Translate this page into:
Evaluation of anti-cancer, anti-microbial and anti-biofilm potential of biosurfactant extracted from an Acinetobacter M6 strain
⁎Corresponding author. youngscholar2013@gmail.com (Abraham Peele Karlapudi)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Biosurfactants are amphiphilic compounds produced by bacteria either extracellularly or as a part of the cell membrane. Biosurfactants have had a profound impact on medical and pharmaceutical biotechnology. In our previous work, we isolated a new biosurfactant produced by Acinetobacter indicus M6 which reduces the surface tension of water from 72.0 to 39.8 mN/m and which showed thermophilic, halophytic and acidophilic stability. The chemical nature was found to be a class of glycolipoprotein. Here, our research presents the extracted biosurfactant’s anti-proliferative activity against lung cancer cells (A549), and anti-microbial and anti-biofilm activity against MRSA. The anti-tumour activity of biosurfactant against lung cancer cells was evaluated in terms of cell viability at different concentrations. The results showed a decrease in the percentage of lung cancer viable cells with increasing biosurfactant concentrations and incubation time, with a significant decrease being observed at 200 µg/ml concentration leading to cell proliferation inhibition at G1 phase. Treatment of biofilms for seven days at 500 µg/ml resulted in up to 82.5% biofilm disruption.
Keywords
Biosurfactant
Acinetobacter indicus M6
Cell viability
Anti-proliferative activity
Anti-biofilm activity
1 Introduction
Biosurfactants are amphiphilic surface-active compounds produced extracellularly by microbes with a profound impact on medicine, food and bioremediation fields. Biofilms comprise an exopolysaccharide (EPS) sheath which protects bacteria from unfavourable conditions. Chemical surfactants have been predominant on the market, while attention has more recently been diverted to extracting lower toxicity and higher biodegradability biosurfactants (Peele, 2016). Biosurfactants exhibit an interesting biological activity profile, and may be useful as anticancer drugs. The molecules are progressing to become highly suitable drug candidates against many infectious diseases; biosurfactants have anti-proliferative in vitro activity against human lung cancer cell lines as well as antimicrobial effects against selected pathogens (Karlapudi et al., 2018). Although many studies have found biosurfactants to be potential drug candidates in the antimicrobial field, their role has been poorly explored in the area of cancer biology. Here, our research explored biosurfactant molecules and resulted in designing powerful non-toxic and biocompatible anticancer agents. Biosurfactants inhibited proliferation of A549 lung cancer cells. The antiproliferative potency had no influence on non-tumour cell cultures. The evaluation of biosurfactants as an active compound showed that they inhibit DNA synthesis in cancer cells and are non toxic. In vitro evaluation of antiproliferative activity against cancer cell lines and cytotoxicity against normal cells was performed. In the next step, in order to identify the molecular mechanism involved in the anti cancer action of the biosurfactant and its effects on DNA synthesis, cell cycle progression was examined.
2 Materials and methods
2.1 Biosurfactant production and recovery
The high-yielding biosurfactant strain Acinetobacter indicus M6 was used to produce biosurfactant (Accession No: KR559749). Acinetobacter indicus M6 culture was grown in LB medium and incubated for 72 h; cells were removed by centrifugation (14000 × g, 20 min, 4 °C), supernatant was mixed with twice volumes of acetone and left overnight at 4 °C; precipitate was collected. The precipitated biosurfactant was dissolved in milli-Q water (pH-7.0) dialyzed at 4 °C (cut-off 6000–8000 Da), and freeze-dried (Peele, 2016). Biosurfactant solution (10 mg/mL) was injected into the chromatographic column at a flow rate of 2 mL/min by using 1 M sodium chloride. Carbohydrate and protein contents were determined according to the standard procedures using phenol-sulphuric acid and Lowry methods. 1H NMR study was performed by dissolving 5 mg/ml in chloroform. LC–MS analysis was conducted on an Ion Trap mass instrument equipped with an ESI source. Twenty microliters of the purified sample, (10 μg/ml in methanol) was injected into the instrument and scanned over the mass range of 100–2000 m/z (Djuric et al., 2017).
2.2 Cell proliferation assay
The A549 lung cancer cell line was purchased from Sigma Aldrich (no: 86012804). DMEM (Hi-media) supplemented with 10% fetal bovine serum was used as the control medium. Cells (A549) were seeded into 96-well plates until reaching a density of 1 × 104 cells ml−1. Culture medium was replaced and A549 cells were exposed to different concentrations of biosurfactant prepared in PBS (25, 50, 100, 200 µg/mL). Non-tumorous mouse fibroblast cell line MC-3T3-E1 was used to evaluate the cytotoxicity of the biosurfactant. Biosurfactant solutions with different concentrations were added to the wells and incubated for 72 h. Cell proliferation was assessed using the MTT method and the product was quantified spectrophotometrically at 570 nm (Duarte et al., 2014).
2.3 Cell cycle
Cells (A549) were grown in 24-well microtiter plates until they reached 2 × 105 cells; spent medium was replaced with fresh medium containing biosurfactant (highest concentration, 0.05 g l−1 surfactin). After the incubation period, cells were trypsinised and the pellet was collected by centrifugation (Duarte et al., 2014). The pellet was resuspended in 1 ml PBS. The cell suspension was mixed with equal volumes of absolute ethanol, and 50 μl of RNase A was added and incubated at room temperature for 20 min. Propidium iodide dye was used and flow cytometry analyses was performed using flow cytometry (Becton Dickinson FACS Caliber) (Yang et al., 2017).
2.4 Anti-microbial properties of biosurfactant
The antimicrobial activity of the biosurfactant was determined by an Agar well diffusion assay. Antibacterial and antifungal activities against different fungal strains (Sabouraud dextrose agar (SDA) and Muller Hinton agar media were checked for 50% and 100% growth inhibition at different concentrations of biosurfactant. Fungal and bacterial cultures were incubated at 27 °C and 37 °C for 48 and 24 h, respectively (El-Gendy et al., 2018). Cells were collected by centrifugation and fixed with 4% (v/v) glutaraldehyde for 2 h. Samples were dehydrated using 70% acetone and stained. Results were viewed using a transmission electron microscopy system (Hickey et al., 2017).
2.5 Anti-biofilm activity of biosurfactant against MRSA
Biofilm was grown in96-well microtiter plates for seven days in LB medium. The wells were washed with phosphate buffer saline to remove non-adherent cells and the biofilm attached to the walls of the wells was exposed to different concentrations of biosurfactant. The plate was incubated at room temperature for 24 h; non-adherent cells were removed by washing twice with PBS. Crystal Violet dye (100 μl of 0.2%) was added to the wells and incubated for 60 min. Excess stain was removed and the biofilm was air dried; ethanol was added to solubilize the Crystal Violet. The optical density was recorded at 600 nm using an Eliza reader (Ramírez et al., 2018).
3 Results and discussion
3.1 Biosurfactant production and characterization
The purified product containing biosurfactant was recovered using chromatography, and fractions containing high carbohydrate and protein contents were pooled, freeze-dried, and stored for further use. The composition of biosurfactant analyzed using 1H NMR spectra indicated the presence of glycolipoprotein. The 1H NMR spectra showed distinguished peaks at 4.2, 4.1, 4.0, 3.8 and 3.5 ppm which indicated the presence of carboxyl, methyl and keto groups (Schneider et al., 1985). The structure of purified biosurfactant elucidated by LC–MS analysis showed the spectrum Fig. 1a) main signal located at m/z 787 and corresponding signals at m/z 653, 562, 525, 473, 383, 219 were identified. The probable peptide combination is assumed as Leu-cys (−219 Da) and Leu-cys-Asp-His (−473 Da), Leu-cys-Asp-His-Trp (−653 Da), Leu-cys-Asp-His-Trp-His (−787 Da) (Dalili et al., 2015).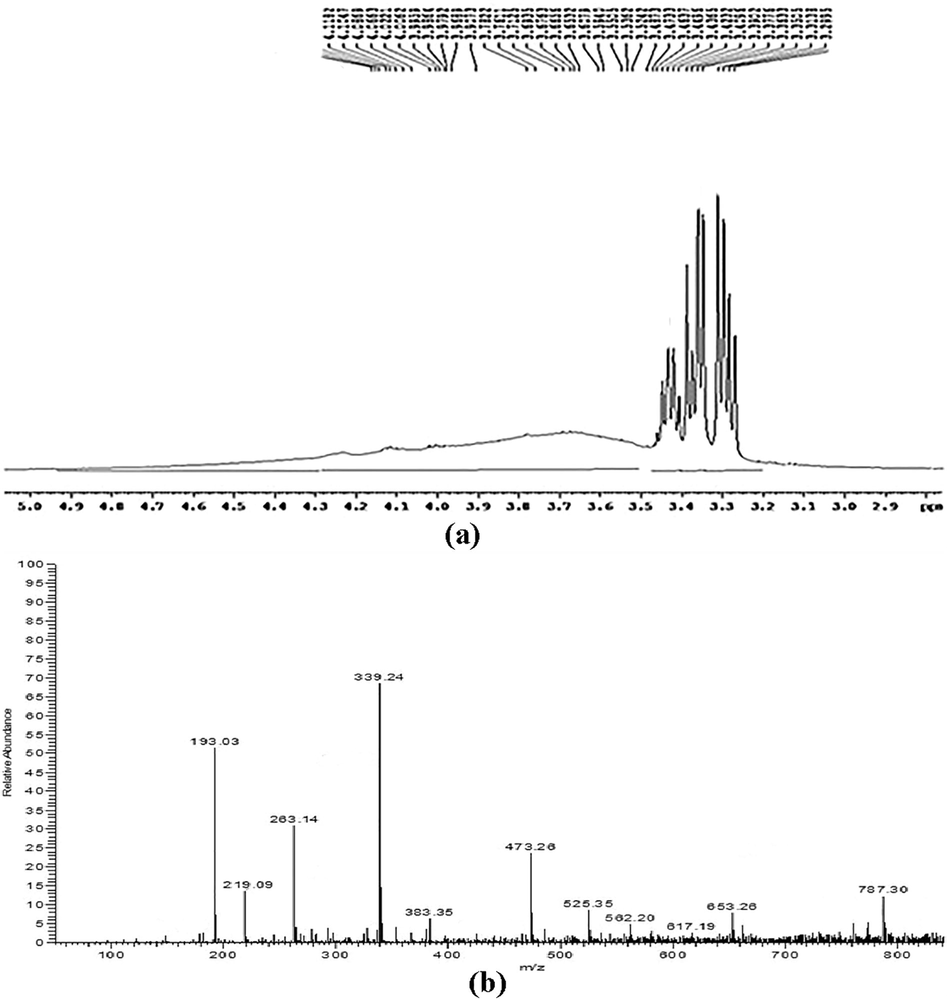
(a) 1H NMR spectrum of Biosurfactant produced by Acinetobacter indicus M6 (b) LC-ESI–MS/MS spectrum of purified biosurfactant Product ion.
3.2 Effect of biosurfactant on cell viability
Biosurfactant was tested in four different concentrations against A541 lung cancer cell lines and non-tumour cell line, MCT-3 T3-E1 at different incubation times to evaluate the toxicity effect Fig. 2). The results obtained clearly showed a decrease in the percentage of lung cancer viable cells with increasing biosurfactant concentrations and incubation times, with a significant decrease being observed at 200 µg/ml concentration. This effect was observed for 72 h as a linear decrease in cell viability. The biosurfactant decreased viable cells by half at 100 µg/ml concentration Fig. 2. Statistical analyses were performed with the use of Graph Pad Prism 5 software.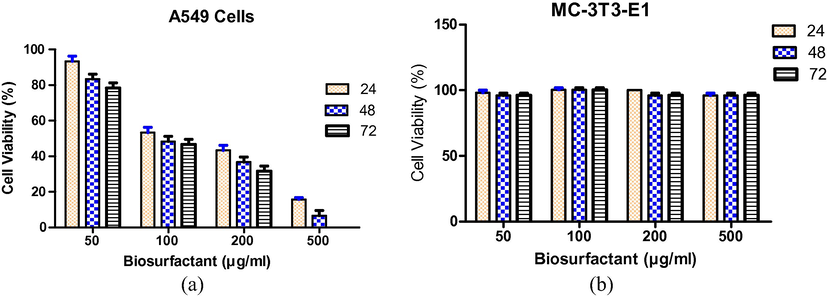
Dose-response curve for (a) A549 cell lines (b) MC-3T3-E1exposed to different concentrations of biosurfactant at different time intervals. Values represent without biosurfactant correspond to 100% cell viability.
The evaluation of cell proliferation through cell cycle analysis was performed by flow cytometry subjected to a 24 h exposure to 500 µg/ml biosurfactant. Biosurfactant showed G1 arrest and decreased the viable cells during S-phase in lung cancer (A549) cell lines which indicated the inhibition of DNA synthesis; the protein moiety of the biosurfactant significantly contributes to the detergent activity affecting the cell membrane, which then leads to lysis. As far as we know, this is the first study on the effect of a glycolipopeptide biosurfactant against A549 lung cancer cell lines. Non-tumour MC-3 T3-E1 cell lines were exposed to different concentrations of biosurfactant and did not particularly affect cell viability, which illustrates the non-toxic nature of the biosurfactant; the cell viability was considered to be 100% at the 0th hour treatment, whereas 100% cell viability was achieved for the control. Because the time of incubation increases, the cell viability reduces and after 72 h of incubation it was observed that viability of cells in the control reduced to 97.5% with 500 µg/ml (maximum concentration), whereas for 200 µg/ml the viability reduced to 85.6% (Table 1).
Sample (µg/ml)
% Cells in cell cycle phases
G0-G1
S
G2-M
Control
52 ± 3.4
34 ± 6.2
20 ± 1.8
200
54 ± 1.4
26 ± 2.6
15 ± 4.4
500
51 ± 2.48
24 ± 3.42
12 ± 1.4
3.3 Anti-biofilm activity
Biosurfactant disrupted the 48-h developed biofilms of MRSA. The anti-biofilm activity of biosurfactant showed a linear relationship with increases in concentration Fig. 3). Treatment of biofilms for seven days at 500 µg/ml resulted in up to 82.5%, with forced removal of the biofilm leading to disruption and indicating that the obtained effect was dose dependent Fig. 4). The results suggested the influence of biosurfactant on bacteria as demonstrated in Fig. 3 A and B, a clear decrease of the biofilm with crystal violet staining was observed during 7th day. The microtiter plate Fig. 3C) assay also confirmed the decrease of the biofilm in the wells treated with biosurfactant.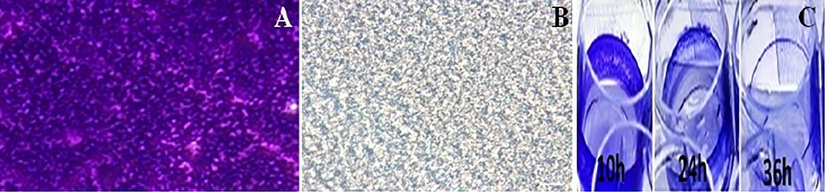
Biofilm formation in 96-well polystyrene plate wells in the absence (A) and presence (B) of biosurfactant at (500 µg/ml) after 7 days of incubation are shown (C) The microtiter plate showing decrease of biofilm with crystal violet staining.
The effect of biosurfactant at different concentrations on seven day developed biofilm.
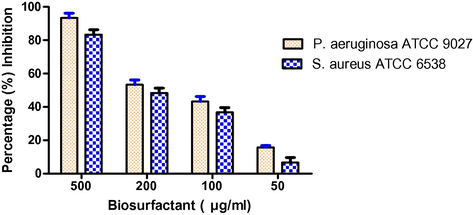
3.4 Anti-microbial activity
Biosurfactant was found to be effective against various pathogenic and non-pathogenic microorganisms to different degrees (Table 2). The purified biosurfactant showed antimicrobial activity against a broad range of pathogenic and non-pathogenic strains, including Gram-positive and Gram-negative bacteria, as well as yeasts. Nearly complete inhibition was observed for different biosurfactant concentrations ranging between 20 and 50 mg ml−1, except for Staphylococcus aureus. The lowest concentration of biosurfactant tested (12.5 mg ml−1) showed the highest degree of inhibition for Escherichia coli. The effect of biosurfactant on the cell membrane of E. coli was detected using TEM Fig. 5). The untreated bacterial cells possess intact cell membranes Fig. 2b). After being exposed to biosurfactant at a particular concentration (12.5 mg/ml), structural changes were observed around the cell membrane.
Microorganism
Biosurfactant concentration
50% growth inhibition (mg/mL)
100% growth inhibition (mg/mL)
E. coli
12.5
20
P. aeruginosa
15
20
S. aureus
50
ND
S. epidermidis
25
50
K. Pneumoniae
40
100
S. pyogenes
30
50
C. albicans
40
100
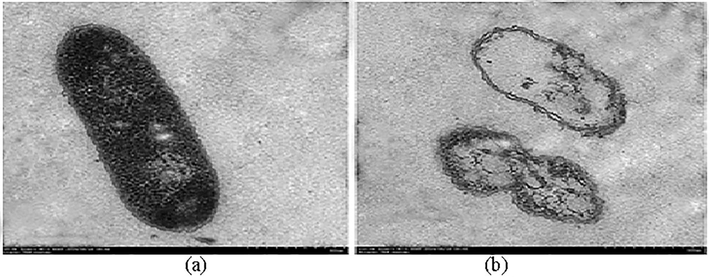
TEM image analysis showing the effect of biosurfactant action at 12.5 mg ml−1 on the cell membrane of E. coli. The untreated (a) and treated (b) samples were observed under the microscope.
4 Conclusion
Biosurfactant isolated from Acinetobacter indicus M6 displayed good anti-proliferative activity against lung cancer cell lines. The most sensitive cell concentration was found to be 1.5 gl−1. The tested concentrations were non toxic for normal fibroblast cultures. Biosurfactants possess strong drug-like properties as well as low toxicity and seem to be particularly promising. They also provide an opportunity for laying the foundation for development of more promising molecules of anticancer potency. In summary, we found that the tested biosurfactant showed anti-proliferative activity at appropriate concentrations and exposure times which decreased cellular proliferation. It is important to note that the biosurfactant produced by Acinetobacter indicus M6 has been isolated and characterized by our research team, so there are no previous reports on its potential anti-tumour activity. The biosurfactant at a particular concentration (500 µg/ml) resulted in up to 82.5% removal of biofilm, and hence may be appropriate for treating infections related to highly resistant pathogenic bacteria. Moreover, the biosurfactant was found to be effective against various selected bacteria, with broad anti-microbial activity against Gram-positive and Gram-negative bacteria. Therefore, it will be interesting in future studies to explore the biosurfactant more in detail in terms of its mechanism of action.
Conflict of the interest
The authors declare no conflicts of interest.
Acknowledgement
The authors would like to acknowledge the support of Vignan’s Foundation for Science Technology and Research (Deemed to be University), Guntur, India.
References
- Emulsifying activity of a biosurfactant produced by a marine bacterium. 3 Biotech. 2016;6(2):177.
- [Google Scholar]
- Brachybacterium sp. CH-KOV3 isolated from an oil-polluted environment–a new producer of levan. Int. J. Biol. Macromol.. 2017;104:311-321.
- [Google Scholar]
- Effects of biosurfactants on the viability and proliferation of human breast cancer cells. AMB Express. 2014;4(1):40.
- [Google Scholar]
- Structural characterization of an immunostimulating polysaccharide from the stems of a new medicinal Dendrobium species: Dendrobium Taiseed Tosnobile. Int. J. Biol. Macromol. 2017:1185-1193.
- [Google Scholar]
- Phylogenetic analysis and biological evaluation of marine endophytic fungi derived from red sea sponge hyrtios erectus. Appl. Biochem. Biotechnol.. 2018;12:1-23.
- [Google Scholar]
- Three-dimensional bright-field scanning transmission electron microscopy elucidate novel nanostructure in microbial biofilms. J. Microscopy.. 2017;265(1):3-10.
- [Google Scholar]
- Role of cell surface composition and lysis in static biofilm formation by Lactobacillus plantarum WCFS1. Int. J. Food Microbiol. 2018
- [Google Scholar]
- Proton NMR analyses, shielding mechanisms, coupling constants, and conformations in steroids bearing halogen, hydroxy, and oxo groups and double bonds. J. Am. Chem. Soc.. 1985;107(24):7027-7039.
- [Google Scholar]
- Isolation and structural characterization of Coryxin, a novel cyclic lipopeptide from Corynebacterium xerosis NS5 having emulsifying and anti-biofilm activity. Colloids Surf., B. 2015;135:425-432.
- [Google Scholar]







