Translate this page into:
Dual phase role of composite adsorbents made from cockleshell and natural zeolite in treating river water
⁎Corresponding author at: Department of Environmental Engineering, Faculty of Civil Engineering, Universiti Teknologi Malaysia (UTM), 81310 Johor, Malaysia. shahab.rezania@sejong.ac.kr (Shahabaldin Rezania)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
In this study, the potential of dual-phase composite adsorbent to determine the removal efficiency of organic compounds such as COD, BOD, TP, and TN was investigated. The combination ratio of cockleshell and natural zeolite was optimized using D-optimal mixture design (DMD). The generated ratio was tested using run test in Easy Care PipeSystem (ECPS). Breakthrough curve was plotted to determine the total removal by composite adsorbent. In addition, linearization of the breakthrough curve by dynamic models was implemented to characterize the adsorption process by the composite adsorbent in ECPS column model. The linearization of breakthrough curve was done using mathematical models, Adam-Bohart, Yoon-Nelson and Thomas model. It was found that the optimal mixture ratio was at 75% cockleshells and 25% natural zeolite. Based on the experiments, the composite adsorbent showed high tendency to higher removal by 90% of targeted value. Based on the results, the composite adsorbent was fitted better with Yoon-Nelson and Thomas model rather than Adam-Bohart model. The generated models were able to characterize the adsorption process using composite adsorbent in the ECPS column system.
Keywords
Composite adsorbents
Cockleshells
Natural zeolites
Adsorption
Mathematical model
1 Introduction
Due to quick population growth, the generation of solid wastes has become a critical issue that needs to be rectified and to alleviate the impact to the environment (Korai et al., 2017; PhunChien et al., 2017). The utilization of waste materials as alternative adsorbents is an attractive solution to reduce the volume of wastes (Abdolali et al., 2014; Dahri et al., 2014). Adsorption is considered as a good option for superior water treatment due to its accessibility, ease of operation, maintenance and simplicity in design (Ademiluyi et al., 2009; Bhatnagar and Sillanpaa, 2010). In addition, this process has been found to be capable of minimizing different types of pollutants and therefore has very wide applicability in pollution control and water treatment (Yagub et al., 2014; Rezania et al., 2016). The development of an adsorbent from waste materials basically depends on several factors. The waste materials are available abundantly which are cheap and biodegradable. It is speculated that the waste materials should contain high level of carbon or oxygen contents to obtain good removal capacities (Lupul et al., 2015). Additional characteristics to improve the quality of adsorbent are high abrasion resistance, high thermal stability, and small pore diameter (Andrejkovičovǎ et al., 2016).
Cockleshells are known as Anadara granosa which is a species of bivalve shellfish, and contains high level of calcium carbonate (CaCO3) (Ezzah-Mahmudah et al., 2016). Bivalve shells are able to adsorb inorganic (Yao et al., 2014; Nan et al., 2016) and organic compounds (Moideen et al., 2016; Daud et al., 2017) from polluted water. Generally, cockleshells comprise 98% to 99% of calcium carbonate in the form of aragonite meanwhile mussle shells consist of the mixture of calcite and aragonite (Kamba et al., 2013). The potential of bivalve shells as adsorbent are deniable since several studies have been proved their effectiveness. For instance, Chowdury and Saha (2010) used bivalve shells to remove Basic Green 4 (Malachite Green) whereas Jalil et al. (2012) applied bivalve shells with the corn leaves (Zea mays) to enhance the adsorption of malachite green. Therefore, the utilization of seashell and eggshells mixture for the adsorption of Malachite Green was conducted (Chowdury and Saha, 2012; Chowdury and Das, 2012).
In the adsorption process, a natural zeolite has been found to be more favorable as it can be used for a wide range of applications including industrial wastewater treatment with source of sorbent for ammonium contents (Misaelides, 2011), heavy metals (Perego et al., 2013) and dyes (Hor et al., 2016). Previously, extensive studies have been carried out to evaluate the ability of natural zeolites as a source of adsorbent due to the presence of hydrated aluminosilicate minerals which has high physicochemical properties such as cation exchange, molecular sieving, catalysis and sorption (Wang and Peng, 2010). Clinoptilolite is a type of natural zeolites which has widely been used as an adsorbent with maximum water/vapor adsorption rate by 8 mmol (Favvas et al., 2016). Clinoptilolite was further applied for removal of salt ion (Wibowo et al., 2017) and ammonium (Martins et al., 2017). According to Sana and Jalila (2016), modeling of breakthrough curves is necessary for the prediction of removal or adsorption using experimental parameters.
The development of a novel dual-phase composite adsorbent has been considered to be a attractive solution for enhancing the removal efficiency in respect of utilizing a single-phase adsorbent material. It was evaluated that the new composite adsorbent have higher affinity towards the organic compounds, also its adsorption capacity was found to be higher than the commercially available activated carbons (Chen et al., 2017). Halim et al. (2011) found that the composite adsorbent consisting of rice-husked carbon wastes, activated carbons and ordinary portland cement are capable of adsorbing ammonia and COD.
Most of the studies focused on the others bivalve shells such as oyster shells (Chen et al., 2013; Alidoust et al., 2015) and mussel shells (Lim and Aris, 2014; El Haddad, 2016). In our previous study, the feasibility of utilizing cockleshells as an adsorbent for wastewater treatment was evaluated (Moideen et al., 2016). Although, in most of the studies the focus was on single type of adsorbent for wastewater treatment. Furthermore, the main focus of this study was to determine the removal efficiency of organic compounds using dual-phase composite adsorbent of cockleshells and natural zeolites. Also, a mathematical model to evaluate the adsorption process which reflects the characteristic of composite adsorbent in adsorbing organic matter from wastewater was developed.
2 Materials and methods
2.1 Preparation of cockleshells
Cockleshells were collected from the Fisheries Research Institute (FRI), Gelang Patah, Johor, Malaysia. Initially, the cockleshells were washed throughly with tap water and then by de-ionized water to remove unwanted particles adhered to the shells. Then, the shells were sun dried for 3 days and further dried in oven at 105 °C for 24 h. Finally, the shells were crushed and sieved into the average size of 1 mm. In order to avoid any additional moisture, the crushed shells were kept in polyethylene bags for further usages.
2.2 Preparation of natural zeolites
The natural zeolite mostly consisted of 65.02% SiO2 which followed by 13.25% Al2O3, 3.65% TFe2O3 and some trace elements such as Na2O, K2O, CaO. Natural zeolites were washed with distilled water in order to remove the debris and unwanted particles. Then, it was dried at room temperature for 24 h and were kept in polyethylene bag to avoid moisture.
2.3 Designing of Easy Care Pipe System(ECPS): Fabricated column model
The Easy Care Pipe System (ECPS) was designed using PVC pipes with the height of 7.5 inch and diameter of 2.0 inch. The system was a closed loop circuit with three columns connected in series. The operation of ECPS started with the pumping in 200 L of wastewater with the flow rate of 11.49 (m3.day−1) and 600 g of adsorbent used for the pertreatment. Wastewater was circulated for a period of 3 days in the column for the adsorbents saturation. The removal of chemical oxygen demand (COD), biochemical oxygen demand (BOD), total phosphorus (TP) and total nitrogen (TN) was measured according to APHA (2005). Sampling done in the interval of 3 days continuously. Fig. 1 shows the schematic diagram of ECPS.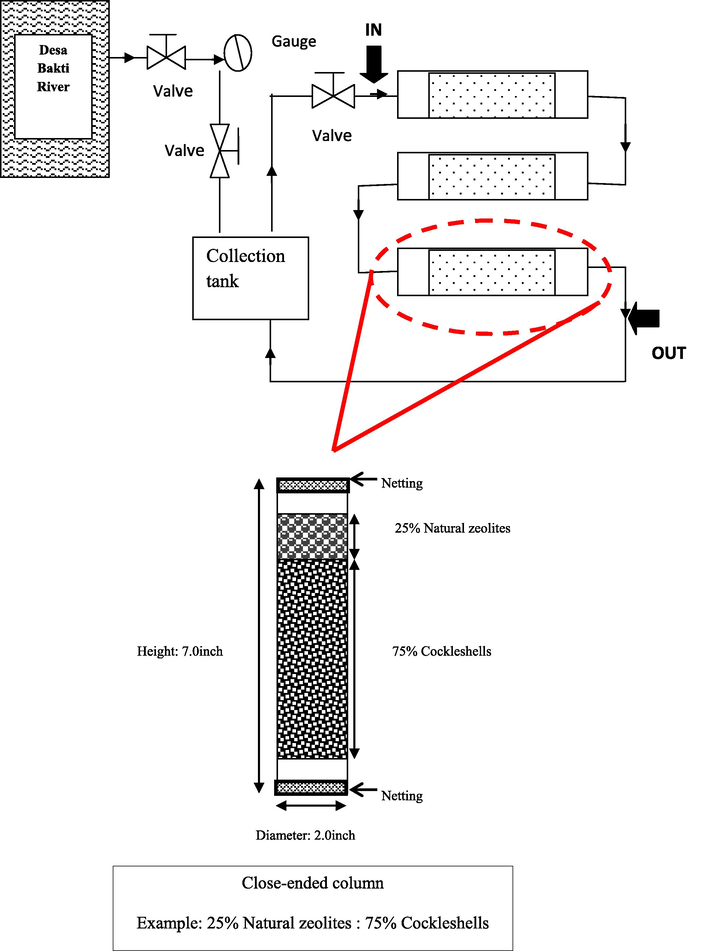
Schematic diagram of ECPS with close-ended column.
2.4 Column test experiment
Total removal of COD, BOD, TP and TN in ECPS with different combination ratio of cockleshells and natural zeolites was conducted to reach the adsorbent to adsorption equilibrium. Table 1 shows the combination ratio of cockleshells and natural zeolites generated by Design Expert software version 7.0. Based on the software, 13 runs of mixture ratio of cockleshels and natural zeolite were generated.
Run
Component
A: Natural Zeolite
B: Cockleshells
1
0.50
0.50
2
0.50
0.50
3
1.00
0.00
4
0.00
1.00
5
1.00
0.00
6
0.00
1.00
7
0.25
0.75
8
1.00
0.00
9
0.00
1.00
10
0.50
0.50
11
0.00
1.00
12
1.00
0.00
13
0.75
0.25
2.5 Analytical procedures
2.5.1 Optimization of combination ratio by D-optimal mixture design (DMD)
To examine the removal of BOD, COD, TP and TN, multiple regression analysis was carried out. As factor for mixture designing, natural zeolite and cockleshells were named as (A) and (B) respectively. The response function was to measure the removal efficiency of natural zeolites and cockleshells and the values described as A and B in the second-degree polynomials. The coefficient of polynomials was defined by constant terms, A and B as linear effects and AB as an interaction effects. The effects and regression coefficient of individual linear and interaction terms were verified with plotted linear graph based on the breakthrough curve. The significance of all the terms in polynomial equation was assessed by computing the probability (P) value. In addition, the desirability function (DF) was generated at the last stage of optimization using DMD. The function suggested the best mix ratio of cockleshells and natural zeolites.
3 Results and discussion
3.1 Mixed ratio optimization using D-optimal mixture design (DMD)
Table 2 shows the removal of BOD, COD, TP and TN after column testing of composite adsorbents in ECPS. The experiments were carried out according to the run number in order to minimize the variability for the values of all responses. Table 3 shows the fit statistics in response to the removal of BOD, COD, TP and TN. Then the results have been analyzed with the responses accordingly.
Run
Mixture ratio
Response
A: Natural Zeolites (%)
B: Cockleshells (%)
BOD removal (%)
COD removal (%)
TP removal (%)
TN removal (%)
1
0.50
0.50
50.00
94.12
80.87
83.33
2
0.50
0.50
48.34
90.12
78.14
81.43
3
1.00
0.00
41.67
70.83
70.83
83.33
4
0.00
1.00
57.14
71.43
98.33
85.71
5
1.00
0.00
41.33
71.33
70.97
84.11
6
0.00
1.00
55.23
68.45
95.77
83.33
7
0.25
0.75
50.00
67.39
53.07
71.30
8
1.00
0.00
43.33
73.11
70.33
80.33
9
0.00
1.00
59.43
73.33
95.04
88.33
10
0.50
0.50
52.76
95.32
82.33
85.11
11
0.00
1.00
58.13
72.33
97.33
83.33
12
1.00
0.00
43.33
71.11
70.83
83.11
13
0.75
0.25
42.86
35.19
55.17
83.33
Model 1: BOD removal (%)
Standard Deviation
2.08
R-Squared
0.9120
Mean
49.43
Adjusted R-Squared
0.9041
C.V.
4.21
Predicted R-Squared
0.8826
PRESS
63.65
Adequate Precision
18.679
Model 2: COD removal (%)
Standard Deviation
14.70
R-Squared
0.2881
Mean
73.39
Adjusted R-Squared
0.0508
C.V.
20.03
Predicted R-Squared
-3.2605
PRESS
11636.45
Adequate Precision
3.9490
Model 3: TP removal (%)
Standard Deviation
11.82
R-Squared
0.4456
Mean
78.39
Adjusted R-Squared
0.3952
C.V.
15.08
Predicted R-Squared
0.2816
PRESS
1991.34
Adequate Precision
5.200
Model 4: TN removal (%)
Standard Deviation
4.08
R-Squared
0.0095
Mean
82.79
Adjusted R-Squared
-0.0805
C.V.
4.93
Predicted R-Squared
-0.3009
PRESS
240.47
Adequate Precision
0.5690
R2 of Model 1 was used to explain the behavior variation of BOD removal using the mixture of natural zeolite and cockleshells (Table 3). For instance, R2 value (0.9120) in Model 1 refers to the removal of BOD which was closer to unity number 1.000 in comparison to other R2 values of different models (Model 2, Model 3 and Model 4).
Adequate precision compares the range of predicted values at the design points to the average simulated error. If the ratio values become more than four, it indicates that the model is adequate (Khalili et al., 2015). In this study, the value of adequate precision of Model 1, 2 and 3 was more than four. However, Model 2 and Model 3 had low R2 value, meanwhile the value of adequate precision was more than four. On the other hands, Model 4 showed unsatisfactory for both R2 and the number for adequate precision. The standard deviation of Model 1 was found to be 2.08 that indicated the model is acceptable with the minimal deviation. In comparison to other models (Model 2, 3 and 4), the value of standard deviation for Model 1 was lower. Coefficient of variation (C.V.) is the standard deviation and higher value of C.V. shows the higher variation in the mean value while the predictive ability of the model is not satisfactory (Lim et al., 2017). Based on Table 3, the Model 1 is more accurate as C.V. was the lowest 4.21. For the other models, CV was significantly higher by 20.03, 15.08 and 4.93 for models 2, 3 and 4, respectively. Based on these findings, the predictive ability for these models is not satisactory.
The optimization of mixture ratio was the key indicator in determining the optimal mixture ratio of natural zeolite and crushed cockleshells to be as the desirability value. From the generated mixture ratios, it was found that the optimal mixture ratio was 75% and 25% for cockleshells and natural zeolite with the desirability value of 0.700. As shown in Fig. 2, the stimulated values of BOD, COD, TP and TN removal with the dual-phase composite adsorbent consisting of 75% of cockleshells and 25% of natural zeolites. It was found that the column tests achieved 90% of the predicted removal. Therefore, it was proven that the mixture ratio of natural zeolites and cockleshells was good to be used for adsorption process.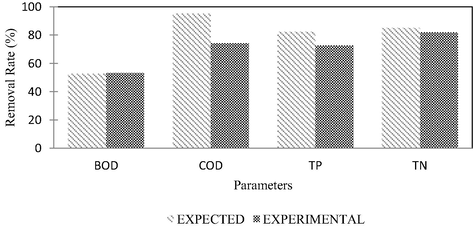
Removal of BOD, COD, TP and TN based on prediction (modelling) and actual experiment.
3.2 Breakthrough curve analysis for the date of column test
In order to analyze the dynamic adsorption in column model, set of breakthrough curve was plotted (Matta et al., 2008). Fig. 3 shows the breakthrough curves for the total removal of BOD, COD, TP and TN obtained from the column test experiments. It was found that the breakthrough points of TN and BOD achieved breakthrough point before COD and TP. As the time increased, the removal rate of TN and BOD together with the removal rates of TP and COD became insignificant. Hence, a minimal increase in the removal by natural zeolite-cockleshell composite adsorbent was observed.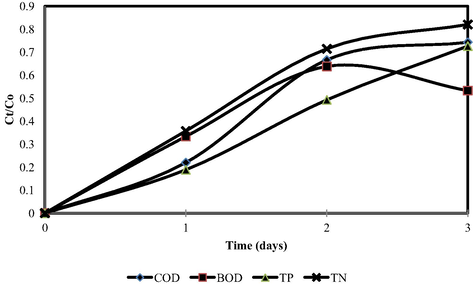
Breakthrough curve plot (Combination ratio: 25% natural zeolites: 75% cockleshells).
Total amount of organic contents adsorbed (mad), total amount of organic matters that passed through the columns (mtotal) and total removal (%) was obtained by calculating the area below the curve. The summary of the results are presented in Table 4.
Volume Influent (L)
Flow (m3 day−1)
Initial Cona (mgL−1)
Exhaustion Time, te (day)
Volume Effluent, Veff (L)
Total amount of organic matter adsorbed, mad
Total amount of organic matter through, mtotal
Removal of organic matter (%)
200
11.49
COD
46
3
34.47
5.99
1585.62
0.38
200
11.49
BOD
12
3
34.47
10.46
413.64
2.53
200
11.49
TP
2.6
3
34.47
8.75
89.62
9.76
200
11.49
TN
14
3
34.47
18.50
482.58
3.83
The analysis of breakthrough curve were in contradict with other studies. According to Samarghandi et al. (2008), by increase in the inlet adsorbate concentration, the slope of breakthrough curve increased while the volume of treated adsorbate reduced. As shown in Fig. 3, as the concentration at the inlet increased, the slope of breakthrough curve decreased and the volume of adsorbate treated also not as much as expected. This may be due to the constant value of flow rate in the system. Hence the value was not similar to the previous study, but this study proved the theory of aragonite shells could remove phosphate from water. As stated by Chen et al. (2013), oyster shell was able to remove phosphate content effectively.
3.3 Breakthrough curve testing using mathematical models; Adam-Bohart, Yoon-Nelson and Thomas model
Mathematical model for dynamic adsorption describes the behaviour of ECPS column to scale up for industrial applications. Three models (Adam-Bohart, Yoon-Nelson and Thomas model) were used to determine the behaviour of adsorption in column model. Table 5 shows the summary of these models.
(a) Adam-Bohart Model
(mg day(m3)-1)
(mg L−1)
R2
0.7091
0.3626
0.2814
0.4107
0.2079
0.2683
0.6587
0.1410
0.0643
0.3782
0.0316
0.0089
(b) Yoon-Nelson Model
(day−1)
Ʈ (day)
R2
0.2641
0.6919
0.1797
0.1485
0
0.3570
0.1019
0.6898
0.0314
0.4253
0.3267
0.5524
(c) Thomas Model
(m3(day.mg)-1)
(mg.g−1)
R2
0.6919
0.2641
0.1797
0
0.1485
0.3570
0.6898
0.1019
0.0314
0.3267
0.4253
0.5524
Table 5 (a), presents the values obtained from the linear regression graph using Adam-Bohart model. kAB is kinetic constant and N0 maximum adsorption capacity which are dependent in the initial concentration. High initial concentration provides the higher value of kAB and N0. This behavior can be explained by the mass transfer between the molecules in water and adsorbent during the dynamic adsorption in the column model experiments as reported by Adhikari et al. (2012). They found that the removal rates for COD, BOD, TP and TN were found to be lower and far from the unity value. This showed that Adam-Bohart model not fitted well with the data due to the inconsistency in flow rates during the experiment (Priya and Radha, 2016).
Table 5 (b), shows the values obtained from linear regression graph using Yoon-Nelson model. The value of kYN was found to be dependent to the initial concentration value. In the other hand, Ʈ value was inversely proportional to kYN. In general, Yoon-Nelson model was developed based on the hypothesis. As the rate of velocity decreased, the adsorption of adsorbate molecule was proportional to the breakthrough on the adsorbate. In addition, the R-squared values of COD, BOD, TP and TN were higher than the value obtained from the Adam-Bohart model. According to Guaya et al. (2015), modified natural zeolite by aluminum oxide (Z-Al), was effective for the adsorption of phosphate and ammonium which was well fitted by Langmuir model.
Table 5 (c) shows the increase in value of q0 which was due to the adsorption of adsorbates into the adsorbent surface and the difference of concentration of organic matters in water. This also indicated that the value of q0 and kTH greatly depends on the initial concentration. The R-value of Thomas model was high and similar to the value obtained from Yoon-Nelson model. Based on the results, the external and internal diffusions of Thomas model were not the limiting step. Therefore, the composite adsorbent consisting of natural zeolites and cockleshells in ECPS column was the best as described by Thomas model. In a studies by Alshameri et al. (2014) and Millar et al. (2016), found that the Freundlich model was the fit for optimum exchange of ammonium ions using natural zeolite. Although, the composite adsorbent of cockleshells and natural zeolites were fitted more to Yoon-Nelson model and Thomas model in compare to Adam-Bohart model. This is mainly due to the column study of ECPS relied on the plug flows that happened inside the pipe column.
4 Conclusion
Based on the results, two significant conclusion can be drawn as follows:
-
The optimal mixture ratio obtained by DMD for the design of composite adsorbent was 75% of cockleshells and 25% of natural zeolites which can be used for treating of river water most effectively.
-
Based on the mathematical model cockleshells and natural zeolites were more fitted to Langmuir isotherm. The dynamic isotherm for column model showed that composite adsorbent was fitted with Yoon-Nelson model and Thomas model.
Acknowledgements
This research was partially supported by W-BRIDGE, in which Waseda University and Bridgestone Corporative are engaged in the collaborative research project with the aim of contributing to mitigating environmental problems (R.J130000.7317.4B247). The authors would like to thank the financial support by Fundamental Research Grant Scheme from the Ministry of Higher Education Malaysia (R.J130000.7817.4F731 & R.J130000.7817.4L516) and Research University Grant from Universiti Teknologi Malaysia (Q.J130000.2517.14H39).
References
- Typical lignocellulosic wastes and by-products for biosorption process in water and wastewater treatment: a critical review. Bioresour. Technol.. 2014;160:57-66.
- [Google Scholar]
- Adsorption and treatment of organic contaminants using activated carbon from waste Nigerian Bamboo. J. Appl. Sci. Environ. Manage.. 2009;13:39-47.
- [Google Scholar]
- Continuous Removal of malathion by immobilised biomass of Bacillus species S14 using a packed bed column reactor. Chem. Spec. Bioavail.. 2012;24:167-175.
- [Google Scholar]
- Mechanism of cadmium biosorption from aqueous solutions using calcined oyster shells. J. Environ. Manage.. 2015;150:103-110.
- [Google Scholar]
- The investigation into the ammonium removal performance of Yemeni natural zeolite: modification, ion exchange mechanism, and thermodynamics. Powd. Technol.. 2014;258:20-31.
- [Google Scholar]
- Standard method for examination of water and wastewater (21st ed.). AWWA, WPCF, Washington: APHA; 2005.
- The effect of natural zeolite on microstructure mechanical and heavy metals adsorption properties of Metakaolin based geopolymers. Appl. Clay. Sci.. 2016;126:141-152.
- [Google Scholar]
- Utilization of agro-industrial and municipal waste materials a potential adsorbents for water treatment-a review. Chem. Eng. J.. 2010;157:277-296.
- [Google Scholar]
- Feasible preparation and characterization of tunable novel montmorillonite/block-copolymers based composites as potential dual adsorbent candidates. Appl. Clay. Sci.. 2017;137:192-202.
- [Google Scholar]
- Equilibrium and kinetic studies of phosphate removal from solution onto a hydrothermally modified oyster shell material. PIoS. One. 2013;8(4):e60243.
- [Google Scholar]
- Utilization of a domestic waste-eggshells for removal of hazardous malachite green from aqeous solutions. Environ. Prog. Sustain. Energy. 2012;31(3):415-425.
- [Google Scholar]
- Sea shell powder as a new adsorbent to remove basic Green 4 (Malachite Green) from aqueous solutions: equilibrium, kinetic and thermodynamic studies. Chem. Eng. J. 2010;164:168-177.
- [Google Scholar]
- Fixed-bed adsorption of malachite green onto binary solid mixture of adsorbents: seashells and eggshells. Toxicol. Environ. Chemist.. 2012;94:1272-1282.
- [Google Scholar]
- Water remediation using low cost adsorbent walnut shell for removal of malachite green; Equilibrium, kinetics, thermodynamic and regeneration studies. J. Environ. Chem. Eng.. 2014;2(3):1434-1444.
- [Google Scholar]
- Adsorption studies of leachate on cockle shells. Int. J. Geomate.. 2017;12(29):46-52.
- [Google Scholar]
- Removal of basic Fuchin dye from water using mussel shell biomass waste as an adsorbent equilibrium, kinetics and thermodynamics. J. Taibah. Uni. Sci.. 2016;10(5):664-674.
- [Google Scholar]
- Synthesis and characterization of Fe2O3/CaO derived from Anadara Granosa for methyl ester production. Energ. Conv. Manage.. 2016;126:124-131.
- [Google Scholar]
- Clinoptilolite, a natural zeolite material: structural characterization and performance evaluation on its dehydration properties of hydrocarbon-based fuels. Micropor. Mesopor. Mater.. 2016;225:385-391.
- [Google Scholar]
- Simultaneous phosphate and ammonium removal from aqueous solution by a hydrated aluminum oxide modified natural zeolite. Chem. Eng. J.. 2015;271:204-213.
- [Google Scholar]
- Ammonia and COD Removal from Synthetic Leachate using Rice Husk Composite Adsorbent. J. Urban. Environ.. 2011;5:24-31.
- [Google Scholar]
- Evaluation of physicochemical methods in enhancing the adsorption performance of natural zeolite as low-cost adsorbent of methylene blue dye from wastewater. J. Clean. Prod.. 2016;118:197-209.
- [Google Scholar]
- Utilization of bivalve shell-treated zea Mays L. (Maize) husk leaf as a low-cost biosorbent for enhanced adsorption of malachite green. Bioresour. Technol.. 2012;120:218-224.
- [Google Scholar]
- Synthesis and characterisation of calcium carbonate aragonite nanocrystals from cockleshell powder (Anadara Granosa) J. Nanomater. 2013:1-9.
- [Google Scholar]
- Optimization of production conditions for synthesis of chemically activated carbon produced from pine cone using response surface methodology for CO2 adsorption. RSC Adv.. 2015;5(114):94115-94129.
- [Google Scholar]
- The feasibility of municipal solid waste for energy generation and its existing management practices in Pakistan. Renew. Sustain. Energ. Rev.. 2017;72:338-353.
- [Google Scholar]
- Run Sum Chart for Monitoring Multivariate Coefficient of Variation. Comput. Indust. Eng.. 2017;109:84-95.
- [Google Scholar]
- A review on economically adsorbents on heavy metals removal in water and wastewater. Rev. Environ. Sci. BioTechno.. 2014;13(2):163-181.
- [Google Scholar]
- Adsorption of Atrazine on Hemp Stem-Based Activated Carbons with Different Surface chemistry. Adsorption.. 2015;21(6):489-498.
- [Google Scholar]
- Ammonium removal from landfill leachate by Clinoptilolite adsorption followed by bioregeneration. J. Environ. Chem. Eng.. 2017;5(1):63-68.
- [Google Scholar]
- Equilibrium studies of ammonium exchange with Australian natural zeolites. J. Wat. Proc. Eng.. 2016;9:47-57.
- [Google Scholar]
- Application of natural zeolites in environmental remediation: a short review. Micropor. Mesopor. Mater.. 2011;144:15-18.
- [Google Scholar]
- Wasted cockle shell (Anadara granosa) as a natural adsorbent for treating polluted river water in the fabricated column model (FCM) Desali. Wat. Treat.. 2016;57(35):16395-16403.
- [Google Scholar]
- Mechanisms of cadmium accumulation (adsorption and absorption) by the freshwater bivalve Corbicula fluminea under hydrodynamics conditions. Envion. Pollut.. 2016;212:550-558.
- [Google Scholar]
- Zeolites and related mesoporous materials for multi-talented environmental solutions. Micropor. Mesopor. Mater.. 2013;166:37-49.
- [Google Scholar]
- Review on the renewable energy and solid waste management policies towards biogas development in Malaysia. Renew. Sustain. Energ. Rev.. 2017;70:988-998.
- [Google Scholar]
- Fixed-bed column dynamics of tetracycline hydrochloride using commercial grade activated carbon: comparison of linear and nonlinear mathematical modeling studies. Desali. Wat. Treat.. 2016;57(40):18964-18980.
- [Google Scholar]
- Effectiveness of Eichhornia crassipes in nutrient removal from domestic wastewater based on its optimal growth rate. Desal. Wat. Treat.. 2016;57(1):360-365.
- [Google Scholar]
- Breakthrough curve analysis for fixed-bed adsorption of azo dyes using novel pine cone-derived active carbon. Adsorp. Sci. Technol.. 2008;32(10):791-806.
- [Google Scholar]
- Combined effect of unsaturated soil condition and soil heterogeneity on methylene blue adsorption/desorption and transport in fixed bed column: experimental and modeling analysis. J. King. Saud. Uni.. 2016;28(4):308-317.
- [Google Scholar]
- Natural zeolites as effective adsorbents in water and wastewater treatment. Chem. Eng. J.. 2010;156:11-24.
- [Google Scholar]
- Reduction of seawater salinity by natural zeolite (Clinoptilolite): adsorption isotherms, thermodynamics and kinetics. Desalin.. 2017;409:146-156.
- [Google Scholar]
- Dye and its removal from aqueous solution by adsorption: a review. Adv. Colloid. Interface. Sci.. 2014;209:172-184.
- [Google Scholar]
- Bivalve shell: not an abundant useless waste but a functional and versatile biomaterial. Criti. Rev. Environ. Sci. Technol.. 2014;44:2502-2530.
- [Google Scholar]







