Translate this page into:
Effect of zinc oxide nanoparticles on Triticum aestivum L. and bioaccumulation assessment using ICP-MS and SEM analysis
⁎Corresponding author at: Department of Botany & Microbiology, College of Science, P.O. Box: 22452, King Saud University, Riyadh 11495, Saudi Arabia. kperveen@ksu.edu.sa (Kahkashan Perveen)
⁎⁎Corresponding author: Department of Botany & Microbiology, College of Science (Girls Campus), King Saud University, Riyadh 11495, Saudi Arabia. 434202646@student.ksu.edu.sa (Alanoud S. Alslimah),
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
The impact of zinc oxide nanoparticles (ZnONPs) on Triticum aestivum L. has been explored in this study. The wheat seedlings were allowed to grow on Hoagland and Knop agar medium supplemented with different concentrations of ZnONPs (2500, 5000, 7500, 10,000, and 15,000 ppm) for three weeks. To determine their effect, the growth parameters, viz., wheat seed germination, seedling growth, and chlorophyll content, were taken into consideration. Moreover, the bioaccumulation of ZnONPs in root cells was determined by means of SEM, and zinc content in ZnO NP treated seedlings was measured by ICP-MS. The abatement in seed germination was observed at all concentrations of ZnONPs used, except at 2500 ppm. The plant growth and chlorophyll content also declined with an increase in ZnONPs concentration, except at 2500 ppm. The maximum reduction in shoot height (23.7%), root length (66.8%), seedling fresh weight (33.8%), and dry weight (54.8%) was noticed at the highest concentration of ZnONPs (15,000 ppm). At 15,000 ppm of ZnONPs, the chlorophyll a, chlorophyll b, and total chlorophyll were reduced by 80.6%, 74.2%, and 78.5%, respectively. An elevation in Zn concentration was noticed with an increase in the concentration as revealed by the ICP-MS analysis. The TEM micrographs exhibit the accumulation of ZnONPs within the root cells of wheat seedlings treated with ZnONPs (15,000 ppm). Thus, nanoparticles may have a destructive effect on the plant, and therefore their considerate use is advisable.
Keywords
Zinc oxide nanoparticles
ICP-MS
Wheat plant
Phytotoxicity
Bioaccumulation

- ZnONPs
-
Zinc oxide nanoparticles
- TEM
-
Transmission electron microscopy
- Chl a
-
Chlorophyll a
- Chl b
-
Chlorophyll b
- Total chl
-
Total chlorophyll
- ICP-MS
-
Inductively coupled plasma mass spectrometry
Abbreviations
1 Introduction
Nanoparticles, being smaller in size, possess special physio-chemical properties than their normal metal and metal oxide forms. The makeup of a nanoparticle is an important factor that defines its reactivity and bioactivity (Teske and Detweiler, 2015; Wang et al., 2016). In the last few years, the utilization of nanoparticles has increased tremendously. In agriculture, nanoparticles of Se, Au, Ag, Si, Mg are utilized as fertilizers, while some, like CuO, Fe3O4, S, chitosan, or Ca-alginate-chitosan nanoparticles, are used as pesticides or pesticide carriers (Ale et al., 2021; Bano et al., 2021; Singh et al., 2021). Furthermore, their usage has broadened to various industries. The extensive use of nanoparticles can have a negative impact on the environment and may be considered a pollutant (Keller and Lazareva, 2013; Rajput et al., 2020). On the one hand, nanoparticles such as CuO, Ag, Zn, and iron, as well as many others, have been shown to promote plant growth, but they have also been linked to phytotoxicity (Akbarnejad-Samani et al., 2020; Fedorenko et al., 2021; Goswami et al., 2019). The behavior of these nanoparticles in different ecosystems varies according to physical and chemical environmental conditions (Javed et al., 2019; Levard et al., 2012). As the usage of nanoparticles has proliferated, this can cause their disposal issues. Furthermore, the use of nanoparticles in soil and water for a variety of purposes may contaminate soil, water, and air. These nanoparticles may be taken up by plants through these contaminated environments, raising concerns about their influence on plants (Verma et al., 2021; Yadav et al., 2014). Moreover, they may cause dangerous implications if they enter the food chain (Hawthorne et al., 2014). Therefore, it is necessary to evaluate the impact of these nanoparticles on plants and other living organisms. Researchers have reported both the positive and negative influences of various nanoparticles on living organisms. However, extensive research is needed in this area because there is a dearth of specific knowledge about the influence of nanoparticles on plants and other organisms.
Zinc (Zn) is an element that is needed by most living organisms, mainly for metabolic activities and enzyme functioning (Auld, 2001). In plants, Zn is an integral part of various enzymes and cofactors that are involved in photosynthesis (Hu and Sparks, 2019). Zinc is categorized as a micronutrient, required by plants in a small amount for their proper growth. In plants, a lack of this element can cause deficiency, but an excess of this element can cause toxicity (Javed et al., 2019; Singh et al., 2018; Tarafdar et al., 2014). The application of zinc oxide nanoparticles in agriculture, tissue culture, and plant protection has increased tremendously. In recent years, zinc nanoparticles have been used as a component in nano fertilizer to improve plant growth and vitality. This has raised concerns about the overuse of ZnONPs in plants. Although ZnONPs have proven to be a replacement for their bulk form, the impact of their higher concentrations on plants and bioaccumulation still needs investigation. Wheat (Triticum aestivum L.), is a staple cereal crop that is cultivated globally and can grow in a variety of cultivation conditions (Aggarwal et al., 2015). Wheat is a good bio-indicator of pollution. Moreover, it is a metal accumulator and can translocate metal from the root to the shoot system. However, the studies on the translocation and bioaccumulation of nanoparticles in plants are meager (Yahyaoui et al., 2017). The current study was undertaken to investigate the effects of ZnONPs on plant growth, Zn concentration, and their accumulation in wheat roots.
2 Materials and methods
2.1 Characterization and suspension preparation of zinc oxide nanoparticles (ZnONPs)
ZnONPs (>30 nm and < 50 nm) were purchased from NANOGRAFI Co. Ltd. (Universiteler Mah Cankaya, Ankara, Turkey). The details on the properties and characteristics of ZnONPs supplied by the manufacturing company are shown in Table 1.
Properties/Characteristics
Specification
Purity
99.5 (%)
Average particle size
30–50 nm
Morphology/ Shape
Nearly spherical
Color
Milky white
Specific Surface Area
70 m2/g
Crystal Phase
Single crystal
True Density
5.5 g/cm3
To further confirm the morphology, i.e., the size and shape of the ZnONPs, transmission electron microscopy (TEM) was used. The ZnONPs powder was mixed with deionized water (2.5 mg/ml) and the solution was sonicated at an ultrasonic water bath (Elmaonic S30H) for 30 min at 35 °C to disperse the nanoparticles properly. A drop of solution was placed on the grid and the image was captured with a TEM (JEM-1400, Japan). The size of the ZnONPs was also measured.
To prepare five concentrations of ZnONPs (2500, 5000, 7500, 10,000, and 15,000 ppm), the pre-calculated amount of ZnONPs was added to the deionized water. For adequate dispersion of nanoparticles, the prepared suspensions were sonicated in an ultrasonic water bath (Elmaonic S30H) for 30 min at 35 °C.
2.2 Effect of ZnONPs on wheat seed germination
Wheat seeds were surface sterilized with 10% sodium hypochlorite for 10 min, and after that, they were rinsed 3 times with deionized water. The surface sterilized seeds were soaked in deionized water for 36 h. Subsequently, five seeds were placed into the petri dish lined with Whatman 40 filter paper. The seeds were irrigated with 5 mL of ZnONPs suspension (2500, 5000, 7500, 10,000, and 15,000 ppm). Deionized water without ZnONPs served as a control. Thereafter, seeds were observed daily till the seeds in the control plates were fully germinated. After a week, the number of seeds germinated was recorded and the percent of germination was calculated.
2.3 Effect of ZnONPs on wheat seedling growth and chlorophyll content
After a week of seed germination in the petri dishes, 2 uniform seedlings were selected and transplanted to a 250 mL glass culture bottle containing 50 mL of sterilized Hoagland & Knop agar medium supplemented with 0, 2500, 5000, 7500, 10,000, and 15,000 ppm of ZnONPs suspension (Miranda-Fuentes et al., 2021). The seedlings were allowed to grow in the laboratory at 23 ± 2 °C under 16/8h light/dark conditions. There were six replicates for each treatment.
2.3.1 Recording the growth parameters of wheat seedlings
After two weeks, the seedlings were harvested from each culture bottle. First the seedlings were washed with water, then they were rinsed three times with deionized water. The growth parameters (length of the root and shoot) were recorded. The fresh weight of the seedling was also measured. For measuring the dry weight/biomass of the seedlings, they were dried at 105 °C for 30 min and at 70 °C for 24 h.
2.3.2 Estimation of chlorophyll content in the leaves
Chlorophyll a (Chl a), Chlorophyll b (Chl b) and total chlorophyll (total chl) content in the seedlings were also measured. The fresh leaf (0.2 g) was ground with ashton (80% acetone) and centrifuged at 5000 rpm for 10 min. After that, the volume of the obtained supernatant was increased to 10 mL by adding ashton, and absorbance at 645 and 663 nm was measured (Perveen et al., 2010).
The chlorophyll content was determined using the formula below.
Chl a (mg/g) = [12.7 (OD663) − 2.69 (OD645)] × V/1000 × w.
Chl b (mg/g) = [22.9 (OD645) − 4.68 (OD663)] × V/1000 × w.
Total Chl (mg/g) = 20.2 × OD645 + 8.02 × OD663 × V/1000 × w.
Where:
OD645 = Absorbance at a wavelength of 645 nm.
OD663 = Absorbance at a wavelength of 663 nm.
V = Final volume; W = weight of leaf tissue.
2.4 Determination of Zn content in the wheat seedling by ICP-MS analysis
An Inductively Coupled Plasma–Mass Spectrometry (ICP MS) analysis was performed to determine the concentration of Zn in ZnONPs treated seedlings. The samples were digested by a microwave digestion system (Milestone ETHOS lab station). A closed-vessel microwave system (Ethos-1600, Milestone, Sorisole, Italy) equipped with a fiber-optic temperature sensor was employed for performing digestions and extractions. A mass of 50 mg of each sample material was transferred to the 50 mL glass vessel, and volumes of 7 mL of diluted nitric acid solutions (65%) and 1 mL of H2O2 (30% v/v) were added. Two-stage heating was done at 200 °C for 10 min each (Alsabhan et al., 2022).
The digested samples were analyzed with ICP-MS analysis at the central laboratory, College of Science, King Saud University, KSA. The external calibration was done by using a multi-element standard of 10 ppm concentration.
2.5 Observation of bioaccumulation of ZnONPs in the wheat root tissue by transmission electron microscopy (TEM)
The roots were also subjected to transmission electron microscopy (TEM) to detect the accumulation of ZnONPs in the root cells (Helliot et al., 2003). Fresh roots sectioned into small pieces. After fixation with 3.5% glutaraldehyde for 24 h, root cells were post-fixed with 1% osmic acid at 4 °C for 4 h and then were dehydrated with ethanol. Next, the samples were implanted in freshly prepared 100% Epon-812 and polymerized at 80 °C for 24 h. For ultrastructural observations, the samples obtained were sectioned into ultrathin sections (60 nm) on an ultramicrotome. The ultrathin sections were transferred to 250-mesh grids and were post-stained with uranyl acetate and lead citrate (Mazumdar and Ahmed, 2011). Finally, the TEM images were obtained with a TEM (JEM-1400, Japan).
2.6 Statistical analysis
ANOVA and Tukey (HSD) post hoc analyses were done through XLSTAT (version. 2021.3.1.1162).
3 Results
The current study determined the effect of ZnONPs on seed germination, seedling growth, chlorophyll content, zinc concentration in the seedlings and bioaccumulation of ZnONPs in the root cells. To conduct the study, ZnONPs were purchased from a manufacturer with a size range of >30 nm to <50 nm. The suspension of nanoparticles in de-ionized water was used to see the morphology and dimensions of ZnONPs through TEM. The image clearly reveals that the nanoparticles were spherical and larger than 30 nm, confirming the manufacturer's assertion regarding their size (Fig. 1).
TEM micrograph of ZnONPs dispersed in De-ionized water.
To determine the effect of ZnONPs on seed germination, wheat seeds were treated with different concentrations of ZnONPs and, after a week, percent seed germination was recorded (Fig. 2). The seed germination was 100% for the seeds that received 2500 ppm of ZnONPs, while there was a non-significant (P ≤ 0.05) decline in the seed germination at 5000 ppm, 7500 ppm, and 10,000 ppm of ZnONPs. Whereas a significant decrement in seed germination (13.6%) was recorded at a ZnONPs with a 15,000 ppm concentration.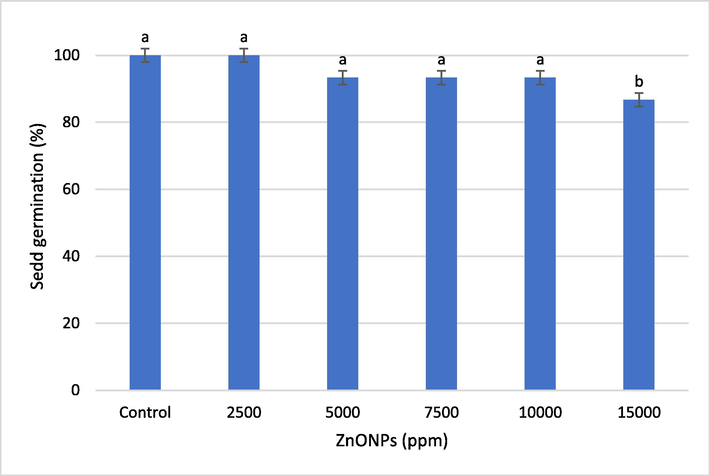
Effect of different concentrations of ZnONPs on wheat seed germination. Bars represent mean ± SE of 5 replicates per treatment. The data marked by the different letters are significantly different (P ≤ 0.05) according to Tukey’s HSD tests.
Wheat seedlings growing on Hoagland and Knop agar supplemented with different concentrations of ZnONPs were harvested after 2 weeks of seed germination. Figs. 3 and 4 show data for the growth parameters of seedlings treated with different concentrations of ZnONPs for three weeks. In general, the higher concentration of ZnONPs has a negative effect on wheat seedlings. However, at 2500 ppm of ZnONPs, a significant (P ≤ 0.05) improvement in the seedling height (6.7%), and fresh (9.2%) and dry weight (17.0%) was noticed when compared to untreated control seedlings. A linear reduction in shoot height, plant fresh and dry weight was observed at and above 5000 ppm ZnONPs. An inversely proportional relationship was found between ZnONPs concentrations and wheat root length. The maximum percent reduction in shoot height (23.7%), root length (66.8%), seedling fresh weight (33.8%), and dry weight (54.8%) was recorded at the highest concentration of ZnONPs (15,000 ppm) (Figs. 3 and 4).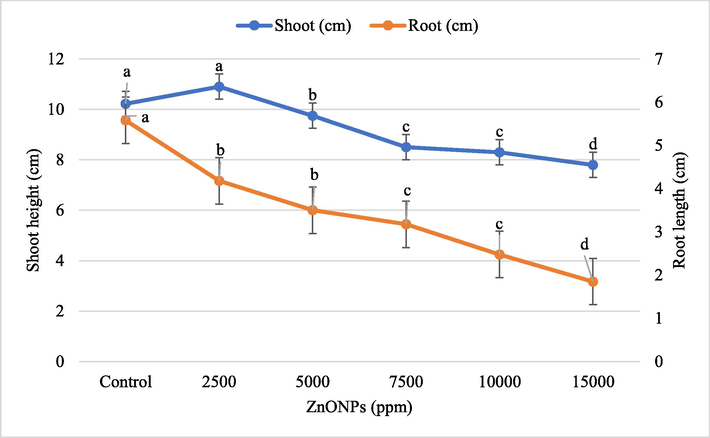
Effect of different concentrations of ZnONPs on wheat seedling height and root length. Bars represent mean ± SE of 6 replicates per treatment. The data marked by the different letters are significantly different (P ≤ 0.05) according to Tukey’s HSD tests.
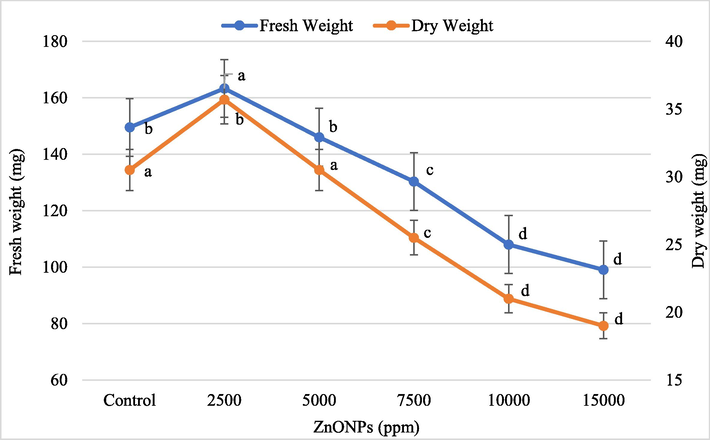
Effect of different concentrations of ZnONPs on wheat seedling fresh and dry weight. Bars represent mean ± SE of 6 replicates per treatment. The data marked by the different letters are significantly different (P ≤ 0.05) according to Tukey’s HSD tests.
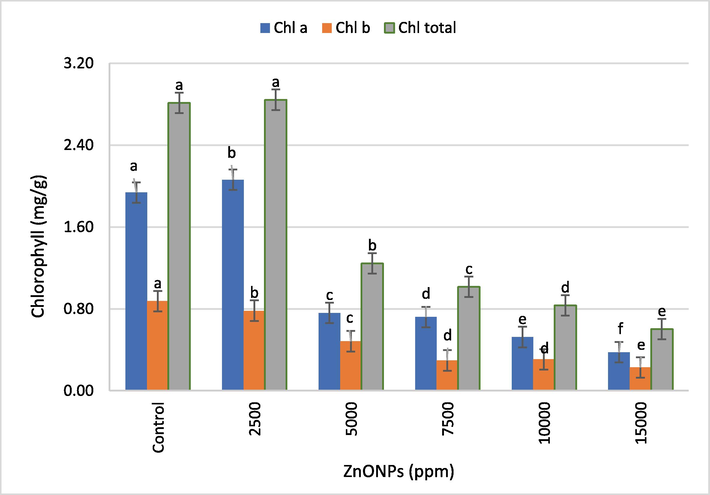
Effect of different concentrations of ZnONPs on wheat seedling chlorophyll a, chlorophyll b and chlorophyll total content. Bars represent mean ± SE of 6 replicates per treatment. The data marked by the different letters are significantly different (P ≤ 0.05) according to Tukey’s HSD tests.
Chlorophyll content (chlorophyll a, chlorophyll b, and chlorophyll total) in three-week old wheat seedlings was also measured. The data showed that the chlorophyll a (6.3%) and chlorophyll total (1.1%) in the wheat seedling were higher at a concentration of 2500 ppm of ZnONPs than the control. However, at 5000 ppm or higher concentrations of ZnONPs, a significant (P ≤ 0.05) decrease in the chlorophyll a, chlorophyll b, and chlorophyll total was recorded. At 15,000 ppm of ZnONPs, the maximum reduction in chlorophyll a, chlorophyll b, and total chlorophyll (80.6%, 74.2%, and 78.5%, respectively) was noted (Fig. 5).
An inductively coupled plasma with mass spectrometry (ICP-MS) analysis was carried out on wheat seedlings to measure the amount of Zn content in ZnONPs exposed samples. The Zn concentration in samples treated with 7500, and 15,000 ppm of ZnONPs was found to be higher than in control seedlings. A linear relationship was noticed between the Zn concentration in seedlings and the treatment dose of ZnONPs. There was a 17.7 and 34.7-fold increase in the Zn amount in the seedlings treated with 7500 and 15,000 ppm of ZnONPs, respectively (Fig. 6).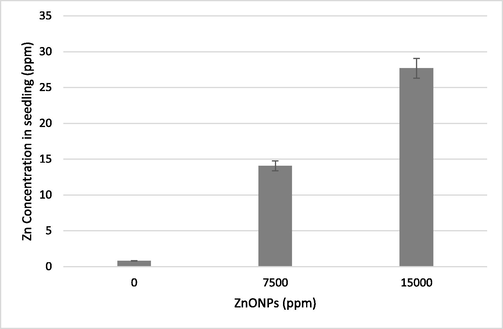
Zn concentration analyzed by ICP-MS in the wheat seedlings exposed to 7500 ppm and 15,000 ppm of ZnONPs.
Wheat seedlings were examined under a transmission electron microscope to detect the bioaccumulation of ZnONPs in the root cells. The aggregation of ZnONPs in the root cells was clearly evident in the micrographs of the root cells exposed to 15,000 ppm ZnONPs. The nanoparticles were detected in the intercellular space, the vacuole, and the cytoplasm with degenerated mitochondria (Fig. 7c and d). In contrast, control root cells were observed to be free of any such attribute (Fig. 7a and b). Moreover, well integrated mitochondria were located in the root cells of control seedlings.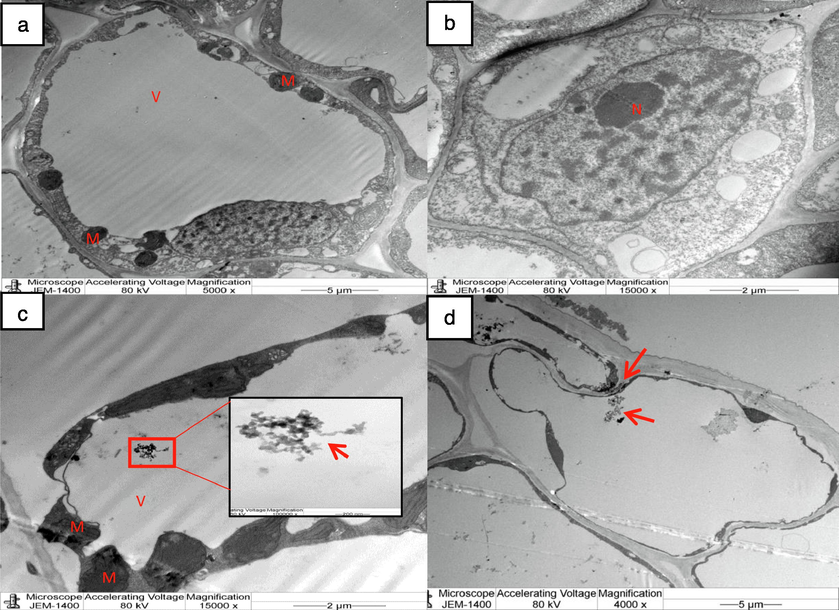
TEM micrograph of root cells of wheat seedlings (a, b) control showing root cells with mitochondria, nucleus, & (c, d) exposed to ZnONPs (15,000 ppm). accumulation of ZnONPs in the intercellular space, vacuole and cytoplasm of the root cells are visible (arrow head). 7 (c) Disintegrated mitochondria can be noticed in the cell subjected to ZnONPs (15,000 ppm). Mitochondrion (M), Vacuole (V), Nucleus (N).
4 Discussion
Plants continuously interact with the physical environment, and they are the major victims of pollution. Since the use of nanoparticles has expanded in the past decades, it’s critical to understand the implications of these nanoparticles on plants since, through plants, these nanoparticles may enter the food chain and may have a deleterious effect on other living organisms. The aggregation of nanoparticles is also a source of worry for soil health (Javed et al., 2019).
In the present study, wheat seeds were treated with various concentrations of ZnONPs and observed for a week, and the percent seed germination was recorded. In addition, seeds were allowed to grow for three weeks to determine the effect of ZnONPs on the growth, chlorophyll, and zinc content of the seedlings as well as the bioaccumulation of ZnONPs in the root cells of the seedlings. At the concentrations of 2500 ppm and 5000 ppm of ZnONPs, an improvement in the growth parameters and chlorophyll content was noticed. The incorporation of ZnONPs (1000 ppm) into peanut plants enhanced the seed germination, stem and root growth (Prasad et al., 2012). An increase in the dry weight of Vigna radiata was recorded when treated with 500, 1000, 2000, and 4000 ppm of ZnONPs (Mahajan et al., 2011). An increase in the growth and chlorophyll content of wheat after seed priming with ZnONPs has also been reported (Munir et al., 2018). Recently, it has been reported that seed priming with a lower level of ZnONPs enhanced seed germination and chlorophyll in wheat (Rai-Kalal and Jajoo, 2021).
The current findings clearly indicate that higher levels of ZnONPs have a negative impact on wheat, and it was observed to be dose dependent. The current findings are consistent with prior studies carried on maize, raddish, lettuce, cucumber (Lin and Xing, 2007), ryegrass (Lin and Xing, 2008), peanut (Prasad et al., 2012), and wheat (Yahyaoui et al., 2017). Given that metal ions are expected to be released in metal and metal oxide nanoparticle suspensions, zinc ions dispersed in nanoparticle suspensions may contribute to their phytotoxicity. The chlorophyll content also followed the same pattern as observed in the plant growth parameters. While, at 2500 ppm of ZnONPs, they didn’t have any deleterious effect on the chlorophyll content. However, a considerable decline in chlorophyll content was noted at a higher level of ZnONPs. There are several reports on the enhancement of chlorophyll content in plants when treated with a lower quantity of ZnONPs (Mahajan et al., 2011; Prasad et al., 2012; Tarafdar et al., 2014). The reduction in chlorophyll content due to ZnONPs has also been reported previously. A decrease in photosynthetic pigments of mung and gram was observed when treated with ZnONPs (Mahajan et al., 2011). Similarly, a reduction in chlorophyll content was noticed in green peas due to ZnONPs (Mukherjee et al., 2014). Another study reported inhibition of chlorophyll content in egg plants at 1000 ppm of ZnONPs (Baskar et al., 2018). The hinderance in chlorophyll production may cause a decrease in wheat seedling biomass. The probable reason for the reduction in chlorophyll content could be the competition of zinc with magnesium in the leaves. In a wheat plant, ZnONPs induced the formation of ROS and a reduction in chlorophyll content (Panda et al., 2003).
The zinc level in the seedlings after treatment with ZnONPs was detected by ICP-MS. A linear rising trend in the zinc content was found in the seedlings treated with increasing doses of ZnONPs. Munir et al. (2018) found an increase in Zn ion concentration in wheat when treated with ZnONPs (Munir et al., 2018). A many-fold increase in zinc ion concentration in Schoenoplectus tabernaemontani was recorded when treated with zinc oxide nanoparticles (Zhang et al., 2015).
The root tissue of the wheat seedlings was also observed to further confirm the accumulation of these ZnONPs in the root cells. TEM micrographs showed the presence of clusters of ZnONPs in the roots of cells treated with 15,000 ppm of ZnONPs. The appearance of ZnONPs in both the cell wall and the cytoplasm might suggest the migration of ZnONPs from the cell wall into the cytoplasm. The uptake of ZnONPs by various plants was documented earlier. Previously, researchers have reported bioaccumulation of ZnONPs in the roots of maize (Zhao et al., 2012), in S. tabernaemontani (Zhang et al., 2015), and ryegrass (Lin and Xing, 2008). The plant requires 0.05 ppm of zinc for normal functioning, and excessive Zn may induce phytotoxicity. Instigation of oxidative stress and lipid peroxidation by ZnONPs might be linked to phytotoxicity (Han et al., 2011). Cytotoxicity in soybean seedlings at 4000 ppm of ZnONPs has been reported (López-Moreno et al., 2010). Uptake and transport of nanoparticles into plants is a vital aspect that determines the influence of nanoparticles on plants. The uptake and translocation of nanoparticles in plants primarily depends on the physio-chemical properties of nanoparticles (Singh et al., 2018). The entry and movement of bigger nanoparticles through the cell is strenuous (Nair, 2016). Moreover, lower translocation of ZnONPs from roots to leaves may have caused the higher accumulation of ZnONPs in the root tissue (Wang et al., 2013). Besides that, as reported, the ZnONPs at higher concentration induces the formation of ROS that may result in cellular damage (Xia et al., 2006). The ZnO NP concentrations had an influence on the wheat seedlings' morphology and physiology. According to certain investigations, these nanoparticles can also affect antioxidants, DNA, protein synthesis, and molecular functioning as well (Goswami et al., 2019; Rajput et al., 2020; Wang et al., 2018). However, the current investigation was placed in a controlled condition, taking one variable into consideration. In natural conditions, however, various biotic and abiotic variables interact, causing the nanoparticles to behave differently and produce distinct results. With that, the capability of different plant species to absorb and translocate these nanoparticles may influence their effect on them. Furthermore, the type, physio-chemical properties, and stability of the nanoparticles are some of the pivotal variables to consider (Handy et al., 2008).
5 Conclusions
As it is evident from the current findings, ZnONPs affected the morphology and physiology of the seedlings. At lower concentrations of ZnONPs, they have a positive effect on the wheat seedlings, but they have a negative impact on them at higher concentrations of ZnONPs. The accumulation of these nanoparticles in the root cells and a many-fold increase in Zn2+ in seedlings shows the phytotoxic effect of ZnONPs. However, additional research is needed to identify the ZnONPs level that can be regarded phytotoxic and the environmental hazards of NPs, taking into account the biota that interact with nanoparticles.
Acknowledgement
The authors would like to acknowledge the support provided by Researchers Supporting Project Number (RSP-2021/229), King Saud University, Riyadh, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Two detoxification mechanisms by external malate detoxification and anti-peroxidation enzymes cooperatively confer aluminum tolerance in the roots of wheat (Triticum aestivum L.) Environ. Exp. Bot.. 2015;120
- [CrossRef] [Google Scholar]
- The toxicity potential of ag nanoparticles synthesized from Cordia myxa L. Adv. Hortic. Sci.. 2020;34
- [CrossRef] [Google Scholar]
- Simulated dryland cotton yield response to selected scenario factors associated with soil health. Front. Sustain. Food Syst.. 2021;4
- [CrossRef] [Google Scholar]
- A.H. Alsabhan K. Perveen A.S. Alwadi Heavy metal content and microbial population in the soil of Riyadh Region, Saudi Arabia J. King Saud Univ. - Sci. 34 1 2022 10.1016/j.jksus.2021.101671 101671 101671.
- Assessment of the effects of metal oxide nanoparticles on the growth, physiology and metabolic responses in in vitro grown eggplant (Solanum melongena) 3 Biotech. 2018;8
- [CrossRef] [Google Scholar]
- The toxic effect of CuO of different dispersion degrees on the structure and ultrastructure of spring barley cells (Hordeum sativum distichum) Environ. Geochem. Health. 2021;43
- [CrossRef] [Google Scholar]
- Goswami, P., Yadav, S., Mathur, J., 2019. Positive and negative effects of nanoparticles on plants and their applications in agriculture. Plant Sci. Today. https://doi.org/10.14719/pst.2019.6.2.502.
- Nano-zinc oxide damages spatial cognition capability via over-enhanced long-term potentiation in hippocampus of Wistar rats. Int. J. Nanomed.. 2011;6
- [Google Scholar]
- The ecotoxicology of nanoparticles and nanomaterials: current status, knowledge gaps, challenges, and future needs. Ecotoxicology 2008
- [CrossRef] [Google Scholar]
- Particle-size dependent accumulation and trophic transfer of cerium oxide through a terrestrial food chain. Environ. Sci. Technol.. 2014;48
- [CrossRef] [Google Scholar]
- Ultrastructural changes associated with cryopreservation of banana (Musa spp.) highly proliferating meristems. Plant Cell Rep.. 2003;21:690-698.
- [CrossRef] [Google Scholar]
- Zinc deficiency inhibits chlorophyll synthesis and gas exchange in ‘stuart’ pecan. HortScience. 2019;26
- [CrossRef] [Google Scholar]
- Effect of accumulation of nanoparticles in soil health- a concern on future. Front. Nanosci. Nanotechnol.. 2019;5:1-9.
- [CrossRef] [Google Scholar]
- Predicted releases of engineered nanomaterials: from global to regional to local. Environ. Sci. Technol. Lett.. 2013;1
- [CrossRef] [Google Scholar]
- Environmental transformations of silver nanoparticles: Impact on stability and toxicity. Environ. Sci. Technol. 2012
- [CrossRef] [Google Scholar]
- Root uptake and phytotoxicity of ZnO nanoparticles. Environ. Sci. Technol.. 2008;42
- [CrossRef] [Google Scholar]
- Phytotoxicity of nanoparticles: Inhibition of seed germination and root growth. Environ. Pollut.. 2007;150:243-250.
- [CrossRef] [Google Scholar]
- Evidence of the differential biotransformation and genotoxicity of ZnO and CeO2 nanoparticles on soybean (Glycine max) plants. Environ. Sci. Technol.. 2010;44
- [CrossRef] [Google Scholar]
- Effect of nano-ZnO particle suspension on growth of mung (Vigna radiata) and gram (Cicer arietinum) seedlings using plant agar method. J. Nanotechnol. 2011
- [CrossRef] [Google Scholar]
- Mazumdar, H., Ahmed, G.U., 2011. Phytotoxicity effect of silver nanoparticles on oryza sativa. Int. J. ChemTech Res. 3.
- Updated characterization of races of plasmopara halstedii and entomopathogenic fungi as endophytes of sunflower plants in axenic culture. Agronomy. 2021;11:268.
- [Google Scholar]
- Physiological effects of nanoparticulate ZnO in green peas (Pisum sativum L.) cultivated in soil. Metallomics. 2014;6
- [CrossRef] [Google Scholar]
- Effect of zinc oxide nanoparticles on the growth and Zn uptake in wheat (Triticumaestivum L.) by seed priming method. Dig. J. Nanomater. Biostructures. 2018;13
- [Google Scholar]
- Nair, R., 2016. Effects of nanoparticles on plant growth and development, in: Plant Nanotechnology: Principles and Practices. https://doi.org/10.1007/978-3-319-42154-4_5.
- Heavy metals induce lipid peroxidation and affect antioxidants in wheat leaves. Biol. Plant.. 2003;46
- [CrossRef] [Google Scholar]
- Effect of Sclerotinia sclerotiorum on the disease development, growth, oil yield and biochemical changes in plants of Mentha arvensis. Saudi J. Biol. Sci.. 2010;17:291-294.
- [CrossRef] [Google Scholar]
- Effect of nanoscale zinc oxide particles on the germination, growth and yield of peanut. J. Plant Nutr.. 2012;35
- [CrossRef] [Google Scholar]
- Priming with zinc oxide nanoparticles improve germination and photosynthetic performance in wheat. Plant Physiol. Biochem.. 2021;160
- [CrossRef] [Google Scholar]
- ZnO and CuO nanoparticles: a threat to soil organisms, plants, and human health. Environ. Geochem. Health. 2020;42
- [CrossRef] [Google Scholar]
- Zinc oxide nanoparticles: a review of their biological synthesis, antimicrobial activity, uptake, translocation and biotransformation in plants. J. Mater. Sci.. 2018;53
- [CrossRef] [Google Scholar]
- Nanoparticles in sustainable agriculture: an emerging opportunity. J. Control. Release.. 2021;329
- [CrossRef] [Google Scholar]
- Development of zinc nanofertilizer to enhance crop production in pearl millet (Pennisetum americanum) Agric. Res.. 2014;3
- [CrossRef] [Google Scholar]
- The Biomechanisms of metal and metal-oxide nanoparticles’ interactions with cells. Int. J. Environ. Res. Public Health. 2015
- [CrossRef] [Google Scholar]
- Verma, A., Roy, A., Bharadvaja, N., 2021. Remediation of heavy metals using nanophytoremediation, in: Advanced Oxidation Processes for Effluent Treatment Plants. https://doi.org/10.1016/b978-0-12-821011-6.00013-x.
- Nanotechnology: a new opportunity in plant sciences. Trends Plant Sci 2016
- [CrossRef] [Google Scholar]
- Fate of ZnO nanoparticles in soils and cowpea (Vigna unguiculata) Environ. Sci. Technol.. 2013;47
- [CrossRef] [Google Scholar]
- Effects of zinc oxide nanoparticles on the growth, photosynthetic traits, and antioxidative enzymes in tomato plants. Biol. Plant.. 2018;62
- [CrossRef] [Google Scholar]
- Comparison of the abilities of ambient and manufactured nanoparticles to induce cellular toxicity according to an oxidative stress paradigm. Nano Lett 2006
- [CrossRef] [Google Scholar]
- Fabricated nanoparticles: current status and potential phytotoxic threats. Rev. Environ. Contam. Toxicol.. 2014;230
- [CrossRef] [Google Scholar]
- Assessment of exposure wheat triticum aestivum L. To zinc oxide nanoparticles (ZNO): evaluation of oxidative damage. Stud. Univ. Vasile Goldis Arad, Ser. Stiint. Vietii. 2017;27
- [Google Scholar]
- Phytotoxicity and bioaccumulation of ZnO nanoparticles in Schoenoplectus tabernaemontani. Chemosphere. 2015;120
- [CrossRef] [Google Scholar]
- Transport of Zn in a sandy loam soil treated with ZnO NPs and uptake by corn plants: Electron microprobe and confocal microscopy studies. Chem. Eng. J.. 2012;184
- [CrossRef] [Google Scholar]






