Translate this page into:
Ipomoea tricolor (Convolvulaceae) in Turkey: New occurrence record and potential spread areas under current climatic conditions
⁎Corresponding author. onenhuseyin@gmail.com (Huseyin Onen)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Background
The invasive alien plant species exert significant economic and ecological impacts in the introduced ranges. The occurrence reports of newly introduced species and assessing their potential spread are important for halting their future spread.
Methods
The presence of Ipomoea tricolor Cav. (Convolvulaceae) in Turkey is confirmed through surveys in Tokat province. The general characteristics and seed germination potential of the plant were determined, and its potential distribution areas in the country were modeled by maximum entropy model. Global habitat suitability of the species was predicted under current climatic conditions and then downscaled to Turkey.
Results
The plant was first recorded on the edge of a field and in the garden of a house in Tokat province (Kat town) of Turkey. The species was transported/introduced here for ornamental purposes. Afterward, the species was detected at two more locations in the residential areas of Tokat province, grown as an ornamental plant. The model predicted that there are plenty of vacant niches with moderate habitat suitability for I. tricolor in Turkey. The Black Sea, Aegean, Mediterranean and some parts of central Anatolia regions of the country are suitable for the spread of this species. Considering the rapid spread of I. purpurea (an ornamental plant species in the same genus) in agricultural and non-agricultural areas of the country, it is predicted that I. tricolor could spread and cause negative impacts on agro-ecosystems.
Conclusions
The potential spread areas of the species should be mapped under future climatic conditions to develop early detection and rapid response system in the country. Rapid surveys should be conducted in the areas predicted as suitable to track the spread of the species and improve models’ prediction. Nonetheless, awareness should be created through citizen science to halt the spread of this species in the current distribution region of the country.
Keywords
Multicolored morning-glory
Ipomoea tricolor
Invasive plant
Distribution
Turkey
1 Introduction
Human-mediated transfer/transport of exotic/alien plant species into new ranges for getting some benefits (agriculture, biocontrol etc.) and ornamental purposes without considering long-term impacts are among the main reasons for their spread (Farooq et al., 2015; Onen and Farooq, 2015). Moreover, globalization have eased the frequent movements of people and goods, resulting in the transport of plant species from native to introduced ranges (Onen et al., 2014; Seebens et al., 2015). Hence, increased number of invasive alien species successfully spread and established into the new geographic regions/habitats (Chapman et al., 2017; Van Kleunen et al., 2015). Increasing global trade is further making the spread of non-native species easier (Humair et al., 2015; Özaslan et al., 2016; Reyjol, 2007).
The diverse topographic features, rich floristic and habitat diversity, and different climatic zones in Turkey facilitates the spread and naturalization of introduced species (Arslan et al., 2015; Onen, 2015; Özaslan et al., 2016). Different alien plant species have already invaded Turkey and it is possible that the number, abundance, and impacts of these species will be accelerated in the future (Farooq et al., 2015; Onen et al., 2016a; Onen and Farooq, 2015; Onen and Ozcan, 2010; Uludag et al., 2017).
Ipomoea L. is the largest genus in the family Convolvulaceae. The genus has ∼810 species mainly distributed throughout the tropics and subtropics of the world (Wood et al., 2020). Many species have been used as food (sweet potato, I. batatas), medicines, in religious rituals, or for ornamental purposes (Meira et al., 2012). However, several Ipomoea species cause significant problems in natural habitats and agro-ecosystems as weeds/invasive plants, both in native and non-native ranges (Hançerli et al., 2018; Maimoni-Rodella and Yanagizawa, 2007; Onen et al., 2021).
Hançerli et al. (2018) recorded six Ipomoea species inTurkey, including I. sagittata Poir., I. stolonifera (Cirillo) J.F. Gmel. (syn: Ipomoea imperati (Vahl) Griseb.), I. purpurea (L.) Roth., I. hederacea Jacq., I. triloba L. and I. hederifolia L.(Davis, 1978; Gönen, 1999; Hançerli et al., 2018; Yazlık et al., 2018). Onen et al. (2021) recently reported the presence of I. coocinea, raising the total number to seven.
Species distribution models predict the potential spread of species in space and time by using occurrence records and climatic data. Huge changes in the distribution of plant species are mediated by climate (Gebrewahid et al., 2020; Ji et al., 2020; Kaky et al., 2020). Climate change is causing large-scale changes in the distribution of native and introduced plants species (Gebrewahid et al., 2020; Kaky et al., 2020; Pecl et al., 2017). Therefore, predicting the distribution of species before their spread could aid in halting further spread. The MaxEnt model is based on the maximum-entropy approach for modeling species niches and distributions. Environmental data along with occurrence records of target species are used to develop an index describing the suitability of species to persist in different grid cells. A higher index is representative of high suitability, whereas the low index values indicate that species is unable to persist in respective grid cell. MaxEnt model has been successfully used to predict the habitat suitability for plenty of species in various geographic regions of the world (Hijmans and Graham, 2006).
Although the plant has been reported as an alien/invasive species in Turkey along with several countries or islands in the world, there is no occurrence for Turkey in GBIF to date (Uludag et al., 2017). In this study we confirm the presence of I. tricolor in Turkey. We provide general information about its morphological characteristics and the habitat in Tokat-Turkey where it was found, as well as explore the potential agricultural risks associated with the species. We also identify potential areas in Turkey that the species could colonize under current climatic conditions. Our results help to inform species management and halt its further spread in the country.
2 Materials and methods
2.1 General characteristics of the plant and seed germination potential
Surveys were carried out in agricultural and residential areas in the Tokat province of Turkey. Voucher specimens and seeds were collected from four populations in three locations where the plant was detected in the survey area. The plants were pictured on site. The general characteristics of the plant were determined, and a germination trial was carried out to find out the germination potential of the collected seeds. The seeds were manually sacrificed, and 25 of them (five replications) were placed between two layers of moistened filter paper in Petri dishes. The seeds were incubated 24 °C, 12 h light/dark conditions (Hardcastle, 1978).
2.2 Habitat suitability and occurrence records
The occurrence records of species at global scale were retrieved from various databases and search engines. Occurrence records were downloaded from GBIF and cleaned. For data cleaning, first the records having coordinate precision < 1 km were deleted. The records having no coordinates, belonging to other species and duplicate entries were deleted in the second step. Furthermore, Scopus (https://www.scopus.com/home.uri) and Web of Science (https://www.webofscience.com/wos/woscc/basic-search) were explored for occurrence records. The occurrence records were combined to get the final data set for model calibration. The occurrence records in Turkey were added to complete the dataset and the final data included 479 occurrences of the species.
2.3 Climatic data
The high-resolution climate data (∼1 km grids) for current climate (1979–2019) was downloaded from CHELSA (https://chelsa-climate.org/ (Karger et al., 2017) and used in predicting the potential spread areas of the species.
2.4 Modeling approach
The model was calibrated in a hierarchical fashion (Petitpierre et al., 2012). Model calibration was done on a global scale using global and local occurrence records. Habitat suitability was then projected globally and for Turkey. Since the Maxent model requires both occurrences and absences, 10,000 pseudo-absences were included in the model. A 1 km distance around each occurrence was buffered while sampling the pseudo-absences.
The values of 18 environmental variables (Table 1) were extracted for each occurrence/absence record. The model was calibrated with 70 % of the data and evaluated on the remaining 30 % (Elith et al., 2011). The model had 10 replications and results were averaged to get habitat suitability. Bio18 was removed due to high auto collinearity.
Variable
Full Form
Abbreviation
BIO1
Annual Mean Temperature
AMT
BIO2
Mean Diurnal Range (Mean of monthly (max temp - min temp)
MDR
BIO3
Isothermality (BIO2/BIO7) × (100)
ISO
BIO4
Temperature Seasonality (standard deviation × 100)
TSE
BIO5
Max Temperature of Warmest Month
MaWM
BIO6
Min Temperature of Coldest Month
MiCM
BIO7
Temperature Annual Range (BIO5-BIO6)
TAR
BIO8
Mean Temperature of Wettest Quarter
MeWtQ
BIO9
Mean Temperature of Driest Quarter
MeDQ
BIO10
Mean Temperature of Warmest Quarter
MeWaQ
BIO11
Mean Temperature of Coldest Quarter
MeCQ
BIO12
Annual Precipitation
AP
BIO13
Precipitation of Wettest Month
PWtM
BIO14
Precipitation of Driest Month
PDM
BIO15
Precipitation Seasonality (Coefficient of Variation)
PS
BIO16
Precipitation of Wettest Quarter
PWtQ
BIO17
Precipitation of Driest Quarter
PDQ
BIO19
Precipitation of Coldest Quarter
PCQ
2.5 Model validation and statistical analysis
The final fitted model was visually validated with global distribution data. The goodness of fit of the model was evaluated by “area under the receiver operating characteristic curve” (AUC) (Swets, 1988). The AUC measures the overall discriminatory ability of the model by quantifying the probability that the model correctly ranks a random presence locality higher than a random background pixel (Phillips et al., 2006). The AUC ranges between 0.5 (model that performs no better than random) and 1 (model with perfect discrimination).
3 Results and discussion
3.1 Species description and occurrence
During field observations, a plant belonging to the genus Ipomoea L. was first encountered on the edge of an agricultural land (orchard) and in the garden of a house in Kat town in Tokat province, Turkey (Figs. 1–4). The plant was subsequently identified as I. tricolor. Although Uludag et al. (2017) listed I. tricolor among the alien taxa in the flora of Turkey, a detailed literature review found no actual records showing the presence of the species in Turkey (Hançerli et al., 2018; Onen et al., 2021; Özkil and Üremiş, 2020). The general characteristics of the plant and the locations where the plant was observed were not mentioned by Uludag et al. (2017). Hançerli et al. (2018) and Özkil and Üremiş (2020) did not report the presence of I. tricolor in Turkey. Furthermore, the species is not listed in the Plants Data Services in Turkey nor in other databases keeping the record of plant distribution in the country.
Ipomoea tricolor plants observed during the exploratory surveys in Tokat province, Turkey.

Ipomoea tricolor A) flowers, B) fruits and C) seeds obtained from the plants recorded during the exploratory surveys.

Ipomoea tricolor plants climbing (A) apple and (B) vine plants on the edge of an orchard.
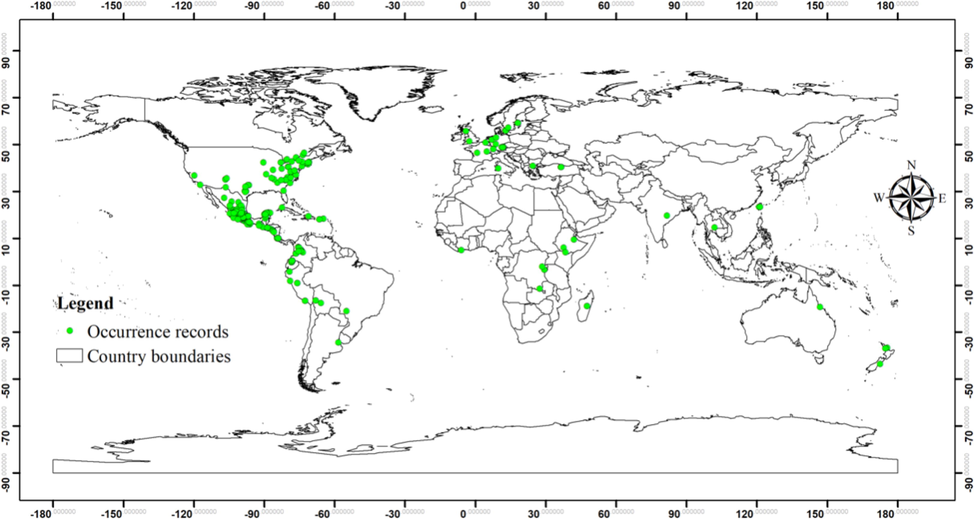
Global occurrence records of Ipomoea tricolor used in the study to predict habitat suitability at global and regional scales (n = 479).
Detailed surveys were carried out in agricultural and residential areas in the region, and the plant was found at two more locations (in the courtyard of a mosque and in a green area). Local people reported that seeds were collected from the garden of the house where the plant first detected due to its extremely dense bloom and stunning flowers. The coordinates of the occurrence sites are given in Table 2.
Name of the site
General characteristics of the site
Coordinates
Specimen collector
Kat town of Tokat Province
Roadside and edge of agricultural lands
40°19′38.5″N 36°20′59.4″E
Hüseyin Önen
Kat town of Tokat Province
On the fence and garden of a house
40°19′39.7″N 36°21′09.5″E
Hüseyin Önen
The residential area of Tokat province
In the courtyard of a mosque
40°20′39.3″N 36°32′07.7″E
Hüseyin Önen
The residential area of Tokat province
In a green area belonging to a residential site
40°20′25.9″N 36°32′52.0″E
Hüseyin Önen
Ipomoea species are generally grown as ornamental plants in Turkey (Onen et al., 2021). Their seeds are often circulated throughout the country, are available from local and foreign sources and can also be purchased via internet from ecommerce websites (Onen, 2015; Onen et al., 2021, 2016b). Therefore, the species has possibly been introduced to the region as an ornamental plant and may spread throughout the country.
The general characteristics of the plant were determined from the voucher specimen collected from the region. It has been observed that the plant is a fast-growing vining and/or twining annual species (Wood et al., 2020). The stems are robust, 4–6 mm broad and up to 3.5–4 m in length, and glabrous. The plant has a deep-going taproot with dense fibrous roots. The leaves are alternate. The leaf blades are petiolate heart-shaped, cordate with rather angular, apex acuminate, 3.5–13.5 cm in length, and 3–14 cm in width. Leaves are entire, glabrous, and green. Auricles are rather angular, apex acuminate. Petioles are 1.5–19 cm long.
Inflorescence is pedunculate (few-flowered axillary cymes). Peduncles are 5–21 cm in length. The large showy flowers are trumpet-shaped (salverform). Sepals are dark green with white margin, oblong-deltoid. Corolla is 5-lobed, 5–7.5 cm long, and vivid bright blue in color with white tube and yellowish throat (Figs. 2 and 3). Sepals are dark green with white margin, oblong-deltoid, glabrous, inner slightly longer than the outer. Pedicels are 2–3 cm. Ovaries are 4-celled, with 1 ovule in each cell. The plant is hermaphrodite and is flowering from July to mid-December. The flowers open in the morning and then fade and drop by evening (Fig. 3A). Fruits are in capsular form, ovoid, glabrous, 9–11 × 5–6 mm, light to dark brown, and contain 1–4 seeds (Fig. 3B). The seeds are reddish-brown to blackish in color, 4.5–7 × 3–4.5 mm, minutely tomentose. Hilum wide is 0.5–1 mm (Fig. 2C). The general descriptions of plants from Tokat populations agreed with the existing definitions in the literature (Park et al., 2004; Wood et al., 2015).
Among the Ipomoea spp. found in Turkey, I. tricolor can be confused with I. purpurea (L.) Roth. However, I. tricolor is completely glabrous, and corolla 5.5–7 cm, blue with yellowish throat and white tube. Sepals are lanceolate, acute with white margins. I. tricolor leaves are entire. But all vegetative parts are softly pubescent in I. purpurea. I. purpureasepals are oblong or lanceolate, corollas are 4–5 cm and usually pink (rarely white or blue). I. purpurealeaves are entire or 3–5-lobed (Wood et al., 2020).
It has been reported that I. tricolor can produce many capsules and accordingly seeds (Fig. 3) like other Ipomoea species (Crowley and Buchanan, 1982; Onen et al., 2021; Thullen and Keeley, 1983). Besides the average seed germination rate of I. tricolor seeds in the current study was quite high (94.67 ± 3.27 %) from Petri dish experiment. Previous studies also revealed that the seeds of the genus have a high germination potential (Egley, 1990; Hardcastle, 1978; Onen et al., 2021). Therefore, it is a common annual species that regenerates via seeds in successive years, in the USA and particularly in cooler regions than its tropical origin of Mexico (Wood et al., 2020).
I. tricolor has allelopathic potential (Anaya et al., 1995), and is one of the Ipomoea species distributed around agro-ecosystems or disturbed habitats near settlements oftropical regions in almost all countries of the Americas, and clearly escapes from garden (Wood et al., 2020). In conclusion, the plant can easily escape, establish and naturalize into new areas, eventually becoming weedy.
3.2 Habitat suitability under current climatic conditions
The AUC values of the model were 0.94, indicating that habitat suitability was predicted with high accuracy. Thus, the results of the model are considered reliable for designing action plans to halt the further spread of the species (Fig. 5).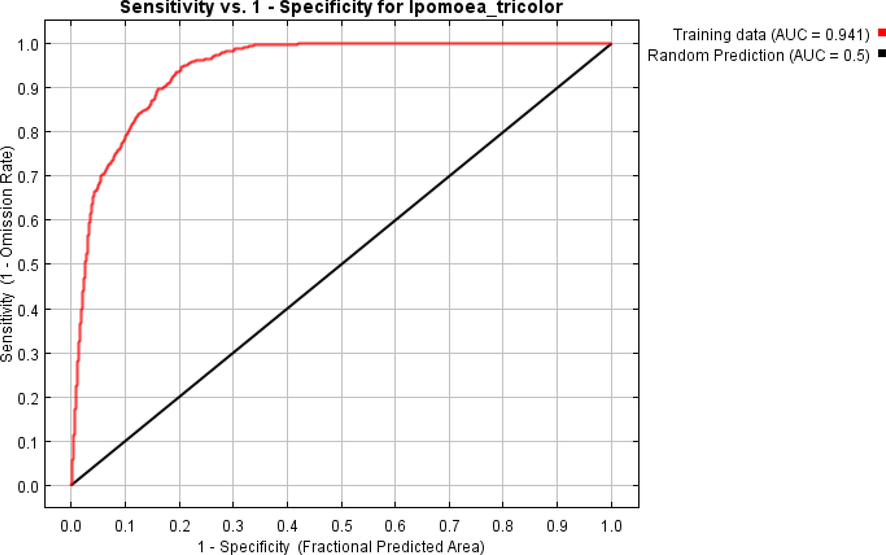
Curves for AUC under the receiver operating characteristic indicating habitat suitability of Ipomoea tricolor under current climatic conditions.
The contributions of the bioclimatic variables used to predict the habitat suitability of I. tricolor under current climatic conditions was evaluated by Jackknife analysis and permutations. The results indicated that mean diurnal range, temperature seasonality, minimum temperature of the coldest month, and mean temperature of coldest and the warmest quarter had the highest contribution towards the model under current climatic conditions (Table 3). These results indicatedthat temperature is the main driver for habitat suitability of I. tricolor under current climatic conditions (Table 3). Temperature was the most influential environmental variable affecting the distribution of the species. These results indicated that temperature changes will exert strong impacts on the further spread of the species in Turkey.
Bioclim variable
Percent contribution
Annual Mean Temperature
1.38
Mean Diurnal Range (Mean of monthly (max temp - min temp)
19.39
Isothermality (BIO2/BIO7) × (100)
7.91
Temperature Seasonality (standard deviation × 100)
16.16
Max Temperature of Warmest Month
2.40
Min Temperature of Coldest Month
20.97
Temperature Annual Range (BIO5-BIO6)
0.00
Mean Temperature of Wettest Quarter
0.28
Mean Temperature of Driest Quarter
0.00
Mean Temperature of Warmest Quarter
11.08
Mean Temperature of Coldest Quarter
13.65
Annual Precipitation
0.22
Precipitation of Wettest Month
0.67
Precipitation of Driest Month
4.23
Precipitation Seasonality (Coefficient of Variation)
0.26
Precipitation of Wettest Quarter
0.01
Precipitation of Driest Quarter
1.31
The model predicted that the areas neighboring to the current distribution range of the species have high suitability for the spread of the species at global scale (Fig. 6). Interestingly, a large portion of Turkey has been predicted as suitable for the spread of the species (Fig. 7). The Black Sea and partly central Anatolia regions were highly suitable, and some areas in Thrace, Aegean, and Mediterranean regions moderately and/or less suitable, whereas the other areas of the country were predicted as unsuitable for the spread of the species.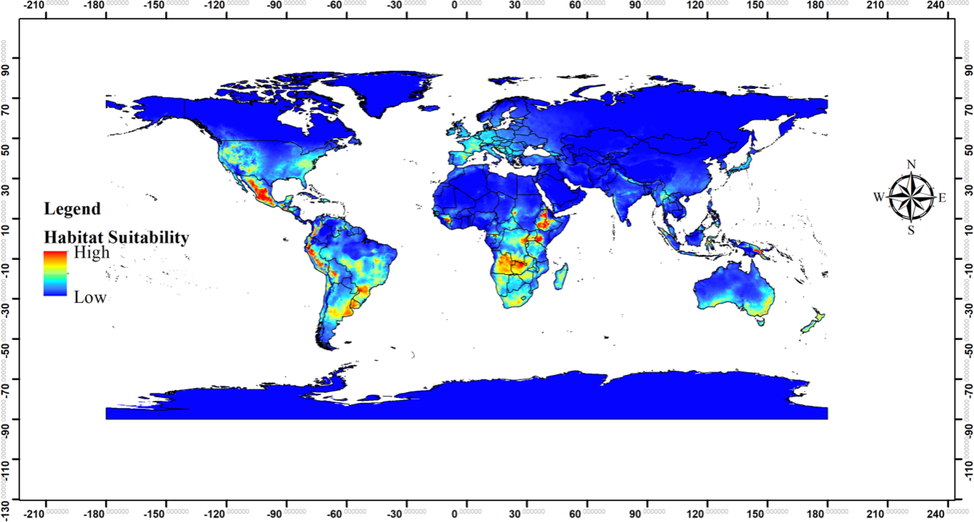
Predicted global habitat suitability of Ipomoea tricolor under current climatic conditions.
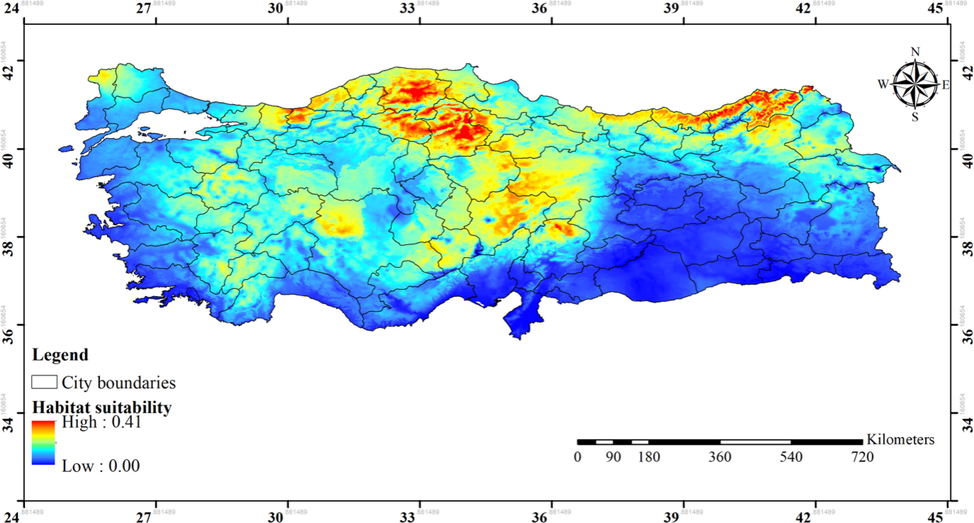
Predicted habitat suitability of Ipomoea tricolor in Turkey under current climatic conditions.
4 Conclusion
The occurrence of I. tricolor in Turkey is confirmed in this study. The species has possibly been introduced in the region as an ornamental plant and it may have a much more widespread distribution throughout the country. Species distribution model indicated plenty of vacant niches for the species in the country. Some other introduced Ipomoea species have already become troublesome weeds and cause problems at a regional scale, especially in certain agro-ecosystems in Turkey. There is also a risk that I. tricolor could extend its occurrence range, naturalize, and invade other areas in the country. The plant can be a problematic weed due to its vigorous growing nature, reproductive capacity and allelopathic characteristics.Therefore, detailed surveys are needed to determine the distribution areas of I. tricolor in other parts of the country. Since some congeners of this species cause significant problems, it should also be considered that early warning and eradication programs against the plant may be needed according to the weed risk assessment to be made.
Acknowledgement
This project was supported by Researchers Supporting Project Number (RSP2023R5) King Saud University, Riyadh, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Allelopathic potential of Ipomoea tricolor (Convolvulaceae) in a greenhouse experiment. J. Chem. Ecol.. 1995;21:1085-1102.
- [CrossRef] [Google Scholar]
- Status of invasive alien plants included in EPPO Lists in Turkey. EPPO Bull.. 2015;45:66-72.
- [CrossRef] [Google Scholar]
- Global trade networks determine the distribution of invasive non-native species. Glob. Ecol. Biogeogr.. 2017;26:907-917.
- [CrossRef] [Google Scholar]
- Variations in seed production and the response to pests of morningglory (Ipomoea) species and smallflower morningglory (Jacquemontia tamnifolia) Weed Sci.. 1982;30:187-190.
- [Google Scholar]
- Flora of Turkey and the East Aegean Islands (6th ed.). Edinburgh University Press; 1978.
- High-temperature effects on germination and survival of weed seeds in soil. Weed Sci.. 1990;38:429-435.
- [Google Scholar]
- A statistical explanation of MaxEnt for ecologists. Divers. Distrib.. 2011;17:43-57.
- [CrossRef] [Google Scholar]
- Farooq, S., Onen, H., Ozaslan, C., 2015. Erken Tanı , Takip ve Bilgi Sistemi, in: Onen, H. (Ed.), Türkiye İstilacı Bitkiler Kataloğu. GIDA, TARIM VE HAYVANCILIK BAKANLIĞI Tarımsal Araştırmalar ve Politikalar Genel Müdürlüğü Bitki Sağlığı Araştırmaları Daire Başkanlığı.
- Current and future predicting potential areas of Oxytenanthera abyssinica (A. Richard) using MaxEnt model under climate change in Northern Ethiopia. Ecol. Process.. 2020;9:6.
- [CrossRef] [Google Scholar]
- Determination of Germination Biology and Morphologic Characteristic to Use Practical Identification with Computer of Summer Growing Weed Species in Çukurova Region of Turkey. Cukurova University; 1999.
- A new weed species record for the Flora of Turkey Ipomoea hederifolia L. (Convolvulaceae) Türkiye Herboloji Derg.. 2018;21:36-38.
- [Google Scholar]
- Influence of Temperature and Acid Scarification Duration on Scarlet Morningglory (Ipomoea coccinea) Seed Germination. Weed Sci.. 1978;26:261-263.
- [CrossRef] [Google Scholar]
- The ability of climate envelope models to predict the effect of climate change on species distributions. Glob. Chang. Biol.. 2006;12:2272-2281.
- [CrossRef] [Google Scholar]
- Predicting the potential distribution of the vine mealybug, Planococcus ficus under climate change by MaxEnt. Crop Prot.. 2020;137:105268
- [CrossRef] [Google Scholar]
- Kaky, E., Nolan, V., Alatawi, A., Gilbert, F., 2020. A comparison between Ensemble and MaxEnt species distribution modelling approaches for conservation: A case study with Egyptian medicinal plants. Ecol. Inform. https://doi.org/10.1016/j.ecoinf.2020.101150.
- Climatologies at high resolution for the earth’s land surface areas. Sci. Data. 2017;4:170122.
- [CrossRef] [Google Scholar]
- Floral biology and breeding system of three Ipomoea weeds. Planta Daninha. 2007;25:35-42.
- [CrossRef] [Google Scholar]
- Review of the genus Ipomoea: traditional uses, chemistry and biological activities. Rev. Bras. Farmacogn.. 2012;22:682-713.
- [CrossRef] [Google Scholar]
- The Black Sea highway: the route of common ragweed (Ambrosia artemisiifolia L.) invasion in Turkey. In: 8th International Conference on Biological Invasions from Understanding to Action. Turkey: Antalya; 2014.
- [Google Scholar]
- Onen, H., Farooq, S., Gunal, H., 2016a. Monitoring and information system : a helpful approach for minimizing ecological impacts of invasive plants. In: COST Action ES1104 White Paper on the Restoration of Drylands.
- Onen, H., Sarı, T., Farooq, S., 2016b. E-Commerce: An Open Gateway for Plant Invasion in Turkey. In: Turkey 6th Plant Protection Congress with International Participation. Konya, p. 821.
- Current Status and Future Prospects of Invasive Plants in Turkey. CIHEAM Watch Lett.. 2015;33
- [Google Scholar]
- Ipomoea coccinea L. (Convolvulaceae): a new introduced alien plant species in Turkey. EPPO Bull.. 2021;51:207-212.
- [CrossRef] [Google Scholar]
- klim Değişikliğine Bağlı Olarak Yabancı Ot Mücadelesi. İklim Değişikliğinin Tarıma Etkileri ve Alınabilecek Önlemle. 2010:336-357.
- [Google Scholar]
- Onen, H., 2015. Türkiye İstilacı Bitkiler Kataloğu, 1st ed. T.C. GIDA, TARIM VE HAYVANCILIK BAKANLIĞI Tarımsal Araştırmalar ve Politikalar Genel Müdürlüğü Bitki Sağlığı Araştırmaları Daire Başkanlığı, Ankara.
- Özkil, M., Üremiş, İ., 2020. Akdeniz Bölgesi Tarım Alanlarında Bulunan Akşam Sefası (Ipomoea spp.) İle Tarla Sarmaşığı (Convolvulus spp.) Türlerinin, Yaygınlık Ve Yoğunluk Durumları. Ege Üniversitesi Ziraat Fakültesi Derg. 71–80. https://doi.org/10.20289/zfdergi.596203.
- An intragenic tandem duplication in a transcriptional regulatory gene for anthocyanin biosynthesis confers pale-colored flowers and seeds with fine spots in Ipomoea tricolor. Plant J 2004
- [CrossRef] [Google Scholar]
- Pecl, G.T., Araújo, M.B., Bell, J.D., Blanchard, J., Bonebrake, T.C., Chen, I.-C., Clark, T.D., Colwell, R.K., Danielsen, F., Evengård, B., Falconi, L., Ferrier, S., Frusher, S., Garcia, R.A., Griffis, R.B., Hobday, A.J., Janion-Scheepers, C., Jarzyna, M.A., Jennings, S., Lenoir, J., Linnetved, H.I., Martin, V.Y., McCormack, P.C., McDonald, J., Mitchell, N.J., Mustonen, T., Pandolfi, J.M., Pettorelli, N., Popova, E., Robinson, S.A., Scheffers, B.R., Shaw, J.D., Sorte, C.J.B., Strugnell, J.M., Sunday, J.M., Tuanmu, M.-N., Vergés, A., Villanueva, C., Wernberg, T., Wapstra, E., Williams, S.E., 2017. Biodiversity redistribution under climate change: Impacts on ecosystems and human well-being. Science (80-.). 355, eaai9214. https://doi.org/10.1126/science.aai9214.
- Climatic Niche Shifts Are Rare Among Terrestrial Plant Invaders. Science (80-.). 2012;335:1344-1348.
- [CrossRef] [Google Scholar]
- Maximum entropy modeling of species geographic distributions. Ecol. Modell.. 2006;190:231-259.
- [CrossRef] [Google Scholar]
- Internet forums as potential invasion routes for exotic plants. Biodivers. Conserv.. 2007;16:2231-2232.
- [CrossRef] [Google Scholar]
- Global trade will accelerate plant invasions in emerging economies under climate change. Glob. Chang. Biol.. 2015;21:4128-4140.
- [CrossRef] [Google Scholar]
- Measuring the Accuracy of Diagnostic Systems. Science. 1988;80. 240:1285-1293.
- [CrossRef] [Google Scholar]
- Germination, Growth, and Seed Production of Ipomoea hederacea when Planted at Monthly Intervals. Weed Sci.. 1983;31:837-840.
- [CrossRef] [Google Scholar]
- Alien flora of Turkey: checklist, taxonomic composition and ecological attributes. NeoBiota. 2017;35:61-85.
- [CrossRef] [Google Scholar]
- Van Kleunen, M., Dawson, W., Essl, F., Pergl, J., Winter, M., Weber, E., Kreft, H., Weigelt, P., Kartesz, J., Nishino, M., Antonova, L.A., Barcelona, J.F., Cabezas, F.J., Cárdenas, D., Cárdenas-Toro, J., Castaño, N., Chacón, E., Chatelain, C., Ebel, A.L., Figueiredo, E., Fuentes, N., Groom, Q.J., Henderson, L., Inderjit, Kupriyanov, A., Masciadri, S., Meerman, J., Morozova, O., Moser, D., Nickrent, D.L., Patzelt, A., Pelser, P.B., Baptiste, M.P., Poopath, M., Schulze, M., Seebens, H., Shu, W.S., Thomas, J., Velayos, M., Wieringa, J.J., Pyšek, P., 2015. Global exchange and accumulation of non-native plants. Nature 525, 100–103. https://doi.org/10.1038/nature14910.
- A foundation monograph of Ipomoea (Convolvulaceae) in the New World. PhytoKeys. 2020;143:1-823.
- [CrossRef] [Google Scholar]
- Impact of alien plants in Turkey assessed by the Generic Impact Scoring System. NeoBiota. 2018;39:31-51.
- [CrossRef] [Google Scholar]







