferric reducing antioxidant power, respectively. In comparison, the aqueous and methanolic extracts of B. rubra showed lower antioxidant potential, with values of 8.73 ± 0.1 and 10.17 ± 0.08 µg/mg AAE, respectively. Additionally, the DPPH assay displayed that the aqueous and methanolic extracts of C. pentandra had IC50 values of 157.32 ± 3.44 and 27.71 ± 1.54 µg/mL, respectively. In contrast, the aqueous and methanolic extracts of B. rubra had higher IC50 values of 661.78 ± 1.8 and 253.76 ± 2.4 µg/mL, respectively. Owing to their significantly higher antioxidant activity, the aqueous and methanolic extracts of C. pentandra leaves also displayed better α-glucosidase inhibitory effects compared to B. rubra, with IC50 values of 109.54 ± 1.72 and 10.78 ± 0.48 µg/mL, respectively. Q-TOF LCMS analysis of C. pentandra’s methanolic extract identified significant bioactive compounds, including m-coumaric acid (1), cis-β-d-Glucosyl-2-hydroxycinnamate (2), luteolin 7-rhamnosyl (1->6)galactoside (3), avenanthramide 2s (4), robinetin 3-rutinoside (5), melanoxetin (6), scutellarein 7-glucoside (7), torosaflavone B 3’-O-β-d-glucopyranoside (8), and 2’’-O-α-l-rhamnosyl-6-C-fucosyl-3’-methoxyluteoiin (9). Molecular docking analysis showed that compounds 7, 8, and 3 were the most active with protein 3A4A, having affinity energies of -9.7, -10.0, and -10.3 kcal/mol, respectively. These phenolic compounds could be safe α-glucosidase inhibitors for diabetes treatment.
Keywords
α-glucosidase inhibitory effect
Antioxidants
Basela rubra
Ceiba pentandra
DPPH
FRAP
in silico
molecular docking
Phytochemical analysis
Q-TOF LCMS
1. Introduction
Cellular damage is a severe condition that increases the presence of unstable molecules known as free radicals in the human body, potentially leading to health problems such as neurodegenerative, type 2 diabetes mellitus (T2DM), cancer, and cardiovascular disorders. This cellular damage triggered by free radicals is commonly referred to as oxidative stress. The long-term effects of oxidative stress can result in metabolic disorders, including diabetes and its complications. The International Diabetes Federation (IDF) statistics reported that 415 million adults worldwide were affected by diabetes mellitus (DM) in 2015, and this number increased to 537 million adults in 2021 and is projected to reach 784 million by 2045. More than 60% of these patients are from Asian countries. While there is no definitive cure for DM, medications can help manage its complications (https://idf.org/). Studies have shown that natural antioxidants are commonly used in diabetes treatment. Antioxidants are crucial in global health for managing chronic diseases like diabetes and cardiovascular conditions. They help neutralize free radicals, reduce cellular damage, and improve overall health outcomes. Alpha-glucosidase inhibitors are a class of compounds that help manage diabetes by slowing down the digestion of carbohydrates, thereby reducing postprandial blood glucose levels. Antioxidants can play a role in this process due to their potential inhibitory effects on alpha-glucosidase. Therefore, investigating the antioxidant properties of herbs with alpha-glucosidase inhibitory effects is essential for preventing and treating diabetes in natural medicine (Sharifi-Rad et al., 2020).
A variety of herbs are widely utilized in traditional medicine systems to heal numerous ailments, including T2DM (Saleh et al., 2018). Among these, the two notable traditional medicinal plants from Indonesia are Ceiba pentandra (L.) Gaertn. (Family: Malvaceae), commonly known as the silk-cotton tree or kapok, and Basella alba Linn Var rubra (Family: Basellaceae), commonly known as Malabar spinach or gondola. Both plants are recognized for their therapeutic properties, including their potential to treat diabetes. C. pentandra can grow up to 15 meters in height (Chan, 2023). In contrast, Basella alba Linn var. rubra is an herbaceous annual or biennial climbing plant found in tropical and subtropical areas. It is a succulent, branched, and smooth vine. Both plants have numerous traditional medicinal uses in Southeast Asian countries. The fresh leaves of C. pentandra are administered compressed to alleviate dizziness, while the boiled roots are used to treat edema. The gum from the tree helps to relieve stomach upset, and tender shoots are used as a contraceptive. Leaf infusions are also used to treat fever, cough, hoarseness, and sore throats (Satyaprakash et al., 2013). However, B. rubra is traditionally used as a detoxification agent and to treat a variety of conditions, including fever, constipation, stomach aches, kidney damage, high blood pressure, diabetes, heart-swelling, vomiting blood (hematemesis), hemorrhoids, rheumatism, intestinal inflammation, wound healing, stomach ulcers, gout, vaginal discharge, liver swelling, and to increase vitality and endurance (Ajiboye et al., 2021; Sharma and Behera, 2022).
C. pentandra bark’s extracts have been shown to improved glucose tolerance in normal and streptozotocin-induced diabetic rats (Satyaprakash et al., 2013). They also have α-glucosidase inhibitory properties (Syihabudin, et al., 2018). Lukiati (2014) reported that the B. rubra leaves ethanolic extract shows antioxidant properties. Moreover, Vita et al. (2019) demonstrated that B. rubra leaves ethanolic extract significantly decreased blood glucose levels in white mice, and Thavamani and Subburaj (2017) discovered that the B. rubra leaves extract possesses significant α-glucosidase and α-amylase inhibitory effects, confirming its role in the management of diabetes. The significant medicinal effectiveness of both traditional plants requires further pharmacological investigations, specifically concerning their antioxidant and α-glucosidase inhibitory properties to meticulously confirm their potential role in diabetes management. Hence, this research aimed to determine and validate C. pentandra and B. rubra leaves potential through phytochemical screening and in vitro assays exploring their antioxidant and α-glucosidase inhibitory capabilities using non-polar and polar extracts. Notably, no comparative study on the antidiabetic and antioxidant properties of C. pentandra and B. rubra leaves extracts using these solvents has been reported in the literature. Hence, this is the first scientific study to compare the antidiabetic and antioxidant potentials of the leaves of both plants using different natures of extracts. This was also done by the identification of bioactive compounds as antioxidants and α-glucosidase inhibitors through Q-TOF LCMS analysis and molecular docking approach.
2. Material and methods
2.1 Sample collection, preparation and determination of herbal samples
The fresh leaves of C. pentandra (1.5 Kg) and B. rubra (850 g) (Fig. 1) were collected from Jakarta, Indonesia, in October 2023. Prof. Agustin Krisna Wardani at the herbarium Bogorinase, located at the Research Center for Biology (BRIN), Indonesia, verified the identity of both plants’ leaves (#154427 for C. pentandra and #154430 for B. rubra). The leaves of both plants were pulverized using a FRITSCH PULVERISETTE 19 Universal Cutting Mill (Germany) to obtain powder material: 300 g of C. pentandra and 100 g of B. rubra.
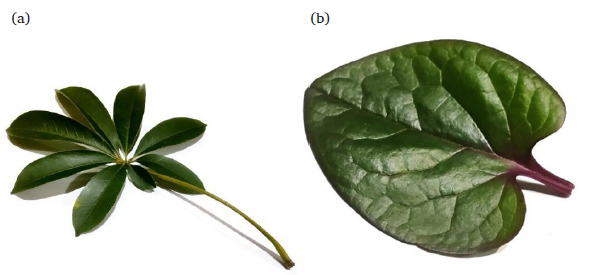
- (a) Ceiba pentandra, (b) Basella rubra.
2.2 Plant samples extraction
Fifty grams of leaf powder from each plant was separately macerated in 150 mL of DCM for 24 h. This process was carried out at room temperature, followed by filtration using Whatman filter paper 1. Subsequently, the plant material residues were subjected to another round of maceration using 150 mL of methanol. The resultant filtrates were concentrated under reduced pressure by the Buchi rotary evaporator at 45°C. Finally, the dried plant residues were macerated using 150 mL of distilled water for 3 cycles of 24 h. The resulting DCM, MeOH, and aqueous extracts were dried in the laboratory dryer at 50°C. After drying, the percentage yield of all extracts was determined using the following formula:
2.3 Phytochemical screening
All extracts were screened for the presence of bioactive phytoconstituents namely, flavonoids, phenolics, hydrolyzable tannins, alkaloids, anthraquinones, steroids,terpenoids, and saponins. Various chemical reagents were prepared, and specific tests were conducted to identify the phytochemicals (Ahmed et al., 2018; Roheem et al., 2020; Raduan et al., 2022).
2.4 Quantitative analysis
2.4.1 Determination of total phenolic content (TPC)
Initially, the extracts were prepared in 30% DMSO and gallic acid was taken as the standard. Then, 20 µL of each extract was mixed with 20 uL of Folin-Ciocalteu reagent in a 96-well plate and shaken for 60 seconds, followed by a five-minute incubation period. After incubation, 200 µL of 7% sodium carbonate and 10 µL of 30% DMSO were added, shaken gently for sixty seconds, and again incubated for 120 minutes in darkness at room temperature. The absorbance was taken using a TECAN Infinite 200 PRO microplate reader and measured at 750 nm. The blank contained the solvent used to prepare the extract and standard. The TPC was expressed as µg gallic acid equivalents (GAE)/mg of dried extract. All determinations were carried out in triplicates (Raduan et al., 2022).
2.4.2 Determination of total flavonoid content (TFC)
The extract, using 30% DMSO and the quercetin standard were prepared using the same treatment.as the extract. 50 µL of the extract was diluted in 100 µL of 30% DMSO, then 20 µL of 10% aluminum chloride was added to a 96-well plate, that was gently shaken and incubated for 3 minutes. After incubation, 60 µL of 30% DMSO and 20 µL of 1M sodium acetate were added. Then, the 96-well plate was incubated for 40 min in darkness at room temperature. Finally, the absorbance was taken using a TECAN Infinite 200 PRO microplate reader and measured at 430 nm. The blank contained the solvent used to prepare the extract and standard. The TFC was shown as µg quercetin equivalents (QE)/mg of dried extract (Raduan et al., 2022).
2.5 Antioxidant activities
2.5.1 Ferric reducing antioxidant power (FRAP) assay
Initially, the above reagent was freshly made by adding 2.5 mL of 10 mM 2,4,6-tris(2-pyridyl)-S-pyrazine in 40 mM HCl, 2.5 mL of 20 mM ferric chloride, and 25 mL of 0.1 M acetate buffer with pH 3.6 in a Schott bottle and incubated for 10 minutes at 37°C. In a 96-well plate, 20 µL of the extract (prepared in 30% DMSO) was mixed with 40 µL of FRAP reagent, followed by the addition of 140 µL distilled water, resulting in the formation of a blue colored complex. The mixture was then incubated for 120 minutes in darkness at 37°C. Finally, the absorbance was taken using a TECAN Infinite 200 PRO microplate reader and measured at 593 nm. The blank contained the solvent used to prepare the extract and standard (ascorbic acid). The FRAP calculation was based on the ascorbic acid calibration curve and shown as µg ascorbic acid equivalents (AAE)/mg of dried extract (Nipun et al., 2021).
2.5.2 Inhibition activity of DPPH (2,2-diphenyl-1-picrylhydrazyl) radicals
The solution (0.2 mM) of DPPH was made by dissolving it (3.94 mg) in ethanol (50 mL). Later, 80 µL of DPPH solution was added to 20 µL of extract in a 96-well plate. The plate was then incubated in darkness for 20 minutes at room temperature. Subsequently, the absorbance was taken using a TECAN Infinite PRO microplate reader and measured at 540 nm. All the determinations were carried out in triplicates. The DPPH radical’s percentage inhibition by the plant extracts was determined by the formula shown below:
% inhibition = [(Ac – As)/Ac] x 100%; where Ac is the absorbance of the control and As is the absorbance of the sample (Sarian et al., 2017).
2.6 α-glucosidase inhibitory activity
p-nitrophenyl-α-D-glucopyranoside (PNPG) as substrate was made after adding 6 mg of 4-Nitrophenyl-β-d-glucopyranoside in 20 mL of 50 mM phosphate buffer. In the 96-well plate, a total of 20 µL of plant’s extract was combined with 100 µL of 30 mM buffer and 10 µL of AG enzyme (0.02) U/µL, then was gently shaken and incubated for 5 minutes at room temperature. After incubation, 50 µL of PNPG was added and incubated for 5 minutes. After the enzyme, substrate and extract reacted, 70 µL of glycine was added to stop the reaction. The plate was incubated again for 10 minutes at room temperature before the measurement using a TECAN Infinite PRO microplate reader at 405 nm. The linear regression analysis was carried out to obtain the IC50 values. Quercetin, as a standard, was prepared using 50% DMSO and 50 mM phosphate buffer. The percentage inhibition activity of the enzyme was determined by the following equation:
% Inhibition = [(Ac – As) / Ac] x 100; where Ac is the absorbance of the control and As is the absorbance of the sample (Sarian et al., 2017).
2.7 LC/MS QTOF analysis of extracts
The LC/MS-QTOF system used in this research included an Agilent 1200 liquid chromatography system with a vacuum degasser, autosampler, binary pump, and 6520 QTOF-MS with an Agilent ESI source. Chromatographic separation was achieved at 40 °C using Agilent ZORBAX Eclipse Plus C18 Rapid Resolution HT (2.1 x 100 mm) 1.8 µm with 0.1% formic acid in dH2O (A) and 0.1% formic acid in acetonitrile (B) for positive mode ionization. The gradient elution program was 0.00 – 18.00 min, 5 - 95% (B); 18 to 23 min; 95% (B); 23.01 min, 5% (B). The total run time was 30 minutes, with 2 minutes re-equilibration before each injection. The sample injection volume was 2 µL, and the mobile phase flow rate was 0.25 mL/min. The MS was operated at 325 °C, with a gas flow of 11 L/min and nebulizer pressure at 35 psi. Agilent MassHunter Qualitative Analysis B.05.00 software (Agilent Technologies, Santa Clara, CA, USA) was used for the data analysis (MS data (.d)). The analysis of chromatographic profiles was done using the METLIN database (Perumal et al., 2021).
2.8 Molecular docking analysis
The main bioactive compounds in C. pentandra extracts were investigated using in-silico molecular docking. The protein model employed was isomaltase (crystal structure) from Saccharomyces cerevisiae (PDB ID: 3A4A) (https://www.rcsb.org/structure/3A4A). The docking technique was optimized by re-docking the native ligand into the receptor on the 3A4A protein. The resulting root-mean-square deviation (RMSD) was less than 2, using the designed grid points X; Y; Z of 20; 26; 30, along with their coordinate grid points X; Y; Z of 21.243, -7.756, and 24.341, respectively. This specific grid box facilitated the docking of the compounds thatwere active and dominantly present in C. pentandra leaves extracts (Ahmed et al., 2018).
2.9 Physico-chemical and pharmacokinetics properties
Bioactive compounds identified through LC/MS-QTOF analysis were evaluated for their influence on drug absorption and distribution, considering factors such as solubility, lipophilicity, and molecular size (http://www.swissadme.ch/index.php, https://biosig.lab.uq.edu.au/pkcsm/prediction) (Ahmed et al., 2018).
2.10 Statistical analysis
The data were collected in three replicates and analyzed for comparative statistics e.g., mean ± standard error, one-way analysis of variance (ANOVA), and Tukey’s post-hoc analysis for a pair-by-pair multiple comparison. Values were considered significant when p<0.05. The Sigma Plot (Systat Software, San Jose, California, USA) and IBM SPSS Statistics for Windows, Version 20.0 Armonk, NY: IBM Corp) were used to perform all statistical analyses.
3. Results and discussion
3.1 Yields of plant extracts
Dried powdered leaves of C. pentandra and B. rubra were successively macerated using dichloromethane, MeOH, and distilled water to obtain DCM, MeOH, and aqueous extracts, respectively. The various conventional and non-conventional extraction techniques employ solvents with varying degrees of polarity to selectively isolate bioactive compounds based on their differing polarities. Therefore, this strategy causes a difference in the resulting yield (Tasnuva et al., 2019). The percentage of yield for each extract is shown in Table 1.
| Plant | Solvent | Percentage yield (%) |
|---|---|---|
| Ceiba pentandra | DCM | 3.93 ± 0.04 |
| MeOH | 7.76 ± 0.29 | |
| H2O | 5.82 ± 0.16 | |
| Basella rubra | DCM | 3.870 ± 0.04 |
| MeOH | 15.00 ± 0.18 | |
| H2O | 15.69 ± 0.10 |
DCM: Dichloromethane, MeOH: Methanol.
The MeOH extract of C. pentandra leaves recorded the highest % yield, followed by the aqueous and DCM extracts. However, in the case of B. rubra, aqueous extract displayed the highest % yield followed by methanolic extract (Fig. 1S) (Supplementary file). According to Abookleesh et al. (2022), the skeletal, structural, and functional groups of secondary metabolites affect their solubility and confer on them a distinctive characteristic suitable for a wide range of medical uses. Similarly, non-polar and polar extracts from the leaves of C. pentandra and B. rubra were prepared using three different solvents with varying degrees of polarity to obtain secondary metabolites of different natures. Dichloromethane, a non-polar organic solvent, was used to extract non-polar secondary metabolites from the plant material; however, MeOH as a polar organic solvent and water as a polar inorganic solvent were employed to extract polar secondary metabolites from the plant material (Alshammari, 2021).
3.2 Phytochemical screening
The phytochemical screening of all plant extracts was done through previously described chemical tests to confirm the presence of secondary metabolites as bioactive compounds. The results displayed in Table 2 reveal that MeOH extract of C. pentandra leaves was the most potent in nature, exhibiting the presence of several secondary metabolites, namely flavonoids, phenolics, hydrolyzable tannins, anthraquinones, steroids, terpenoids, and saponins. DCM extracts of both plants showed the presence of alkaloids. However, alkaloids were found to be absent in the MeOH and aqueous leaf extracts of C. pentandra and B. rubra.
| Phytochemical screening | Ceiba pentandra | Basella rubra | ||||
|---|---|---|---|---|---|---|
| DCM | MeOH | H2O | DCM | MeOH | H2O | |
| a) Flavonoids | ||||||
| 1. Shinoda’s test | + | + | + | + | + | - |
| 2. Zinc-HCl test | + | + | + | + | + | - |
| b) Phenolics | + | + | + | + | + | + |
| c) Hydrolyzable Tannins | + | + | + | + | + | - |
| d) Alkaloids | + | - | - | + | - | - |
| e) Anthraquinones | - | + | - | - | - | - |
| f) Steroids | + | + | - | + | + | - |
| g) Terpenoids | + | + | + | + | - | - |
| h) Saponins | - | + | + | - | + | + |
DCM: Dichloromethane, MeOH: Methanol. +: Positive, -: Negative
3.3 Total phenolic and total flavonoid contents (TPC & TFC)
The C. pentandra and B. rubra leaves MeOH extracts showed the highest TPC (165.30 ± 5.86 and 54.86 ± 1.57 µg/mg GAE), followed by aqueous (46.82 ± 1.63 and 27.00 ± 0.35 µg/mg GAE), and DCM extracts (10.87 ± 0.14 and 6.27 ± 0.15 µg/mg GAE). It has been confirmed by several studies that TPC has significantly correlated to the free radical scavenging effect. Polar solvents lead to the extraction of phenolic compounds, which could be one of the chief reasons for the methanol extracts exhibiting higher TPC than DCM (Alara et al. 2021).
The results of TFC and TPC are shown in Table 3 and Fig. 2S and 3S. From these results, it was observed that the DCM leaf extracts of C. pentandra and B. rubra had the highest flavonoid content (72.58 ± 1.23 and 59.27 ± 1.02 µg/mg QE) followed by methanolic extracts (15.36 ± 0.15 and 18.82 ± 0.10 µg/mg QE), and aqueous extracts (2.90 ± 0.05 and 3.00 ± 0.01 µg/mg QE). Generally, a high TPC correlates with a high TFC owing to the occurrence of several kinds of flavonoids in the plant extracts (Hikmawanti et al., 2021). However, the results of our study revealed a slightly different trend for the presence of phenolic compounds compared to previous findings. This trend was not found to be proportionate for methanolic and aqueous extracts, which could be ascribed to the existence of different types of phenolic substances, other than flavonoids, in the plant material. Furthermore, the flavonoids detected in the DCM extract are likely to have hydrophobic groups or be methoxylated, acetylated, prenylated or have a smaller number of hydroxyl groups as substituents, thereby making them less polar in nature (Sarian et al., 2017).
| Plants | Solvents | TFC (µg/mg QE) | TPC (µg/mg GAE) |
|---|---|---|---|
| Ceiba pentandra | DCM | 72.59 ± 1.23a | 10.87 ± 0.14a |
| MeOH | 15.36 ± 0.15b | 165.30 ± 5.86b | |
| H2O | 2.90 ± 0.05c | 46.82 ± 1.63c | |
| Basella rubra | DCM | 59.27 ± 1.02d | 6.27 ± 0.15d |
| MeOH | 18.82 ± 0.10e | 54.86 ± 1.57e | |
| H2O | 3.00 ± 0.01c | 27.00 ± 0.35f |
Note: Mean values (± standard deviation), triplicates, a to f significant difference (p<0.05). DCM: Dichloromethane, MeOH: Methanol.
Generally, phenolic compounds, including flavonoids, are a major group of secondary metabolites that serve as primary antioxidants. Variations in flavonoid or phenolic structures and group substitution influence the stability of phenoxy radicals, thus influencing the flavonoids’ antioxidant properties (Shamsudin et al., 2022). Apart from the typical antioxidant properties, phenols (flavonoids in particular) can decrease their membrane fluidity, which stabilizes membranes, reduces the lipid membrane peroxidation, and consequently limits the diffusion of free radicals (Kulbat, 2016).
3.4 Biological activities: Antioxidants and α-glucosidase inhibitors
Plants’ antioxidant properties are crucial in evaluating their medicinal effectand benefits. Researchers are actively investigating new plant sources to assess their efficacy, considering them possibly desirable alternatives to conventional medicines due to their minimum deleterious properties. Antioxidants isolated from plant-based extracts have been shown to be effective in counteracting a wide range of disorders, including cardiovascular disease, neurological disorders, cancer, and diabetes (Ahmed et al., 2020). These antioxidant effects are chiefly attributed to the existence of polyphenolic compounds, including flavonoids and tannins, which are the main polyphenolic compounds present in medicinal plants (Shamsudin et al., 2022). Several research studies have shown the antioxidant effects of aerial parts and roots extracts, including vegetables which denote a promising source of antioxidants with potential health benefits. However more research studies are still warranted to appropriately acknowledge and comprehend their bioavailability and medicinal functions (Alhassan et al., 2019).
This study determined the potency of the leaf extracts of C. pentandra and B. rubra in terms of antioxidant and α-glucosidase inhibitory properties. Both pharmacological properties are considered essential for using these traditional medicinal plants in treating diabetes and the complications associated with it. Therefore, the antioxidant and antidiabetic properties of leaves DCM, MeOH, and aqueous extracts of C. pentandra and B. rubra were determined through the in vitro DPPH, FRAP, and α-glucosidase tests, respectively (Table 4; Fig. 4S, 5S, 6S). The results of antioxidant assays displayed that the leaf methanol extracts of both plants exhibited the strongest antioxidant effects. Table 4 shows that the highest FRAP values are associated with the methanolic leaf extracts of C. pentandra (53.58 ± 0.25 µg/mg AAE) and B. rubra (10.17 ± 0.08 µg/mg AAE). Notably, the aqueous leaf extract of C. pentandra showed lower FRAP activity compared to its methanolic extract. The FRAP experiment evaluates the antioxidant effect of a compound by its ability to reduce ferric (Fe3⁺) to ferrous (Fe2⁺) ions. The results are often expressed in terms of ascorbic acid equivalents (AAE), typically in µg/mg. Comparison of FRAP values of the plant extract with those of a known standard helps to understand the extract’s antioxidant capacity. In general, high FRAP values indicate a strong antioxidant effect, and low FRAP values suggest a weaker antioxidant effect (Sarian et al., 2017). Olugbami et al. (2014) mentioned that an ideal IC50 value for the DPPH assay should preferably be under 50 µg/mL to demonstrate higher antioxidant activity. A higher IC50 value would suggest a lower or inconsequential antioxidant effect with no therapeutic implications. Antioxidants play a significant role in inhibiting α-glucosidase, which can be useful for managing blood sugar levels and potentially preventing diabetes and its associated complications (Shamsudin et al., 2022). The correlation between antioxidants and α-glucosidase inhibition is particularly intriguing in the context of diabetes management. Studies have verified a positive correlation between the antioxidant activity of certain phenolic compounds and their capability to inhibit α-glucosidase. For instance, flavonoids, known for their potent antioxidant effects, have been shown to effectively hinder the action of α-glucosidase on the carbohydrates obtained from diet (Alhassan et al., 2019). This inhibition mechanism typically involves the binding of these antioxidative compounds to the active or allosteric sites of the α-glucosidase, thereby decreasing its action of the hydrolysis of carbohydrates (Sarian et al., 2017).
| Samples | Solvents | Antioxidant activities | α-glucosidase | |
|---|---|---|---|---|
|
FRAP (µg/mg AAE) |
DPPH (IC50 µg/mL) |
IC50 (µg/mL) | ||
| Ceiba pentandra | DCM | 3.92 ± 0.03 | 649.77 ± 4.56 | NA |
| MeOH | 53.58 ± 0.25 | 27.71 ± 1.54 | 10.78 ± 0.48 | |
| H2O | 17.66 ± 0.70 | 157.32 ± 3.44 | 109.54 ± 1.72 | |
| Basella rubra | DCM | 7.76 ± 0.28 | 380.65 ± 2.63 | NA |
| MeOH | 10.17 ± 0.08 | 253.76 ± 2.40 | 500.82 ± 3.38 | |
| H2O | 8.73 ± 0.10 | 661.78 ± 1.81 | 500 ± 5.94 | |
| Ascorbic acid | - | 3.96 ± 0.03 | - | |
| Quercetin | - | - | 9.59 ± 0.29 | |
Note: Mean values (± standard deviation) are for triplicate assay; NA: Not Active; FRAP: Ferric reducing antioxidant power; DPPH: 2,2-diphenyl-1-picrylhydrazyl; DCM: Dichloromethane, IC: Inhibitory concentration, MeOH: Methanol.
The one-way ANOVA showed that the plant-solvent interaction had a significant effect on the FRAP antioxidant assay (µg/mg AAE) (Plant * Solvent; df = 2; F = 2731.707; p<0.001). To understand the differences among various solvents in greater detail, Tukey’s post-hoc analysis was employed. It revealed that the MeOH extract had the highest FRAP values in comparison to the other two solvents. The MeOH extract had a significantly higher FRAP value than the DCM extract (mean difference = 26.0333; p < 0.001) followed by the aqueous extract (mean difference = 18.7167; p < 0.001) (Table 1S).
The one-way ANOVA showed that the plant-solvent interaction had a significant effect on the DPPH assay (IC50 µg/mL) (Plant * Solvent; df = 2; F = 10502.459; p<0.001). To understand the differences among various treatments in greater details, Tukey’s post-hoc analysis was employed (Table 2S). The pair-by-pair comparison through the post-hoc analysis confirmed the significantly stronger antioxidant activity of C. pentandra leaves. It had the strongest DPPH values in comparison to the other B. rubra extracts. The C. pentandra extracts had significantly lower DPPH values than the B. rubra extracts (mean difference = -153.7967; p < 0.001). In addition to this, the MeOH extract had the highest activity, followed by the aqueous extract (mean difference = -268.8183; p < 0.001) and the DCM extract (mean difference = -374.4783; p < 0.001). It was also found that the control (ascorbic acid) had a significantly higher antioxidant activity than the plant extracts (Table 2S).
The one-way ANOVA showed that the plant-solvent interaction had a significant effect on the α-glucosidase IC50 (µg/mL) assay (Plant * Solvent; df = 1; F = 152.043; p<0.001). To understand the differences among various treatments in greater detail, Tukey’s post-hoc analysis was employed (Table 3S). The post-hoc analysis further confirmed the significantly stronger C. pentandra leaves activity on α-glucosidase. It had the strongest IC50 (µg/mL) values in comparison to B. rubra extract. The C. pentandra extract had a significantly lower IC50 value than B. rubra extract (mean difference = -456.00; p < 0.001). The analysis further revealed that in addition to this, the MeOH extract had the higher activity than the aqueous extract (mean difference = -59.7133; p < 0.001). The results also confirmed that the control (Quercetin) had significantly higher α-glucosidase activity than the plant extracts (Table 3S).
3.5 Identification of the bioactive compounds using LC-MS QTOF
A cutting-edge QTOF LC-MS was used to identify the potent bioactive substances possessing antioxidative and α-glucosidase inhibitory effects present in the C. pentandra leaves. This advanced, sophisticated analytical method links the separation capabilities of liquid chromatography (LC) to mass spectrometry (MS) using a quadrupole time-of-flight (TOF) analyzer. This technique is generally employed to quantify, characterize and identify bioactive agents present in plant extracts with higher accuracy, precision and confidence (Irakli et al., 2021). Moreover, this method also allows for the exact quantification of the biologically active agents, which is necessary to understand the potency and effectiveness of the therapeutically active plant-based extracts (Mustikasari et al., 2024). Additionally, it provides powerful quantitative and qualitative abilities and is widely considered one of the most modern and promisingly accurate mass instrumentation methods available (Zhang et al., 2015). Evaluating the levels of the major compounds present in the C. pentandra leaves aqueous and methanolic extractscontributes to a better comprehension of their potential health benefits associated with antioxidant activity and α-glucosidase inhibitory results. The chromatograms are displayed in Fig. 2, and the predicted compounds are summarized in Table 5.
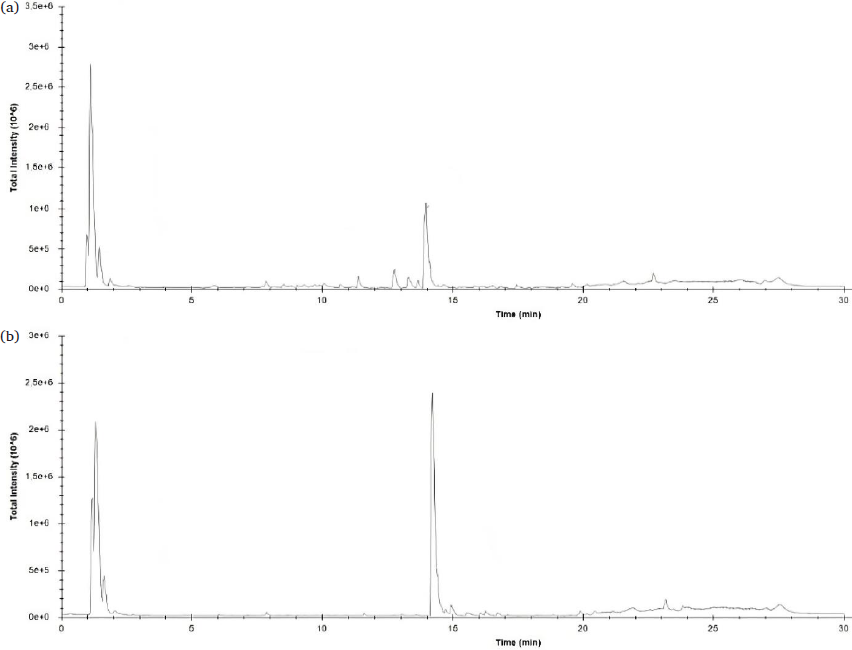
- LC-MS QTOF Chromatogram: (a) C. pentandra leaves methanol extract; (b) C. pentandra leaves aqueous extract. LC-MS QTOF: Liquid chromatography-mass spectrometry with quadrupole time-of-flight.
| No | Rt | Compounds | Score | M/Z | Levels | |
|---|---|---|---|---|---|---|
| CPM | CPW | |||||
| 1 | 1.147 | 4-Methylaminobutyrate | 99.75 | 118.0862 | 19744726 | - |
| 2 | 1.334 | Isoamyl nitrite | 99.30 | 118.0865 | - | 8363255 |
| 3 | 1.412 | 11-amino-undecanoic acid | 95.75 | 202.1804 | - | 142352 |
| 4 | 1.439 | m-Coumaric acid | 97.47 | 182.0811 | 164399 | - |
| 5 | 1.482 | L-Leucine | 98.47 | 132.1015 | 1885364 | 761606 |
| 6 | 1.639 | Citric acid | 96.36 | 210.0604 | - | 360712 |
| 7 | 5.886 | cis-β-d-Glucosyl-2-hydroxycinnamate | 97.86 | 344.1337 | 201237 | - |
| 8 | 7.896 | Luteolin 7-rhamnosyl(1->6)galactoside | 98.02 | 595.1652 | 272497 | 174210 |
| 9 | 8.549 | Avenanthramide 2s | 99.13 | 360.1081 | 192313 | - |
| 10 | 9.168 | Robinetin 3-rutinoside | 93.2 | 611.1592 | 36431 | - |
| 11 | 10.097 | Melanoxetin | 96.49 | 303.0499 | 141642 | - |
| 12 | 10.098 | Scutellarein 7-glucoside | 98.98 | 449.1076 | 126568 | - |
| 13 | 11.378 | Torosaflavone B 3’-O-β-d-glucopyranoside | 97.43 | 593.1872 | 775330 | - |
| 14 | 11.600 | 2’’-O-α-L-Rhamnosyl-6-C-fucosyl-3’-methoxyluteoiin | 98.93 | - | 142678 | |
| 15 | 12.758 | 11-hydroperoxy-12,13-epoxy-9-octadecenoic acid | 99.31 | 346.2585 | 509040 | - |
| 16 | 13.305 | α-9(10)-EpODE | 98.66 | 295.2272 | 182743 | - |
| 17 | 13.311 | 5,8,12-trihydroxy-9-octadecenoic acid | 99.04 | 348.2747 | 331585 | - |
| 18 | 13.671 | 9-keto palmitic acid | 93.72 | 271.2255 | 168196 | - |
| 19 | 13.99 | Xestoaminol C | 98.2 | 230.248 | 3856468 | 363783 |
| 20 | 14.215 | C16 Sphinganine | 95.3 | 274.2752 | 4415674 | 15195404 |
| 21 | 14.365 | Phytosphingosine | 98.9 | 318.3003 | - | 502697 |
| 22 | 14.646 | C17 Sphinganine | 95.52 | 288.2894 | 141404 | 535367 |
| 23 | 26.084 | Docosanedioic acid | 98.62 | 371.3158 | 171753 | - |
Note: “CPM” referred to the methanol extract of Ceiba pentandra and “CPW” referred to the aqueous extract of Ceiba pentandra. RT: Retention time, M/Z: Mass-to-charge ratio.
Based on the QTOF LC-MS results (Table 5), some compounds that are present in the C. pentandra leaves methanolic extract are similar to those in the water extract. For instance, L-leucine, luteolin 7-rhamnosyl(1->6)galactoside, xestoaminol C, C16 sphinganine, and C17 sphinganine were detected in both polar extracts. However, most of the flavonoids and their derivates were present in the methanolic extract. Aqueous extract of C. pentandra contained isoamyl nitrite, 11-amino-undecanoic acid, citric acid, 2’’-O-α-L-Rhamnosyl-6-C-fucosyl-3’-methoxyluteoiin, and phytosphingosine, which were not present in the methanol extract (Table 5). These compounds might influence the extract’s antioxidant activity and ability to inhibit the α-glucosidase. The MeOH extracts of barks and leaves of C. pentandra and their fractions highlighted many plant polyphenols (Nelly et al. 2023; Orabi et al. 2024). Table 4 depicts that the leaf MeOH extract of C. pentandra shows noteworthy potential in terms of antioxidant activity and α-glucosidase inhibition.
Based on Table 5, flavonoids and their derivates were identified in the C. pentandra leaves methanolic extract. According to Chen et al. (2021), flavonoids and phenolic compounds are secondary metabolites of plants, known for their remarkable bioactive properties. Flavonoids, which are polyphenols, are considered some of the most bioactive molecules and exhibit inhibitory activities against α-glucosidase (Dirir et al., 2022). The structures of different identified flavonoids are shown in Fig. 3.
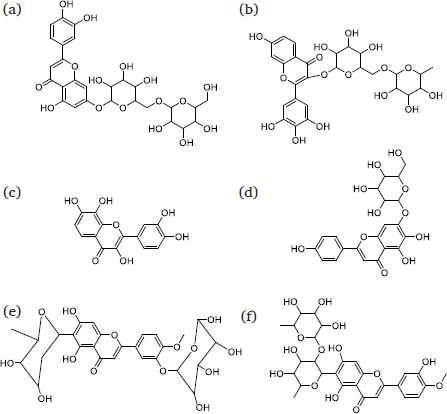
- The identified flavonoids of C. pentandra leaf’s extract. (a) Luteolin 7-rhamnosyl (1->6) galactoside; (b) Robinetin 3-rutinoside; (c) Melanoxetin; (d) Scutellarein 7-glucoside; (e) Torosaflavone B 3’-O-β-D-glucopyranoside; (f) 2’’-O-α-L-Rhamnosyl-6-C-fucosyl-3’-methoxyluteoiin.
The leaf methanolic extract of C. pentandra contains five different flavonoids (a-e), while the aqueous extract only contains two: luteolin 7-rhamnosyl(1->6)galactoside and 2’’-O-α-L-Rhamnosyl-6-C-fucosyl-3’-methoxyluteoiin (Fig. 3). In vitro assessments suggest that the C. pentandra leaf methanolic extract demonstrates the highest effect in inhibiting DPPH. stable free radical and α-glucosidase enzyme. The C. pentandra leaves aqueous extract demonstrates moderate effect in both DPPH (IC50: 157.32 ± 3.44 µg/mL) and α-glucosidase inhibitions (IC50: 109.54 ± 1.72 µg/mL). Especially, luteolin 7-rhamnosyl(1->6)galactoside has earlier been reported to be present in the active extract showing enzyme inhibitory effect against α-glucosidase enzyme (Ali et al., 2020), while 2’’-O-α-L-Rhamnosyl-6-C-fucosyl-3’-methoxyluteoiin has not yet been reported by any research to exhibit enzyme inhibitory effects against carbohydrate hydrolyzing enzymes including α-glucosidase enzyme.
The five different flavonoids identified in the C. pentandra leaf methanolic extract (Fig. 3) may be one of the factors responsible for the significant inhibition of both DPPH. radical and α-glucosidase. In this regard, melanoxetin has been reported to be potent in preventing diabetes via several pathways including the inhibition of α-glucosidase (Rocha et al., 2024). Moreover, another flavonoid named robinetin 3-rutinoside was identified in the extract of M. buxifolia through ultrahigh performance liwuid chromatography – Mass spectrometry (UHPLC-MS) in negative ion mode. This further proved the plant’s ability to reduce the carbohydrate hydrolyzing action of both α-amylase and α-glucosidase (Ali et al., 2020). The structural features of torosaflavone B 3’-O-β-d-glucopyranoside illustrate potential antioxidant and α-glucosidase inhibition effects owing to their resemblance with other flavonoid glycosides, for example, quercetin-3-O-β-d-glucopyranoside. An aglycon part of quercetin may be responsible for the manifestation of antioxidative and other biological properties, while the attached glucopyranoside moiety glucopyranoside might have interacted with the α-glucosidase’s active site, thereby inhibiting enzyme interaction and reducing carbohydrate breakdown/hydrolysis. Though other two flavonoids, namely luteolin 7-rhamnosyl (1->6)galactoside and scutellarein 7-glucoside have not yet been extensively evaluated for their biological effects through any studies, the stronger α-glucosidase inhibitory activity of the methanolic extract of M. buxifolia could likely be due to the presence of both of these. (Ali et al., 2020). Therefore, further discussion post molecular docking analysis will elaborate on the identified compounds in C. pentandra extract as α-glucosidase inhibitors.
3.6 The prediction of the identified active compounds as α-glucosidase inhibitors using an in-silico approach
Active compounds identified from the LC-MS based analysis were evaluated for their interaction with the 3A4A protein. The identified compounds from the potent extracts of this herb are reported in Table 5. The molecular docking analysis results, which are provided in Table 6, clearly explain the re-docking of the native ligand, which produced an affinity binding energy (ABE) of -6.0 kcal/mol, with a RMSD (root mean square deviation) of 0.491. This finding was found to be like and even better than the previous finding published by Nipun et al. (2020), in which an RMSD value of 0.633 was reported.
| Retention Time (min) | Dominant identified compounds of CPM | Retention time (min) | Dominant identified compounds of CPW | ||
|---|---|---|---|---|---|
| Methanol extract |
Affinity (kcal/mol) |
Aqueous extract |
Affinity (kcal/mol) |
||
| - | Native ligand (ADG) | -6.0 | - | Quercetin | -8.4 |
| 1.147 | 4-Methylaminobutyrate | -4.5 | 1.334 | Isoamyl nitrite | -5.1 |
| 1.439 | m-Coumaric acid | -8.9 | 1.412 | 11-amino-undecanoic acid | -5.8 |
| 1.679 | L-Leucine | -5.3 | 1.639 | Citric acid | -6.0 |
| 5.886 | cis-β-d-Glucosyl-2-hydroxycinnamate | -8.1 | 11.600 | 2’’-O-α-l-Rhamnosyl-6-C-fucosyl-3’-methoxyluteoiin | -8.9 |
| 7.896 | Luteolin 7-rhamnosyl(1->6)galactoside | -10.3 | 14.215 | C16 Sphinganine | -6.1 |
| 8.549 | Avenanthramide 2s | -8.3 | 14.365 | Phytosphingosine | -5.8 |
| 9.168 | Robinetin 3-rutinoside | -8.6 | 14.646 | C17 Sphinganine | -6.1 |
| 10.097 | Melanoxetin | -8.9 | |||
| 10.098 | Scutellarein 7-glucoside | -9.7 | |||
| 11.378 | Torosaflavone B 3’-O-β-d-glucopyranoside | -10.0 | |||
| 12.758 | 11-hydroperoxy-12,13-epoxy-9-octadecenoic acid | -6.8 | |||
| 13.305 | α-9(10)-EpODE | -6.7 | |||
| 13.311 | 5,8,12-trihydroxy-9-octadecenoic acid | -6.6 | |||
| 13.671 | 9-keto palmitic acid | -6.0 | |||
| 13.990 | Xestoaminol C | -5.9 | |||
| 26.084 | Docosanedioic acid | -6.4 | |||
Note: “CPM” referred to the methanol extract of Ceiba pentandra and “CPW” referred to the aqueous extract of Ceiba pentandra.
Table 6 shows that quercetin can interact with 3A4A protein with an ABE of -8.4 kcal/mol. Compounds from C. pentandra extract were chosen based on having an ABE of less than -8.00 kcal/mol, as quercetin’s activity as an α-glucosidase inhibitor, with an IC50 of 9.59 ± 0.30 µg/mL sets a benchmark. Probably, the selected compounds with ABEs less than -8.00 kcal/mol are active and comparable to quercetin. According to Table 6, nine compounds have ABE values less than -8.00 kcal/mol. These compounds include m-coumaric acid (hydroxycinnamic acid), cis-β-d-Glucosyl-2-hydroxycinnamate, luteolin 7-rhamnosyl(1->6)galactoside, avenanthramide 2s, robinetin 3-rutinoside, melanoxetin, scutellarein 7-glucoside, torosaflavone B 3’-O-β-d-glucopyranoside, and 2’’-O-α-L-Rhamnosyl-6-C-fucosyl-3’-methoxyluteoiin.
Generally, flavonoids and their derivates are active in inhibiting α-glucosidase, as reported in Table 6. As previously explained, luteolin 7-rhamnosyl(1->6)galactoside, scutellarein 7-glucoside, torosaflavone B 3’-O-β-D-glucopyranoside, and 2’’-O-α-L-Rhamnosyl-6-C-fucosyl-3’-methoxyluteoiin have not been studied extensively. This study has identified new potent flavonoid derivates as α-glucosidase inhibitors. Therefore, it is recommended that future research should focus on isolating and synthesizing these compounds.
Furthermore, m-coumaric acid, at 100 mg/kg bw, reduced blood glucose levels in rats (Amalan et al., 2016). Additionally, cis-β-d-glucosyl-2-hydroxycinnamate shares a chemical structure with m-coumaric acid, a cinnamic acid derivative. However, biological testing is still required to confirm its medicinal potential, specifically to evaluate its anti-diabetic, antioxidant and anti-inflammatory effects. Avenanthramide 2s, a unique phenolic compound that functions as an antidiabetic agent and may assist in diabetes management by controlling postprandial glucose levels (Zhouyao et al., 2022). Additionally, these untested compounds should be validated by interacting with the catalytic sites of the 3A4A protein.
Table 7 also shows how the selected compounds interact with amino acid residues. The native ligand (α-D-glucose) forms hydrogen bonds with several catalytic residues, including ASP352 and ASP69; ARG442 (Fig. 4a). While luteolin 7-rhamnosyl(1->6)galactoside lacks catalytic residues that interact through hydrogen bonds, it does have two catalytic residues that bind via other interactions (Table 7). The interactions of the selected compounds of this herb are shown in Fig. 4b. Moreover, torosaflavone B 3’-O-β-d-glucopyranoside is another potent α-glucosidase inhibitor that interacts with three catalytic sites. Fig. 4c illustrates how the catalytic residues interact with torosaflavone B 3’-O-β-d-glucopyranoside via hydrogen bonding. The pyranose structure of torosaflavone B 3’-O-β-d-glucopyranoside interacts with two catalytic residues, potentially enhancing its inhibitory effects on α-glucosidase.
| Compounds | Compound types | Amino acids interaction | |||
|---|---|---|---|---|---|
| Hydrogen binding | Bond distance (Å) | Other bindings | Bond distance (Å) | ||
| Native ligand (ADG) | Control 1 | ARG213; ASP352; HIS351; ARG442; ASP69; HIS112 | 6.46; 2.05&4.52; 2.04; 1.86; 1.74&2.89; 2.51 | ||
| Quercetin | Control 2 | ASP215 | 2.86 | ARG442; ASP352; PHE303 | 3.77; 4.30; 5.14&4.97 |
| The selected compounds | |||||
| m-Coumaric acid | Cinnamic acid derivate | TYR158 | 2.88 | TYR158; ASP352; ARG442; PHE178; HIS112; SER240 | 2.76, 3.99, 4.66&4.61; 4.36; 3.55; 3.73; 4.37; 3.53 |
| cis-β-d-Glucosyl-2-hydroxycinnamate | Cinnamic acid derivate | GLU411; ARG442; GLN353; ASP307; | 3.37; 2.29; 3.73; 3.22 | ARG315 | 4.67 |
| Luteolin 7-rhamnosyl(1->6)galactoside | Flavonoid derivate | ASN415; GLN279; GLN353 | 1.90&2.65; 2.42&2.61; 2.98 | GLY160; LYS156; PHE314; ARG315; ASP352; ASP215; PHE178 | 3.77; 5.25; 4.71; 4.48&5.27; 2.70; 3.40; 2.97 |
| Avenanthramide 2s | Phenolic alkaloids | ASP352; PRO312; ARG315; LEU313; LYS156 | 2.28; 2.95; 3.15; 2.62; 2.28; 1.96 | PHE178; GLU277; PHE152; PHE303; GLN353; PHE314; ARG442 | 4.47; 2.70; 4.98; 4.37; 3.63; 5.79; 4.08 |
| Robinetin 3-rutinoside | Flavonoid derivate | ASP215; GLN353; THR306 | 2.00; 1.68; 2.31 | TYR158; ARG442; ASP352; PHE303 | 3.56&3.69; 3.77&346; 4.43&4.67; 3.50 |
| Melanoxetin | Flavonoid | ASP352; GLU277; ASP307; HIS280 | 2.14&2.54; 2.12; 2.27; 2.43 | TYR158; ARG315; ARG442 | 5.67; 4.78&4.31; 4.76 |
| Scutellarein 7-glucoside | Flavonoid derivate | ASP69; ASP307 | 2.91; 2.18 | VAL216; ASP352; PHE303; ARG315 | 5.32; 4.19; 4.87&5.40; 2.22&1.63 |
| Torosaflavone B 3’-O-β-d-glucopyranoside | Flavonoid derivate | ASP69; ASP215; GLU277; ARG315; SER157; ARG442 | 1.65; 2.55; 1.41; 2.14; 2.20; 6.25 | HIS351; TYR72; TYR158; ASP215; ARG315; | 4.49; 3.98; 5.18&4.95; 3.27; 3.56 |
| 2’’-O-α-L-Rhamnosyl-6-C-fucosyl-3’-methoxyluteoiin | Flavonoid derivate | LYS156; TYR158; ASP215 | 2.37; 2.94; 2.25 | SER240; TYR158; ARG442; ASP352; PHE178; HIS112 | 3.49; 2.94, 4.64, 4.72&3.96; 3.48; 4.39; 3.78; 4.27 |
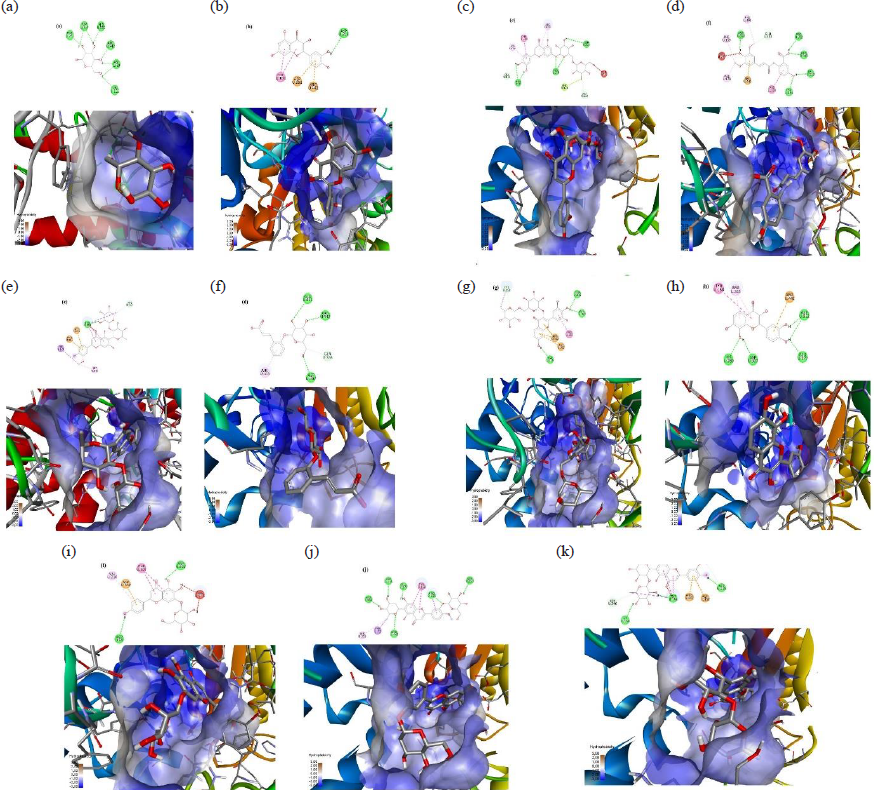
- The interaction of the selected compounds with 3A4A protein: (a) Native ligand; (b) Quercetin; (c) m-Coumaric acid; (d) cis-β-D-Glucosyl-2-hydroxycinnamate; (e) Luteolin 7-rhamnosyl(1->6)galactoside; (f) Avenanthramide 2s; (g) Robinetin 3-rutinoside; (h) Melanoxetin; (i) Scutellarein 7-glucoside; (j) Torosaflavone B 3’-O-β-D-glucopyranoside; (k) 2’’-O-α-L-Rhamnosyl-6-C-fucosyl-3’-methoxyluteoiin.
Fig. 5 illustrates how the selected compounds interact within the catalytic sites. Luteolin 7-rhamnosyl(1->6)galactoside and torosaflavone B 3’-O-β-d-glucopyranoside are the most potent compounds, with affinity binding energies of -10.3 and -10.0 kcal/mol, respectively. Furthermore, Fig. 5 shows the interactions of multiple compounds. Compounds localized in the catalytic site of the 3A4A protein inhibit α-glucosidase activity. An in vitro investigation demonstrated that the C. pentandra leaf methanolic extract inhibits α-glucosidase activity, similar to quercetin (Table 4). Hence, it may have exhibited its antioxidant and α-glucosidase inhibitory effects due to the existence of these and other unknown bioactive compounds possessing α-glucosidase inhibitory effects.
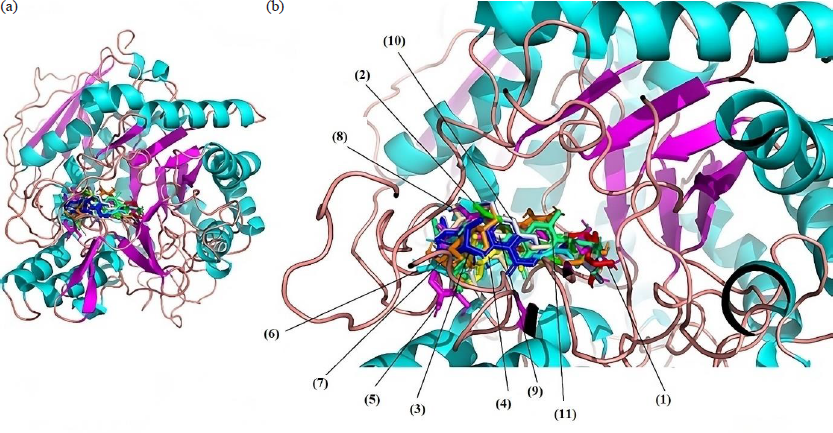
- Super impose of the interaction of the selected compounds with 3A4A protein: (a) normal protein; (b) zoom 20 Å. (1) Native ligand; (2) Quercetin; (3) m-Coumaric acid; (4) cis-β-d-Glucosyl-2-hydroxycinnamate; (5) Luteolin 7-rhamnosyl(1->6)galactoside; (6) Avenanthramide 2s; (7) Robinetin 3-rutinoside; (8) Melanoxetin; (9) Scutellarein 7-glucoside; (10) Torosaflavone B 3’-O-β-d-glucopyranoside; (11) 2’’-O-α-l-Rhamnosyl-6-C-fucosyl-3’-methoxyluteoiin.
3.7 Physico-chemical properties and pharmacokinetics of the selected compounds of C. pentandra extracts
Compounds from C. pentandra extracts have been predicted to inhibit α-glucosidase through strong interactions with the 3A4A protein, as reported previously. These compounds were studied further for their physicochemical and pharmacokinetic properties to determine their potency for human use. Table 8 shows that the selected compounds from C. pentandra extracts include m-coumaric acid, which has a high absorption rate (>90%). Furthermore, the methanolic leaf extract of C. pentandra contains numerous potent compounds with drug-like properties. All selected compounds were found to be devoid of hepatotoxic effects, indicating they are safe for human use.
| Compound types | Properties | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Physicochemical | Pharmacokinetic | |||||||||||
| Log P | NRB | H-Ac | H-Do | DL | NVL | IA | AT | LD50 | MTD | HT | ||
| m-Coumaric acid | 1.49 | 2 | 3 | 2 | Yes | 0 | 92.864 | No | 2.232 | 1.232 | No | |
| cis-β-d-Glucosyl-2-hydroxycinnamate | -2.37 | 5 | 8 | 4 | Yes | 0 | 16.283 | No | 2.174 | 0.764 | No | |
| Luteolin 7-rhamnosyl(1->6)galactoside | -2.42 | 7 | 16 | 10 | No | 3 | 13.585 | No | 2.478 | 0.464 | No | |
| Avenanthramide 2s | 2.46 | 6 | 6 | 4 | Yes | 0 | 42.313 | No | 2.648 | 0.606 | No | |
| Robinetin 3-rutinoside | -1.69 | 6 | 16 | 10 | No | 3 | 20.596 | No | 2.49 | 0.443 | No | |
| Melanoxetin | 1.99 | 1 | 7 | 5 | Yes | 0 | 74.413 | No | 2.485 | 0.522 | No | |
| Scutellarein 7-glucoside | -0.24 | 4 | 11 | 7 | No | 2 | 36.191 | No | 2.544 | 0.598 | No | |
| Torosaflavone B 3’-O-β-d-glucopyranoside | -0.37 | 6 | 14 | 8 | No | 3 | 31.35 | Yes | 2.548 | 0.822 | No | |
| 2’’-O-α-l-Rhamnosyl-6-C-fucosyl-3’-methoxyluteoiin | -0.02 | 5 | 14 | 8 | No | 3 | 40.663 | No | 2.538 | 0.585 | No | |
Log P: Predicted octanol/water partition coefficient; NRB: No. of rotatable bonds; H-Ac: No. of hydrogen bonds; H-Do: No. of hydrogen donor; DL: Drug likeness; NVL: No. of Lipinski’s rule violations; IA: Internal absorption; AT: AMES toxicity; LD50: Oral rat acute toxicity, mol/kg; MTD: Maximum tolerated dose for human (log mg/kg/day); HT: Hepatotoxicity.
According to Table 8, a few compounds in the C. pentandra leaves extract do not comply with the Lipinski Rule of 5. This implies that these compounds do not possess drug-like properties and cannot easily cross the cell membrane, rendering them ineffective as natural drugs. Andhiarto et al. (2022) found that the ability of drugs to penetrate the cell membrane follows at least two Lipinski’s rules. Besides, Tijjani et al. (2022) stated that drugs administered orally should not followmore than one of the Lipinski rules. Table 8 shows that C. pentandra leaf extracts contain active compounds such as m-coumaric acid, cis-β-d-Glucosyl-2-hydroxycinnamate, avenanthramide 2s, and melanoxetin, which have drug-likeness properties. Melanoxetin is a hydroxylated flavonoid that has earlier been shown to demonstrate antioxidant, anti-inflammatory, and antidiabetic properties (Rocha et al., 2024). It also lacks the toxicity exhibited by AMES toxicity. Melanoxetine has a similar structure to quercetin. Quercetin showed no toxic properties based on AMES toxicity or mutagenic effects (Amar, 2017). Furthermore, m-coumaric acid and melanoxetin are predicted to be antidiabetic agents based on their physicochemical and pharmacokinetic properties.
4. Conclusions
The results revealed that both non-polar and polar extracts from C. pentandra and B. rubra leaves demonstrated antioxidant and alpha-glucosidase inhibitory effects. Notably, the methanolic and aqueous extracts of C. pentandra exhibited potent antioxidant and α-glucosidase inhibitory effects. The methanolic extract of C. pentandra was more effective in inhibiting DPPH and α-glucosidase than the aqueous extract. Based on this study’s findings, luteolin 7-rhamnosyl(1->6)galactoside, torosaflavone B 3’-O-β-d-glucopyranoside and scutellarein 7-glucoside are predicted to be α-glucosidase inhibitors due to their lowest affinity binding energies of –10.3 kcal/mol, -10.0 kcal/mol and -9.7 kcal/mol., respectively. These flavonoids could serve as leads for the discovery of safe antidiabetic agents acting as α-glucosidase inhibitors. Further research is needed to isolate these compounds and evaluate their in vivo antidiabetic effects.
Acknowledgement
This study was partially supported in part by CRIGS UKM-IIUM-UiTM-UPM Research Collaboration Grant 2024 (C-RIGS24-017-0023), Research Management Centre, International Islamic University Malaysia (IIUM), Malaysia. All authors are grateful to the KOP for their research collaborations and iPROMISE to carry out the Q-TOF LCMS analysis of plant samples. This work was funded by Researchers Supporting Project number (RSP2025R414).
CRediT authorship contribution statement
Zahradifa Kaniabila Ananda: Literature Search, Experimental Studies, Data Acquisition, Data Analysis, Manuscript Preparation; Taslima Begum: Manuscript Editing and Review, Experimental Studies; Mustofa Ahda: Experimental Studies, Data Acquisition, Data Analysis, Manuscript Editing and Review; Mohd Salleh Rofiee: Data Acquisition, Manuscript Editing and Review; Syed Adnan Ali Shah: Data Acquisition, Manuscript Editing and Review; Mohd Zaki Salleh: Data Acquisition, Manuscript Editing and Review; Bader O Almutairi: Manuscript Editing and Review; Syed Najmul Hejaz Azmi: Data Analysis, Statistical Analysis, Manuscript Editing and Review; Pankaj Sah: Data Analysis, Statistical Analysis, Manuscript Editing and Review; Agustin Krisna Wardani: Manuscript Editing and Review; Alfi Khatib: Manuscript Editing and Review; Syed Atif Abbas: Manuscript Editing and Review; Md. Abdur Rashid Mia: Manuscript Editing and Review; Qamar Uddin Ahmed: Concepts, Design, Experimental Studies, Data Acquisition, Data Analysis, Statistical Analysis, Manuscript Editing and Review. All authors approved the final version of the manuscript.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Declaration of Generative AI and AI-assisted technologies in the writing process
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Appendix A. Supplementary material
Supplementary material to this article can be found online at https://dx.doi.org/10.25259/JKSUS_16_2024
References
- Potential antiviral action of alkaloids. Molecules. 2022;27:903.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Medicinal potential of isoflavonoids: Polyphenols that may cure diabetes. Molecules. 2020;25:5491. https://doi.org/10.3390/molecules25235491
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Methylation and acetylation enhanced the antidiabetic activity of some selected flavonoids: In vitro, molecular modelling and structure activity relationship-based study. Biomolecules. 2018;8:149. https://doi.org/10.3390/biom8040149
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Ameliorating activity of polyphenolic-rich extracts of Basella rubra L. leaves on pancreatic β-cell dysfunction in streptozotocin-induced diabetic rats. J. Complement Integr. Med.. 2021;19:335-344. https://doi.org/10.1515/jcim-2020-0304
- [CrossRef] [PubMed] [Google Scholar]
- Extraction of phenolic compounds: A review. Current Res. Food Sci.. 2021;4:200-214. https://doi.org/10.1016/j.crfs.2021.03.011
- [CrossRef] [Google Scholar]
- A new sulphated flavone and other phytoconstituents from the leaves of Tetracera indica Merr. and their alpha-glucosidase inhibitory activity. Natural Product Res.. 2019;33:1-8. https://doi.org/10.1080/14786419.2018.1437427
- [Google Scholar]
- Metabolic fingerprinting, antioxidant characterization, and enzyme-inhibitory response of Monotheca buxifolia (Falc.) A. DC. extracts. BMC Complement Med. Ther.. 2020;20:313. https://doi.org/10.1186/s12906-020-03093-1
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Extraction of natural products using deep eutectic solvents. University of Leicester; 2021. UK. Doctor of Philosophy Thesis
- In silico analysis and admet prediction of flavonoid compounds from syzigium cumini var. album on α-glucosidase receptor for searching anti-diabetic drug candidates. Pharmacogn J.. 2023;14:736-743. https://doi.org/10.5530/pj.2022.14.161
- [Google Scholar]
- Antidiabetic and antihyperlipidemic activity of p-coumaric acid in diabetic rats, role of pancreatic GLUT 2: In vivo approach. Biomed Pharmacother. 2016;84:230-236. https://doi.org/10.1016/j.biopha.2016.09.039
- [CrossRef] [PubMed] [Google Scholar]
- In silico pharmacodynamics, toxicity profile and biological activities of the Saharan medicinal plant Limoniastrum feei. Braz. J. Pharm. Sci.. 2017;53 https://doi.org/10.1590/s2175-97902017000300061
- [CrossRef] [Google Scholar]
- Ceiba pentandra (L.) Gaertn.: An overview of its botany, uses, reproductive biology, pharmacological properties, and industrial potential. J. Applied Biology Biotech.. 2023;11:1-7. http://dx.doi.org/10.7324/JABB.2023.110101
- [Google Scholar]
- Comparison of phenolic and flavonoid compound profiles and antioxidant and α-glucosidase inhibition properties of cultivated soybean (Glycine max) and Wild Soybean (glycine soja) Plants. 2021;10:1-14. https://doi.org/10.3390/plants10040813
- [Google Scholar]
- Oxidative stress and antioxidants in diabetes mellitus. Asian Pac. J. Trop. Med.. 2020;13:431. https://doi.org/10.4103/1995-7645.291036
- [CrossRef] [Google Scholar]
- A review of alpha-glucosidase inhibitors from plants as potential candidates for the treatment of type-2 diabetes. Phytochem Rev.. 2022;21:1049-1079. https://doi.org/10.1007/s11101-021-09773-1
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Total flavonoids content of polar extracts of cayratia trifolia leaves. IOP Conf. Ser.: Earth Environ. Sci.. 2021;819:012056. https://doi.org/10.1088/1755-1315/819/1/012056
- [CrossRef] [Google Scholar]
- LC-MS identification and quantification of phenolic compounds in solid residues from the essential oil industry. Antioxidants. 2021;10:2016.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- The role of phenolic compounds in plant resistance. Biotechnology and Food Sci.. 2016;80:97-108.
- [Google Scholar]
- Determination of antioxidant activity and total phenol content of gendola leaf extract (Basella rubra Linn) and binahong leaf (Anredera cordifolia Stennis) as herbal drug candidates. In Proceedings Biology Education Conference: Biology, Science, Enviromental, and Learning. 2014;11:195-200.
- [Google Scholar]
- Antioxidant, α-glucosidase inhibitory, and cytotoxic activities of Mangifera rufocostata extract and identification of its compounds by LC-MS/MS analysis. Arabian J. Chem.. 2024;17:105391. https://doi.org/10.1016/j.arabjc.2023.105391
- [Google Scholar]
- Chemical constituents of the methanolic extracts of Ceiba pentandra (L.) Gaertn and the antioxidant activities of its aqueous, ethanolic and hydro-ethanolic extracts. J. Pharmacognosy Phytochemistry. 2023;12:103-118.
- [CrossRef] [Google Scholar]
- Characterization of α-glucosidase inhibitors from psychotria malayana jack leaves extract using LC-MS-Based multivariate data analysis and in-silico molecular docking. Molecules. 2020;25:5885. https://doi.org/10.3390/molecules25245885
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Preliminary phytochemical screening, in vitro antidiabetic, antioxidant activities, and toxicity of leaf extracts of psychotria malayana jack. Plants (Basel). 2021;10:2688. https://doi.org/10.3390/plants10122688
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Investigation of α-glucosidase inhibitory metabolites from tetracera scandens leaves by GC-MS metabolite profiling and docking studies. Biomolecules. 2020;10:287. https://doi.org/10.3390/biom10020287
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- In vitro evaluation of the antioxidant potential, phenolic, and flavonoid contents of the stem bark ethanol extract of Anogeissus leiocarpus. African J. Medicine Med. Sci.. 2014;43:101-109.
- [Google Scholar]
- Ceiba pentandra ethyl acetate extract improves doxorubicin antitumor outcomes against chemically induced liver cancer in rat model: A study supported by UHPLC-Q-TOF-MS/MS identification of the bioactive phytomolecules. Front Pharmacol.. 2024;15:1337910. https://doi.org/10.3389/fphar.2024.1337910
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Antioxidants profile of Momordica charantia fruit extract analyzed using LC-MS-QTOF-based metabolomics. Food Chem.: Molecular Sci.. 2021;2:100012. https://doi.org/10.1016/j.fochms.2021.100012
- [CrossRef] [Google Scholar]
- Antioxidant capabilities of Litsea garciae bark extracts and their relation to the phytochemical compositions. MABJ. 2022;51:99-118.
- [CrossRef] [Google Scholar]
- Melanoxetin: A hydroxylated flavonoid attenuates oxidative stress and modulates insulin resistance and glycation pathways in an animal model of type 2 diabetes mellitus. Pharmaceutics. 2024;16:261.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Assessment of free radical scavenging and digestive enzyme inhibitory activities of extract, fractions and isolated compounds from Tetracera macrophylla leaves. J. Herbal Med.. 2020;22:100351. https://doi.org/10.1016/j.hermed.2020.100351
- [Google Scholar]
- Salacca zalacca: A short review of the palm botany, pharmacological uses and phytochemistry. Asian Pacific J. Tropical Med.. 2018;11:645-652.
- [Google Scholar]
- Antioxidant and antidiabetic effects of flavonoids: A structure-activity relationship based study. BioMed Res. Int.. 2017;2017:1-14. https://doi.org/10.1155/2017/8386065
- [Google Scholar]
- Hypoglycemic and antihyperglycemic effect of Ceiba pentandra L. Gaertn in normal and streptozotocin-induced diabetic rats. Ghana Med. J.. 2013;47:121-127.
- [Google Scholar]
- Flavonoids as antidiabetic and anti-inflammatory agents: A review on structural activity relationship-based studies and meta-analysis. Int. J. Mol. Sci.. 2022;23:12605. https://doi.org/10.3390/ijms232012605
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Lifestyle, oxidative stress, and antioxidants: Back and forth in the pathophysiology of chronic diseases. Front Physiol.. 2020;11:694. https://doi.org/10.3389/fphys.2020.00694
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- A review on upodika (Basella rubra Linn.) - An ayurvedic nutraceutical with enormous medicinal value. World J. Pharmaceutical Res.. 2022;11:237-259.
- [Google Scholar]
- Inhibitory activity of α-glucosidase of bark of Ceiba pentandra Linn. Indones. J. Pharm.. 2018;29:206-213.
- [Google Scholar]
- Repurposed antiviral drugs for COVID-19 - Interim WHO solidarity trial results. N. Engl. J. Med.. 2021;384:497-511. https://doi.org/10.1056/NEJMoa2023184
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- α-glucosidase inhibitors isolated from Mimosa pudica L. Nat. Prod. Res.. 2019;33:1495-1499. https://doi.org/10.1080/14786419.2017.1419224
- [CrossRef] [PubMed] [Google Scholar]
- In vitro studies on Basella rubra different extracts as inhibitors of key enzymes linked to diabetes mellitus. Pharmacognosy J. 2017;9:107-111.
- [Google Scholar]
- In silico insight into the interaction of 4-aminoquinolines with selected SARS-CoV-2 structural and nonstructural proteins. In: Egbuna C., ed. Coronavirus drug discovery. Elsevier; 2022. p. :313-333. https://doi.org/10.1016/B978-0-323-95578-2.00001-7
- [Google Scholar]
- Antidiabetic effects of gendola leaf extract (Basella rubra L.) on blood sugar levels of white rats. J. Veterinary Med.. 2019;2:127-132.
- [Google Scholar]
- Sacrificial synthesis of supported ru single atoms and clusters on n-doped carbon derived from covalent triazine frameworks: A charge modulation approach. Adv. Sci. (Weinh). 2020;8:2001493. https://doi.org/10.1002/advs.202001493
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- The inhibition of intestinal glucose absorption by oat-derived avenanthramides. J. Food Biochem.. 2022;46:e14324. https://doi.org/10.1111/jfbc.14324
- [CrossRef] [PubMed] [Google Scholar]







