Translate this page into:
Chemical composition, in vitro antibacterial and antioxidant potential of Omani Thyme essential oil along with in silico studies of its major constituent
⁎Corresponding author. shahalam@nu.edu.om (Shah Alam Khan) sakhan@omc.edu.om (Shah Alam Khan)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Abstract
Chemical profiling of Omani Thymus vulgaris species was done by GC-MS analysis. The GC-MS analysis of thyme essential oil established it to be a carvacrol chemotype. Thyme essential oil exhibited better antimicrobial spectrum than ampicillin. Thyme essential oil showed significant in vitro free radical scavenging activity in DPPH assay method. In silico PASS prediction studies revealed carvacrol to possess useful bioactivities including cytotoxicity.
Abstract
Thyme is an evergreen shrub that has been utilized in traditional medicine and culinary packages for centuries because of its aroma and medicinal properties. The medicinal properties of thyme leaves are attributed to its volatile constituents. The study aimed to identify the volatile constituents in the oil extracted from the Thyme sp grown in Oman and to evaluate its in vitro antibacterial and antioxidant activity. Fresh Thyme leaves were collected from Muscat, Sultanate of Oman in the month of September 2018. Thyme oil was isolated from leaves by hydro-distillation. The volatile chemical constituents present in the thyme oil were analyzed by Gas Chromatograph coupled with Mass Spectrometer. The ability of isolated oil to scavenge free radicals was evaluated by an in vitro DPPH assay method while antimicrobial activity was tested against S. aureus and E. coli bacterial strains by disc diffusion method. The bioactivity of the major constituent of the oil was predicted with the help of PASS and CLC-pred software. Molecular docking studies were performed by docking server. GC–MS analysis of thyme oil revealed the presence of 11 components. Carvacrol and γ-terpinene were identified as the major volatile constituents in thyme oil. Thyme oil inhibited 71.57% of DPPH radicals at 40 µg/mL concentration. Thyme oil displayed the better antimicrobial activity than the standard drug ampicillin against both the bacteria. Antiseptic, antiinfective, reductant and antimutagenic bioactivities of carvacrol as predicted by PASS support the in vitro experimental results. CLC-pred showed carvacrol to be active against Metastatic melanoma, Oligodendroglioma and Non small cell lung cancer cell lines. The antioxidant and antimicrobial activity of Omani thyme oil could be due to its high content of carvacrol. Omani thyme oil seems to be a viable alternative source of natural antimicrobial agent(s) and warrants further studies to ascertain its therapeutic spectrum of biological studies.
Keywords
Antimicrobial
Antioxidant
DPPH
Essential oil
Hydro-distillation
Thyme
1 Introduction
Essential oils are volatile constituents of plants which are responsible for plant aroma (Ali et al., 2015). The volatile oil content varies from plant to plant but significant quantity is present in the flowers and aerial parts as these plant parts have more number of oil producing glands. The volatile oil owing to its strong and pungent aroma primarily functions as an insect repellant and thus helps to protect the plant from insects. Volatile oils have been used by the mankind since ancient time in food, cosmetics and for healing common ailments. These odorous constituents of plant oils are used as; flavoring agent in food and drug industry, starting material for the synthesis of complex medicinal compounds, therapeutic agent for skin and upper respiratory diseases, lipophilic solubility enhancer, carrier of drugs, cosmetic and in fragrance industries, etc (Shrivastava et al., 2010).
Thyme, also known as Zaatar in Arabic is an aromatic evergreen shrub belonging to the Lamiaceae family. There are over 350 species of thyme grown in many parts of the world but is more indigenous to Europe and the Mediterranean basin. It has been used in medicine and culinary packages for many centuries. Thymus vulgaris is local to the Mediterranean region and is the common species planted in Arabic world in addition to certain African countries including Egypt. Thyme in Oman is commonly used as a flavoring agent in Arabic breads, cheese pizza, pickle and in some other snacks. It is also used in the alternative system of medicine since ancient time as a remedy for cough, toothache, bronchitis, constipation, warts, helminthes, alopecia, spasm, indigestion, inflammation, skin infections and for its diuretic properties (Basch et al., 2004). Thyme volatile oil is also used in mouth washes and gargles because of its aroma and antimicrobial properties (Mohammed et al., 2016). Several experimental studies have also demonstrated that Thyme essential oil exhibits significant antimicrobial, muscle relaxant as well as strong antioxidant properties (Rota et al., 2008; Grigore et al., 2010; Boskabady et al., 2006) Thyme oil has commercial importance partly because of its aroma and thus frequently added to different kinds of nutriment and drinks. It is also used as a natural food preservative in food industry. It is commonly used in preparation of soaps, perfumes, beautifying products and some aromatic solutions in fragrance and cosmetic industries (Cosentino et al., 1999; Schulz et al., 2003).
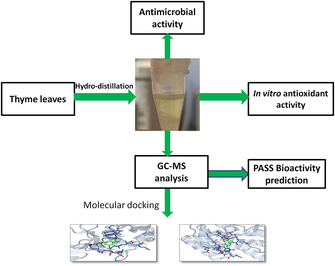
Thus, in lieu of the numerous published reports on its beneficial effects and its regular consumption as part of Omani food, we thought to examine the volatile chemical constituents, antioxidant effect and antimicrobial spectrum of the Omani thyme essential oil. In silico prediction and molecular docking studies of the major chemical constituents are also carried out to support and account for the experimental results. The study would pave a way to identify the major volatile constituents in Omani Thyme essential oil vis a vis alternative sources of natural antioxidants and antibacterial agents having applications in the food and drug industry.
2 Materials and methods
2.1 Chemicals and bacterial strains
Ampicillin antibiotic disc, chemicals like DPPH, anhydrous sodium sulphate, solvents (DMSO, ethyl acetate) and Muller Hinton agar (MHA) media of high purity were obtained from the local supplier. Cultures of gram positive (S. aureus) and gram negative (E. coli) bacterial strains were procured from the microbiology labs of Natural Sciences Department, College of Pharmacy, Sultanate of Oman.
2.2 Collection and identification of Thyme leaves
Omani Thyme leaves were collected from Al-Mawaleh farm in the month of September 2018. The leaves were cleaned with water to remove the dust particles. The Pharmacognosy faculty member of College of Pharmacy authenticated the thyme leaves sample. A voucher specimen of thyme was also deposited for reference purpose in the herbarium of Pharmacy lab, College of Pharmacy, NU, Oman.
2.3 Extraction of Thyme essential oil (TEO)
The leaves of T. vulgaris (180 g), freshly collected and uniform in size, were washed under running water and blended using a domestic mixer and grinder. The paste of thyme leaves was taken in a round bottom (rb) flask of 500 mL capacity and then sufficient amount of water was added to cover the leaves paste. The Clevenger assembly was set up and the hydro-distillation was carried out for 4.5 h. TEO was separated from aqueous layer, collected in Eppendorf tube and finally traces of water droplets were removed by drying over the anhydrous Na2SO4. The dried TEO was set aside at 4 °C in refrigerator prior to its use for analysis and biological testing. The percentage yield of TEO was calculated based on the dry weight of leaves and oil obtained.
2.4 Gas chromatography-mass spectrometry analysis of TEO
Perkin Elmer Clans 600 GC System was used to perform the GC-MS analysis at Higher College of Technology, AlKhuwair, Muscat. It was fitted with HP-5MS capillary column (30 m × 0.25 mm (internal diameter) × 0.25 μm film thickness). The highest temperature of the column in oven for the elution was set to maximum 350 °C. The system was connected to a Perkin Elmer Clarus 600C MS detector for identification of chemicals. The flow rate (1.0 mL/min) of carrier gas helium (He) of ultra-high purity (99.9999%) was maintained constant during the whole separation process. The temperatures were set at 290, 280 and 270 °C for injection, transfer line and ion source, respectively. The 70 eV chemical ionization energy was used for MS. Auto-tune was used to produce Electron multiplier (EM) voltage. The mass spectral data were collected in the scan range of 40–550 amu by running the full-scan mass. The volatile oil was diluted and the 1 µL of sample was injected at a split ratio of 150:1. The temperature of the oven was programmed at 60 °C and was increased gradually to 280 °C with a constant rate of 3 °C/min. The chemical class and nature of the compounds was established by matching the mass spectral data of TEO with the reference mass spectra.
2.5 Investigation of antioxidant activity by DPPH assay method
TEO in the concentration range of (10–40 μg/mL) was investigated for its ability to scavenge free radicals by using 0.01 mM DPPH in ethyl acetate as per the reported method of Al-Breiki et al. (2018). The experiment was performed in triplicate. The % inhibition of DPPH radical was computed by the following formula; where, Actl = Absorbance of control without sample and Asam = Absorbance of TEO
2.6 In vitro antibacterial activity
The antimicrobial potential of the TEO was evaluated against two bacterial strains of S. aureus and E. coli. The microbial culture was grown on standard Muller Hinton agar (MHA) nutrient media. The inhibition of bacterial growth was evaluated by the disc diffusion method. Briefly, holes of uniform dimensions were made in the plastic plates containing media and then 5 & 10 μL of TEO were added to the pre labeled wells. A disc of standard Ampicillin antibiotic (25 μg/disc) was also placed in the petri plate for comparing the results. To assess the inhibitory effect of TEO and antibiotic on the microbial growth, the plates were incubated at an optimum temperature of 37 °C for a day. The antibacterial activity was evaluated by measuring the diameter of zone of inhibition (mm) around each well (Al-Aamri et al., 2018).
2.7 In silico prediction of bioactivity and molecular docking studies
The various bioactivities and cytotoxicity potential of the major chemical constituent present in the TEO were predicted with the help of PASS and CLC-pred online software (Nath et al., 2014). Molecular docking studies of the carvacrol were also performed online on dockingserver (www.dockinserver.com) using enzyme xanthine oxidase (PDB: 3NRZ) and antibacterial protein (PDB: 4TZK) in order to know the binding affinity and various ligand –receptor interactions to account for the antibacterial and antioxidant activity of TEO.
3 Results and discussion
3.1 Percentage yield and chemical composition of isolated volatile oil
Thyme oil was obtained in 0.44% v/w yield and is slightly higher than the amount reported in a study previously done in Oman (Mohammed et al., 2016). The variation in yield and chemical composition of volatile oil are dependent on various factors including environment, time of collection and cultivation practices (Hudaib and Aburjai, 2007).
A total of 11 volatile components representing 99.71% of the total detected constituents were identified in TEO with the help of GC–MS analysis (Fig. 1). The chemical composition of TEO is shown in Table 1. Carvacrol (59.29%) and γ-terpinene (29.12%) were identified as the two major constituents in Omani TEO. The other components were present in a total amount of less than 11.5%. Thus, based on the analysis results, Omani thyme was established as carvacrol chemotype. Three monoterpenoids constituted approximately 59.93% of TEO composition while seven monoterpenes and one sesquiterpene accounted for 39.17% and 0.90% respectively (Fig. 2). An earlier study done on Omani Thyme leaves reported the presence of 18 chemical compounds in its essential oil with durenol (56.2%) as the major constituent. Though, in current study we did not detect any δ-terpinene but it was found to be 5.24% in their study (Mohammed et al., 2016). The chemical composition of Omani TEO in our study is also found to be significantly different from that previously reported in Morocco and Spain for the same species of Thyme (Imelouane et al., 2009; Ballester-Costa et al., 2013). Similar studies in Poland, Iran, Spain and Italy, respectively, reported p-cymene, γ-terpinene and thymol as major compounds in the TEO (Rota et al., 2008; De Lisi et al., 2011; Pirbalouti et al., 2013). TEO from the same species can have diverse chemical composition. They are reported to contain up to 30 monoterpenes which lead to identification of different chemotypes. Chemotypes of T. vulgaris species grown in Southern France contain geraniol, α-terpineol, thuyanol-4, linalool, carvacrol, and thymol as the major constituents, whereas Spanish chemotype has 1,8-cineol being the main component (Guillen and Manzanos, 1998).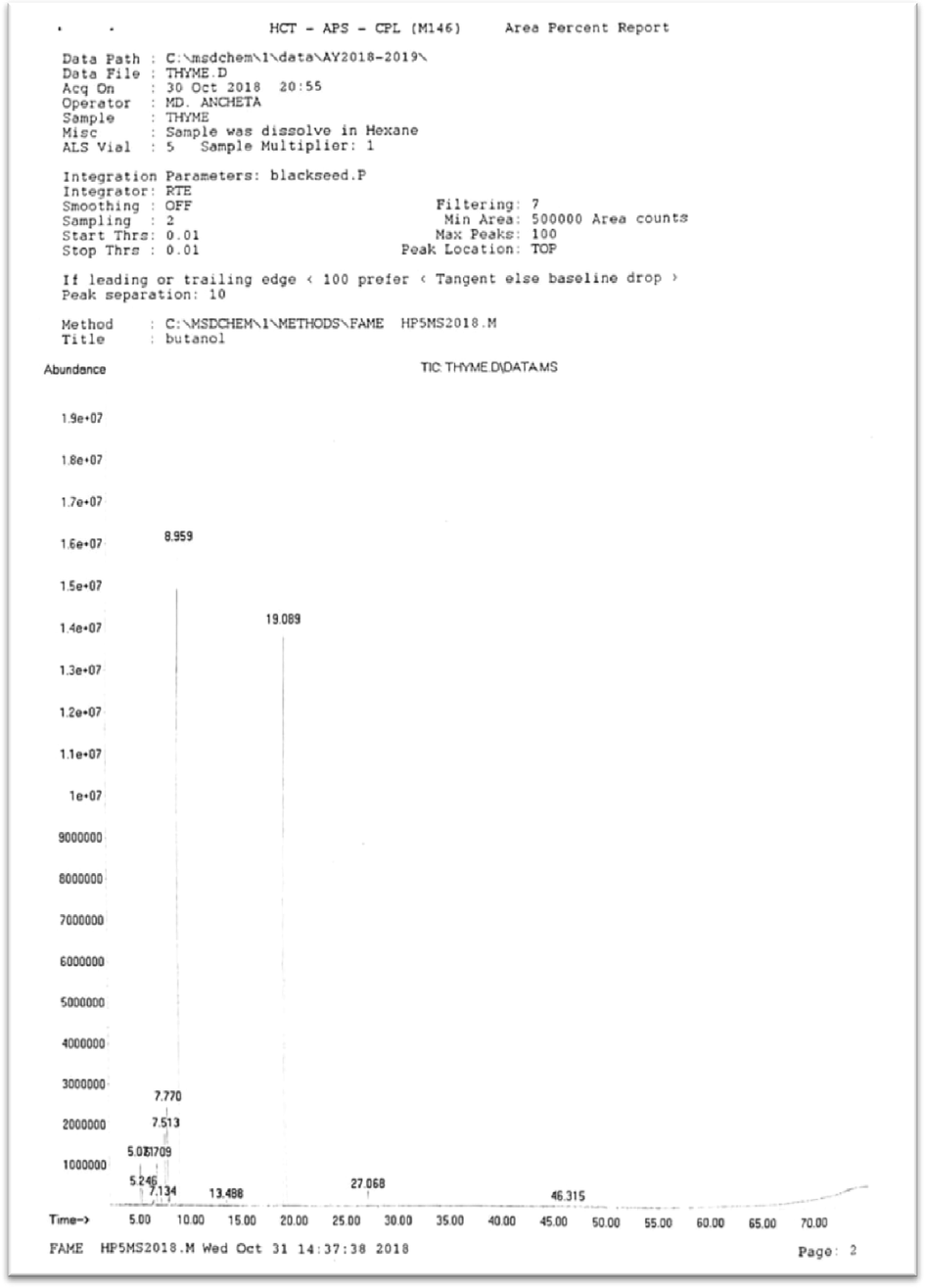
Gas chromatogram of TEO.
S. No
Retention time (min)
Area %
Identified compound
Structure
1.
5.071
1.25
α-Thujene
2.
5.246
0.47
α-Pinene
3.
6.709
1.55
β-Myrcene
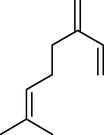
4.
7.134
0.27
Phellandrene
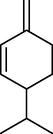
5.
7.513
2.79
α-Terpinene
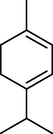
6.
7.770
3.72
p-Cymene
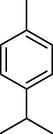
7.
8.959
29.12
γ-Terpinene
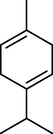
8.
13.488
0.33
4-Terpineol
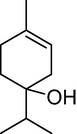
9.
19.089
59.29
Carvacrol
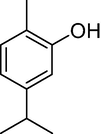
10.
27.068
0.90
β-Bisabolene
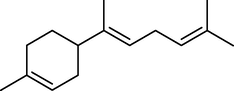
11.
46.315
0.31
Carvone
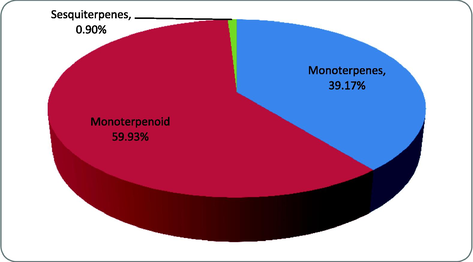
% composition of various terpenes in TEO.
3.2 In vitro antioxidant activity
Antioxidants play an important role in the prevention and promotion of health in humans against the harmful free radicals that cause many age-related diseases. It is considered that the essential oils rich in oxygenated monoterpenes and/or sesquiterpenes show significant antioxidative properties (Schelz et al., 2008). The antioxidant activity of TEO against 1,1 diphenyl picrylhydrazyl (DPPH) radical was determined by in vitro colorimetric assay. This method was adopted to study antioxidant activity because of the stability and unique purple color of free radicals of DPPH at room temperature. The intensity of purple color (λmax 517 nm) diminishes after being reduced by electron or a hydrogen radical donated by an antioxidant compound. Therefore, the antioxidant power of plant extract/chemical compound/volatile oil is determined by their ability to reduce DPPH radical which is measured by recording the lowering in absorbance values. The DPPH scavenging activity of TEO was measured at three different concentrations 10 µg/mL, 20 µg/mL and 40 µg/mL, which showed concentration dependent inhibition of DPPH (25.88–71.57%) (Table 2; Fig. 3). The IC50 value of TEO was calculated as 23.64 μg/mL indicating it to have good antioxidant potential. The TEO IC50 result for DPPH assay in our study is much lower than the 2.03 mg/mL obtained by Dasthti et al., for TEO from Irani species (Dashti et al., 2015). Antioxidant activity exhibited by TEO justifies its traditional uses. The phenolic chemotype are known to be strong scavengers of free radicals, therefore, the antioxidant activity of TEO could be attributed to its major phenolic constituent carvacrol. Based on the results, it can be proposed that TEO can be used as an alternative source of natural antioxidants.
S. No
Concentration (μg/mL)
% Inhibition (mean ± SD)
1.
10
25.88 ± 3.5
2.
20
51.18 ± 1.7
3.
40
71.57 ± 1.9
4
IC50
23.64 μg/mL
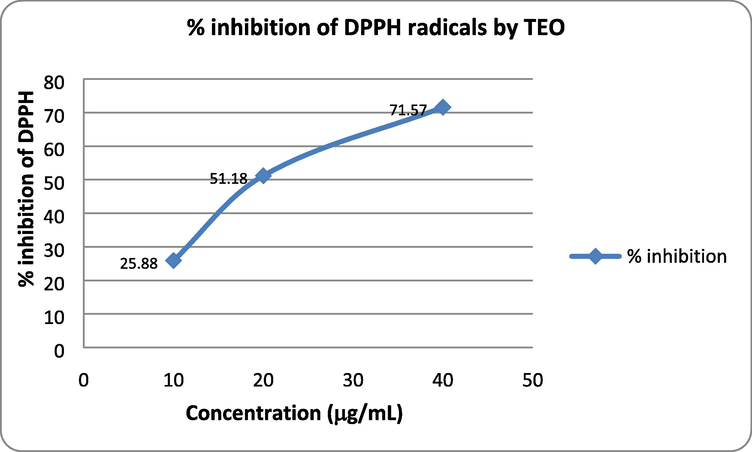
In vitro antioxidant activity of TEO.
3.3 Antibacterial activity of TEO
The antimicrobial spectrum of TEO was assessed by recording the zone of inhibition (mm) against S. aureus and E. coli bacteria at 5 μL and 10 μL concentrations. TEO showed antibacterial activity against both pathogenic microorganisms (Gram −ve and Gram+), but the growth inhibitory effect was more pronounced on E. coli (Table 3). Interestingly, TEO exhibited better antibacterial spectrum than standard Ampicillin drug against both the strains even at low concentration of 5 μL. The results of this study highlight the ability of TEO to inhibit bacterial growth and its potential as a powerful antimicrobial agent. A study by Khan et al. (2017) also evaluated the antibacterial activity of Pakistani variety of TEO at 15 μL and obtained the zone of inhibitions (30 mm and 25 mm) against E. coli and S. aureus. The results of our study are in agreement with their findings (Khan et al., 2017). Yamazaki et al., have reported that antibacterial effects of TEO is associated with the presence of phenolic compounds such as thymol and carvacrol. They found thyme oil to exhibit greatest antibacterial activity against E. coli O157:H7 (Yamazaki et al., 2004). Thus, it can be proposed that the antimicrobial activity of Omani TEO is predominantly due to the presence of phenolic compound and other hydrocarbons present in it. The phenolic compounds present in essential oil such as carvacrol have the ability to disrupt the cytoplasmic membrane eventually leading to bactericidal effect (Xu et al., 2008).
Microorganism
Diameter of Zone of inhibition (mm)
Thyme essential oil
Ampicillin
5 μL
10 μL
25 μg
S. aureus
18
24
15
E. coli
22
36
10
3.4 In silico studies of carvacrol
Prediction of activity spectra for substances (PASS) is a free online cheminformatic software that predicts the biological activities of chemical compounds based on the structural similarity to the large data base of active molecules. The bioactivity score is given in terms of Pa and Pi values. A predicted compound is possibly be active if its Pa value i.e. chances to be active is more than the Pi (chances to be inactive) values. Carvacrol is predicted to exhibit diverse bioactivities (Pa > 0.5) such as antiseptic, anti-infective, reductant and antimutagenic, etc. The Pa values for predicted bioactivities lies in between 0.861 and 0.513 (Table 4). Furthermore, the cytotoxicity potential of carvacrol as predicted by CLC-pred tool showed it to be active against three types of cancer cell lines. The cancer cell-line prediction results (Pa > 0.5) are obtained in the following order; Metastatic skin melanoma (Pa = 0586; Pi = 0.005; Cell line SK-MEL-1) > Oligodendroglioma of brain (Pa = 0574; Pi = 0.033; Cell line Hs 683) > Non small cell lung cancer, 3 stage (Pa = 0532; Pi = 0.035; Cell line NCI-H838).
S. No.
Pa
Pi
Predicted Biological activity
1.
0.898
0.003
Antiseptic
2.
0.884
0.002
Carminative
3.
0.784
0.005
Antiinfective
4.
0.748
0.010
Oxidoreductase inhibitor
5.
0.722
0.004
Antihelmintic (Nematodes)
6.
0.700
0.005
Antipyretic
7.
0.691
0.007
Anesthetic general
8.
0.673
0.019
Anti-inflammatory
9.
0.637
0.018
Cytoprotectant
10.
0.621
0.004
Urease inhibitor
11.
0.590
0.009
Antinociceptive
12.
0.562
0.015
Reductant
13.
0.523
0.020
Antiviral (Influenza)
14.
0.513
0.014
Antimutagenic
Molecular docking studies of carvacrol were also performed at the xanthine oxidase enzyme and antibacterial protein to account for the observed antioxidant and antibacterial activities. Xanthine oxidase (PDB ID: 3NRZ) is involved in the generation of reactive oxygen species (ROS) during metabolic phase while enoyl acyl carrier protein reductase (PDB ID: 4TZK) is considered as a potential target to inhibit bacterial growth. The binding energy of carvacrol was observed to be −5.84 kcal/mol and −4.16 kcal/mol for 4TZK and 3NRZ respectively. Docked structure of carvacrol with 3NRZ showed polar interaction with Gln 102 and hydrophobic interactions with Val 88, 121 and Pro 118 protein residues (Fig. 4). The binding interaction of carvacrol ligand on the 4TZK receptor protein also showed hydrogen bonding interaction with Thr 39 while hydrophobic interactions with Ile95 16 and Val65 residues (Fig. 5). Thus based on the binding interaction, it is evident that carvacrol is slightly better in exhibiting antibacterial activity than antioxidant activity.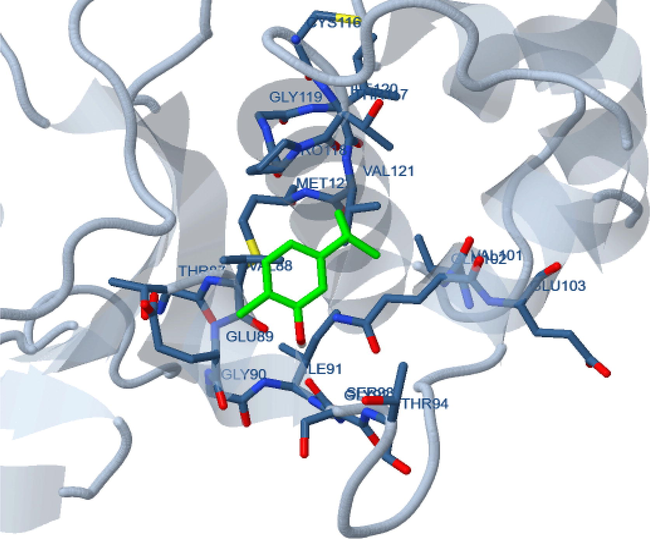
Molecular docking of carvacrol with xanthine oxidase enzyme.
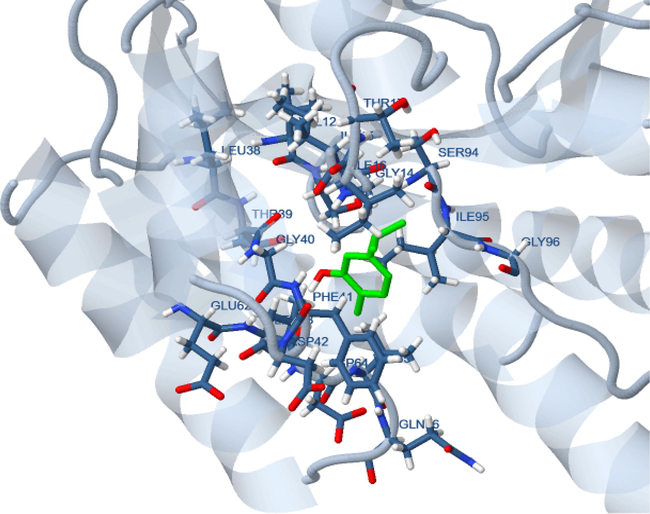
Molecular docking of carvacrol with enoyl acyl carrier protein reductase.
4 Conclusion
Thyme plays an important role in human health due to the presence of phytochemicals which contribute to many health benefits. The amount of these phytochemicals in thyme varies depending upon geographical and climatic conditions. In the current study, TEO isolated from an Omani cultivar was examined for its chemical composition, antioxidant and antimicrobial activity to provide a justification of its health benefits of daily consumption in this part of the world.
TEO exhibited moderate in vitro free radical scavenging activity in DPPH assay method. However, TEO exerted potent antimicrobial activity against both S. aureus and E. coli and is categorized as bactericidal. The antioxidant and antimicrobial activity of TEO is attributed to its chemical composition and primarily due its major chemical constituent carvacrol. The experimental results are also supported with the in silico prediction and molecular docking studies of its major constituent, carvacrol. It has been approved by the authorities to use in food stuff as it is generally recognized as safe (GRAS). TEO because of its powerful antibacterial activity can be explored further as source of efficacious antibacterial agents either alone or in combination with currently used antibiotics to overcome the emergence of the drug resistance.
Acknowledgements
The authors acknowledge the support of HCT technical staff for recording the GC-MS of volatile oil. Authors are also thankful to NU management for providing infrastructure and other facilities to carry out this research work.
Declaration of Competing Interest
We, the authors of the manuscript entitled “Chemical composition, in vitro antibacterial and antioxidant potential of Oman” submitted solely to the JKSU-Sci declare that there is no conflict of interest among us.
References
- Chemical composition and in-vitro antioxidant and antimicrobial activity of the essential oil of Citrus aurantifolia L. leaves grown in Eastern Oman. J. Taibah Univ. Med. Sci.. 2018;13:108-112.
- [Google Scholar]
- Comparative GC-MS analysis, in-vitro antioxidant and antimicrobial activities of the essential oils isolated from the peel of Omani lime. Chiang Mai J. Sci.. 2018;45:1782-1795.
- [Google Scholar]
- Essential oils used in aromatherapy: a systemic review. Asian Pac. J. Trop. Biomed.. 2015;5:601-611.
- [Google Scholar]
- Chemical composition and in vitro antibacterial properties of essential oils of four Thymus species from organic growth. Ind. Crop. Prod.. 2013;50:304-311.
- [Google Scholar]
- Relaxant effect of Thymus vulgaris on guinea-pig tracheal chains and its possible mechanism(s) Phytother. Res.. 2006;20:28-33.
- [Google Scholar]
- In vitro antimicrobial activity and chemical composition of Sardinian Thymus essential oils. Lett. Appl. Microbiol.. 1999;29:130-135.
- [Google Scholar]
- Antioxidant effect of thyme essential oil on oxidative stability of chicken nuggets. Int. J. Food Eng.. 2015;1:115-120.
- [Google Scholar]
- Chemical characterization of Thymus populations belonging from Southern Italy. Food Chem.. 2011;125:1284-1286.
- [Google Scholar]
- Chemical composition and antioxidant activity of Thymus vulgaris L. volatile oil obtained by two different methods. Rom. Biotech. Lett.. 2010;15:5436-5443.
- [Google Scholar]
- Study of the composition of the different parts of a Spanish Thymus vulgaris L. plant. Food Chem.. 1998;63:373-383.
- [Google Scholar]
- Volatile components of Thymus vulgaris L. from wild-growing and cultivated plants in Jordan. Flavour Fragr. J.. 2007;22:322-327.
- [Google Scholar]
- Chemical composition and antimicrobial activity of essential oil of Thyme (Thymus vulgaris) from Eastern Morocco. Int. J. Agric. Biol.. 2009;11:205-208.
- [Google Scholar]
- Antimicrobial activities of thyme essential oil cultivated in Quetta, Balochistan. Pakistan Int. J. Biosci.. 2017;10:105-110.
- [Google Scholar]
- Composition of essential oil of Thymus vulgaris grown in Oman. JEOP. 2016;19:475-478.
- [Google Scholar]
- Computer aided screening of natural products in search of lead molecules for design and development of potent anti-inflammatory agents. Sch. Acad. J. Pharm.. 2014;3(6):496-503.
- [Google Scholar]
- Essential oil and chemical compositions of wild and cultivated Thymus daenensis Celak and Thymus vulgaris L. Ind. Crop. Prod.. 2013;48:43-48.
- [Google Scholar]
- Antimicrobial activity and chemical composition of Thymus vulgaris, Thymus zygis and Thymus hyemalis essential oils. Food Control.. 2008;19:681-687.
- [Google Scholar]
- Antimicrobial and antiplasmid activities of essential oils. Fitoterapia. 2008;77:279-285.
- [Google Scholar]
- Rapid evaluation and quantitative analysis of thyme, origano and chamomile essential oils by ATR-IR and NIR spectroscopy. J. Mol. Struct.. 2003;661–662:299-306.
- [Google Scholar]
- Plant volatiles-based insect pest management in organic farming. Crit. Rev. Plant Sci.. 2010;29:123-133.
- [Google Scholar]
- The antibacterial mechanism of carvacrol and thymol against Escherichia coli. Lett. App. Microbiol.. 2008;47:174-179.
- [Google Scholar]
- Enhancement of antilisterial activity of essential oil constituents by nisin and diglycerol fatty acid ester. Food Microbiol.. 2004;21:283-289.
- [Google Scholar]







