Translate this page into:
Lipid composition and oxidative changes in diabetes and alcoholic diabetes rats
⁎Corresponding author. fanyingxa@sina.com (Yingying Fan)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Background
The investigating of study was expected to the lipid composition of diabetes and alcoholic diabetes in plasma and erythrocyte membrane biochemical profile in rats. Diabetic male Wistar Streptozotocin (STZ)-induced rats were used experimental models. Control rats (C) were maintained group I, received glucose (i.e., caloric equivalent), diabetic induced rats (STZ) group II, and group III alcoholic treated and IV diabetic and alcoholic treated rats which received 20 % (v/v) alcohol in water, administered through stomach tube (5 g/kg body weight/day).
Results
STZ-induced diabetic hepatic damage in rats was observed which leads to the increased plasma lipid peroxidation, nitrate and nitrite levels. Diabetic and administration alcohol rats also suggestively lesser the activities of antioxidants, glutathione peroxidase, glutathione S-transferase, superoxide dismutase, catalase and reduced glutathione when related with control rats (group I). Plasma enzymes are normal levels and renovate the enzymic and non-enzymatic antioxidants level in experimental groups.
Conclusion
The present data point out that the nitric oxide scavenging levels increased and may possibly protect and adjacent to oxidative stress and free radicals in diabetic hepatopathy rats and histopathological studies were identified in regular hepatic cortex of Group II, III and IV of animals with diabetes, alcoholic and alcohol-induced diabetes rats.
Keywords
Diabetic
Alcoholic
Nitric oxide
Lipid peroxidation
1 Introduction
The most broadly used and regularly abused psychoactive drug is alcohol in the world with stern communal and well-being insinuations (Xu et al., 2005). As per the worldwide position based on the 2004 report of alcohol (WHO) exposed that extra two billion people have been intense alcohol worldwide with increase in the amount with the adding of new drinkers each year counting adolescent youngsters and young woman (World Health Organization, 2004; Lieber, 2000). DM (diabetes mellitus) is a chronic metabolic illness categorized by imbalances in carbohydrate, protein and lipid metabolisms, due to faulty or shortage in insulin action and secretion (Lu and Cederbaum, 2008). The near relation amid ethanol and liver damage is mostly owing to the fact that 80 % of swallowed liquor is absorbed in the liver. Throughout alcohol/ethanol absorption numerous sensitive oxygen species are produced via cytochrome P450 (Tuma and Casey, 2003). The aim of work was to expand an investigational model of Streptozotocin (STZ) treated to diabetic male Wistar rats were used as investigational rats, control and Diabetes and alcoholic treated and diabetic + alcoholic treated (body wt/day g/kg 5) for sixty days. Diabetes alcoholic treated and diabetic + alcoholic treated hepatocyte/retinopathy damage. Nitric oxide (NO) is a significant intermediary of a lot of functions of physiological, and its position in the pathogenesis of several diseases is attainment in the identification. Diabetic, alcoholic treated and diabetic + alcoholic treated utilization increased levels of NO and may show the mode to toxicity by nitrites of peroxides, a strong oxidant. Therefore, the work investigates and evaluate the changes in various plasma biochemical parameters SGOT, SGPT and lipid peroxidation. NO is a significant go-between of a lot of physiological function, and its role in the pathogenesis of a lot of disease is attainment credit (Pacher et al., 2007). Diabetic, alcoholic treated and diabetic + alcoholic treated consumption increases NO level and may lead to toxicity by peroxynitrite, a potent oxidant (Venkatraman et al., 2004). Thoughtless nitrogen species/reactive oxygen species (RNS/ROS) let go of overproduction might take place when its production in an arrangement exceed the system’s capability to counterbalance and abolish them.
Lipid peroxidation, antioxidant levels of rats to appreciate the position and importance of nitric oxide in diabetes, alcoholic and diabetes + alcoholic treated rats with plasma and erythrocyte membrane chemical and physical alterations and may it lead hepatic damage. In wide-ranging and toxicity of these drugs, with its numerous troubles at a time, have an effect on altered organs causes some disorders. The numerous pathogenicity of diabetic, alcoholic treated and diabetic + alcoholic treated burden many modes of healing come near to fight and adjacent to such trouble by modulating activities of enzymes, metabolism, receptor performance, transduction of signal mechanism and scavenge free radicals at a mixture of levels (Trinder, 1969). There has been a look for safer customary nutritional supplementation of inhabitant plant extracts containing some principles for healing reason with multiple targets for treating multiple pathologies of diabetic plus alcoholic rats.
2 Methods
2.1 Subjects for study and experimental design
60 days male Wistar rats, about 120–140 g weighing, are maintained in animal house. They were feed among pellet diet and tap water ad libitum. The rats were separated into four groups of 8 rats in each. Group I normal rats (C), which inward sugar in its place of diabetic/alcohol (caloric equal), group II diabetic Streptozotocin (STZ) induced rats, III group (5 g/kg body weight/day) alcohol treated rats, and group IV diabetic plus alcohol administered from side-to-side abdomen tube procured from animal house in creature cages in an AC room (25 ± 1 ℃) with daylight from 7:00 a.m to 7:00p.m. Hence the present work determined on the result of diabetes and alcohol induces oxidative injure/alteration in plasma with pressure on its machinery. The study was established by institutional ethical committee. The study for the night fasted blood sample use from subjects. The dose of the sample is set based on our previous study (Allian et al., 1974; Reddy et al., 2009). Administered way out two months by using intragastric pipe every day. Foodstuff and water eating of every rat was record every day and maintained mass on every other day. At the end of the trial time, the rats in each set were fasted during the night and then kill by cervical dislocation.
2.2 Blood collection and determinations of plasma nitric oxide scavenge action
By Trinder process (Sreejayan and Rao, 1997) glucose was estimated spectrophotometrically by kits. NO estimation by Greiss reaction (Ohkawa et al., 1979) and generated from sodium nitroprusside was measured. NO Scavengers, compete with oxygen important to summary assembly of NO. Phosphate buffered saline in sodium nitroprusside (5 mM) were mixed with various concentration and incubate at 150 min in 25 °C. Greiss reagent (5 % o-phosphoric acid, 1 % sulfanilamide and 0.01 % naphthylethylene diamine) and the samples from the above were reacted. The absorbance of the chromophore produced throughout diazotization of nitrite with sulfanilamide and subsequent mixture with napthylethylene diamine was examined in UV–visible spectrophotometer at 546 nm.
2.3 In vivo assays
Tissues dissect out from the liver, wash and weighed with way out of saline with ice cold. Muscles were crushed and homogenized (10 % w/v) in Tris–HCl buffer (0.1 M; pH 7.4) and centrifugation at 10,000 g for 4 °C in 20 min. The resultant upper part was use for different assays.
2.4 Estimation of thiobarbituric acid (TBA) and protein carbonyls
TBARS was calculated by the configuration of malondialdehyde (MDA) by the process of Ohkawa et al. (Reznick and Packer, 1994). The absorption of protein carbonyls was calculated using 2, 4-dinitrophenylhydrazine (DNPH) evaluate as describe formerly (Abei, 1988).
2.5 Antioxidant importance, nitrite and nitrate analysis were calculated
Abei (Mishra and Fridovich, 1972) catalase (CAT) was evaluated as deliberate. The expression of CAT action was as nmol. SOD was assay utilize the process of Mishra and Fridovich (Rotruck et al., 1973). Enzyme was articulated as 50 % reserve of NBT reduction/min/mg protein. The processes describe by Rotruk (Habig et al., 1974) and action measured GPx (glutathione peroxidase). GPx action was articulated lmol. GST action was calculated according to the method of Habig et al. (Ellman, 1959). GST action articulated as lmol. Whole GSH content was intended by Ellman’s (Sastry et al., 2002). By Sastry et al. (Lowry et al., 1951) nitrite and nitrates were determined. Absorption of protein was determined by Lowry et al. (Pigeolot et al., 1990) method. Plasma transaminases, glutamate oxalo acetate transaminase (SGOT) and glutamate pyruvate transaminase (SGPT) were measured by Reitman and Frankel (1957) methods. The histological sections of the hepatocytes of rats were taken by adopting the procedure as described by Humason (1972).
3 Results and discussion
Information presented in plasma enzymes (SGPT) glutamate pyruvate trasminase levels were not changed and (SGOT) glutamate oxalo acetate trasminase propose that an important raise in Table 1, the levels were significantly decreased in diabetes treated and alcoholic and diabetes + alcoholic, administrated rats. Na+-K+-ATPase actions in erythrocytes were sightly increased the group II and group III, IV experimental rats with group I control rat’s summarization and glycolated enzymes in erythrocyte and no alter of hexokinase in groups of (group II and group III, IV). Table 3 revealed the differences in plasma parameters viz., plasma glucose, plasma LPO, nitrate and nitrite, in normal male and experimental subjects. Additionally, the effect of diabetic rats and alcoholic rats and diabetic + alcoholic on the on top of parameter supervision at certain quantity in male rats. Reported antioxidant enzymes decreased significantly namely GST, GPx, CAT, SOD and GSH the contented of in liver were specific in Table 2. A significant (P < 0.05) in the performance was observed decreased in treated diabetes, alcoholic and diabetic + alcoholic rats compare to control of antioxidant enzymes. All Table values are expressed as Mean ± SEM, in each column followed by the same letter are not significantly different (P ≤ 0.05) from each other according to Duncan’s Multiple Range (DMR) test, n = 8. All Table values are expressed as Mean ± SEM, in each column followed by the same letter are not significantly different (P ≤ 0.05) from each other according to Duncan’s Multiple Range (DMR) test, n = 8. All Table values are expressed as Mean ± SEM, in each column followed by the same letter are not significantly different (P ≤ 0.05) from each other according to Duncan’s Multiple Range (DMR) test, n = 8.
Parameter
Groups
Controls Rats
Diabetes treated rats
Alcohol administration rats
Diabetes with alcohol administration rats
Glutamate oxalo acetate transaminase (GOT) (IU/L)
51.6 ± 2.60a
54.2 ± 2.20b
57.6 ± 2.38b
56.5 ± 2.40b
Glutamate Pyruvate Trasminase) (GPT) (IU/L)
24.6 ± 1.64a
22.2 ± 1.40a
24.5 ± 1.32a
24.2 ± 1.21b
Hexokinase (IU/gm Hb)
0.93 ± 0.01a
0.95 ± 0.24a
0.94 ± 0.36a
0.95 ± 0.35a
Na + -K + ATPase (µg pi liberated/min/mg/protein)
1.31 ± 0.03a
1.42 ± 0.05b
1.40 ± 0.04b
1.41 ± 0.04b
Parameter
Groups
Controls Rats
Diabetes treated rats
Alcohol administration rats
Diabetes with alcohol administration rats
Superoxide Dismutase (SOD) (Units/min/mg Hb)
5.7 ± 0.62a
5.4 ± 0.76b
5.3 ± 0.54b
5.5 ± 0.55b
Catalase (CAT)
(IU/104/gm Hb)8.2 ± 0.42a
7.6 ± 0.81a
7.7 ± 0.62b
7.6 ± 0.63b
Red Cell Reduced glutathione (GSH) (µ moles/gm Hb)
3.4 ± 0.16a
3.3 ± 0.09b
3.4 ± 0.07b
3.4 ± 0.08b
Glutathione S-transferase (^mol/mg/min)
2.89 ± 0.49
2.87 ± 0.44
2.69 ± 0.47
2.59 ± 0.42
Glutathione peroxidase(G-Px)
(IU/gm Hb)16.5 ± 1.04a
15.2 ± 1.21b
14.3 ± 1.20b
14.7 ± 1.22b
Parameter
Groups
Controls Rats
Diabetes treated rats
Alcohol administration rats
Diabetes with alcohol administration rats
Plasma glucose (mg/dl)
81.12 ± 0.17a
102.16 ± 0.30b
85.12 ± 0.32b
104.12 ± 0.35b
Plasma lipid peroxidation (p mole of MDA formed/mg protein)
1.92 ± 0.01a
2.11 ± 0.20b
2.15 ± 0.28b
2.40 ± 0.26b
Plasma NO2 (µ mole /L)
2.53 ± 0.01a
3.82 ± 0.16b
4.50 ± 0.42b
4.17 ± 0.40b
Plasma NO3 (µmole /L)
24.66 ± 0.01a
25.74 ± 0.01b
32.52 ± 0.01b
39.62 ± 0.01b
Enzymes of free radical scavenge like GPx, SOD, GST and CAT, are the major line of defence against the injury of oxidatives were the catalase and superoxide ions (SOD) scavenges transfer H2O2 to water. In this study it is observed that there is a less impact of GPx, SOD and CAT, at the same time GSH in diabetes, diabetic treated rats, alcoholic treated rats and diabetes + alcoholic tested rats while compare with control rats. Owing to the oxidative inactivation of the enzyme there is a reduction in activity of SOD therefore as result there is an excessive oxygen species generation (Wilce and Parker, 1994). The isozymes that catalyze the conjugation of GSH to a diversity of electrophilic compound where GSTs are a multigene family of isozymes, whereby it as decisive role in protecting cellular touching ROS (Hayes and Pulford, 1995; Alin et al., 1985). Since the GST activity is reduced there is a compromise detoxification of a toxic aldehyde, 4-hydroxynonenal and a creation of LPO. Hence it leads to decrease in enzyme action or appearance may supply to drugs hepatotoxicity specifically targeting GST isoenzymes by drugs its metabolic products (Gueeri, 1995). As GSH preservation of usual cell arrangement and function from side-to-side detoxification reaction and its redox (Das, 1997). Thus, GSH acting a major role in coordinate the antioxidant protection procedure as it is the central non-protein thiols. Glutathione is a tripeptide there in approximately all the cells and acting a significant position in metabolism, cellular production and transport beside oxidation by reactive oxygen and free radical’s intermediates in investigational groups II, III and IV with compare to group I control (Das et al., 1997; Saxena et al., 1993). These reactive oxygen class levels of are required by viz., glutathione reductase antioxidant enzymes (Oberley, 1998); catalyzing the reduce of GSSG to GSH and NADPH utilize, make in path of HMP shunt, glutathione peroxidase catalyzing the reduce of organic hydro peroxides and hydrogen peroxide (Jacob, 1995; Dincer et al., 2002) by SOD, GSH catalyzes the reduce of superoxide radical to hydrogen peroxide (Ashour et al., 1999) and CAT the reduction of hydrogen peroxide to water (Deisseroth and Dounce, 1970) and multifunctional protein, glutathione-S-transferase is catalyzing the conjugation between GSH with a lane variety of minor substrates and contribute, an very important place in the detoxification of xenobiotics (Denake and Fanburg, 1989). The endogenous glutathione and glutathione peroxidase association and catalase are significant antioxidants and cytoprotective apparatus in the hepatocytes expose to on top of drugs. GR is worried with the preservation of cellular level of concentrated GSH.
Catalase and GR performance are enlarged as importance of drugs exposure. Diabetes alcoholic and diabetes + alcoholic induced injuries by oxidative and heart failure rat have been reported to associated with risk of cardiovascular diseases. Rats were constantly exposed to 3 months constantly per day by oral gavages into circulation and change in cardiac markers. The present study was aimed to diabetes and alcoholic and diabetes + alcoholic induced lipid composition changes in male albino Wistar rats, demonstrates an important raise in the level in liver activities and asparate transaminase, alanine transaminase in serum with subsequent decrease with cardiac markers, and also show an increase significant in the levels of glucose in blood. Hence, the results of our study demonstrate that it makes lipid composition changes and lipid toxicity. Fig. 1 showed the histopathological examination changes observed in the diabetes, alcohol treated and diabetes with alcohol treated rats compared to control rats in hepatocytes cells.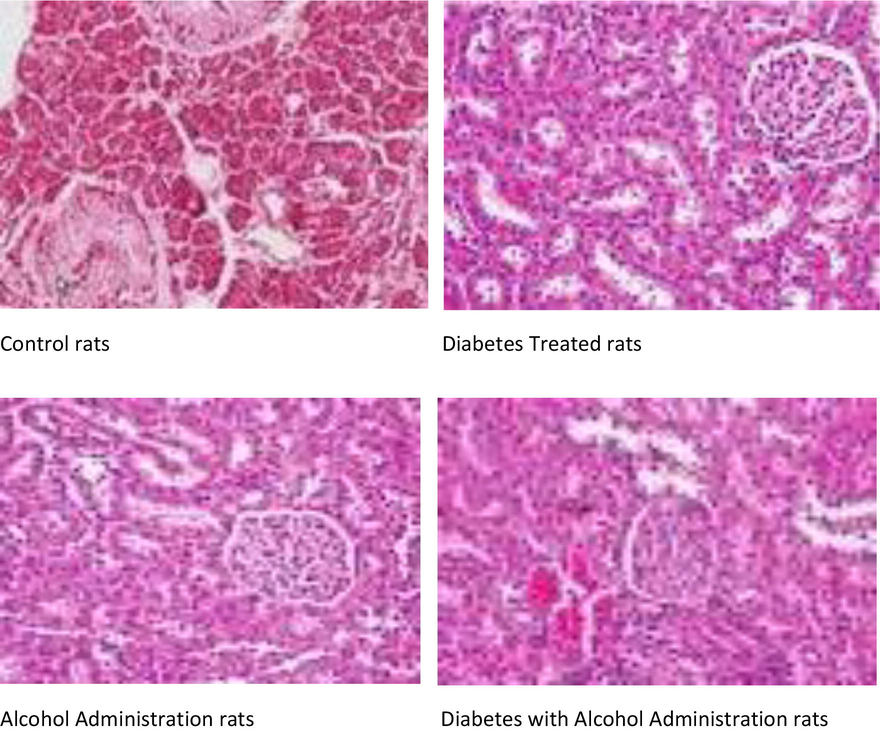
Histopathological changes in Hepatocytes.
4 Conclusion
Increase glucose in blood levels and significant lift in serum SGOT, SGPT values were decrease drastically in diabetes treated and alcoholic and diabetes + alcoholic, administrated rats, NO scavenging compounds presently increased and may protect beside free radical mediate in rat hepatocytes oxidative stress. Reduce act of GPx CAT and SOD as well GSH in diabetes treated and alcoholic and diabetes + alcoholic treated rats compared to control rats. Further, the biochemical activities have been supported by the histopathological studies. Additional study is essential to associate the toxic property of long-lasting make use of diabetes treated and alcoholic and diabetes + alcoholic on rat lipid composition.
5 Contributions of authors’
All authors were concerned in the scientific assessment, analytic for the cases describe in this document. All authors read and accepted the concluding version.
Funding
The authors extend their appreciation to the Researchers Supporting Program for funding this work through Researchers Supporting Project number (RSP-2021/371), King Saud University, Riyadh, Saudi Arabia. This work also financially supported by Yunnan Province Clinical Research Center for Metabolic Diseases (202102AA100056), Science and Technology Innovation Team of Diagnosis and Treatment for Glucolipid Metabolic Diseases in Kunming Medical University (CXTD202106) and The National Natural Science Foundation of China (Grant No 82160164).
Acknowledgements
The authors are highly thankful and acknowledge the funding support by the Researchers Supporting Project number (RSP-2021/371), King Saud University, Riyadh, Saudi Arabia. The authors also thankful for financial support to Yunnan Province Clinical Research Center for Metabolic Diseases (202102AA100056), Science and Technology Innovation Team of Diagnosis and Treatment for Glucolipid Metabolic Diseases in Kunming Medical University (CXTD202106) and The National Natural Science Foundation of China (Grant No 82160164).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 4-Hydroxyalk-2-enals are substrates for glutathione transferase. FEBS Lett.. 1985;179:267-270.
- [Google Scholar]
- Ashour, M., Salem, S., Hassaneen, H., EL. Gadban, H., Elwan, N., Awad A., Basu T.K. 1999. Antioxidant status and insulin dependent diabetes mellitus. Journal of Clinical Biochemistry. 29, 99-107.
- Hydroxy radical is the major causative factor in stress induced gastric ulceration. Free Radic. Biol. Med.. 1997;23:8-18.
- [Google Scholar]
- Glutathione status of some homeothermic vertebtate species and its relation with diabetogenesis. Indian J. Exp. Biol.. 1997;35:66-662.
- [Google Scholar]
- Catalase: physical and chemical properties. Mechanism of catalysis and physiological role. Physiological review.. 1970;50(3):319-375.
- [Google Scholar]
- Regulation of cellular glutathione. American Journal ofPhysiology.. 1989;257(1163–1):173.
- [Google Scholar]
- Susceptibility of glutathione and glutathione related antioxidant activity to hydrogen peroxide in patients with type 2 diabetes: effect of glycemic control. Clin. Biochem.. 2002;35:297-301.
- [Google Scholar]
- Influence on prolonged ethanol intake on the level and turnover of alcohol and aldehyde dehydrogenase and glutathione. Adv Exp Med Biol.. 1995;23:12-14.
- [Google Scholar]
- The first enzymatic step in mercapturic acid formation. J Biol Chem. 1974;249:7130-7139.
- [Google Scholar]
- The glutathione S-transferase supergene family: regulation of GST and the contribution of the isoenzymes to cancer chemoprotection and drug resistance. Crit Rev Biochem Mol Biol.. 1995;30:445-600.
- [Google Scholar]
- Animal tissue techniques (3rd.). San Francisco, CA: Freeman; 1972.
- Alcohol and the liver: metabolism of alcohol and its role in hepatic and entrahepatic diseases. The mount Sinai journal of medicine.. 2000;67(1):84-94.
- [Google Scholar]
- CYP2E1 and oxidative liver injury by alcohol. Free Radic Biol Med. 2008;44:723-738.
- [Google Scholar]
- The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem. 1972;247:3170-3175.
- [Google Scholar]
- Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351-358.
- [Google Scholar]
- Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87:315-424.
- [Google Scholar]
- Glutathione peroxidase, superoxide dismutase and catalase inactivation by peroxide and oxygen derived radicals. Mech Ageing Dev.. 1990;51:283-297.
- [Google Scholar]
- Modulatory role of Emblica officinalis against alcohol induced biochemical and biophysical changes in rat erythrocyte membranes. FoodChem Toxicol. 2009;47:1958-1963.
- [Google Scholar]
- Reitman, S., Frankel, S., 1957. Transaminases. Am. J. Clin. Pathol. 28, 56. In: Methods of Enzymatic Analysis, 2 1x1 edn. Bergmeyer, H.U. (ed.), vol 2, Verlag Chemie Weinheim, Academic Press Inc, New York, pp. 735–739, 760–764.
- Oxidative damage to proteins: spectroscopic method for carbonyl assay. MethodsEnzymol. 1994;233:357-363.
- [Google Scholar]
- Selenium: biochemical role as a component of glutathione peroxidase. Science. 1973;179:588-590.
- [Google Scholar]
- Spectrophotometric determination of serum nitrite and nitrate by copper-cadmium alloy. Anal Biochem. 2002;306:79-82.
- [Google Scholar]
- Impaired antioxidant status in diabetic rat liver. Effect of vanadate. Biochemistry Pharmacology.. 1993;45(3):569-572.
- [Google Scholar]
- Dangerous byproducts of alcohol breakdown-focus on adducts. Alcohol Res Health. 2003;27:285-290.
- [Google Scholar]
- The role of iNOS in alcohol-dependent hepatotoxicity and mitochondrial dysfunction in mice. Hepatology. 2004;40:565-573.
- [Google Scholar]
- Structure and function of glutathione s-transferases. Biochim Biophys Acta.. 1994;1205:1-18.
- [Google Scholar]
- World Health Organization. 2004. Global status report on alcohol: alcohol policy: Geneva: World Health Organization.
- Natural medicines for alcoholism treatment: a review. Drug Alcohol Rev.. 2005;24:525-536.
- [Google Scholar]







