Translate this page into:
Current trends and future perspectives on dental nanomaterials – An overview of nanotechnology strategies in dentistry
⁎Corresponding authors. drvidhyarekha@gmail.com (Vidhya Rekha Umapathy), prabhuperio@gmail.com (Prabhu Manickam Natarajan)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
The field of nanotechnology or nanoscience encompasses research and development in the applied sciences at the atomic or molecular levels and has a huge impact on nearly every aspect of human health and well-being, including pharmacological studies, clinical diagnosis, and supplemental immune system treatments. The numerous dental uses of nanotechnology have led to the development of the field of nanodentistry. The efficacy of dental operations and the field’s possibilities have grown greatly over the years as a result of extensive research in the fields of biomaterials and nanotechnology. Materials with nanoscale dimensions can display features not found in their larger-scale equivalents. Endodontic procedures are time-consuming and usually require multiple visits to attain the best results. The eradication of primary bacterial infection from dental root canals is still a serious concern in dentistry. Nanoparticles have been shown to be more efficient than standard materials and to have superior capabilities in terms of surface chemistry and bonding. Their antimicrobial characteristics are also promising in all medical procedures, particularly in endodontics. Because of their versatility, nanomaterials are a useful tool in dental clinics for a variety of operations including pulp regeneration, drug administration, root restoration, cleaning, obturation, and canal filling. This review provides an overview of the synthesis and characteristics of nanomaterials and their potential applications in dentistry and describes future perspectives of nanotechnology in dentistry with an updated literature review.
Keywords
Nanodentistry
Nanoparticles
Nanomaterials
Therapeutics

1 Introduction
Nanotechnology is now being used in a wide range of scientific fields since it offers a variety of practical solutions to scientific and medical problems. Nanotechnologies work at dimensions smaller than 100 nm. Viruses can be as small as 100 nm, and glucose molecules can be as small as 1 nm. Thus, the technology encompasses the study of molecular- and atomic-scale structures (Khatami et al., 2016). Dendrimers, nanotubes, nanocapsules, nanoshells, nanorings, nanobelts, nanospheres, fullerenes, nanowires, nanorods, liposomes, and quantum dots are examples of diverse nanomaterials that can be classified based on their shape and the existence of nanopores. In the last 20–30 years, most research has concentrated on nanoparticles; this trend indicates that nanotechnologies and knowledge of the properties of materials at these scales are highly sought after. According to the data obtained by nanotechnological means, it is possible to determine the surface area of one kilogram (1000 gm) of a powder at various spherical sizes, and these data reveal that the surface area per kilogram rises exponentially below 100 nm. The surface energy per gram of the material increases because of this phase change. An increase in surface area can be used for a variety of applications (Wei and Ma, 2008) (see Table 1)**.
Name of the material
Purpose of use
Advantages of this material
Toxicity/side effect known
Reference
Carbon nanotubes
Tooth filling, a coating of the tooth surface.
Increased surface area, bringing active substances to living cells, quickly attaching to the tooth and dentin/cementum surface.
The reactivity of carbon nanotubes (CNTs) is strongly influenced by their structure, size, surface, and purity. Crossing membrane barriers using nanotubes can cause inflammatory and fibrotic reactions in rare cases.
(Akasaka et al., 2009)
Graphene
Tooth coating, suitable for implantation, biofilm reduction.
Economical, fracture-resistant, low-density, forms a homogeneous crystal lattice, and effectively treats bacterial biofilms.
The toxicity of graphene is dependent on its structure, size, and oxidative state. Metallic impurities may be introduced during postsynthesis processing, eliciting a range of toxicological reactions.
(Yin et al., 2015)
Hydroxyapatite (HAp)
Reduces dental hypersensitivity, also acts as a cavity filler, delays secondary demineralization, and repairs enamel surfaces.
HAp nanoparticles are easily incorporated into tooth tubules. Comparable to tooth and bone in composition, biocompatible, adheres to the tooth enamel, protects the tooth by forming a coating of fake enamel around it, and corrects periodontal deficiencies.
They have the ability to interact with proteins and form protein–particle complexes that are subsequently destroyed by macrophages in tissues. Blood carries these particles to and distributes them throughout the lungs, spleen, and liver. Nanoparticle toxicity can have an effect on the inflammatory response, signaling system, and oxidative stress.
(Besinis et al., 2012)
Zirconia
Reduces bacterial adhesion to the tooth surface, protects against dental caries, and acts as an excellent polisher.
Similar to the tooth in terms of mechanical properties and color, these materials exhibit low cytotoxicity, good biocompatibility, and great fracture resistance.
Zirconium oxide nanoparticles have been shown to cause considerable DNA damage in human T cells, induce apoptosis, and decrease cell proliferation in human mesothelioma and rodent fibroblast cell lines. Additionally, zirconia was discovered to promote cellular oxidative stress, resulting in cell death. These NPs have been shown in studies to be capable of halting the cell cycle and crossing numerous physiological barriers, resulting in detrimental effects.
(Asadpour et al., 2016)
Silica
Dental filling agent, dental hypersensitivity treatment, antibacterial agent, dental caries prevention, tooth polishing.
It is biocompatible, has a minimal toxic effect, a low density, a high adsorption capacity, and is, most significantly, cost-effective. When used as a polishing agent, the roughness of the tooth surface is reduced.
Toxic effects are dependent on the route of entry and the physiochemical characteristics of the substance. Recent studies have shown that silica nanoparticles, like crystalline particles, may cause lung cancer and silicosis. SiNPs are cytotoxic. Additionally, they can cause oxidative stress and mediate apoptosis, depending on the size and dose. Additionally, Research shows that SiNPs are genotoxic (DNA damage, regulation of genes that control apoptosis and autophagy) and immunotoxic (immunotoxicity).
(Yoshida et al. 1999)
Titania
Mainly dental implants.
Long-term effect on dental implants, surface modification resulted in additional benefits such as decreased bacterial adherence and increased hardness.
Nanoparticles provide a greater risk than smaller particles. They enter the body via the nose or mouth. Cancer is prevalent among workers in TiO2 manufacturing plants (as revealed in epidemiological studies). In the hippocampus and cortex, TiO2 nanoparticles that have crossed the blood–brain barrier may aggregate. TiO2 exposure activates microglia, produces reactive oxygen species (ROS), and activates signaling pathways involved in cell death and inflammation.
(Shi et al., 2013)
Silver
Dental restorative material, dental implants and dental prostheses, are all examples of antimicrobial agents.
It has been shown to reduce bacterial colonization and improve dental health. Because of its tiny size, it may easily penetrate bacterial membranes. It is biocompatible and has long-lasting antibacterial activity.
AgNPs are poisonous. Chronic silver exposure can cause argyria. AgNPs are toxic because they generate reactive oxygen species (ROS). Silver ions along with silver nanoparticles both contribute to the toxicity. Ag nanoparticles are implicated in the generation of genotoxicity and oxidative stress, activation of lysosomal AcP, actin disruption, stimulation of hemocyte phagocytosis, and inhibition of Na-K-ATPase.
(Katsumiti et al., 2015)
Carbon nanotubes
Tooth filling, a coating of the tooth surface.
Increased surface area, bringing active substances to living cells, quickly attaching to the tooth and dentin/cementum surface.
The reactivity of carbon nanotubes (CNTs) is strongly influenced by their structure, size, surface, and purity. Crossing membrane barriers using nanotubes can cause inflammatory and fibrotic reactions in rare cases.
(Akasaka et al., 2009)
Graphene
Tooth coating, suitable for implantation, biofilm reduction.
Economical, fracture-resistant, low-density, forms a homogeneous crystal lattice, and effectively treats bacterial biofilms.
The toxicity of graphene is dependent on its structure, size, and oxidative state. Metallic impurities may be introduced during postsynthesis processing, eliciting a range of toxicological reactions.
(Yin et al., 2015)
Hydroxyapatite (HAp)
Reduces dental hypersensitivity, also acts as a cavity filler, delays secondary demineralization, and repairs enamel surfaces.
HAp nanoparticles are easily incorporated into tooth tubules. Comparable to tooth and bone in composition, biocompatible, adheres to the tooth enamel, protects the tooth by forming a coating of fake enamel around it, and corrects periodontal deficiencies.
They have the ability to interact with proteins and form protein–particle complexes that are subsequently destroyed by macrophages in tissues. Blood carries these particles to and distributes them throughout the lungs, spleen, and liver. Nanoparticle toxicity can have an effect on the inflammatory response, signaling system, and oxidative stress.
(Besinis et al., 2012)
Zirconia
Reduces bacterial adhesion to the tooth surface, protects against dental caries, and acts as an excellent polisher.
Similar to the tooth in terms of mechanical properties and color, these materials exhibit low cytotoxicity, good biocompatibility, and great fracture resistance.
Zirconium oxide nanoparticles have been shown to cause considerable DNA damage in human T cells, induce apoptosis, and decrease cell proliferation in human mesothelioma and rodent fibroblast cell lines. Additionally, zirconia was discovered to promote cellular oxidative stress, resulting in cell death. These NPs have been shown in studies to be capable of halting the cell cycle and crossing numerous physiological barriers, resulting in detrimental effects.
(Asadpour et al., 2016)
Silica
Dental filling agent, dental hypersensitivity treatment, antibacterial agent, dental caries prevention, tooth polishing.
It is biocompatible, has a minimal toxic effect, a low density, a high adsorption capacity, and is, most significantly, cost-effective. When used as a polishing agent, the roughness of the tooth surface is reduced.
Toxic effects are dependent on the route of entry and the physiochemical characteristics of the substance. Recent studies have shown that silica nanoparticles, like crystalline particles, may cause lung cancer and silicosis. SiNPs are cytotoxic. Additionally, they can cause oxidative stress and mediate apoptosis, depending on the size and dose. Additionally, Research shows that SiNPs are genotoxic (DNA damage, regulation of genes that control apoptosis and autophagy) and immunotoxic (immunotoxicity).
(Yoshida et al. 1999)
Titania
Mainly dental implants.
Long-term effect on dental implants, surface modification resulted in additional benefits such as decreased bacterial adherence and increased hardness.
Nanoparticles provide a greater risk than smaller particles. They enter the body via the nose or mouth. Cancer is prevalent among workers in TiO2 manufacturing plants (as revealed in epidemiological studies). In the hippocampus and cortex, TiO2 nanoparticles that have crossed the blood–brain barrier may aggregate. TiO2 exposure activates microglia, produces reactive oxygen species (ROS), and activates signaling pathways involved in cell death and inflammation.
(Shi et al., 2013)
Silver
Dental restorative material, dental implants and dental prostheses, are all examples of antimicrobial agents.
It has been shown to reduce bacterial colonization and improve dental health. Because of its tiny size, it may easily penetrate bacterial membranes. It is biocompatible and has long-lasting antibacterial activity.
AgNPs are poisonous. Chronic silver exposure can cause argyria. AgNPs are toxic because they generate reactive oxygen species (ROS). Silver ions along with silver nanoparticles both contribute to the toxicity. Ag nanoparticles are implicated in the generation of genotoxicity and oxidative stress, activation of lysosomal AcP, actin disruption, stimulation of hemocyte phagocytosis, and inhibition of Na-K-ATPase.
(Katsumiti et al., 2015)
Dentin, periodontal ligament, pulp, cementum, and enamel are all components of teeth. Food is easily swallowed as well as digested when chopped and crushed by teeth. Teeth also boost one's self-esteem and overall well-being. Consequently, tooth loss from illness or decay can impede a person's ability to eat, speak, or laugh. There are many ways to safeguard teeth in dentistry (Boelen et al., 2019). However, these attempts have several drawbacks that necessitate new tactics and cutting-edge technology in modern dentistry (Moradpoor et al., 2021).
Antibacterial agents used in the mouth function by degrading or preventing the production of biofilms on the tooth surface. By incorporating silver, gold, or titanium nanoparticles into these biomaterials, their antibacterial characteristics may be enhanced (Abou Neel et al., 2015). Metallic nanoparticles (metallic NPs) have a high surface area, enhancing their antimicrobial activity. Metallic nanoparticles also improve mechanical qualities like strength and durability (Rezaei et al., 2019). Inorganic nanoparticles predominantly composed of metals and metal oxides were proved to have potency used as fillers in dental nanocomposites (Schabes-Retchkiman et al., 2006). Pathogenic microorganisms like Streptococcus mutans (S. mutans) may develop colonies between enamel and dental restorations, resulting in tooth loss. The incorporation of nano encapsulated antimicrobials in dental restorative material enhances the efficacy.
Unlike other biomaterials, nanoparticles have unique bioactivities that could be used in emerging dentistry uses such as endodontics, implantology, and oral malignancies. Nanoparticles have a lot of potential because they are antibacterial, antiviral, and antifungal. To eliminate microleakage and secondary caries, nanoparticles are used in dental composites to inhibit biofilm formation on top of the material. Bacteria are not completely removed from root canals, but reducing their number is essential for successful dental treatment. As a result, NPs effectively minimize microleakage in canal spaces when used against root canal infective species. Restoration materials containing these nanoparticles have improved mechanical qualities and increased binding strength to dentin and biomaterials. Adhesives containing nanoparticles can prevent white spot lesions during orthodontic therapy. When these nanoparticles are incorporated into dental ceramics, in vitro studies have shown that they can prevent fracture and increase the toughness of dental ceramics, thus preventing cracking of the porcelain restoration (e.g., crown or bridge) (Thomas et al., 2018; Salas et al., 2018). The inclusion of nanoparticles in dental biomaterials has shown that they can be helpful; however, long-term evidence is required to support their use for clinical purposes. In addition to determining the advantages of nanoparticles, clinical applications require study of the long-term results in vivo, techniques of nanomaterials inclusion and characterization, and evidence of their long-term antimicrobial activities (Soares et al., 2020).
NPs used in dentistry can comprise a variety of materials, including dendrimers, hydrogel, hydroxyapatite, solid lipids, various polymers, carbon-based NPs, silica, and metals/metal oxides. NPs have been detected in roughly 3500 dental materials (Slavin et al., 2017). Because of their broad-spectrum bactericidal capabilities, NPs used in dentistry in the past decade have primarily included noble metals like silver, gold, platinum, as well as metal oxide NPs such as zirconia, titania, zinc oxide, and iron oxide. As a result, much recent study has focused on the properties of NPs. Several researches have indicated in periodontal apparatus regeneration, including the activation of periodontal ligament cells.
Additionally, recent advancements with in utilization of NPs for dental tissue regeneration and denture foundations have demonstrated satisfying results (Schmalz et al., 2017). In the case of replacement dentures, the dispersion of nanoparticles to the polymers use for tissue conditioning systems could help to reduce the risk of denture stomatitis. Many reviews of nanoparticle applications in dentistry are available, but most articles discuss only a few materials. In this review, we extensively discuss all the nanomaterials and both their advantages and challenges. We also consider nanotechnology-based approaches to dental materials with improved qualities that can be used in the future, as shown in Fig. 1.
Schematic representation of nanoscale materials used to improve the practice of dentistry.
2 Classification of nanomaterials used in dentistry on the basis of shape and composition
Nanomaterials are categorized based on their function and composition. The shape and content of nanomaterials are used to classify them in this instance. Nanoparticles, nanotubes, and nanoplatelets are three subcategories of nanomaterials that come in various shapes.
2.1 Nanoparticles
Nanoparticle-based nanomaterials can be either conventional or unconventional. Metal NPs and metal oxide NPs are examples of conventional nanoparticles, and the uses of metallic and metal oxide NPs have been studied for decades. The latest generation of fillers for innovative dental materials include unconventional NPs (nanodiamonds, nanoshells, and quantum dots) that can easily be customized for different purposes (Fig. 2).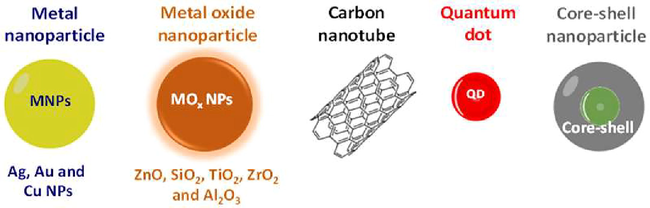
Classification of nanomaterials in dental applications.
2.2 Metallic nanoparticles
Metallic nanoparticles are made by reducing larger particles to smaller ones and then sputtering the resulting nanoparticles onto a surface (Fig. 3). Metal nanoparticles with antibacterial characteristics have the potential to combat bacteria as well as microorganisms in the treatment of dental problems.
Potential uses of metal nanoparticles in dental treatment.
2.3 Silver nanoparticles (AgNPs)
The antibacterial properties of silver were recognized since ancient times. Its antiviral and antifungal activities have also been demonstrated. Silver ions kill microorganisms by inactivating enzymes that prevent DNA replication, resulting in cell death (Samuel and Guggenbichler, 2004). The antibacterial properties of silver have drawn the attention of scientists and because of this properties it has been used in dental materials (Yoshida et al. 1999). The antibacterial characteristics of AgNPs as a filler in dental restorative materials have been demonstrated in recent publications. When utilized as a filler, AgNPs improve dental composites' aesthetics and mechanical qualities (Bürgers et al., 2009) without compromising either. AgNPs in a dental composite were shown in a recent study to minimize the development of lactic acid and the growth of biofilms on teeth, preventing secondary caries (Cheng et al., 2012). Using AgNPs in an endodontic sealer (Gutta-Flow Sealer) with gutta-percha powder in a single-use capsule form showed significant antibacterial efficacy (Punia et al., 2011). To obtain dual-action capabilities like remineralization and antibacterial properties, researchers have looked into the potential of AgNPs in combination with other bioactive substances. Recent studies have shown that the antibacterial activity and strength of dental composites using AgNPs were significantly improved (Weir et al., 2012). Earlier work found that a gel containing AgNPs had substantial antibacterial action against an E. faecalis biofilm (Wu et al., 2014). While testing commercially available dental composite products, researchers found that the dual-action features of these materials had higher mechanical parameters such as flexural strength and elastic modulus.
2.4 Gold nanoparticles (AuNPs)
Gold's inert, biocompatible, and antibacterial qualities have captivated scientists for decades. Gold nanoparticles have been investigated as a possible nanodrug delivery system for treating and detecting cancers in recent research investigations. In these investigations, gold nanostructures such as nanospheres and nanorods were synthesized for usage as photothermal agents, contrast agents, and nanodrug delivery carriers (Pourjavadi et al., 2020). AuNPs were utilized as osteoinductive agents by Heo et al. to immobilize the titanium surfaces of dental implants. As osteoinductive agents for dental implants, gold nanoparticles on dental implant surfaces helped to stimulate bone growth and preserve nascent bone formation around dental implants (Heo et al., 2016). AuNPs can be made in a variety of ways, including chemical functionalization, which renders them less dangerous than other metal nanoparticles.
2.5 Copper nanoparticles (CuNPs)
Copper appears to be an important component in the metabolic activity of plant and animal cells. This is a necessary component of over 30 proteins and is found in practically every cell. When copper creates hydroxyl radicals, it upsets the cellular membrane equilibrium, resulting in membrane leakage and cell death in bacteria and other microbes. Cell membrane destabilization is caused by CuNPs binding to amine and carboxylic groups on the microorganism's surface, which results in cell death (Ruparelia et al., 2008). Free radicals produced by copper ions can alter microorganism DNA replication and protein synthesis (Kishen et al., 2008). For example, according to a recent study, the inclusion of CuNPs provided good antibacterial activity against S. mutans, preventing the adhesive interface from degrading without a significant change in the formulation's mechanical qualities. Metal nanoparticles with antibacterial characteristics have the potential to combat microorganisms and bacteria in the treatment of dental problems.
2.6 Metal oxide nanoparticles
In their oxide, Metal particles are more stable form than as metal particles alone. Metal oxide nanoparticles have recently been the subject of extensive research into their potential as antibacterial agents and dental fillings (Fig. 4).**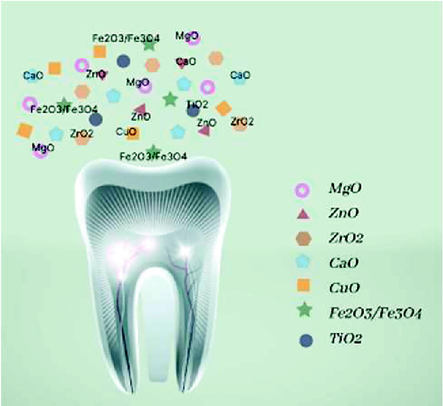
Metal oxide nanoparticles commonly used in dental applications.
2.7 Zinc oxide nanoparticles (ZnO NPs)
The antibacterial efficacy of zinc at the nanoscale is greatly increased compared to that at the macroscale. In the presence of zinc ions, the bacterial cell membrane becomes more permeable, leading to the cell's death (Gutiérrez et al., 2017; Aydin Sevinç and Hanley, 2010). An increase in oxygen radical production and Zn2+ ion production by zinc nanoparticles (ZnNPs) causes an increase in oxidative stress, which inhibits the growth of microorganisms. Human embryonic lung fibroblast (HELF) cell lines have also been used to study ZnO NPs (Yuan et al., 2010). Additionally, ZnNPs exhibited fewer cytotoxic effects on gastric epithelial cell lines (GES-1), increasing when supplied with ascorbic acid (Wang et al., 2014). Antibacterial activity against Lactobacillus and Streptococcus mutans has been demonstrated in dental composite resins comprising nanoparticles of ZnO and nanoparticles of Ag, according to Kasraei et al. (2014). Dental resin composites have been found to benefit from the mechanical and antibacterial features of cellulose nanocrystal and zinc oxide nanoparticle (ZnO NP) nanohybrids, as revealed by Wang et al. (2019). In dental materials, antibacterial properties of ZnO NPs can be beneficial.
2.8 Titanium dioxide nanoparticles (TiO2 NPs)
The exceptional features of titanium alloys, like their high strength along with corrosion resistance, and excellent biocompatibility (Özcan and Hämmerle, 2012), make them widely employed in dentistry. UV rays cause TiO2 (titanium dioxide) to produce reactive oxygen (OH and H2O2) radicals that cause bacterial cell lysis by interfering with their phosphorylation and creating an imbalance in the micro-osmotic organism's pressure. An earlier study found that human gingival fibroblasts were inflamed when exposed to high concentrations of TiO2 NPs (Garcia-Contreras et al., 2015). In oral squamous cell carcinoma cell lines, GIC modification with TiO2 showed moderate biocompatibility (Padovani et al., 2015). Streptococcus mutans and Streptococcus sanguinis colony counts were reduced significantly when TiO2 nanoparticles were added to composite resins (Sodagar et al., 2017). Compared to pure titanium’s smooth and rough surfaces, a chemical etching approach was used to achieve nanoscale modification of the titanium surface in order to quantify the proliferation of murine pre-osteoblastic cells in vitro. There is still much work to be done before TiO2 can be used in oral applications.
2.9 Zirconium dioxide nanoparticles (ZrO2 NPs)
Zirconia has been used for decades in dental materials and its importance cannot be underestimated. In rigidity, capacity, fatigue resistance, good wear resistance, and biocompatibility, zirconia (sometimes known as “ceramic steel”) far outstrips the competition. Because of its close resemblance in characteristics and color to the natural tooth, it is an excellent choice for cosmetic purposes. Using laser vaporization techniques, it is possible to make zirconia nanoparticles as small as 20–50 nm (Meng et al., 2013). In terms of dental implants and prosthetics, they have been demonstrated to have biocompatibility, osteoconductivity, and the inclination to decrease plaque buildup, and the mechanical characteristics of PMMA can be improved by using ZrO2 NPs as a filler to reinforce the matrix (Enomoto et al., 2017). Zirconia-based ceramics outperformed alumina ceramics in terms of strength and bending resistance. Dentists can utilize ZrO2 NPs as a filler in dental nanocomposites for improving the materials' mechanical properties, radio-capacity, as well as appearance. The fracture toughness of dental composites using zirconia nanoparticles has been improved. The compressive strength of GIC restorative cement was improved, and cracks within the cement matrix were reduced with ZrO2 NPs. Color stability, High strength, along with low thermal and electrical conductivity were some of the benefits observed when using ZrO2 NPs in the manufacture of dentures (Gad et al., 2016a).
2.10 Aluminum oxide nanoparticles (Al2O3 NPs)
Aluminum oxide nanoparticles can be manufactured using a variety of procedures, including decomposition and reduction process, bursting wire, laser ablation, mechanical ball-milling, and wet-chemical synthesis. Because Al2O3 NPs are sensitive to sunlight, heat, and moisture, special precautions must be taken to keep them safe. Additionally, Al2O3 NPs should be stored in dry and cool environment in a vacuum. Alumina ceramics have better aesthetics, a more polished surface, wear resistance, hardness, and good biocompatibility with the surrounding oral tissues compared to other materials. Al2O3 NPs can improve the mechanical strength of inadequate dental materials. Low flexural strength and impact strength are two drawbacks of polymethylmethacrylate (PMMA). Adding Al2O3 NPs to a PMMA matrix significantly improved the resin's mechanical and thermal properties and reduced water absorption and solubility. In 2012, UC3M (University Carlos III of Madrid)created an orthodontic bracket made of polysulfone integrated with alumina nanoparticles that has excellent strength, reduced friction as well as mechanical resistance (Llorente et al., 2016).
2.11 Silicon dioxide nanoparticles (SiO2 NPs)
Using silicon dioxide NPs as filler can improve the mechanical qualities of dental restorative materials. As a general dental polishing agent, silica fine powder is used to smooth rough surfaces on teeth to avoid food collection or plaque buildup and thus keep teeth clean. A silicon oxide with the chemical formula SiO2, usually referred to as silica, is most widely distributed in nature as quartz, a form of SiO2 NPs. The use of SiO2 NPs in various biological and dental applications is on the rise. Silica nanoparticles can be loaded with a variety of medicinal compounds. For their capability, low toxicity, biocompatibility, surface area, size, and adsorption SiO2 NPs serve an essential function in dentistry. It is possible to seal dentinal tubules, which cause hypersensitivity when exposed, with mesoporous SiO2. When HA (hydroxyapatite) nanoparticles and silica nanoparticles were combined, they increased calcium phosphate compounds’ concentration in the dentin as well as the volume of mineral in the dentin that had been demineralized (Besinis et al., 2012).
3 Unconventional nanoparticles in dentistry
Nanoparticles have been getting investigated for their special characteristics in the advent of sophisticated dental nanomaterials. Nanoparticles can be used to create dental materials that are robust, nontoxic, and antimicrobial, among other things. In this regard, nanotechnology allows the exploration of numerous nanoparticles for dental applications and testing. Different approaches to integrating nanoparticles in dental materials have been implemented depending on the particle characteristics.
3.1 Nanodiamonds
The hardest natural substance on the planet, diamond, is well known to the general public. Small diamonds, known as nanodiamonds (NDs), are less than 100 nm in diameter. Their outstanding surface and chemical nature in dental nanocomposite manufacturing make them an excellent filler choice. Root canal treatments have recently been improved by developing an amoxicillin-loaded nanodiamond gutta-percha composite (NDGP-AMC) (Lee et al., 2015a). Fixed temporary dental prostheses with ND-integrated PMMA performed better overall. In the field of restorative dentistry, nanodiamonds can be used in a wide variety of ways. The main applications of NDs in dentistry include directed tissue regeneration, polymer reinforcement, and drug administration to treat infections and cancer. It also used as bioactive or antibacterial dental implant coatings.
3.2 Quantum dots
A quantum dot is a semiconductive nanoparticle like indium sulfide, zinc sulfide, or lead sulfide, that may emit light when exposed to a specific amount and wavelength of light. Exposure to an external magnetic light or field can alter the dots’ semiconductive characteristics. In addition, these materials can be employed as nanocarriers for drug or genetic treatments. Through conjugation with photosensitizers and cancer-targeting medicines, quantum dots could be used for cancer treatment. It also used in therapeutic purpose and in diagnostic imaging. Cancer cells can be more easily attached if they are coated with particular chemicals and UV light is emitted, the diagnosis of oral malignancies is improved. As a result, quantum dots can be employed to treat head and neck illnesses by delivering drugs and correcting genetic errors. They can also help to prevent oral cancer (Kanaparthy and Kanaparthy, 2011).
3.3 Nanoshells
An inner dielectric core is encased in a thin metal shell to form nanoshells. In dentistry, nanoshells can be employed for various therapeutic purposes. When stimulated with infrared light, the metal coverings of nanoshells can be used to destroy oral cancer cells by causing a tremendous amount of heat to build up around the nanoshells and eventually kill the cells (El-Sayed et al., 2006). For example, the ligation of the vessels can be utilized for reducing angiogenesis, promoting wound healing, as well as limiting internal bleeding,. With this treatment, blood loss in trauma victims can be reduced while preserving neighboring vital tissues. Targeted medication delivery can be achieved by using nanoshells filled with proteins, antibodies, or other cell-targeting agents (El-Sayed et al., 2006).
3.4 Quaternary ammonium methacrylate (QAM) nanoparticles
The antibacterial properties of quaternary ammonium methacrylate nanoparticles make them suitable for usage in restorative dental materials. This antibacterial chemical destroys the target cells by causing cytoplasmic leakage in microorganism cell walls. There are positively and negatively charged surfaces on QAM resins and bacteria, favoring ionic attachment and increasing osmotic pressure in the cell membrane, ultimately leading to cell death (Li et al., 2014). The capacity of QAM resins to block 3D biofilms is an exciting feature. When bacteria are exposed to QAM's antimicrobial properties, they become more sensitive to apoptosis. The bond strength and antibacterial properties of QAM resins may also be relevant in the development of more advanced dental adhesive restorative solutions with QAM resins (Cheng et al., 2013).
3.5 Quaternary ammonium polyethyleneimine (QPEI) nanoparticles
It is possible to prevent root canal infections by using antibacterial sealers. The antibacterial properties of QPEI NPs have led to their use in commercially available endodontic sealers such as Gutta-flow, Epiphany, and AH plus. There were no significant unfavorable effects on mechanical characteristics when QPEI NPs were used in resin composites, and they demonstrated high antibacterial activity (Beyth et al., 2008). QPEI NPs have also shown stability and great antibacterial potency without generating any byproducts with the inclusion of the latter two products.
3.6 Amorphous calcium phosphate nanoparticles (ACP NPs)
Dentin or enamel remineralization can have a good effect on oral health. Restoring materials using a mineralizing chemical can be highly beneficial. Generally, minimally invasive techniques are utilized to remove deep carious lesions for protecting the pulp along with retain the tooth structure. In restorative dental materials, the antimicrobial effects of nanoparticles can be combined with the remineralization of the decayed tooth by their inclusion in these materials. Nanoparticles of amorphous calcium phosphate in dental composites release calcium and phosphate ions to maintain pH levels in acidic conditions (Xu et al., 2011).
4 Nanotube-based nanomaterials
4.1 Carbon nanotubes (CNTs)
Carbon nanotubes (CNTs) offer exceptional mechanical and electrical properties. It has also been suggested that CNTs be used to strengthen dental composites. SWCNTs (single-walled carbon nanotubes) are graphene-coated cylinders that have attracted the research community. Dental resins including SWCNTs as a filler have shown great flexural strength and satisfactory outcomes. In addition, CNTs can be used to cover titanium dental implants. Previous research has suggested that the epidermal growth factor (EGF) is a promising anticancer medication delivery vehicle for CNTs (Bhirde et al., 2009). Various nanomaterials have had their mechanical qualities boosted by the inclusion of carbon nanotubes (CNTs) and carbon nanofibrils (CNFs). Cooper et al. used a dry powder mixing process to make the composites with varying amounts of CNTs or carbon nanofibrils integrated into a PMMA matrix. The authors of (Cooper et al., 2002) found that composites had high impact strength and improved mechanical properties.
4.2 Halloysite nanotubes (HNTs)
Nanomaterials can be derived from clay. In dentistry, HNTs can be used as dental fillers and nanodrug delivery agents, making them acceptable alternatives. HNTs have a nanotubular structure with a typical nanometer size. Because of their natural milky white color, elastic modulus, and high strength, they are suitable fillers in dental composite manufacturing. A nanofiller that can prevent the production of oral biofilm and hence prevent the growth of secondary dental caries can be created by loading HNTs with antibacterial drugs (Avani et al.,2020).
4.3 Nanoplatelet-based nanomaterials
Materials made from nanoplatelets such as nanosheets or flakes are called nanomaterials. Nanoplatelet-based nanomaterials can use graphene. The unique features of graphene oxide nanoplatelets make them ideal for dental applications.
4.4 Graphene oxide nanoplatelets
Carbon atoms organized in a hexagonal honeycomb lattice make up graphene, which has unique properties. When graphene nanopowder is used to manufacture dental nanomaterials, new opportunities arise (Sava et al., 2015). When using graphene in dental nanocomposites, the mechanical properties of the composite were significantly improved. According to several studies, incorporating graphene into dental materials can serve as both filler as well as an antibacterial agent (Das et al., 2011). Dentists may benefit from using graphene-based nanoplatelets, which have shown high antibacterial action against Streptococcus mutans. There must be no cytotoxic effect on healthy tissues for tissue engineering to be successful. Fibrin and graphene oxide (GO) showed no detrimental effect on periodontal ligament stem cells (PDLSCs) in recent studies, which indicates that GO-based tissue scaffolds have significant potential in regenerative dentistry. Bioactivity, increased bone production, and decreased inflammatory reactions have been attributed to graphene oxide (GO)-coated Ti (GO-Ti) membranes. Graphene's excellent biocompatibility was demonstrated by the attachment and proliferation of dental pulp stem cells (DPSCs) on a GO-based substrate (Rosa et al., 2016). Nanomaterials such as graphene and its derivatives can be used to increase the performance of dental implants, and they can be employed as high-performance coatings for implants.
5 Nanomaterials used in dentistry applications
Nanomaterials are efficiently used for various dentistry applications such as prosthodontic, endodontic, restorative, periodontal, orthodontic, and implantology treatments, as shown in Fig. 5.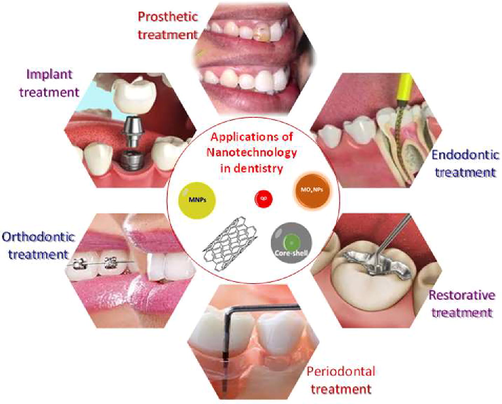
Applications of nanomaterials in different fields of dentistry.
5.1 Prosthodontics
TiO2 nanoparticles were added to a 3 dimensional poly-methylmethacrylate (PMMA) denture foundation to enhance its antimicrobial and structural features (Totu et al., 2017). According to FTIR, SEM, and antimicrobial efficiency tests against Candida species, significant changes in the structural and chemical features were identified. A nanozirconium-oxide-modified heat-cured PMMA was also investigated (Ahmed and Ebrahim, 2014). The inclusion of zirconium oxide nanoparticles greatly enhanced the denture base's hardness, flexibility, and fracture toughness because of their superior dispersion qualities, decreased aggregation potential, and biocompatibility with the organic polymer. The transverse strength of a restored denture base was also increased by nanozirconium during the building phase (Gad et al., 2016b). According to the research results, a three-point bending test revealed that the greatest transverse strength in repairs was made with an auto-polymerized resin modified with 2 or 5% zirconium oxide. According to researchers, removable prosthodontics is just one of the numerous fields where nanomodified zirconium oxide particles can be used in resin matrices.
For denture soft liners and obturators, researchers tested the antifungal properties of a chlorhexidine coating with varied nanoparticle additions. One or more nanoparticles was used: chlorhexidine combined with TP or HMP, sodium triphosphate (TP), or tri-metaphosphate (TMP). The introduction of nanoparticles had no effect on the hydrophilicity or water absorption of denture silicons after 16 weeks in artificial saliva. Because of the nanomodified chlorhexidine coatings, artificial saliva included soluble chlorhexidine, with chlorhexidine–TP and chlorhexidine–TMP being the most concentrated forms. Candida albicans metabolic activity was the most effectively inhibited by the chlorhexidine–HMP coating. In the future, these coatings could become therapeutically necessary for extending the lifespan of dental prosthetics and promoting oral health while also saving patients money.
Nanoparticle-impregnated luting cement was found to be much better at rising the bond strength to dentin and enamel than regular luting cement. Dentin has a greater elastic modulus and less polymerization shrinkage because it may go deeper into the dentin tubules. (Sadat-Shojai et al., 2010). The compression and tensile strength of zinc polycarboxylate were improved in 2011 by integrating ZnO and MgO nanoparticle encapsulation (Zebarjad, 2011). Zinc polycarboxylate cement was shown to have physical and mechanical properties that were superior to typical zinc polycarboxylate cement. One study found substantial differences between a standard zinc polycarboxylate cement and a nanomodified zinc polycarboxylate cement in terms of cement strength. The compressive, tensile, and biaxial flexural strengths of glass ionomer cement were significantly improved when nanohydroxyapatite/fluorapatite particles were added (Moshaverinia et al., 2008).
The recently launched resin nanoceramic CAD/CAM blocks also showed improved tribological properties. The LavaTM ultimate resin nanoceramic blocks made by 3 MTM ESPE were superior (Chen et al., 2014). A resin matrix with a nanoceramic impregnation that can be easily adjusted and tailored after milling was developed, resulting in materials with qualities superior to those manufactured from only resin or ceramics. When it comes to clinical applications, researchers must ensure the safety of nanoparticles by investigating their long-term biocompatibility, mechanical characteristics, toxicity, as well as other physical and chemical features.
5.2 Endodontics
Endodontic sealers can incorporate bioceramic nanoparticles like bioglass, zirconia, and glass ceramics, which are all examples of nanotechnology in action. Adhesive nanoparticles have been found to improve the adhesive's ability to respond to nano-irregularities and its fast setting time, insolubility of tissue fluid, dimensional stability and chemical connection to tooth tissue and osteoconductivity. A recent study found a novel bioactive endodontic sealer that showed antibacterial properties against endodontic biofilm, strong bonding to dentin, along with ionic release of phosphate and calcium when employed. Di-methylamino hexadecyl methacrylate (DMAHDM), ACP (amorphous calcium phosphate) nanoparticles, MPC (2-methacryloyloxyethyl phosphorylcholine), and were used to create the sealer (NACP). Nanoparticles have been particularly helpful in expediting the remineralization as well as improving the bonding strength of the sealer to dentin, which prevented the creation of endodontic stresses.
Enterococcus faecalis growth was suppressed by calcium hydroxide intracanal medication with silver nanoparticle solution, which was evaluated both short- and long-term (Utneja et al., 2015). This was more efficient against E. faecalis than calcium hydroxide alone or calcium hydroxide paired with chlorhexidine. After a week, nanosilver particles had a solid antibacterial impact, but they had no meaningful effect after a month. Researchers found that nanosilver particles worked quickly and effectively against E. faecalis bacteria. Therefore, researchers came to the conclusion that nanosilver particles were ineffective in suppressing E. faecalis in an in vitro investigation done in 2014 (Mozayeni et al., 2014). A root canal sealant containing nanosilver particles was prepared and compared to chlorhexidine and triple antibiotic paste; this nanosilver gel was less effective in preventing the spread of E. faecalis. According to researchers, because of its production process and gel consistency, the nanosilver gel was less effective than chlorhexidine and triple antibiotic pastes because nanoparticles could not be released from the gel. Gutta-percha (GP) was also studied to incorporate nanodiamond particles for improvement (Lee et al., 2015b). The use of nanodiamond-impregnated GP in obturation following a traditional process showed superior chemical properties, biocompatibility, and mechanical qualities, as indicated by digital radiography and microcomputed tomography. Nano-GP has the potential to be an enhanced endodontic filler because of its high-quality adaptation to canal walls and minimal void formation.
5.3 Periodontics, implantology, and regenerative dentistry
The use of triclosan- or tetracycline-loaded nanoparticles has allowed scientists to develop new drug delivery systems to treat periodontal disease. Because of their homogeneous distribution, these nanoparticles can remain in contact with an affected area for an extended period (Sharma et al., 2016). For example, chemically stable nonionic vesicles known as “niosomes” that are both precise and effective can be used to deliver drugs to specific areas of the body, especially whenever the particles are smaller than 100 nm (Pradeepkumar et al., 2012).. Many possible uses for fullerenes, including drug delivery, have been thoroughly investigated. Fullerenes are variously shaped hollow carbon molecules (spheres, tubes, and ellipsoids). In 1980s, the first and most stable fullerene, buckminsterfullerene (C60), was discovered and named after Buckminster Fuller because it resembled the geodesic domes he built. As per the literature review, a stable fullerene structure can be achieved by assembling the fullerenes from larger atoms rather than building them atom by atom (Pradeepkumar et al., 2012). Fullerenes are also employed as antioxidants and radical scavengers in the medical field.
Bone grafting using a light-curable methacrylate resin matrix and nACP was shown to be advantageous, according to the research. nACP crystallizes back into hydroxyapatite a few minutes after injection (Pradeepkumar et al., 2012. An implant's osseointegration is expected to be improved if the extracellular matrix surface topography in native tissue is duplicated on the implant surface that is generally 10–100 nm in size. To increase the adherence of fibrin, nanoparticles including titanium oxide, silver, gold, and hydroxyapatite nanoparticles have been proven to be useful; mechanical nanofeatures like nanogrooves or nanopillars have also been demonstrated to be effective.
5.4 Challenges faced by emerging dental nanomaterials
Oral care products like dentifrice and mouthwash contain suspensions of nanomaterials for cleansing and remineralization, whereas dental composites are composed of powdered nanoparticles. Silver nanoparticles in composites are likely to be considered a medicinal product. When considering the potential benefits and hazards of these new nanomaterials, patients' oral tissues and homeostasis must be considered. For example, silver nanoparticles directly integrated into resin-based composites have been shown to rapidly leach, and ROS generation from these accumulating nanoparticles could contribute to enhanced pro-inflammatory responses and oxidative stress.
The oral epithelium may become hypersensitive to nanoparticles trapped in saliva's mucous production, which can interact with salivary components and cause a local hypersensitivity reaction. These nanoparticles have been demonstrated to alter the conformation of lysozyme and amylase, compromising their ability to operate as enzymes. Further inquiry is needed into the possibility of enhanced absorption rates if these nanomaterials are accidentally consumed ingested nanoparticles of titanium dioxide, for example, could enter the bloodstream via the gastrointestinal tract, posing a risk to the body's internal organs. In addition to the dangers to patients, dental practitioners have also been exposed to aerosols while drilling into a nanocomposite.
6 Concluding remarks
Nanomaterials have shown promising outcomes for various current and future dental uses. Researchers were able to enhance the physical and mechanical qualities of current materials with the help of nanotechnology and to create new materials. Nanoparticles, for example, can strengthen polymer composites and increase the surface area for cell adhesion in tissue engineering scaffolds. There is much ongoing research in this field worldwide, and the field is receiving a lot of funding. Dental materials science will likely undergo substantial changes as new nanomaterials are discovered and developed. It is hoped that promising nanomaterials will supply a wide range of dental materials in the coming decade.
7 Future perspectives
In general, nanotechnology should deliver solutions and “go green” in terms of health and safety. Green nanotechnology should carefully weigh its potential benefits against its possible costs to society, including environmental, public, and occupational health risks. As a result, the environmental and societal benefits will be maximized and health and cost savings. Recently developed nanomaterials and nanotechnology can assist in shed light on commercial applications of nanomaterials for the “real” regeneration of periodontal apparatus as a whole, comprising dentine, cementum, periodontal ligaments, and bone. Tissue engineering triads and scaffolds impregnated with nanoparticles can simulate an extracellular matrix to help stimulate the creation of host tissues in animals. Therefore, low toxicity, antibacterial properties, and better protein–surface interactions make them well-suited for a wide range of dental applications. They're Dentists are excited about the prospect of using these materials to create new and superior biomaterials in a variety of ways. The advancements in nanotechnology and the improvement of conventional treatment modalities have the potential to improve dental care.
Acknowledgements
The authors would like to acknowledge Sree Balaji Dental College and Hospital, Pallikarani, Chennai, India for providing financial support and facilities for the completion of this work.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Nanotechnology in dentistry: prevention, diagnosis, and therapy. Int. J. Nanomed.. 2015;10:6371-6394.
- [Google Scholar]
- Effect of zirconium oxide nano-fillers addition on the flexural strength, fracture toughness, and hardness of heat-polymerized acrylic resin. World J. Nano Sci. Eng.. 2014;2:31-36.
- [Google Scholar]
- Modification of the dentin surface by using carbon nanotubes. Bio-med Mater Eng.. 2009;19:179-185.
- [Google Scholar]
- Oxidative stress-mediated cytotoxicity of zirconia nanoparticles on PC12 and N2a cells. J. Nanoparticle. Res. 2016;18:14.
- [Google Scholar]
- Vancomycin loaded halloysite nanotubes embedded in silk fibroin hydrogel applicable for bone tissue engineering. Int. J. Polym. Mater. 2020;69:32-43.
- [Google Scholar]
- Antibacterial activity of dental composites containing zinc oxide nanoparticles. J. Biomed. Mater. Res. B Appl. Biomater.. 2010;94:22-31.
- [Google Scholar]
- Infiltration of demineralized dentin with silica and hydroxyapatite nanoparticles. Dent. Mater.. 2012;28:1012-1023.
- [Google Scholar]
- Surface antimicrobial activity and biocompatibility of incorporated polyethylenimine nanoparticles. Biomaterials 2008:4157-4163.
- [Google Scholar]
- Targeted killing of cancer cells in vivo and in vitro with EGF-directed carbon nanotube-based drug delivery. ACS. Nano. 2009;3:307-316.
- [Google Scholar]
- Matrix metalloproteinases and inhibitors in dentistry. Clin. Oral. Investig.. 2019;23:2823-2835.
- [Google Scholar]
- The anti-adherence activity and bactericidal effect of microparticulate silver additives in composite resin materials. Arch. Oral. Biol. 2009;54:595-601.
- [Google Scholar]
- The fracture resistance of a CAD/CAM Resin Nano Ceramic (RNC) and a CAD ceramic at different thicknesses. Dental. Mater.. 2014;30:954-962.
- [Google Scholar]
- Dental primer and adhesive containing a new antibacterial quaternary ammonium monomer dimethylaminododecyl methacrylate. J. Den.. 2013;41:345-355.
- [Google Scholar]
- Lin-Gibson, S. Effect of amorphous calcium phosphate and silver nanocomposites on dental plaque microcosm biofilms. J Biomed Mate.r Res. B Appl. Biomater.. 2012;100:1378-1386.
- [Google Scholar]
- Wagner HD. Distribution and alignment of carbon nanotubes and nanofibrils in a polymer matrix. Compos. Sci. Technol. 2002;62:1105-1112.
- [Google Scholar]
- Synthesis of silver nanoparticles in an aqueous suspension of graphene oxide sheets and its antimicrobial activity. Colloids Surf. B Biointerfaces. 2011;2011(83):16-22.
- [Google Scholar]
- Selective laser photo-thermal therapy of epithelial carcinoma using anti-EGFR antibody conjugated gold nanoparticles. Cancer. Lett.. 2006;239:129-135.
- [Google Scholar]
- Unique hydrophobization and hybridization via direct phase transfer of ZrO2 nanoparticles from water to toluene producing highly transparent polystyrene and poly(methyl methacrylate) hybrid bulk materials. Macromolecules. 2017;50:9713-9725.
- [Google Scholar]
- Influence of incorporation Of ZrO2 nanoparticles on the repair strength of polymethyl methacrylate denture bases. Int. J. Nanomed.. 2016;11:5633-5643.
- [Google Scholar]
- The reinforcement effect of nano-zirconia on the transverse strength of repaired acrylic denture base. Int. J. Dentistry. 2016;7094056
- [Google Scholar]
- Alteration of metabolomic profiles by titanium dioxide nanoparticles in human gingivitis model. Biomaterials. 2015;57:33-40.
- [Google Scholar]
- The role of copper nanoparticles in an etch-and-rinse adhesive on antimicrobial activity, mechanical properties and the durability of resin-dentine interfaces. J. Dent.. 2017;61:12-20.
- [Google Scholar]
- Titanium dental implants surface-immobilized with gold nanoparticles as osteoinductive agents for rapid osseointegration. J. Colloid. Interface. Sci.. 2016;469:129-137.
- [Google Scholar]
- Kasraei, S., Sami, L., Hendi,S., AliKhani,M.Y., Rezaei-Soufi, L., Khamverdi Z.,2014. Antibacterial properties of composite resins incorporating silver and zinc oxide nanoparticles on streptococcus mutans and lactobacillus. Restor. Dent. Endod. 109.
- Mechanisms of toxicity of ag nanoparticles in comparison to bulk and ionic ag on mussel hemocytes and gill cells. PloS One. 2015;10:e0129039.
- [Google Scholar]
- Plant-mediated green synthesis of silver nanoparticles using Trifolium resupinatum seed exudate and their antifungal efficacy on Neofusicoccum parvum and Rhizoctonia solani. IET Nanobiotechnol.. 2016;10:237-243.
- [Google Scholar]
- An investigation on the antibacterial and antibiofilm eflcacy of cationic nanoparticulates for root canal disinfection. J. Endod. 2008:1515-1520.
- [Google Scholar]
- Nanodiamond-Gutta percha composite biomaterials for root canal therapy. ACS Nano. 2015;9:11490-11501.
- [Google Scholar]
- Nanodiamond–gutta percha composite biomaterials for root canal therapy. ACS. Nano. 2015;9:11490-11501.
- [Google Scholar]
- Effect of charge density of bonding agent containing a new quaternary ammonium methacrylate on antibacterial and bonding properties. Dent. Mater.. 2014;30:433-441.
- [Google Scholar]
- Nanoindentation and wear behavior of thermally stable biocompatible polysulfone–alumina nanocomposites. RSC. Adv.. 2016;102:100239-100247.
- [Google Scholar]
- Effects of hierarchical micro/nano-textured titanium surface features on osteoblast-specific gene expression. Implant Dent.. 2013;22:656-661.
- [Google Scholar]
- An overview of recent progress in dental applications of zinc oxide nanoparticles. RSC. Adv.. 2021;11:21189-21206.
- [Google Scholar]
- Modification of conventional glass-ionomer cements with N-vinylpyrrolidone containing polyacids, nano-hydroxy and fluoroapatite to improve mechanical properties. Dent. Mater.. 2008;24:1381-1390.
- [Google Scholar]
- Antimicrobial effects of four intracanal medicaments on enterococcus faecalis: an in vitro study. Iranian Endod. J. 2014;2014(9):195-198.
- [Google Scholar]
- Titanium as a reconstruction and implant material in dentistry: Advantages and pitfalls. Materials. 2012;5:1528-1545.
- [Google Scholar]
- Padovani, G.C., Feitosa, V.P., Sa,;uro, S., Tay, F.R., Durán, G., Paula, A.J.,2015.. Advances in dental materials through nanotechnology: Facts, perspectives and toxicological aspects. Trends. Biotechnol. 33, 621–636.
- Synthesis of micelles based on chitosan functionalized with gold nanorods as a light sensitive drug delivery vehicle. Int. J. Biol. Macromol.. 2020;149:809-818.
- [Google Scholar]
- Saravanan A. Current research in Nano periodontics. SRM J. Res. Dental Sci.. 2012;3:46.
- [Google Scholar]
- An in vitro assessment of apical microleakage in root canals obturated with gutta-flow, resilon, thermafil and lateral condensation: A stereomicroscopic study. JCD. 2011;14:173-177.
- [Google Scholar]
- The Role of Nanomaterials in the Treatment of Diseases and Their Effects on the Immune System. Open Access Maced J Med Sci.. 2019;7(11):884-1890.
- [Google Scholar]
- Graphene oxide-based substrate: Physical and surface characterization, cytocompatibility and differentiation potential of dental pulp stem cells. Dent. Mater. 2016;32:1019-1025.
- [Google Scholar]
- Strain specificity in antimicrobial activity of silver and copper nanoparticles. Acta. Biomater.. 2008;4:707-716.
- [Google Scholar]
- Hydroxyapatite nanorods as novel fillers for improving the properties of dental adhesives: Synthesis and application. Dental. Mater.. 2010;26:471-482.
- [Google Scholar]
- Prevention of catheter-related infections: The potential of a new nano-silver impregnated catheter. Int. J. Antimicrob. Agents. 2004;23:75-78.
- [Google Scholar]
- The study of new composites with graphene used in dentistry. Chemia: Studia Universitatis Babes-Bolyai; 2015. p. :60.
- Biosynthesis and characterization of Ti/Ni bimetallic nanoparticles. Opt. Mater.. 2006;29:95-99.
- [Google Scholar]
- Titanium dioxide nanoparticles: a review of current toxicological data. Particle Fibre Toxicol.. 2013;10:15.
- [Google Scholar]
- Metal nanoparticles: understanding the mechanisms behind antibacterial activity. J. Nanobiotechnol.. 2017;3:5(1):65.
- [Google Scholar]
- Raman spectroscopy-multivariate analysis related to morphological surface features on nanomaterials applied for dentin coverage. Spectrochim. Acta A. 2020;5:228-117818.
- [Google Scholar]
- Elhaminejad. F. Effect of TiO2 nanoparticles incorporation on antibacterial properties and shear bond strength of dental composite used in orthodontics. Dental. Press. J. Orthod. 2017;22:67-74.
- [Google Scholar]
- Autism and primary care dentistry: parents' experiences of taking children with autism or working diagnosis of autism for dental examinations. Int. J. Paediatr. Dent.. 2018;28:226-238.
- [Google Scholar]
- Poly (methyl methacrylate) with TiO 2 nanoparticles inclusion for stereolitographic complete denture manufacturing− the fututre in dental care for elderly edentulous patients? J. Dent.. 2017;59:68-77.
- [Google Scholar]
- Current perspectives of bio-ceramic technology in endodontics: calcium enriched mixture cement - review of its composition, properties and applications. Restorative. Dentistry. Endod.. 2015;40:1-13.
- [Google Scholar]
- Strong antibacterial dental resin composites containing cellulose nanocrystal/zinc oxide nanohybrids. J. Dent.. 2019;80:23-29.
- [Google Scholar]
- Combined toxicity study of zinc oxide nanoparticles and vitamin C in food additives. Nanoscale. 2014;24:15333-15342.
- [Google Scholar]
- Nanostructured Biomaterials for Regeneration. Adv. Funct. Mater.. 2008;18:3566-3582.
- [Google Scholar]
- Remineralization of demineralized enamel via calcium phosphate nanocomposite. J. Dent. Res.. 2012;2012(91):979-984.
- [Google Scholar]
- Evaluation of the antibacterial eflcacy of silver nanoparticles against enterococcus faecalis biofilm. J. Endod. 2014;40:285-290.
- [Google Scholar]
- Nanocomposite containing amorphous calcium phosphate nanoparticles for caries inhibition. Dent. Mater.. 2011;27:762-769.
- [Google Scholar]
- Design, synthesis, and characterization of graphene–nanoparticle hybrid materials for bioapplications. Chem. Rev.. 2015;115:2483-2531.
- [Google Scholar]
- Antibacterial activity of resin composites with silver-containing materials. Eur. J. Oral. Sci. 1999;107(4):290-296.
- [Google Scholar]
- Determination, characterization and cytotoxicity on HELF cells of ZnO nanoparticles. Colloids Surf. B: Biointerfaces. 2010;76:145-150.
- [Google Scholar]
- Synthesis and characterization of nanoparticles and nanocomposite of ZnO and MgO by sonochemical method and their application for zinc polycarboxylate dental cement preparation. Int. Nano Lett.. 2011;1:43-51.
- [Google Scholar]






