Translate this page into:
2,4-Di-tert-butylphenol (2,4-DTBP) purified from Streptomyces sp. KCA1 from Phyllanthus niruri: Isolation, characterization, antibacterial and anticancer properties
⁎Corresponding author. mrkactinos@gmail.com (Radhakrishnan Manikkam)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Objectives
The present study reports the bioactivity of 2,4-Di-tert-butylphenol (2,4-DTBP) purified from an endophytic Streptomyces species KCA1 isolated from the leaves of Phyllanthus niruri and its antibacterial and anticancer properties.
Methods
The extracellular metabolites were produced from the strain KCA1 by submerged fermentation using ethyl acetate. The crude extract was further evaluated for antibacterial activity against set of pathogens. Active metabolite from the extract was purified using chromatography techniques and detected its antibacterial activity by disc diffusion assay. The molecular structure of the active molecule was identified through various spectral study. Moreover, the bioactive metabolite 2,4-DTBP was analyzed antibacterial and anti-proliferative activity.
Results
Strain KCA1 was identified as Streptomyces sp. In the preliminary screening, the crude extract exhibited broad spectrum activity against various bacterial pathogens. Based on the spectral properties, the active metabolite was identified as 2,4-Di-tert-butylphenol. The MIC of active compound 2,4-DTBP inhibited E. coli ATCC 25922 and S. aureus ATCC 29213 at 50 μg/ml and 0.78 μg/ml, respectively. The IC50 value of 2,4-DTBP was found to be 11.0 µg/ml and 116.8 µg/ml, against breast cancer cell line (MCF7) and normal VERO cell line, respectively.
Conclusions
This study concluded that 2,4-DTBP, produced from the endophytic Streptomyces sp. KCA1, is the potential candidate to develop as promising antibacterial and anticancer agent.
Keywords
Endophytic Streptomyces
2,4-Di-tert-butylphenol
Antibacterial
Antiproliferative activity
1 Introduction
Infectious diseases caused by the drug-resistant bacterial pathogens are the most significant health concerns (Serwecinska, 2020; Gasperini et al., 2021). Every year, an estimated 7 million people die due to the failed antimicrobial drugs, and the percentage is constantly increasing (Ukuhor, 2021). By 2050, a lack of concerted efforts to address AMR is estimated to result in 10 million deaths annually worldwide, making AMR an even more prevalent reason of mortality than cancer disease (Mahoney et al., 2021). The loss of antibiotic treatment would threaten our capacity to combat infectious diseases. Once first- and second-line antibiotic drugs are reduced due to resistance or lack of availability, doctors are compelled to utilize toxic medicines, which are often more costly but also less efficient (Terreni et al., 2021; Serwecinska, 2020). As a result, finding new medicines to combat such dangerous infections is critical.
Most of the antibiotics in present clinical practice are derived mostly from natural products or inspired by them (Vuong, 2021; Atanasov et al., 2021). Among various diverse sources of natural antibiotics, microorganisms, especially members of the phylum actinobacteria remains in the top (De Simeis and Serra, 2021). Actinobacteria, particularly the species Streptomyces, are estimated to have produced around two-thirds of all natural antibiotics (Maiti et al., 2020; Takahashi and Nakashima, 2018). Also, it has been reported that actinobacteria from rare sources like plants, the so called endophytic actinobacteria, are the promising source for new metabolites to develop as clinically relevant antibiotics (Singh and Dubey, 2018).
With this view, the present study was under taken for the purification of bioactive metabolites from actinobacterial strain KCA1, isolated from the leaves of Phyllanthus niruri and their evaluation for antibacterial and anticancer properties.
2 Methods and materials
2.1 Streptomyces strain KCA1
Using starch casein nitrate agar media, the endophytic strain KCA1 was isolated from the leaves of the medicinal plant Phyllanthus niruri. Viability of the strain KCA1 was maintained in ISP2 agar medium slants and 20% glycerol broth at 4 °C and −80 °C, respectively. Based on 16SrRNA gene sequencing, strain KCA1 was identified as species of the genus Streptomyces (data not shown).
2.2 Production of bioactive metabolites
The fresh culture of KCA1 was inoculated into 50 mL of ISP2 (International Streptomyces Project) broth medium used as mother culture aseptically and incubated at 28 °C in a rotary shaker for 72 h with 120 rpm. Then 5% of seed culture was transferred to a 1L conical flask containing 200 mL of ISP2 broth as the production medium. This production medium incubated for 7 days at 28 °C in a rotary shaker with 120 rpm. The cell-free supernatant (CFS) was separated after fermentation by centrifugation at 5000 rpm for 30 min at 4 °C (Eppendorf AG, Hamburg, Germany). The extracellular bioactive metabolites from the CFS were extracted using ethyl acetate (1:1 v/v) with occasional manual shaking for 24 h. The crude organic extract was obtained by collecting the top organic layer and drying it using a rotary evaporator.
2.3 Preliminary antibacterial activity
The crude extract of Streptomyces KCA1 was tested antibacterial activity against various bacterial pathogens such as Staphylococcus aureus ATCC 29213, Bacillus cereus, Escherichia coli ATCC 25922, Pseudomonas aeruginosa ATCC 27853 and Klebsiella pneumoniae ATCC 13882 by adopting disc diffusion method. Sterile filter paper disc (Himedia) with 5 mm diameter was impregnated with 20 µl of ethyl acetate extract (100 µg/ml) and kept for drying under sterile condition. The panel of bacterial pathogens, were grown in nutrient broth and set equivalent to 0.5 McFarland standards. After incubation, the bacterial pathogens were inoculated into Muller Hinton Agar (MHA) plates. The crude extract impregnated disc was placed over the pathogens inoculated MHA plates and incubated at 37 °C. The zone of inhibition was measured and represented in millimeter after 24 h of incubation (Radhakrishnan et al., 2021).
2.4 Purification of active metabolites
The active metabolite was purified from the crude ethyl acetate extract of Streptomyces KCA1 using column chromatography (35 × 1.0 cm) packed with silica powder (100–200 mesh size, Himedia) and eluted by various concentrations of hexane: and ethyl acetate. Totally 44 fractions were collected, concentrated and checked for antibacterial activity against E. coli ATCC 25922 and S. aureus ATCC 29213 as described earlier. The active antibacterial fractions were pooled together and re-purified using the column (20 × 1.0 cm) with methanol as the high polar eluent. Again, antibacterial activity was evaluated for the 11 re-purified fractions. Based on the results of antibacterial assay, active fractions were pooled together and further purified using semi-preparative UHPLC (Thermo Scientific UltiMate 3000). The flow rate was maintained at 3 mL/min and the UV detection was absorbed at 440 nm. The purified fractions were concentrated and evaluated for antibacterial activity.
2.5 Characterization and structure elucidation
Physicochemical characteristics such as appearance, colour, and solubility of purified metabolite was determined by adopting standard procedures. The Infrared (IR) spectral of the purified sample combined with potassium bromide (KBr) was measured using a high-quality Perkin Elmer Spectrum One FT-IR spectrometer. NMR spectrum was recorded on 500 MHz AVANCE III Bruker spectrometer. LC-Mass spectrum in positive ion mode was taken to determine the molecular weight using Thermo/Finnigan Surveyor system. All the spectral analysis was carried out at CSIR-National Chemical Laboratory, Pune and India. Chemical structure of the purified metabolite was determined based on their spectral properties.
2.6 Minimum inhibitory concentration (MIC) of purified metabolite
The purified metabolite was evaluated for MIC against the pathogens E. coli ATCC 25922 and S. aureus ATCC 29213 by the micro broth dilution technique. The two-fold diluted concentrations of the purified sample (100–0.78 μg/ml) were prepared in 10% DMSO. Pathogenic bacteria viz. E. coli ATCC 25922 and S. aureus ATCC 29213 were grown to logarithmic phase (0.1 OD at 600 nm) and mixed with 100 µl of different concentrations of purified sample. 100 μl of test sample at various concentrations was mixed with 100 μl of nutrient broth served as control. The positive control well had 100 μl of pathogenic culture and 100 μl of nutrient broth, whereas the negative control had 200 μl of nutrient broth alone and incubated at 37 °C for 24 hrs. The optical density (OD) was calculated at 595 nm using microplate reader (Epoch 2, BioTek Instruments, Agilent Technologies, CA, USA).
2.7 Antiproliferative activity of purified metabolite
2.7.1 Cell lines maintenance and growth condition
Antiproliferative activity of purified metabolite against MCF7 (breast cancer cell line) and VERO (normal cell line) was determined by adopting MTT assay.
2.7.2 MTT assay
In a sterile flat bottom 96-well plate, 5000 cells per well concentration was maintained and incubated for 48 h. Each well received 20 µl of purified metabolite. The final concentration range was between 100 and 0.78 µg/ml. For this experiment, sterile DMSO at 0.05 % (v/v) was administered as a negative control. The microtitre plate was further incubated for 72 h. Later, 50 µl of MTT (Sigma) solution (5 mg/ml) was added to each well. The plates were further incubated in CO2 incubator for 4 h at 37 °C with 5% CO2 and 95% air. After gently aspirating the medium, to dissolve the formazan crystals, DMSO (100 µl) was added. Using a microplate reader, the absorbance of dissolved formazan product was measured at 570 nm.
The following formula was used to compute the proportion of viable cells: cell viability (%) = (Treated cells absorbance/Untreated cells absorbance) × 100.
3 Results
3.1 Characterization and taxonomy of actinobacterial strain KCA1
Actinobacterial strain KCA1 produced powdery colonies when culturing on YEME agar plates with gray color aerial mycelium pale brown color reverse side pigment. The presence of aerial and a branched substrate mycelium was also noted under bright filed microscopic observation. The 16SrRNA gene sequencing of KCA1 showed 99.45% similarity with Streptomyces sp. and had a query length of 1452 bp. This partial sequence was submitted to GenBank with the accession number of MW470667.
3.2 Production and preliminary screening for antibacterial activity
In submerged fermentation, 47 mg of crude extract was obtained from 100 mL of ISP2 medium using ethyl acetate. In antibacterial assay, the crude extract was exhibited broad spectrum activity against bacterial pathogens. Maximum of 20.0 ± 0.3 mm zone of inhibition was observed against S. aureus ATCC 29213 followed by 19.83 ± 0.4 mm against B. cereus and 19.0 ± 0.3 mm against K. pneumoniae. Also, the crude extract showed least inhibition against E. coli ATCC 25922 (13.5 ± 0.8 mm).
3.3 Purification and characterization of active molecule
In the process of purification, totally 44 fractions were collected from the silica gel column chromatography in which 14 fractions (F13-F26) eluted with hexane: ethyl acetate was showed antibacterial activity against E. coli ATCC 25922 and S. aureus ATCC 29213. These 14 active fractions were concentrated after being pooled together based on their similar TLC patterns. Re-purification through silica gel column chromatography, the F5 and F6 fraction that indicated antibacterial activity were pooled again before being subjected to semi-preparative UHPLC. The fraction collected at 18.817 min retention time was exhibited antibacterial activity. The active peak was subsequently chromatographed using acetonitrile: water (90:10), yielding a single peak with a retention time of 16.242 min, indicating the metabolite purity.
The active metabolite (4.3 mg/L) was found to be yellow in color. It is soluble in water, chloroform, ethyl acetate and methanol. FT-IR spectra showed a peak at 3233–3302 cm−1, indicating the stretch of O–H phenol group. Furthermore, the C–C stretch of the alkyl group and C–O stretch of phenol group was confirmed by the peaks at 2849–2914 cm−1 and 1246 cm−1, represented. The aromatic C–C stretch was confirmed by the peaks at 1465–1730 cm−1 (Fig. 1). Therefore, the above functional groups by FT-IR analysis confirmed the phenol group of molecules. LC–MS analysis was used to determine the molecular weight, with a mass of [M+H]+ at m/z 209, matching to the primary peak, yielding an accurate mass of 206.17 in the positive ion (Fig. 2).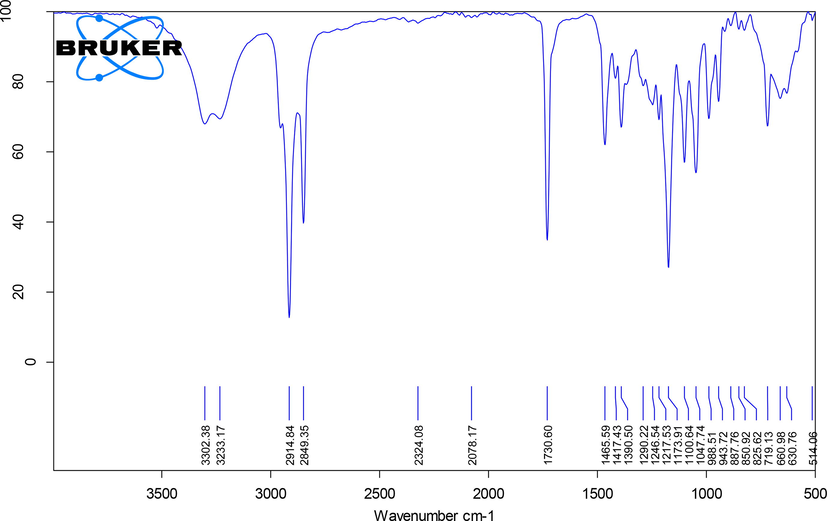
FT-IR spectrum of 2,4-DTBP isolated from Streptomyces sp. KCA1.
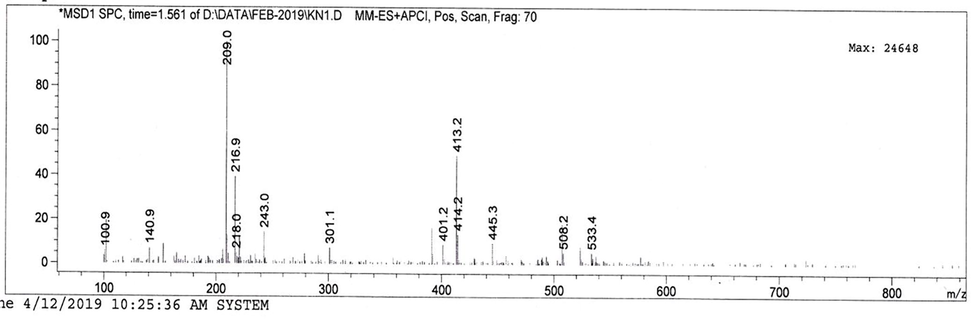
LC-MS analysis of 2,4-DTBP isolated from Streptomyces sp. KCA1.
The presence of two singlets at 1.31 ppm and 1.33 ppm in the 1H NMR data resembles di-substituted tertiary butyl groups (Fig. 3a). A singlet at 4.66 ppm indicates the presence of phenolic hydrogen. The remaining three protons were identified in the aromatic downfield area in the range of 6.60–7.31 ppm, indicating that the metabolite was tri-substituted aromatic. The 13C NMR spectra indicated the existence of ten carbon signals, six of which were downfield carbon signals observed between 115.90 and 151.76 ppm in the aromatic region. In the up-field area, the remaining four carbon signals were discovered between 29.35 and 34.71 ppm. The aromatic substitution of six carbons was confirmed by the presence of three quaternary carbons at 135.17 ppm, 142.97 ppm, and 151.76 ppm. At 151.76 ppm, the presence of a phenolic OH group at the quaternary carbon was found in the furthest downfield position. Tertiary carbons were identified in two of the four carbon signals found in up-field locations (34.31 ppm and 34.26 ppm, respectively), whereas methyl signals were found in the other two (31.61 ppm and 29.65 ppm, respectively). Thus, the spectrum indicates that the molecule has two tert-butyl replacements on the aromatic ring's last quaternary carbons, at 135.17 ppm and 142.97 ppm, respectively (Fig. 3b). Based on the FT-IR (Fig. 1), LC–MS (Fig. 2), and NMR studies (Fig. 3a, 3b and 3c), the chemical structure of the molecule was observed to be 2,4-Di-tert-butylphenol (C14H22O) (Fig. 3d).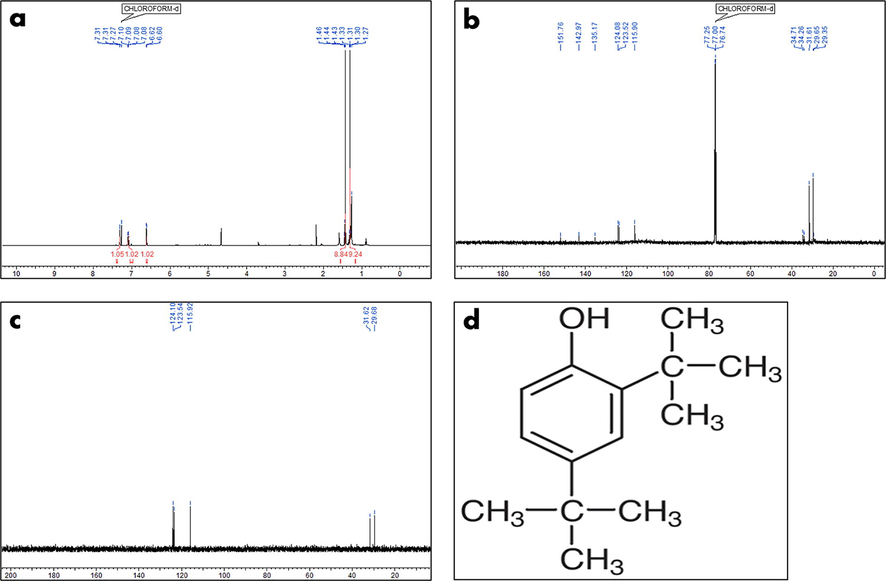
NMR analysis and structural prediction of 2,4-DTBP (a) 1H NMR, (b) 13C NMR, (c) DEPT NMR and (d) chemical structure.
3.4 MIC of 2,4-DTBP against bacterial pathogens
The purified metabolite 2,4-DTBP was shown to be more efficient against S. aureus ATCC 29213, with MIC of 0.781 g/ml, compared to E. coli ATCC 25922, having a MIC of 50 g/ml.
3.5 Cytotoxicity assay of 2,4-DTBP
The MTT assay was used to investigate the cytotoxicity of 2,4-DTBP against MCF7 and VERO cell lines has been depictured in Fig. 4 and Fig. 5. The better effect of 2,4-DTBP on cell viability was observed, and the IC50 value was calculated. As shown in Table 1, 2,4-DTBP showed comparable cytotoxicity against MCF7 and VERO cell lines with IC50 values of 11.0 and 116.8 µg/ml, respectively.![Morphology of breast cancer cell line MCF7 after treatment with 2,4-DTBP at different concentrations. Comparison of the morphological features of MCF7 after the 72hrs with 2,4-DTBP at respective concentrations [(a) control, (b) 3 µg/ml, (c) 30 µg/ml, (d) 100 µg/ml].](/content/185/2022/34/5/img/10.1016_j.jksus.2022.102088-fig4.png)
Morphology of breast cancer cell line MCF7 after treatment with 2,4-DTBP at different concentrations. Comparison of the morphological features of MCF7 after the 72hrs with 2,4-DTBP at respective concentrations [(a) control, (b) 3 µg/ml, (c) 30 µg/ml, (d) 100 µg/ml].
![Morphology of normal VERO cell line after treatment with 2,4-DTBP at different concentrations. Comparison of the morphological features of VERO after the 72hrs with 2,4-DTBP at respective concentrations [(a) control, (b) 3 µg/ml, (c) 30 µg/ml, (d) 100 µg/ml].](/content/185/2022/34/5/img/10.1016_j.jksus.2022.102088-fig5.png)
Morphology of normal VERO cell line after treatment with 2,4-DTBP at different concentrations. Comparison of the morphological features of VERO after the 72hrs with 2,4-DTBP at respective concentrations [(a) control, (b) 3 µg/ml, (c) 30 µg/ml, (d) 100 µg/ml].
Concentrations (μg/ml)
Breast cancer (MCF7)
Normal cells (VERO)
% of cell death
% Live cell
% of cell death
% Live cell
200
80.91 ± 0.83
19.09
59.61 ± 3.01
40.39
100
73.28 ± 0.24
26.72
21.01 ± 0.66
78.99
50
69.65 ± 0.05
30.35
20.14 ± 0.14
79.86
25
56.85 ± 0.59
43.15
12.54 ± 1.14
87.46
12.5
43.20 ± 2.76
56.80
7.82 ± 2.02
92.18
6.25
37.71 ± 0.05
62.29
6.08 ± 1.66
93.92
3.125
26.51 ± 0.53
73.49
6.51 ± 0.12
93.49
1.562
14.83 ± 1.06
85.17
6.07 ± 0.68
93.93
0.781
7.63 ± 1.02
92.37
3.97 ± 0.21
96.03
4 Discussions
Streptomyces has been proven to be a dependable source of secondary metabolites having healthcare applications, therefore much research is being put into isolating novel Streptomyces strains for the isolation of clinically relevant bioactive metabolites (Parte, 2018; Anderson and Wellington, 2001). In the present study, an attempt has been made to identify the bioactive potential of the endophytic Streptomyces sp. KCA1 isolated from Phyllanthus niruri. According to literature, endophytic actinobacteria from medicinal plants are found to exhibit promising bioactivity and bioactive metabolite production. In this study also, during primary screening, the ethyl acetate extract of endophytic actinobacterial strain KCA1 was showed broad spectrum antibacterial activity. Findings of certain earlier studies also showed that the ethyl acetate extract of Streptomyces exhibited broad spectrum activity (Al Farraj et al., 2020). The strain was found to exhibit antimicrobial activities against a set of pathogenic strains. An important finding of this present study was the purification of the active metabolite 2,4-DTBP from endophytic Streptomyces sp. KCA1. This metabolite is also produced by other bacterial strains such as Sphingobium fuliginis, Flavobacterium johnsoniae and Pseudomonas putida. (Toyama et al., 2010; Sang and Kim, 2012; Dharni et al., 2014). In addition, some fungal species such as Termitomyces heimi, Pleurotus sajorcaju and Hericium erinaceus are also reported to produce 2,4-DTBP (Malek et al., 2009). Similar to this study 2,4-DTBP produced by Streptomyces sp. KB1 and Streptomyces mutabilis isolated from air and soil sample from different geographical habitat showed antimicrobial and anticancer activity (Chawawisit et al., 2015a, 2015b; Belghit et al., 2016).
In this present study the purified metabolite 2,4-DTBP possessed antibacterial activity against S. aureus ATCC 29213 and E. coli ATCC 25922 with MICs of 0.78 μg/ml and 50 μg/ml, respectively. Similarly, 2,4-DTBP isolated from Streptomyces sp. KB1 showed antibacterial activity against MRSA (methicillin-resistant Staphylococcus aureus) with the MIC of 31.25 μg/ml (Chawawisit et al., 2015b). In addition, the molecule 2,4-DTBP isolated from S. mutabilis showed antimicrobial activity with higher MIC of 30 μg/ml and >100 μg/ml, against S. aureus ATCC 29213 and E. coli ATCC 25922, respectively (Belghit et al., 2016).
Another important finding of this present study was the purified metabolite 2,4-DTBP showed moderate antiproliferative activity against the breast cancer cell line MCF-7 and less inhibition to the normal VERO cell line. This finding is also in agreement with the previous report by Chawawisit et al. (2015b). The cytotoxic property of 2,4-DTBP has been reported by different research groups against various cancer cell lines such as MCF7 (IC50 5.75 mg/ml), A549 (IC50 6 mg/ml), CasKi cells (IC50 4.5 mg/ ml) and HeLa cell lines (IC50 10 mg/ml) (Malek et al., 2009; Varsha et al., 2015). This metabolite 2,4-DTBP is also well recognized for their antioxidant, anti-inflammatory, insecticidal, nematocidal, antiviral, antimalarial and anti-phytopathogenic activities (Zhao et al., 2020).
5 Conclusion
Based on the findings of this study, it can be concluded that the endophytic Streptomyces sp. KCA1 from Phyllanthus niruri produces 2,4-Di-tert-butylphenol, which possesses antibacterial and anticancer properties. To move this compound further, more research is needed on its mechanism of action, in-vivo cytotoxicity, and pharmacodynamics and pharmacokinetics.
Author contributions
Conceived and designed the experiments: RM. Performed the experiments: AS, MK, AKS. Analysis/interpretation of data: MS, SGD. Drafting of the manuscript: AS, MK. Supervising the work: RM, SGD. Critical revision of the manuscript: RM.
Acknowledgements
Authors acknowledge the management of Sathyabama Institute of Science and Technology (SIST), Chennai Tamil Nadu, India and CSIR-National Chemical Laboratory, Pune, India for the research facilities provided.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Antibiotics production in optimized culture condition using low cost substrates from Streptomyces sp. AS4 isolated from mangrove soil sediment. J. King Saud Univ. Sci.. 2020;32(2):1528-1535.
- [Google Scholar]
- The taxonomy of Streptomyces and related genera. Int. J. Syst. Evol. Microbiol.. 2001;51:797-814.
- [Google Scholar]
- Natural products in drug discovery: advances and opportunities. Nat. Rev. Drug Discov.. 2021;20(3):200-216.
- [Google Scholar]
- Activity of 2,4-Di-tert-butylphenol produced by a strain of Streptomyces mutabilis isolated from a Saharan soil against Candida albicans and other pathogenic fungi. J. Mycol. Med.. 2016;26(2):160-169.
- [Google Scholar]
- Chawawisit K., Bhoopong P., Phupong W., Lertcanawanichakul M., 2015a. 2,4-Di-tert-butylphenol, the bioactive compound produced by Streptomyces sp. KB1. J. Appl. Pharm. Sci. 5,7-12.
- Anti-MRSA activity, mode of action and cytotoxicity of 2, 4-Di-tert-butylphenol produced by Streptomyces sp. KB1. Int. J. Pharm. Sci. Rev. Res.. 2015;35:114-119.
- [Google Scholar]
- Actinomycetes: A never-ending source of bioactive compounds-An overview on antibiotics production. Antibiotics. 2021;10(5):483.
- [Google Scholar]
- Purification, characterization, and in vitro activity of 2,4-Di-tert-butylphenol from pseudomonas monteilii psf84: Conformational and molecular docking studies. J. Agric. Food Chem.. 2014;62(26):6138-6146.
- [Google Scholar]
- Multidrug-resistant bacterial infections in geriatric hospitalized patients before and after the COVID-19 outbreak: Results from a retrospective observational study in two geriatric wards. Antibiotics. 2021;10:95.
- [Google Scholar]
- The silent pandemic: Emergent antibiotic resistances following the global response to SARS-CoV-2. IScience. 2021;24(4)
- [Google Scholar]
- Streptomyces sp SM01 isolated from Indian soil produces a novel antibiotic picolinamycin effective against multi drug resistant bacterial strains. Sci. Rep.. 2020;10:10092.
- [Google Scholar]
- Cytotoxic components of Pereskia bleo (Kunth) DC. (Cactaceae) leaves. Molecules. 2009;14:1713-1724.
- [Google Scholar]
- LPSN—List of prokaryotic names with standing in nomenclature (bacterio.net), 20 years on. Int J. Syst. Evol. Microbiol. 2018;68:1825-1829.
- [Google Scholar]
- Bioprospecting of actinobacteria from selected mangrove ecosystems, India effective against carbapenem resistant Klebsiella pneumoniae ATCC BAA-1705. J. Appl. Pharm. Sci.. 2021;11:89-94.
- [Google Scholar]
- The volatile-producing Flavobacterium johnsoniae strain GSE09 shows biocontrol activity against Phytophthora capsici in pepper. J. Appl. Microbiol.. 2012;113(2):383-398.
- [Google Scholar]
- Diversity and applications of endophytic actinobacteria of plants in special and other ecological niches. Front. Microbiol.. 2018;9:1767.
- [Google Scholar]
- Actinomycetes, an inexhaustible source of naturally occurring antibiotics. Antibiotics. 2018;7:45.
- [Google Scholar]
- New antibiotics for multidrug-resistant bacterial strains: latest research developments and future perspectives. Molecules. 2021;26:2671.
- [Google Scholar]
- Isolation and characterization of 4-tert-butylphenol-utilizing sphingobium fuliginis strains from Phragmites australis rhizosphere sediment. Appl. Environ. Microbiol.. 2010;76(20):6733-6740.
- [Google Scholar]
- The interrelationships between antimicrobial resistance, COVID-19, past, and future pandemics. J. Infect. Public Health. 2021;14(1):53-60.
- [Google Scholar]
- 2,4-Di-tert-butyl phenol as the antifungal, antioxidant bioactive purified from a newly isolated Lactococcus sp. Int. J. Food Microbiol.. 2015;211:44-50.
- [Google Scholar]
- Natural products and their derivatives with antibacterial, antioxidant and anticancer activities. Antibiotics. 2021;10(1):70.
- [Google Scholar]
- Natural sources and bioactivities of 2,4-di-tert-butylphenol and its analogs. Toxins. 2020;12:35.
- [Google Scholar]







