Translate this page into:
Thermal adaptation involves higher expression levels of the lethal(2)-essential-for-life-like (l(2)efl) among the honeybee Apis mellifera L. subspecies
⁎Corresponding author. yalattal@ksu.edu.sa (Yehya Zaki Alattal)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Apis mellifera yemenitica is the native honeybee of the Arabian Peninsula. It demonstrates unique morphological, behavioral, and molecular aspects to survive extreme summer temperatures dominating the region. In this study, relative expression of Apis mellifera lethal(2)-essential-for-life-like (l(2)efl) gene family (ID: 724405; 724488; 234274) were measured in A. m. yemenitica and A. m. carnica under desert (Riyadh) and semiarid (Baha) conditions of Saudi Arabia. Results demonstrated significantly higher day-long expression levels of l(2)efl mRNAs in A. m. yemenitica than in A. m. carnica under the same conditions. Under desert conditions of Riyadh, fold changes in expression levels of l(2)efl mRNAs were ∼100× higher in A. m. yemenitica than the calibrator. In Baha (semiarid conditions), l(2)efl expression levels were very modest compared to those in Riyadh with significant interaction between location and subspecies. In conclusion, expression of l(2)efl (mRNAs) (724405; 724488; 234274) can be considered as a key component of A. m. yemenitica response to extreme desert temperatures characterizing the Riyadh region. On the other hand, the semi-arid conditions (Baha) are a more suitable habitat for A. m. carnica compared to Riyadh.
Keywords
Lethal(2)-essential-for-life-like (l(2)efl)
Apis mellifera yemenitica
Saudi Arabia
Heat stress
qPCR

1 Introduction
The common honeybee Apis mellifera comprises many ecological subspecies that are highly adapted to their native habitat (Ruttner, 1988; Ilyasov et al., 2020). Yet, outside their natural distribution ranges, the survival of exotic A. mellifera subspecies is strongly impacted by extreme environmental conditions compared to the native ones (Ruttner, 1988). Under extreme desert conditions of Saudi Arabia, most colonies of the exotic A. mellifera subspecies (i.e. A. m. carnica and A. m. ligustica and their hybrids) die during the first summer season compared to the native honeybee subspecies A. m. yemenitica (Alqarni, 2006; Alattal and Alghamdi, 2015). In addition to high summer mortalities, A. mellifera winter survival is significantly influenced by previous summer temperatures “researchers refer to as “GoldiKocks” (Calovi et al., 2021; Insolia et al., 2022). In Saudi Arabia, the term over-summering describes the ability of the honeybee colonies to pass the extreme summer temperatures (Alattal and Alghamdi, 2015), a phenomenon that is restricted to regions with extreme summer temperatures. A. m. yemenitica is one of the most thermotolerant A. mellifera subspecies worldwide (Ruttner, 1988; Alqarni, 2006; Alattal and Alghamdi, 2015, Alqarni et al., 2017).
Many morphological, behavioral, and molecular aspects of A. m. yemenitica are key components of thermal adaptation. For example, smaller body size, cuticle anatomy and chemistry, efficient expression of HSPs, reduced metabolism and efficient water management are some aspects of A. m. yemenitica adaptive thermotolerance (Ruttner, 1988; Ali, 2011; Alattal et al., 2014; Alattal and Alghamdi, 2015; Porcelli et al., 2017; Alqarni et al., 2019; Alghamdi and Alattal, 2023). Additionally, as an eusocial insect, A. mellifera colony performs many cooperative functions such as fanning, migratory swarming, clustering, reduced foraging times, and hive cooling to withstand hot summer conditions (Southwick, 1983; Ruttner, 1988; Severson et al., 1990; Zhao and Jones, 2012; Zhao et al., 2021; Xinyu et al., 2023). On the other hand, beekeepers regularly employ management practices such as shading nets and providing colonies with sufficient water sources to alleviate losses during the summer months, which is considered the most critical period during the colony seasonal cycle under the desert climate of Saudi Arabia (Alghamdi and Nuru, 2013).
At the cellular level, heat shock proteins (HSP) are produced to keep protein integrity, a well-documented molecular mechanism of A. mellifera thermotolerance (Severson et al., 1990; Elekonich, 2009; Zhao and Jones, 2012; Perez and Aron, 2020). Molecular thermotolerance of A. m. yemenitica has been briefly discussed, HSP70 was reported as the main heat shock protein induced in A. m. yemenitica after exposure to heat stress with higher response thresholds compared to A. m. carnica and A. m. ligustica (Alqarni et al., 2019; Alghamdi and Alattal, 2023). A recent study reported significantly higher relative expression levels of HSP70, HSC70, HSP90, HSP83, HSP10, and HSP28 (mRNAs) in A. m. yemenitica than in A. m. carnica under the same conditions with an epigenetic layer of HSPs transcriptional regulation (Alattal and Alghamdi, 2015; Alattal and Alghamdi, 2023).
The lethal(2)-essential-for-life-like (l(2)efl) gene family encodes several cellular processes, including protein homeostasis and proteostasis (Kurzik-Dumke and Lohmann, 1995; Taylor, et al., 2014; Bakthisaran et al., 2015; Qin et al., 2019). The lethal(2)-essential-for-life-like (l(2)efl) gene family occurs in the perinuclear region of cytoplasm and is expressed in adult honeybee heads; hearts; mid-guts and in embryonic/larval muscle system (NCBI: https://www.ncbi.nlm.nih.gov). Higher levels of l(2)efl genes (724488, 724405, 724367, 724274, 724449, and 410087(a and b)) transcripts were documented in the mid-guts of A. mellifera adults exposed to 45 °C for 4 h, than in the control (Shih et al., 2021). After exposure to heat stress, A. m. carnica foragers exhibited much higher expression levels of l(2)efl mRNAs than in the control (McMenamin et al., 2020; Runtuwene et al., 2020). Yet, activation or silencing of HSP genes in A. mellifera is regulated by many factors and may require specific enzymes acting on histone remolding (Binda, 2013). Apis mellifera histone-lysine-N-methyltransferase trithorax (trx)) and Apis mellifera polycomb protein Su(z)12 are antagonistic regulators of gene transcription (Binda, 2013; Bakthisaran et al., 2015), the earlier (trx) modulates gene transcription, while the latter (Su(z)12) suppresses transcription, and their abundance is associated with the type of histone remodeling at target genes. Chromatin remolding and histone modifications are very common in A. mellifera and play a key role in many biological functions such as cast differentiation (Dickman et al., 2013; Allis and Jenuwein, 2016) and HSP expression (Alattal and Alghamdi, 2023).
In this study a day-long expression levels of three l(2)efl genes (724405; 724488; 234274), Apis mellifera histone-lysine N-methyltransferase trithorax (trx)) and Apis mellifera polycomb protein Su(z)12 (mRNAs) were measured and compared in A. m. yemenitica (thermotolerant subspecies) and A. m. carnica (thermosusceptible subspecies) under natural heat stress of desert and semiarid conditions of Saudi Arabia.
2 Materials and methods
2.1 Study sites
Apiaries were established in two thermogeographical areas within Saudi Arabia: Riyadh (Educational Apiary, King Saud University 24°73′80, “N 46°62′09″E), with perfect hot and dry desert conditions, and Baha (Baha Beekeepers Association 19°85′17″N, 41°58′59″E), with a semiarid climate (https://en.wikipedia.org/wiki/Climate_of_Saudi_Arabia) (Table 1). Riyadh represents the central region of Saudi Arabia, which is characterized by extremely hot summer temperatures exceeding 46 °C. Baha is part of a mountain range called Sarawat (Sarah) with an altitude of 2270 m above sea level, it is characterized by milder temperatures during summer months, offering a better habitat for A. m. carnica (https://www.latlong.net).
Sampling Position
Altitude (m)
Annual Min. Temp. (°C)
Annual Max. Temp. (°C)
Annual Preci-pitation (mm)
Longitude (E)
Longitude (N)
Riyadh
600
20
44
101
46.71
24.71
Baha
2270
14
26
630
41.63
20.30
2.2 Honeybee colonies preparations
Sixteen colonies of A. m. yemenitica were established from a certified in-house population (Bee Research Unit, King Saud University). Another sixteen queenless package bees were headed by imported purebred certified Carniolan queens (LOKACIJA, Slovenia) to establish sixteen A. m. carnica colonies. After 90 days of colony establishment, standard morphometric analysis was conducted at the University’s Bee Research Unit to confirm subspecies affiliation for each colony following standard protocols including body size, pigmentation, and fore-wing venation (Meixner et al., 2013; Bouga et al., 2011). Reference morphometric data of A. m. yemenitica and A. m. carnica were included in the analysis (Oberursel Bee Research Institute, Frankfurt, Germany). Established colonies belonging to each subspecies were distributed evenly between the two thermogeographical regions (8 colonies/subspecies/region). After colony standardization, every colony consisted of 7–8 frames of adult bees including 3–4 brood frames. Colonies were moved to their assigned locations in March and were treated alike according to APIMONDIA guidelines for performance testing until sampling (Ruttner, 1972).
2.3 Forager honeybee sampling
Established colonies (n = 16 of A. m. carnica and 16 of A. m. yemenitica) were sampled at 3 time periods during June: in the morning after sunrise (about 7:00 a.m.); mid-day (12:00 p.m. "noon"); and before sunset (at 5:00p.m.). Each sample consisted of ten foraging bees collected at the colony entrance using small forceps, then directly dipped in liquid nitrogen and kept frozen at −80 °C. Samples of the same subspecies, region, and sampling period were pooled to form one sample. In total, 12 pooled samples were obtained (2 subspecies× 2 regions × 3 time periods). Each pooled sample contained 80 bees representing 8 colonies. In the end, pooled samples were used to examine gene expression. Ambient temperatures in the study sites were recorded.
2.4 RNA Extraction and cDNA Synthesis
TRIzol™ Plus RNA Purification Kit (Invitrogen, California, USA) was used to extract RNA from each pooled sample (heads with thoraces) following the manufacturer’s instructions. Additional purification step of extracted RNA was conducted using Qiagen RNeasy column (Qiagen, Germantown, TN, USA). SuperScriptTM III First-Strand Synthesis Super Mix (Invitrogen, California, USA) was used to synthesize first-strand cDNA according to the manufacturer’s instructions. The produced cDNA was then used as a template in real-time PCR.
2.5 Primer Design and Real-Time PCR
Nucleotide sequences of the lethal(2)-essential-for life-like (l(2)efl) genes (ID:724405; 724488; 234274); Apis mellifera histone-lysine N- methyltransferase trithorax (trx)(ID: 408716) and Apis mellifera polycomb protein Su(z)12 (ID: 409170) were explored from the Honeybee Genome Consortium (NCBI Database), then the software Geneious® Prime-v.2019.2.3 (https://www.geneious.com, Biomatters Ltd., Newark, NJ, USA) was used for primer design (Table 2). Briefly, qPCRs were prepared using SYBR GREEN (SYBR® GREEN PCR Master Mix; API: Applied Biosystems, Carlsbad, CA, USA) and were run using the Applied Biosystems 7500 Real-Time PCR (Applied Biosystems, Carlsbad, CA, USA). The reaction mix had a final volume of 25 μl (13 μl of the 2X master mix, 2 μl of each primer (2 pmol each), 2 μl sample cDNA, and 7 μl nuclease-free water). The Apis mellifera β-actin was selected as an endogenous control for relative expression analyses. Real-time PCR reactions were conducted in triplicate using the following qPCR parameters: 95 °C for 5 min., 40 cycles of 95 °C for 10 s, 30 s at 57 °C, 72 °C for 10 s, and 95 °C for 20 s.
Gene ID/Gene Name
Location (LG2)/length
Primers
lethal(2)-essential-for-life-like (l(2)efl) (A. mellifera) ID:724405
(LG2):4,831,304…4,832,577
/1274ntF-TGCGACATCGATCAAGCGTCC
R-TTGCGCATCGCACGGTTTCC
lethal(2)-essential-for-life-like (l(2)efl) (A. mellifera) ID:724488
(LG2):4,837,466…4,838,343
/878ntF-ACCTTGGGGTGAACTTCTGCG
R-TCCCCTCGACGACAACACAC
lethal(2)-essential-for-life-like (l(2)efl) (A. mellifera ID:724274
(LG2):4,823,146…4,824,181
/1063ntF-TCACCGAGCCGATTGGAGTTATGT
R-AACTGCCTCTGTCACCACGAAAC
A. mellifera histone-lysine N-Methyl Transferase (trx) ID: 408716
(LG2):4633633…4650053, complement
F-TGCAGCTAGATTCATTAATCATTCAT
R-CATGGAATCTTGATATCCTCGAAAG
A. mellifera polycomb protein Su(z)12, mRNA ID: 409170
(LG10)11912175…11918682
F-ATGCTCTGCCCAAGCAACTATTACG
R-CGGAACCTCCATCTTGTTACATAAA
2.6 Statistical analysis
Analyses of relative expression and calculations of fold changes were done using qPCR Ct values for every gene as per the sampling plan. Related actin cycle thresholds (Ct) were used for the calculation of the relative expression levels (relative expression = 2 ct (actin-gene)) accordingly. Average cycle thresholds (Ct) of A. m. carnica from the semiarid region (Baha) were used (as a calibrator) to calculate fold changes in gene expression of different genes (fold change = 2 ct (reference-(actin-gene)) (Schmittgen and Livak, 2008). Significant differences among study groups were measured based on expression means using the Statistical Analysis System software suite (SAS Institute: https://www.sas.com). Mixed random-effect models for subspecies, location, and sampling times followed by Multiple comparisons based on the Bonferroni post hoc test, p < 0.05. Figures were prepared using GraphPad Prism 9.
3 Results
The results revealed highly significant differences in expression levels of the lethal(2)-essential-for-life-like (l(2)efl) genes (ID: 724405; 724488; 234274) and Apis mellifera polycomb protein Su(z)12 (ID: 409170) in A. m. yemenitica compared to A. m. carnica (Table 3). The expression of the histone-lysine N-MT (trx) (ID: 408716) was also increased in A. m. yemenitica (Table 3). The highest average fold change was reported for l(2)efl (ID:724488) in A. m. yemenitica located in Riyadh with ∼100× more expression folds compared to that of A. m. carnica in Baha (calibrator) (Fig. 1). The results also showed that absolute expression levels of l(2)efl (ID: 724405; 724488; 234274) in A. m. carnica were very modest (Fig. 1) under the study conditions.
Variation Factor
Subspecies
Location
Sampling Time Periods
Subspecies by Location
Gene ID/Gene Name
F
P > F
F
P > F
F
P > F
F
P > F
l(2)efl (ID:724405)
38.86
0.00
83.88
0.000
33.48
0.000
31.18
0.000
l(2)efl (ID:724488)
6.00
0.02
31.16
0.000
5.67
0.010
4.00
0.058
l(2)efl (ID:724474)
7.41
0.01
36.58
0.000
4.71
0.020
4.90
0.038
trx (ID: 408716)
2.11
0.16
3.41
0.079
2.48
0.107
0.00
0.979
Su(z)12 (ID: 409170)
35.43
0.0
127.2
0.000
1.35
0.280
31.83
0.000
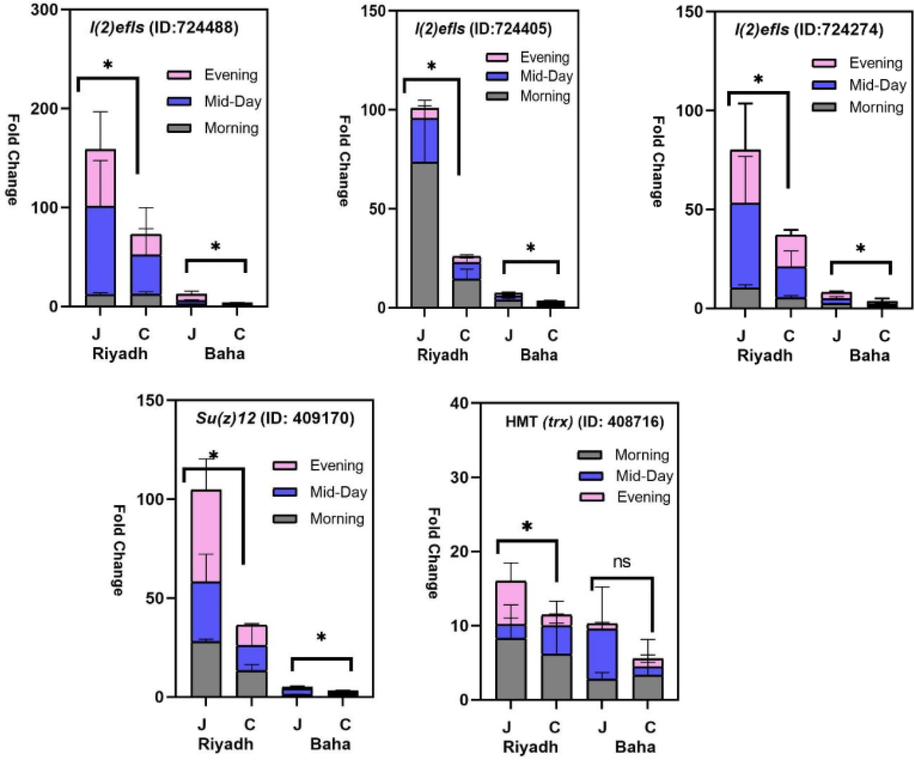
Fold changes of (mRNA) relative expression levels for Apis mellifera lethal(2)-essential-for-life-like (l(2)efl) genes (ID: 724405; 724488; 234274); Apis mellifera histone-lysine N-MT (trx) transcript variant X4 (ID: 408716), and Apis mellifera polycomb protein Su(z)12 (ID: 409170) in A. m. yemenitica (local) and A. m. carnica (carnica) at two thermogeographical regions (Riyadh: R and Baha: B) and the three foraging times (7:00 a.m.: after ≈1 h of foraging; 12:00 p.m.; and 5:00 p.m. (≈1 h before sunset)). Δct was calculated by using actin as endogenous control, and the fold change (fold change = 2ct (reference-treatment)) was calculated using the relative expression in A. m. carnica in the Baha region as reference (Calibrator). Significant fold change difference was determined based on average gene expression of each group using a three-way ANOVA analysis followed by Multiple comparisons based Bonferroni post hoc test, p < 0.05. (*) = significant variation and (ns) = non-significant variation.
When comparing data by locations (Riyadh and Baha), the results likewise revealed significantly higher relative fold changes in expression levels for all l(2)efl genes (ID: 724488; 724405; 234274) under desert conditions (Riyadh) than in semi-arid conditions (Baha) (Table 3). The interaction between subspecies and location was significant for l(2)efl (ID: 724405; 234274) and Apis mellifera polycomb protein Su(z)12 (ID: 409170) (Table 3). Under Riyadh climatic conditions mean fold changes were significantly higher in A. m. yemenitica than in A. m. carnica for all genes under study (Fig. 1, Table 4). Under semiarid conditions of Baha, mean fold changes of genes were significantly higher in A. m yemenitica than in A. m. carnica except for Apis mellifera histone-lysine N-MT (trx) (ID: 408716) (Table 5). Although differences in expression fold changes between A. m. yemenitica and A. m. carnica within the same locality were significant, differences in fold changes between both subspecies were much higher under desert conditions (Riyadh) (Fig. 1).
Variation Factor
Fold change in expression levels
Evening
Overall average
The Morning
Mid-day
Gene ID
Local
Carnica
Local
Carnica
Local
Carnica
Local
Carnica
l(2)efl (ID:724405)
33.6a
8.6b
73.4a
14.5b
22.1b
8.4b
5.2b
3.1b
l(2)efl (ID:724488)
52.9a
24.2b
12.0b
12. b
89.0a
39.8ab
57.7ab
20.8ab
l(2)efl (ID:724474)
26.7a
12.3b
10.3ab
5.4b
43.0a
15.5ab
26.8ab
16.1ab
trx (ID: 408716)
5.3a
3.8b
8.3a
6.1ab
1.9bc
3.9bc
5.9ab
1.5c
Su(z)12 (ID: 409170)
34.9a
12.1b
27.9ab
13.3b
30.4ab
12.8b
46.4a
10.2b
Variation Factor
Fold change in expression levels
Overall average
The Morning
Mid-day
Evening
Gene ID
Local
Carnica
Local
Carnica
Local
Carnica
Local
Carnica
l(2)efl (ID:724405)
2.4a
1.1b
3.8a
1.1c
2.2b
1.1c
1.4c
1.1c
l(2)efl (ID:724488)
4.1a
1.2b
2.5b
1.0b
3.5ab
1.5b
6.3a
1.0b
l(2)efl (ID:724474)
2.7a
1.2b
2.3ab
1.1b
2.4ab
1.0b
3.3a
1.5b
trx (ID: 408716)
3.4a
1.8a
2.8a
3.3a
6.8a
1.1a
0.8a
1.1a
Su(z)12 (ID: 409170)
1.6a
1.0b
1.2ab
1.0ab
2.8a
1.0ab
0.9b
1.0ab
Under the pertaining temperatures at the three sampling time periods (In Riyadh, temperatures were 31, 42 and 39 °C, In Baha, they were 22, 30 and 25 °C; in the morning, mid-day and evening, respectively), fold changes in expression levels were higher in A. m. yemenitica than in A. m. carnica at all time periods (Fig. 1). Relative expression levels (increase/decrease) were not consistent for either subspecies with the sampling time (Fig. 1). For two genes (ID:724405 ID: 408716) the highest expression levels occurred in the morning while for others at mid-day (ID:724488 and ID:724274) or evening (ID: 409170). Nevertheless, the maximum fold change among all time periods occurred for l(2)efl (ID:724488).
4 Discussion
The higher expression levels of Apis mellifera lethal(2)-essential-for-life-like (l(2)efl) genes (724405; 724488; 234274) in A. m yemenitica than in A. m. carnica under extreme temperature conditions (Riyadh) can be allied with the thermal adaptation of A. m. yemenitica. Above that, the daily variation in relative expression levels of l(2)efl (ID: 724405; 724488; 234274) between A. m yemenitica and the calibrator (A. m. carnica in Baha) were very high (∼100×), which demonstrate an efficient response and revealed robust transcriptional activation of these genes in A. m. yemenitica under natural thermal stress of Riyadh. Additionally, these fold changes and expression levels were as high as that of Hsp70 mRNA and other constitutively expressed Hsps reported in a previous study (done at the same locations) for both subspecies (Alqarni, et al., 2019; Alghamdi and Alattal, 2023). Consequently, these higher expression levels of l(2)efl (ID: 724405; 724488; 234274) genes can be considered key elements of survival for A. m. yemenitica under desert conditions of Riyadh.
Under semi-arid conditions of Baha, differences in expression levels between both subspecies were very modest indicating minimal stress-inducing conditions being a more suitable habitat for A. m. carnica than Riyadh. Nevertheless, significant variation in fold changes of expression levels (mRNAs) between both subspecies under Baha conditions might indicate a quicker and earlier response in A. m. yemenitica to accommodate expected changes in daily ambient temperature. Generally, the real forager body temperature is higher than the ambient temperature in many foraging insects, which is connected with foraging distance as well (Kovac et al., 2014; Davis and Moyle, 2020). The significant interaction between subspecies and location indicates that A. m. carnica had a severe locality disadvantage at Riyadh, but only minimal disadvantage at Baha, this may explain -in part- previously published data on survival rates of both subspecies under Riyadh and Baha regions, reporting that most of A. m. carnica colonies in Riyadh died within the first summer season of establishment (Alattal and Alghamdi, 2015). Consequently, keeping A. m. carnica under Baha conditions can be more reasonable than in Riyadh.
The increase/decrease of expression levels was not consistent with daytime. This was clearer in the Riyadh region which may indicate continuous adjustment of l(2)efl transcription responding to daily dynamics in ambient temperatures. Indeed, there is no data on the response threshold associated with l(2)efl gene expression under daily temperature stresses among different honeybee subspecies. Nevertheless, we cannot predict if the thermo-tolerance of A. m. yemenitica will be impacted by the global intensification of climate change including Saudi Arabia (Chen et al., 2011). Likewise, relative expression levels of A. mellifera polycomb protein Su(z)12 (ID: 409170) and Apis mellifera histone-lysine N-MT (trx) (ID: 408716) were higher in Riyadh than in Baha, which can reflect an epigenetic layer of regulation on active transcription of l(2)efls associated with heat stress. We recommend investigating expression thresholds of l(2)efl genes among different A. mellifera subspecies and try to explore how thermotolerance impacts honeybee productivity under different heat regimes. Many reports documented higher productivity but lower survival rates of exotic honeybee subspecies compared to A. m. yemenitica under hot and dry conditions in Saudi Arabia (El-kazafy and Al-kahtani, 2019), which entails the need for selection and breeding programs toward increasing productivity without impacting the thermo-adaptability of A. m. yemenitica.
5 Conclusion
In conclusion, expression of l(2)efls (mRNAs) (724405; 724488; 234274) genes can be considered a key component of A. m. yemenitica survival adaptation under extreme summer temperatures characterizing the Riyadh region. On the other hand, the semi-arid conditions of the Baha region are a more suitable habitat for A. m. carnica compared to Riyadh.
CRediT authorship contribution statement
Yehya Zaki Alattal: Conceptualization, Funding acquisition, Data curation, Writing original draft, Writing review & editing, Visualization, Investigation, Validation, Formal analysis, Methodology, Supervision, Resources, Project administration, Software. Ahmad Abdallah Alghamdi: Conceptualization, Funding acquisition, Data curation, Writing original draft, Writing review & editing, Visualization, Investigation, Validation, Formal analysis, Methodology, Supervision, Resources, Project administration, Software.
Acknowledgments
The authors extend their appreciation to the Deputyship for Research and Innovation, “Ministry of Education” in Saudi Arabia for funding this research (IFKSUOR3-599-1).
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Impact of temperature extremes on survival of indigenous and exotic honey bee subspecies, Apis mellifera, under desert and semiarid climates. Bull. Insectol.. 2015;68(2):219-222.
- [Google Scholar]
- Linking histone methylation states and hsp transcriptional regulation in thermo-tolerant and thermo-susceptible A. mellifera L. subspecies in response to heat stress. Insects. 2023;14(3):225.
- [Google Scholar]
- Characterization of the native honey bee subspecies in Saudi Arabia using the mtDNA COICOII intergenic region and morphometric characteristics. Bull. Insectol.. 2014;67(1):31-37.
- [Google Scholar]
- Expression levels of heat-shock proteins in Apis mellifera yemenitica and Apis mellifera carnica foragers in the desert climate of Saudi Arabia. Insects. 2023;14(5):432.
- [Google Scholar]
- Beekeeping in the Kingdom of Saudi Arabia: past and present practices. Bee World. 2013;90(2):26-29.
- [Google Scholar]
- Comparative study for evaluating two honey bee races, Apis mellifera jementica (indigenous race) and Apis mellifera carnica (Carniolan race) in brood production, population development and foraging activity under the environmental conditions of the central region of the Kingdom of Saudi Arabia. Ann. Agric. Sci.. 2011;56(2):127-134.
- [Google Scholar]
- Tolerance of summer temperature in imported and indigenous honeybee Apis mellifera L. races in Central Saudi Arabia. Saudi J. Biol. Sci.. 2006;13:123-127.
- [Google Scholar]
- Osmotic concentration in three races of honey bee, Apis mellifera L. under environmental conditions of arid zone. Saudi J. Biol. Sci.. 2017;24:1081-1085.
- [Google Scholar]
- Expression of heat shock proteins in adult honey bee (Apis mellifera L.) workers under hot-arid subtropical ecosystems. Saudi J. Biol. Sci.. 2019;26:1372-1376.
- [Google Scholar]
- Small heat shock proteins: role in cellular functions and pathology. Biochim. Biophys. Acta Proteins Proteomi.. 2015;1854:291-319.
- [Google Scholar]
- A review of methods for discrimination of honey bee populations as applied to European beekeeping. J. Apic. Res.. 2011;50:51-84.
- [Google Scholar]
- Summer weather conditions influence winter survival of honey bees (Apis mellifera) in the northeastern United States. Sci. Rep.. 2021;11:1553.
- [Google Scholar]
- Rapid range shifts of species associated with high levels of climate warming. J. Sci.. 2011;333:1024-1026.
- [Google Scholar]
- Constitutive and plastic gene expression variation associated with desiccation resistance differences in the Drosophila americana species group. Genes. 2020;11:146.
- [Google Scholar]
- Extensive histone post-translational modification in honey bees. Insect Bioch. Mol. Biol.. 2013;43:125-137.
- [Google Scholar]
- Extreme thermotolerance and behavioral induction of 70-kDa heat shock proteins and their encoding genes in honey bees. Cell Stress Chaperones. 2009;14:219-226.
- [Google Scholar]
- Comparison of the activity and productivity of Carniolan (Apis mellifera carnica Pollmann) and Yemeni (Apis mellifera jemenitica Ruttner) subspecies under environmental conditions of the Al-Ahsa oasis of eastern Saudi Arabia. Saudi J. Biol. Sci.. 2019;26(4):681-687.
- [Google Scholar]
- A revision of subspecies structure of western honeybee Apis mellifera. Saudi J. Biol. Sci.. 2020;27:3615-3621.
- [Google Scholar]
- Honey bee colony loss linked to parasites, pesticides and extreme weather across the United States. Sci. Rep.. 2022;12:20787
- [Google Scholar]
- Metabolism and upper thermal limits of Apis mellifera carnica and Apis mellifera ligustica. Apidologie. 2014;45:664-677.
- [Google Scholar]
- Sequence of the new Drosophila melanogaster small heat-shock-related gene, lethal(2) essential for life [l(2) ef], at locus 59F4,5. Gene. 1995;154:171-175.
- [Google Scholar]
- The heat shock response in the Western honey bee (Apis mellifera) is antiviral. Viruses. 2020;12(245):1-21.
- [Google Scholar]
- Standard methods for characterizing subspecies and ecotypes of Apis mellifera. J. Apic. Res.. 2013;52(4):1-9.
- [Google Scholar]
- Adaptations to thermal stress in social insects: recent advances and future directions. Biol. Rev.. 2020;95:1535-1553.
- [Google Scholar]
- Local adaptation of reproductive performance during thermal stress. J. Evol. Biol.. 2017;30:422-429.
- [Google Scholar]
- Changes in cold tolerance during the overwintering period in Apis mellifera ligustica. J. Apic. Res.. 2019;58(5):702-709.
- [Google Scholar]
- The Lethal(2)-essential-for-life [L(2)EFL] gene family modulates dengue virus infection in Aedes aegypti. Int. J. of Mol. Sci.. 2020;21(20):7520.
- [Google Scholar]
- Biogeography and Taxonomy of Honeybees. Berlin, Germany: Springer-Verlag; 1988.
- Ruttner, H., 1972. Technische Empfehlungen zur Leistungsprüfung von Bienenvölkern. In :Proceedings of the Paarungskontrolle und Selektion bei der Honigbiene: Internationales Symposium, Lunz am See, Austria.
- Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc.. 2008;3:1101-1108.
- [Google Scholar]
- Heat stress induced enhancement of heat shock protein gene activity in the honey bee (Apis mellifera) Experientia. 1990;46:737-739.
- [Google Scholar]
- Honey bee sHSP are responsive to diverse proteostatic stresses and potentially promising biomarkers of honey bee stress. Sci. Rep.. 2021;11:22087
- [Google Scholar]
- The honey bee cluster as a homeothermic superorganism. Comp. Biochem. Physiol. Part A Physiol.. 1983;75:641-645.
- [Google Scholar]
- A. Systemic stress signalling: understanding the cell non-autonomous control of proteostasis. Nat. Rev. Mol. Cell Biol.. 2014;15:211-217.
- [Google Scholar]
- Honeybees (hymenoptera: apidae) adapt to the shock of high temperature and high humidity through changes in sugars and polyols and free amino acids. J. Insect Sci.. 2023;23(1):4.
- [Google Scholar]
- Expression of heat shock protein genes in insect stress responses. Invertebr. Surviv.. 2012;9:93-101.
- [Google Scholar]






