Translate this page into:
Synthesis, Jahn-Teller labeled via crystal structure in trans-(ClO4)2CuII(Me2N-Py)4 complex: S9/S6/C-H…O synthons, thermal, physicochemical and 1BNA-docking
⁎Corresponding authors. warad@najah.edu (I. Warad), shaukathara@yahoo.co.in (S.A. Khanum)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
The synthesis of the trans-(ClO4)2CuII(Me2N-Py)4 complex (where Me2N-Py = N,N-dimethylpyridin-4-amine ligand) in significant yield involved the reaction of excess Me2N-Py ligand with Cu(ClO4)2·6H2O in MeOH solvent. The progression of the complex synthesis was monitored by UV–Vis, FT-IR, EDX, XRD, MS, SEM, CHN-EA, and TG/DTG. The resulting complex crystallized in the Triclinic space P . In the solid state, the desired complex structure discrete a neutral trans-(ClO4)2CuII(Me2N-Py)4 formula, with copper(II) adopting a distorted octahedral environment formed by four Me2N-Py ligands occupying the square planar position Cu(Py)4, meanwhile, the two ClO4 are in linear trans-position. The XRD measurements proved the existence of elongation Jahn-Teller distortion in the synthesized complex. Furthermore, a comprehensive analysis of the desired complex, including. XRD/HSA interactions revealed the presence of two S9 and S6 main 2D-synthons primarily formed through Cph-H….OClO3 and CMe-H…OClO3 non-classical H-bonding interactions. Thermal stability assessments through TG/DTG indicated that the complexes with the most stable behavior underwent two degradation steps. The molecular docking of the desired complex against 1BNA-DNA was also evaluated.
Keywords
[Cu(Py)4]
XRD/HSA
Docking
Jahn-Teller
Thermal
1 Introduction
Several inorganic chemists have designed octahedral copper(II) complexes using monodentate ligands obtained from pyridine derivatives (Santana et al., 2020; Masternak et al., 2021; Patel et al., 2010; Handy et al., 2017; Warad et al., 2018; Nnabuike et al., 2023). The redox characteristics of Cu(II) complexes have been thoroughly evaluated because of their biological relevance to imitateprotein functions (Fathy et al., 2022; Adhikari et al., 2022; Graur et al., 2023; Alem et al., 2023; Fathima et al., 2020; Andrejević et al., 2023; Jiang et al., 2007; Czaikowski et al., 2022; Balakrishnan et al., 2017; Fu et al., 2015; Jevtovic et al., 2023; Puchoňová et al., 2017; Anupama et al., 2017). [Copper(II)/Py]/[Copper(II)/phen] and their derivative complexes are of great importance among these complexes because of their flexibility to alter structurally and their wide range as DNA-binding, antibacterial, and COX/LOX-inflammation (AlAli et al., 2023; AlAli et al., 2023; Zabiulla et al., 2023; Begum et al., 2014). Supramolecular interactions, such as π-π stacking and hydrogen bonding, are essential in crystal engineering as they enable precise molecular recognition and facilitate the self-assembly of distinct supramolecular synthons (Balakrishnan et al., 2017). Octahedral Cu(II) complexes with d9 centres are commonly recognized to have the ability to display a Jahn-Teller distortion. This distortion is made possible by the existence of unpaired electrons located in the d-orbital. In the theorem of Jahn–Teller, the degeneracy in the orbitals (d-orbitals spin-splitting) results in a simultaneous compression or elongation along one Z-axis as in Scheme 1, which depicts the resultant trans-O2CuN4 complex electrical configuration. It is common practice to investigate these complexes' structures and any Jahn-Teller effect distortions using experimental methods including X-ray crystallography and spectroscopy.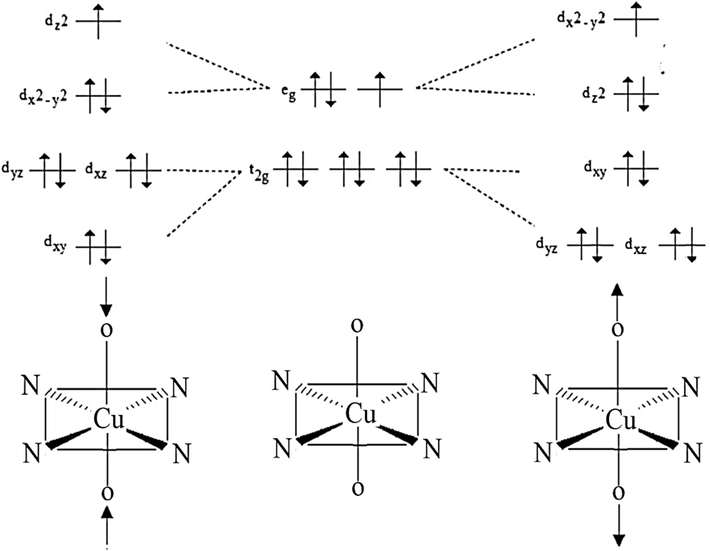
The elongation/compression Jahn–Teller diagram in the trans-O2CuN4 complex.
Molecular docking is a powerful tool for understanding the interaction between organic/inorganic material and DNA or enzymes. By utilizing non-covalent interactions, several studies offer vital insights into the mechanism of action by inserting a molecule at the binding site of a specific area on DNA or enzyme (Warad et al., 2017). The utilization of in silico computational docking methods is crucial in the development of novel inorganic chemotherapeutic complexes that mimic the cisplatin drug. In silico docking analysis of various structural complexes enabled the exploration of energy, hydrogen bonding, and hydrophobic interactions, assisting in developing chemical therapies or understanding the behaviours of proposed drugs before they are introduced into biological cells.
This study details the synthesis of the trans-(ClO4)2CuII(Me2N-Py)4 complex utilizing 4-dimethylaminopyridine ligands. An XRD analysis was performed to confirm the presence of the distorted octahedral elongated Jahn-Teller complex. The HSA-compoution and exp. XRD reflected the excistence of 1 and 2D-synthons stabilized mainly by C-H…OCl H-bonds. In addition, various physicochemical and thermal analyses were employed to demonstrate the intricate structure. Subsequently, the complex was assessed using in silico molecular docking utilizing 1BNA-DNA.
2 Experimental analysis
2.1 Chemical and physical analyses
The Elementar Varrio EL analyzer was employed solely for comprehensive CHN microanalysis. The acquisition of the FT-IR spectra involved the utilization of a spectrophotometer manufactured by 621-Perkin-Elmer. The UV–Vis spectra measurements are obtained using a spectrophotometer manufactured by 4060-LKB-Biochrom. The MS analysis was performed with a ThermoScientific-TM TSQ-Altis-TM Triple Quadrupole Mass Spectrometer. Sigma–Aldrich provided the necessary quantity of solvents and compounds and TG analysis were recorded via TGA-7 PerkinElmer.
2.2 Preparation of trans-(ClO4)2CuII(Me2N-Py)4
0.5 g (1.34 mmol) of Cu(ClO4)2·6H2O were dissolved in 30 ml of EtOH, and then 4 equivalents of N,N-dimethylpyridin-4-amine were added to the mixture. The reaction was performed in a closed system and stirred for 60 min until the blue colour transitioned into a greenish-blue. Subsequently, the reaction mixture was evacuated to a final volume of ∼2 ml, and 40 ml of diethyl ether was added to cause the desired complex to precipitate. Following filtration, the resultant complex was extensively rinsed with 40 ml of CH2Cl2, yielding 83 %.
2.2.1 Trans-(ClO4)2CuII(Me2N-Py)4
MS m/z 751.2 [M+] (theo. 752.4), m.p. = 308.5 °C, molecular formula C28H40Cl2CuN8O8 Cal: C, 44.77; H, 5.37; and N, 14.92 %. Found C, 44.51; H, 5.22; N, 14.87 %, IR (νcm−1): 3052 (νC-H of py), 2960–2840(νC-H of Me), 1555 (νN=C), 510–680 (νCu-N) and (νCu-O), and 1092 (νClO4), UV–Vis in MeOH: λmax (ε/M−1cm−1): 277 nm (1.2 × 104) and 698 nm (8.2 × 102).
2.3 HSA and XRD determinations
The HSA study was conducted utilizing CrystalExplorer 17.1 (Warad et al., 2017). The X-ray intensity data collection of the synthesized copper metal complex was conducted using graphite monochromatized Mo-Kα radiation (λ = 0.7107 Å). The complex's structure is determined using the intrinsic phasing approach, namely the SHELXT (Wolff et al., 2007). The refining process is carried out in Olex2 using SHELXL-2015, utilizing least-squares minimization against F2 (Sheldrick, 2008). The non-hydrogen atoms were identified in the structures using different Fourier maps, and their anisotropic thermal characteristics were revised. The hydrogen atoms were immobilized in a fixed geometric arrangement and refined using the riding model. The maximum positive value of the complex number in the final Fourier difference map is 0.547 eÅ−3. The crystallographic data and the refinement details are provided in Table 1.
Empirical formula
C28H40Cl2CuN8O8
Formula weight
751.12
Temperature (K)
293
Wavelength
0.71073
Crystal system, Space group, Z
Triclinic, P1, 4
a/b/c (Å)
11.059(4)/16.743(6)/20.251(7)
α/β/γ (°)
97.360(5)/104.087(16)/102.733(8)
Volume (Å3)
3481(2)
ρ calc, Mg/cm3
1.433
Absorption coefficient (μ/mm−1)
0.839
F(0 0 0)
1564
2θ range for data collection (°)
3.10 to 23.00
Index ranges
−12 ≤ h ≤ 10, −18 ≤ k ≤ 18, −22 ≤ l ≤ 22
Reflections collected
13,810
Independent reflections
9589
Refinement method
Full matrix least-squares on F2
Absorption correction
Semi-empirical from equivalents
Data/restraints/parameters
9589/120/955
Goodness-of-fit on F2
1.05
Final R indexes [I > 2σ (I)]
R1 = 0.0756, wR2 = 0.1883
Final R indexes [all data]
R1 = 0.1215, wR2 = 0.2286
Largest diff. Peak/hole (eÅ−3)
0.547 and −0.616
3 Results and discussion
3.1 Synthesis and analysis of trans-(ClO4)2CuII(Me2N-Py)4 complex
Trans-(ClO4)2CuII(Me2N-Py)4 complex was made available in high yields via treating of Cu(ClO4)2·6H2O with 4xMe2N-Py ligand in alcoholic medium at RT condition as seen in Scheme 2. The cis-(ClO4)2CuII(Me2N-Py)4 possible isomer was not detected, possibly because to its lower stability caused by steric hindrance, resulting in considerable internal repulsion compared to the trans-isomer. Under RT stirred condition, the reaction processed smoothly since the mixture colour was changed from light blue to green bluish within one hour, resulting in the formation of the desired complex. In the solid state, the trans-(ClO4)2CuII(Me2N-Py)4 complex was found to has a high melting point, blue in colour, and stable in an O2-open atmosphere. Furthermore, its structure was proved via SEM, CHN-EA, UV–Vis, TG/DTG, GC–MS, EDX, FT-IR, and XRD-crystal.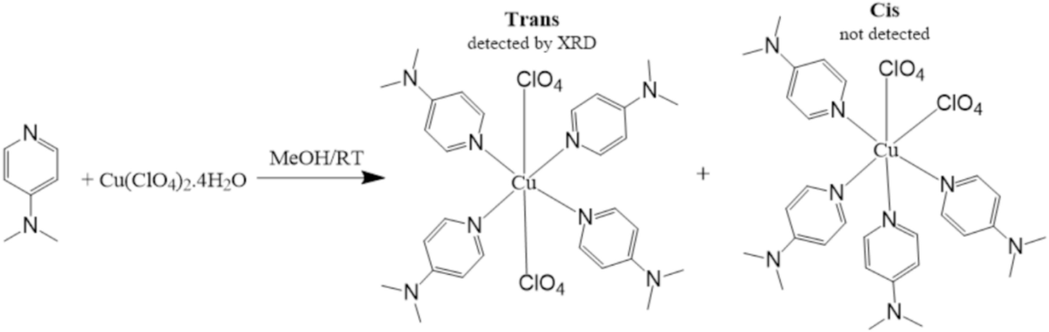
Synthesis of trans-(ClO4)2CuII(Me2N-Py)4.
The GCMS of the desired complex showed an excellent weak peak at m/z 751.2 (theoretical 752.4) supporting the formula weight of [CuII(Me2N-Py)4(ClO4)2]+ complex, peak at m/z 652.2 can be attributed to one ClO4 anion losing to form the mono-cation [CuII(Me2N-Py)4ClO4]+ stable fragment complex, losing of another ClO4 to form the di-cation [CuII(Me2N-Py)4]++ fragment complex can be cited to 552.2 m/z peak (Fig. 1a). The atomic composition of the desired complex has been confirmed via EDX reflected the appearance of O, N, C, Cu and Cl atoms only indicating the purity of the crystal structure (Fig. 1b). Moreover, the crystallinity and morphology were exterminated by SEM reflecting block particles as high crystalline rod-like morphology and complex purity (Fig. 1c). Nevertheless, SEM reflected a high crystalline structure and free of any irregularity, agglomeration, defect, and high particle stability had been recorded (Fig. 1c).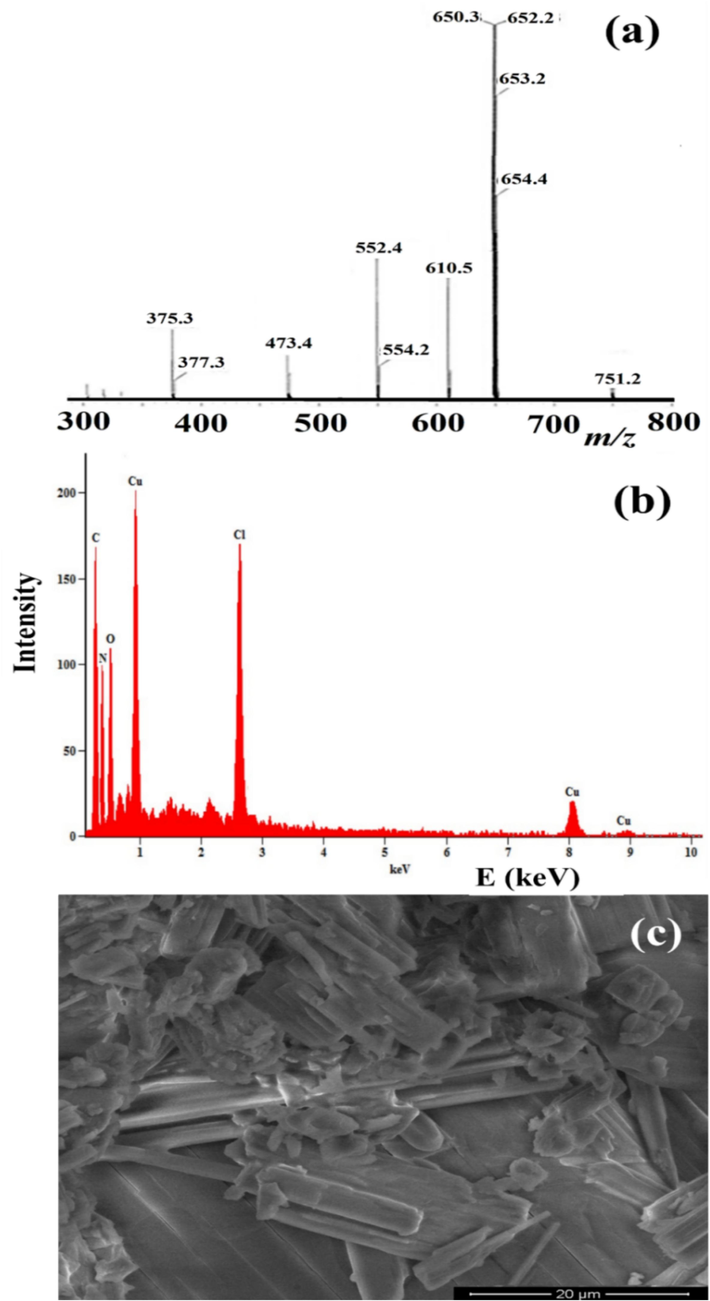
(a) GC–MS, (b) EDX, and (c) SEM of the trans-(ClO4)2CuII(Me2N-Py)4 complex.
In solid-state FT-IR (Fig. 2a), the appearance of the stretching vibration strong band at 1092 cm−1 confirmed the existence of ClO₄ anion ligands in the complex backbone; moreover, the band at 3052 ν(Cph-H), and 2960–2840 ν(CMe-H) corresponded to Me2N-Py ligands coordination. Additionally, peaks at 510–680 cm−1 ν(Cu-N)/ν(Cu-O) supported the formation of Cu–N and Cu–O new bonds via L/M complexation process (Zeisel et al., 2019; Haynes et al., 1987; Spodine et al., 1993; Kongchoo et al., 2016). On the other hand, the UV–Vis behavior in MeOH reflected the trans-(ClO4)2CuII(Me2N-Py)4 complex with two absorption broadbands (Fig. 2b), one in the UV area with λmax = 277 nm (ε = 1.2 × 104 M−1cm−1) attributed to π-to-π e-transition and band in the visible region with λmax 698 nm (ε = 8.2 × 102 M−1cm−1) attributed to d-to-d e-transition. The presence of such visible signal corresponded to the blue color of the solution, confirming the occurrence of N-centers of Me2N-Py bonding to the Cu(II) center.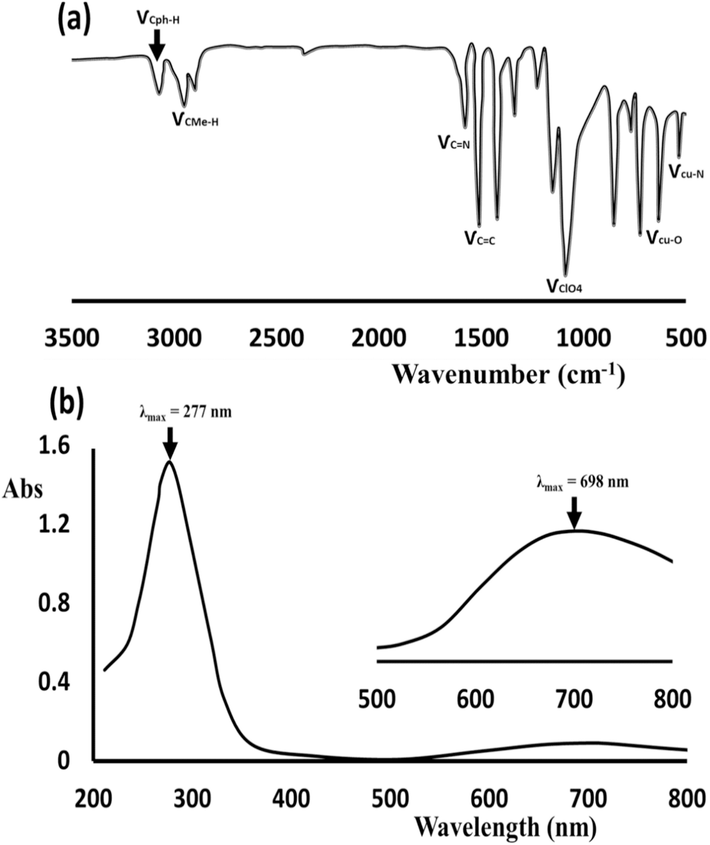
(a) Solid state FT-IR, and (b) UV–Vis of 1 × 10−4 M of trans-(ClO4)2CuII(Me2N-Py)4 dissolved in MeOH.
3.2 XRD of the trans-(ClO4)2CuII(Me2N-Py) complex
The crystal and molecular structure of trans-(ClO4)2CuII(Me2N-Py)4 are depicted in Fig. 3a and Table 2, which contains selective angles and bond lengths. The Cu(II) ion in the desired complex adopts an elongated octahedral (Oh) geometry (Zeisel et al., 2019; Haynes et al., 1987; Spodine et al., 1993; Kongchoo et al., 2016), with six ligands coordinating it in a centrosymmetric manner (Fig. 3b). This structure consists of four Me2N-Py ligands with Cu–N bond lengths of Cu1-N1 = 2.000(6) Å, Cu1-N4 = 2.003(7) Å, Cu1-N5 = 1.994(6) Å, Cu1-N6 = 2.011(6) Å, Cu2-N10 = 2.011(6) Å, Cu2-N11 = 2.009(5) Å, Cu2-N12 = 2.664(11) Å and Cu2-N13 = 2.007(5) Å on the square planar. Additionally, there are two perchlorates positioned along the long z-axis, with a Cu–O bond length of Cu1-O1 = 2.676 (6) Å, Cu1-O15A = 2.73 (3) Å, Cu2-O14 = 2.733(6) Å and Cu2-O5B = 2.73(3) Å. The XRD analysis revealed that the Cu–O bond length around the Cu(II) central atom increased by 8 % compared to similar systems (Kongchoo et al., 2016; Warad et al., 2019). This provided evidence for the occurrence of the widely recognized elongation of the Jahn-Teller distortion. The elongation ratio between the average axial and equatorial bonds was found to be approximately 20 %. Moreover, Oh indicated by the sum of the non-classical Cpy-H…OClO3 and CMe-H…OClO3 H-bond intermolecular forces within the 4 molecules existed in the unit cell as seen in Fig. 3c. Moreover, H-bonds angles of the basal plane measuring 362.27° (Cu1/N3/N4/N5/N6) and 361.68° (Cu2/N1/N2/N13/N10) and the angle subtended by the atoms in the apical position for O1-Cu1-O15A and O14-Cu2-O5B is found to be 168.35° and 158.55°, respectively.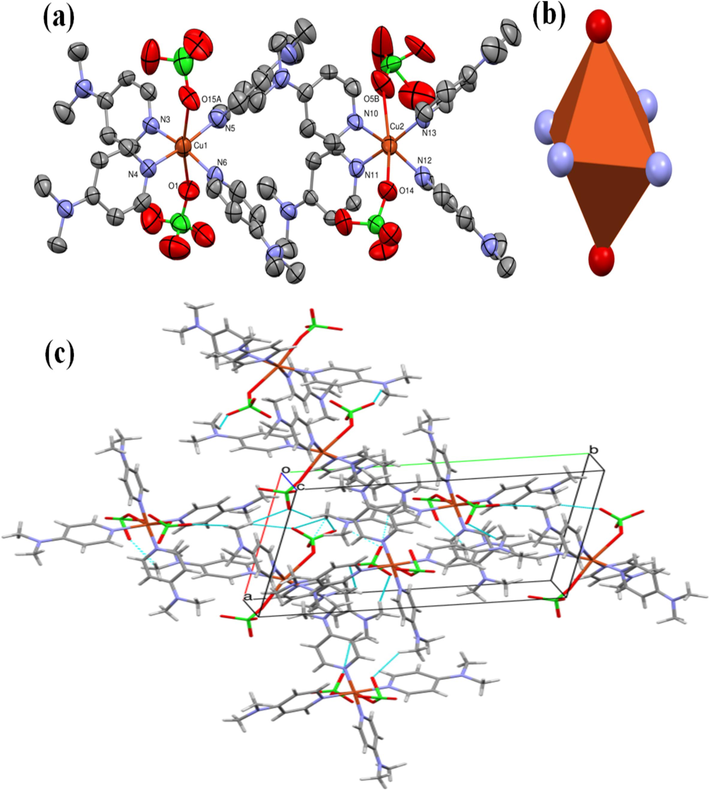
(a) ORTEP, (b) elongation Jahn-Teller distortion, and (c) 3D-molecular intermoleculars.
No.
Bond
Å
No.
Angle
(o)
1
Cu01
O007
2.737(6)
1
O007
Cu01
N008
87.2(2)
2
Cu01
N008
2.017(5)
2
O007
Cu01
N009
82.0(2)
3
Cu01
N009
2.012(5)
3
O007
Cu01
N00G
98.7(2)
4
Cu01
N00G
2.008(6)
4
O007
Cu01
N00I
91.0(2)
5
Cu01
N00I
2.015(6)
5
O007
Cu01
O2
176.5(3)
6
Cu01
O2
2.63(1)
6
N008
Cu01
N009
169.0(2)
7
Cl03
O007
1.429(6)
7
N008
Cu01
N00G
90.8(2)
8
Cl03
O00A
1.408(7)
8
N008
Cu01
N00I
89.2(2)
9
Cl03
O00P
1.413(7)
9
N008
Cu01
O2
90.2(3)
10
Cl03
O00R
1.411(6)
10
N009
Cu01
N00G
89.0(2)
11
N008
C00V
1.34(1)
11
N009
Cu01
N00I
92.8(2)
12
N008
C01L
1.324(8)
12
N009
Cu01
O2
100.7(3)
13
N009
C012
1.351(9)
13
N00G
Cu01
N00I
170.3(2)
14
N009
C01A
1.35(1)
14
N00G
Cu01
O2
83.6(3)
15
N00E
C00N
1.35(1)
15
N00I
Cu01
O2
86.7(3)
3.3 XRD/HSA-interactions investigation
The presence of ClO4 anions as multi O contents plays a critical role in building several H-bonds interaction in the trans-(ClO4)2CuII(Me2N-Py)4 complex lattice as in Fig. 4. The structural analysis revealed that the crystal packing is stabilized by the non-covalent interactions, especially the intermolecular non-classical H-bond interactions. The neighbouring molecules for the symmetry-independent molecule in the asymmetric unit are generated by non-classical Cpy-H…O and CMe-H…O intermolecular H-bonds (Table 3) resulting in the formation of 2 and 1D-dimensional supramolecular synthons molecular arrangement.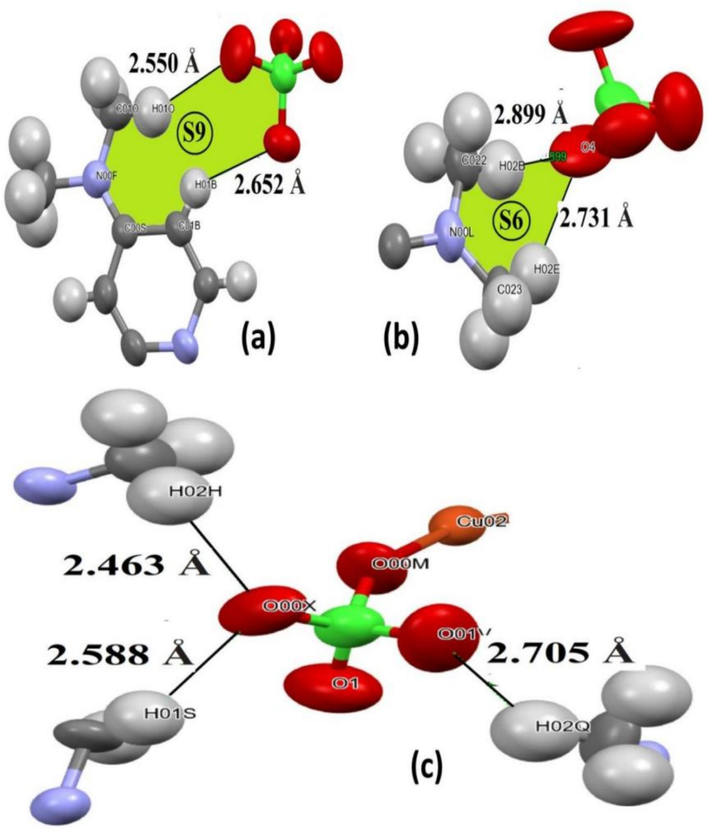
(a) 2D-S9, (b) 2D-S6 synthons, and (c) 1D-interactions around ClO4 anion ligand.
D-H…A
D-H (Å)
H…A (Å)
D…A (Å)
D-H…A (°)
C2-H2A…O2
0.96
2.55
3.473(12)
162
C2-H2B…O15
0.96
2.56
3.422(10)
149
C15-H15…O1
0.93
2.55
3.446(19)
161
C20-H20A…O13
0.96
2.55
3.420(12)
151
C36-H36A…O6
0.96
2.45
3.347(13)
155
C33-H36C…O2
0.96
2.53
3.271(12)
135
C15-H15…O1
0.93
2.56
3.056(11)
114
C5-H5…O5
0.93
2.28
3.037(17)
138
C12-H12…O7
0.93
2.55
3.446(19)
161
C34-H34…O13
0.93
2.54
3.313(10)
141
C47-H47…O14
0.93
2.55
3.156(10)
123
C50-H50…O12
0.93
2.48
3.105(13)
125
The crystal lattice of trans-(ClO4)2CuII(Me2N-Py)4 has two primary 2-D synthons. The S9 synthon was produced through non-classical Cpy–H···Operchlorate and CMe–H···Operchlorate hybrid hydrogen bonds, with bond lengths of 2.652 and 2.550 Å, respectively. In addition, another S6 synthon was formed by combining non-classical CMe–H···Operchlorate and Cpy–H···Operchlorate with distances of 2.731 and 2.899 Å, respectively (Fig. 4b). In addition, the ClO4 anions in trans-(ClO4)2CuII(Me2N-Py)4 also engaged in interactions with adjacent complex molecules. For instance, one of the ClO4 anions formed a non-classical pure 3CMe–H···Operchlorate interactions, leading to the formation of 1D-intermolecular interactions all directions among the molecules, resulting an additional lattice stability, as depicted in Fig. 4c.
The HSA is the most effective computational method for clarifying the intermolecular interactions in the XRD-resolved lattice of a certain complex structure (Warad et al., 2019; Al-Zaqri et al., 2020; Warad et al., 2019; Warad et al., 2018; Saleemh et al., 2017; Hema et al., 2020). The 3D-dnorm surfaces are graphed using a consistent color scale ranging from −0.3283 to 1.887 range. The examination of trans-(ClO4)2CuII(Me2N-Py)4 using HSA has verified the presence of multi-distinct red spots within the complex surface, the surface are with 14 spots as depicted in the dnorm surface (Fig. 5a). Significantly, the greatest spots are located near the ClO4 counterions, exhibiting non-classical Cpy-H….O, and CMe-H….O H-bond short interactions where no other h-bonds like H….N types have been recorded. In addition, the existence of electrophilic hydrogen atoms (blue) and nucleophilic terminal O (red) of the ClO4 counterions (Fig. 5b) on the molecule surface clarified the creation of these H-bonds. The high H….O contribution ratio of 17.5 % compared to the low H….N ratio of 0.8 % supported only the existence of C-H….O nonclassical H-bonds in the complex lattice, the other atom-to-atom (A-to-A) contribution ratios are illustrated in Fig. 5c. The HSA-computational result agreed well with the experimental XRD one.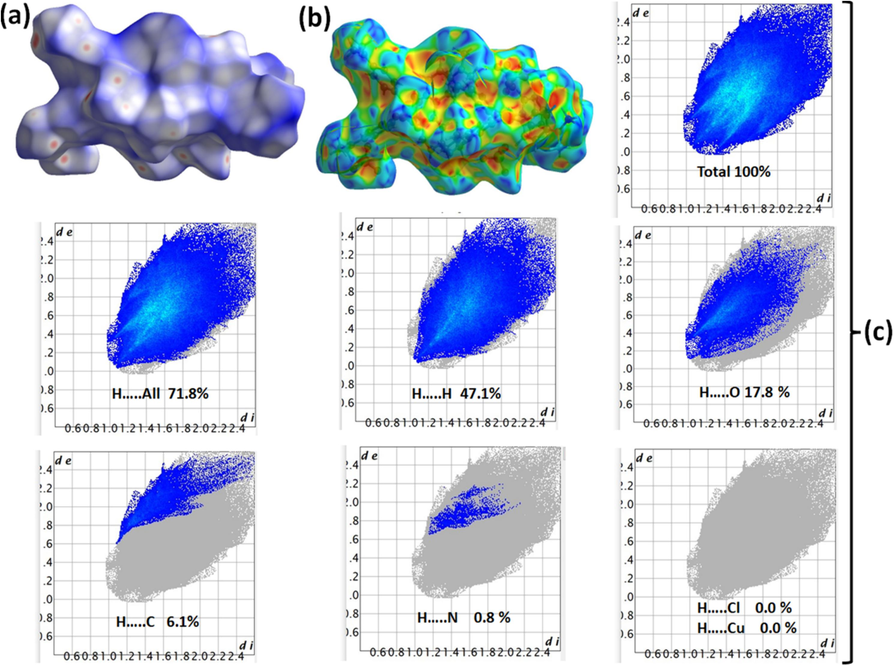
(a) dnorm, (b) shape-index, and (c) A-to-A interactions ratios in trans-(ClO4)2CuII(Me2N-Py)4.
3.4 TGA analysis
TG-DTG was performed in an open O2-atmosphere to examine the thermal characteristics of trans-(ClO4)2CuII(Me2N-Py)4 complex (Fig. 6). The TGA analysis showed that the complex undergoes a mass loss of 65.4 % (Cal, 64.8 %) in the temperature range of 210–395 °C and with TDTG = 260 °C, this step was corresponding to the de-structured or decomposition of the Me2N-Py organic ligands in the trans-(ClO4)2CuII(Me2N-Py)4 to light gasses (CO2 and NOx) producing the nacked Cu(ClO4)2 complex. As the temperature increases, the Cu(ClO4)2 product experiences a further weight reduction of 23.2 % (Cal, 22.9 %) between temperatures of 580–710 °C and with TDTG = 636 °C since ClO4. The remaining residue corresponding to Cu = O final product (yielding 10.8 %) was proved by FT-IR (Fig. 2a).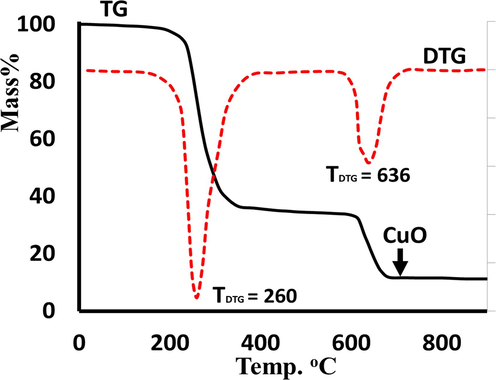
TG/DTG of trans-(ClO4)2CuII(Me2N-Py)4 complex.
3.5 1BNA DNA docking of trans-(ClO4)2CuII(Me2N-Py)4
Molecular docking was used to investigate the electrostatic, hydrophobic, and energetic interactions between trans-(ClO4)2CuII(Me2N-Py)4 and DNA (PDB:1BNA), since the 1BNA is simple, short and free available. The desired complex occupied the major groove of the 1BNA DNA, and the intricate structure smoothly merged with complex structure volume (Fig. 7a). Significantly, the complex showed binding behaviour similar to the cisplatin complex mode of interaction, as it bonded to both DNA helix through short three hydrogen bonding and one π-π interaction (Fig. 7b). The protons of Pyridine ligands did not show any binding contributions, meanwhile, the protons of Me of dimethyl functional groups played the critical role in the formation of the non-classical H-bonds like DNA:A: DA6:OP2….H-CH2N with 2.93 Å bond length and DNA:B: DT19:O4….H-CH2N with 2.88 Å bond length (Fig. 7c) reflecting a positive correlation between the number of formed H-bonds and the binding energy of the complex, which was observed to be −9.45 kcal/mol.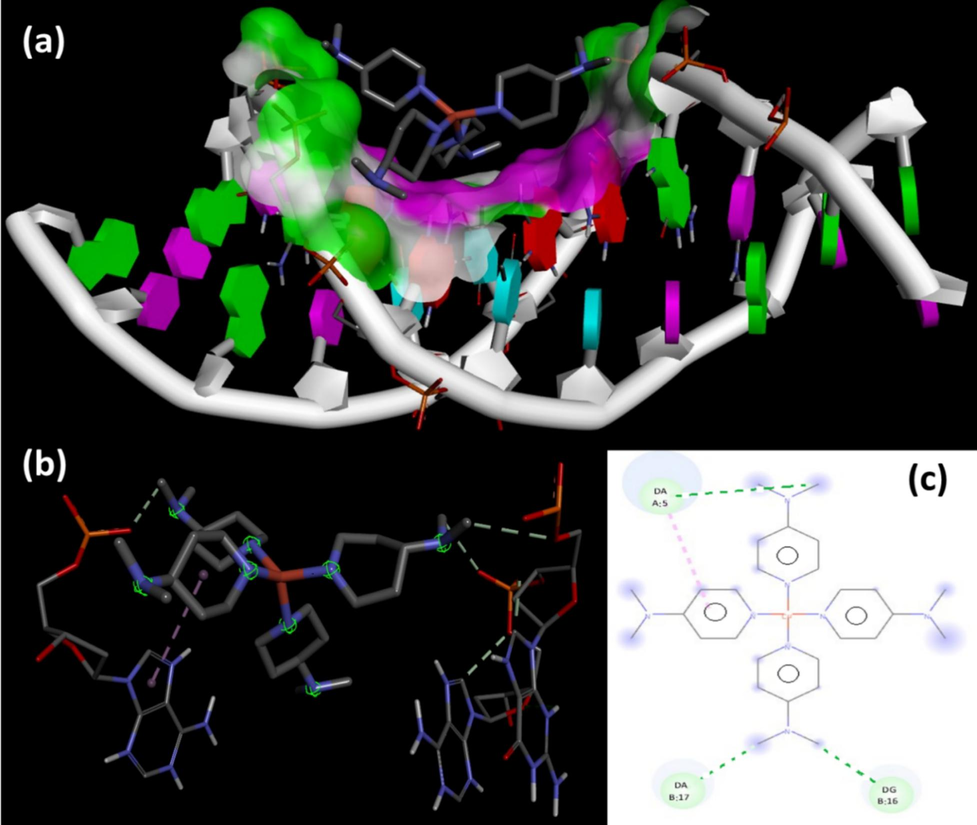
1BNA DNA docking, (a) Major groove, (b) cisplatin mode, and (c) 2D-H-bonds interactions.
4 Conclusion
A novel trans-(ClO4)2CuII(Me2N-Py)4 complex was synthesized and investigated utilizing several physicochemical techniques in the current research study. The structural analysis determined that the desire complex exhibited crystalline properties in a triclinic crystal system with the P1 space group. The XRD of Cu(II) ion reflected a distorted Oh geometry, with two perchlorate ions positioned axially and four Me2N-Py ligands organized in a planar shape. Additionally, XRD study has shown the presence of a dynamic Jahn-Teller elongation distortion, which has resulted in a 20 % increase in the Cu–O bond lengths. The crystal packing is stabilized by various S9 and S6 synthons intermolecular hydrogen bonding constructed via CMe-H…O and Cph-H…O non-classical interactions. The TG/DTG represented the complex with high thermal stability and two steps degradation mechanism. Furthermore, the docking technique revealed the complex to be a good 1BNA major grove positional binder, showing 2H-bonds and −9.45 kcal/mol binding energy.
CRediT authorship contribution statement
A. AlAli: Writing – original draft, Methodology, Data curation. A. AlObaid: Writing – original draft, Methodology, Data curation. B.S. Chethan: Methodology, Investigation. K. Shalalin: Writing – original draft, Validation, Resources. N. Alzeqri: Supervision, Software, Funding acquisition. K. Alkanad: Validation, Investigation, Formal analysis. N.K. Lokanath: Writing – review & editing, Visualization, Investigation. A. Zarrouk: Visualization, Supervision, Data curation. I. Warad: Writing – review & editing, Writing – original draft, Supervision, Methodology, Data curation. S.A. Khanum: Writing – original draft, Software, Resources, Methodology.
Acknowledgements
We extend our appreciation to the Researchers Supporting Project number (RSP2024R381), King Saud University, Riyadh, Saudi Arabia.
References
- Pyridine-based NNS tridentate chitosan thiosemicarbazones and their copper(II) complexes: Synthesis, characterization, and anticancer activity. ACS Omega. 2022;7:30978.
- [CrossRef] [Google Scholar]
- A. AlAli, M. Al-Noaimi, A. AlObaid, H. A. Khamees, A. Zarrouk, K. Kumara, I. Warad, S. A. Khanum, Jahn-Teller distortion in SP-like [Cu(bipy)(triamine)].2BF4 complexes with novel NH… F/CH… F synthon: XRD/HSA-interactions, physicochemical, electrochemical, DFT, docking and COX/LOX inhibition, J. Mol. Liq. 387 (2023) 122689. doi: 10.1016/j.mol liq.2023.122689.
- One-pot reproducible Sonosynthesis of trans-[Br(N∩N’)Cu(μBr)2Cu(N∩N’)Br] dimer:[H….Br S(9)] synthons, spectral, DFT/XRD/HSA, thermal, docking and novel LOX/COX enzyme inhibition. J. Mol. Struct.. 2023;1275:134626
- [CrossRef] [Google Scholar]
- Cytotoxicity and antibacterial potentials of mixed ligand Cu(II) and Zn(II) complexes: a combined experimental and computational study. ACS Omega. 2023;8:13421.
- [CrossRef] [Google Scholar]
- Synthesis, physicochemical, thermal, and XRD/HSA interactions of mixed [Cu(Bipy)(Dipn)](X)2 complexes: DNA binding and molecular docking evaluation. J. Coord. Chem.. 2020;73:3236.
- [CrossRef] [Google Scholar]
- Copper (II) and silver (I) complexes with dimethyl 6-(pyrazine-2-yl) pyridine-3, 4-dicarboxylate (py-2pz): the influence of the metal ion on the antimicrobial potential of the complex. RSC Adv.. 2023;13:4376.
- [CrossRef] [Google Scholar]
- Synthesis, spectral characterization, DNA/protein binding, DNA cleavage, cytotoxicity, antioxidative and molecular docking studies of Cu (II) complexes containing Schiff base-bpy/phen ligands. J. Fluoresc.. 2017;27:953.
- [Google Scholar]
- Biocatalysis, DNA–protein interactions, cytotoxicity and molecular docking of Cu (II), Ni(II), Zn(II) and V(IV) Schiff base complexes. Appl. Organomet. Chem.. 2017;31:3776.
- [CrossRef] [Google Scholar]
- Synthesis, characterization, biological and catalytic applications of transition metal complexes derived from Schiff base. Bioorg. Med. Chem. Lett.. 2014;24:3559.
- [CrossRef] [Google Scholar]
- Generation and aerobic oxidative catalysis of a Cu (II) superoxo complex supported by a redox-active ligand. J. Am. Chem. Soc.. 2022;144:15569.
- [CrossRef] [Google Scholar]
- Synthesis, characterization and biological evaluation of novel 2, 2′-((1, 2-diphenylethane-1,2-diylidene)bis(azanylylidene)) bis (pyridin-3-ol) and metal complexes: molecular docking and in silico ADMET profile. Struct. Chem.. 2020;31:521.
- [CrossRef] [Google Scholar]
- Anionic ligands tune the structural and catalytic properties of quinoxaline-based copper (II) complexes as mimetics of copper-containing oxidase protein. J. Mol. Struct.. 2022;1250:131809
- [CrossRef] [Google Scholar]
- Cu (II)–dipeptide complexes of 2-(4′-thiazolyl) benzimidazole: Synthesis, DNA oxidative damage, antioxidant and in vitro antitumor activity. J. Inorg. Biochem.. 2015;143:77.
- [CrossRef] [Google Scholar]
- Novel copper(II) complexes with N 4, S-diallylisothiosemicarbazones as potential antibacterial/anticancer drugs. Inorg.. 2023;11:195.
- [CrossRef] [Google Scholar]
- Structural comparison of copper (II) thiocyanate pyridine complexes. Inorg. Chim. Acta.. 2017;456:64.
- [CrossRef] [Google Scholar]
- Structure and magnetic exchange in poly-bis (pyrazine) bis (methanesulfonato-o)-copper (II). One-dimensional exchange in a two-dimensional polymer. Can. J. Chem.. 1987;65:420.
- [CrossRef] [Google Scholar]
- XRD/DFT/HSA-interactions in Cu(II)Cl/phen/ß-diketonato complex: physicochemical, solvatochromism, thermal and DNA-binding analysis. J. Mol. Struct.. 2020;1210:128000
- [CrossRef] [Google Scholar]
- The effect of metal ions (Fe Co, Ni, and Cu) on the molecular-structural, protein binding, and cytotoxic properties of metal pyridoxal-thiosemicarbazone complexes. Int. J. Mol. Sci.. 2023;24:11910.
- [CrossRef] [Google Scholar]
- Redox reactions of copper complexes formed with different β-amyloid peptides and their neuropathalogical relevance. Biochem.. 2007;46:9270.
- [CrossRef] [Google Scholar]
- Synthesis, crystal structure, and spectroscopic properties of Cu (II) complex with 14-membered hexaazamacrocyclic ligands. J. Chem. Crystallogr.. 2016;46:222.
- [CrossRef] [Google Scholar]
- Synthesis and structure of novel copper (II) complexes with N, O-or N, N-donors as radical scavengers and a functional model of the active sites in metalloenzymes. Int. J. Mol. Sci.. 2021;22:7286.
- [CrossRef] [Google Scholar]
- Copper (II) complexes containing derivative of aminobenzoic acid and nitrogen-rich ligands: synthesis, characterization and cytotoxic potential. J. Mol. Struct.. 2023;1279:135002
- [CrossRef] [Google Scholar]
- Synthesis, characterization, crystal structures, and superoxide dismutase activity of copper (II) octahedral complexes containing tri-and monodentate ligands. J. Coord. Chem.. 2010;63:586.
- [CrossRef] [Google Scholar]
- Synthesis, spectral, magnetic properties, electrochemical evaluation and SOD mimetic activity of four mixed-ligand Cu (II) complexes. Inorga. Chim. Acta. 2017;455:298.
- [CrossRef] [Google Scholar]
- Diethylenetri-amine/diamines/copper(II) complexes [Cu(dien)-(NN)]Br 2: Synthesis, solvatochromism, thermal, electrochemistry, single crystal, Hirshfeld surface analysis and antibacterial activity. Arab. J. Chem. 2017;10:845.
- [CrossRef] [Google Scholar]
- An oxalate-bridged copper (II) complex combining monodentate benzoate, 2, 2′-bipyridine and aqua ligands: synthesis, crystal structure and investigation of magnetic properties. Molecules. 2020;25:1898.
- [CrossRef] [Google Scholar]
- G.M. Sheldrick, A short history of SHELX, Acta Crystallogr., Sect A64 (2008) 112. doi: 10.1107/S0108767307043930.
- E. Spodine, J. Manzur, M. T. Garland, J. P. Fackler Jr, R. J. Staples, and B. Trzcinska-Bancroft, Copper complexes with di-pyridylmethane. The synthesis and X-ray structures of bis (di-pyridylmethane) copper (I) perchlorate, bis (di-pyridylmethane) copper (II) perchlorate and dichloro-μ-dichloro-bis (di-pyridylmethane) dicopper (II), Inorg. Chim. Acta. 203 (1993) 73. doi: 10.1016/S0020-1693(00)82907-2.
- New isomeric Cu (NO2-phen) 2Br] Br complexes: crystal structure, Hirschfeld surface, physicochemical, solvatochromism, thermal, computational and DNA-binding analysis. J. Photochem. Photobiol. b, Biol.. 2017;171:9.
- [CrossRef] [Google Scholar]
- Synthesis, spectra and X-ray crystallography of dipyridin-2-ylmethanone oxime and its CuX2(oxime)2 complexes: thermal, Hirshfeld surface and DFT analysis. J. Mol. Struct.. 2018;1154:619.
- [CrossRef] [Google Scholar]
- Ultrasound-assisted synthesis of two novel [CuBr(diamine)2.H2O] Br complexes: solvatochromism, crystal structure, physicochemical, Hirshfeld surface thermal, DNA/binding, antitumor and antibacterial activities. Ultrason. Sonochem.. 2018;48:1.
- [CrossRef] [Google Scholar]
- Design, structural, C-H…. H–C supramolecular interactions and computational investigations of Cd (N∩ N ″) X2 complexes based on an asymmetrical 1, 2-diamine ligand: physicochemical and thermal analysis. J. Coord. Chem.. 2019;72:3285.
- [CrossRef] [Google Scholar]
- Ultrasonic synthesis of Oct. trans-Br 2Cu(N∩N)2 Jahn-Teller distortion complex: XRD-properties, solvatochromism, thermal, kinetic and DNA-binding evaluations. Ultrason. Sonochem.. 2019;52:428.
- [CrossRef] [Google Scholar]
- Crystal explorer 2.1. Perth: University of Western Australia; 2007.
- Molecular docking, synthesis and antimicrobial evaluation of metal complexes with schiff base. Results Chem.. 2023;5:100650
- [CrossRef] [Google Scholar]
- 2-Methylsubstituted monotetrazoles in copper(II) perchlorate complexes: manipulating coordination chemistry and derived energetic properties. New J. Chem.. 2019;43:609.
- [CrossRef] [Google Scholar]
Appendix A
Supplementary data
The crystallographic data has been submitted to the Cambridge Crystallographic Data Centre and assigned the additional publication number CCDC 2287195. To receive copies of this information, you can visit the website www.ccdc.cam.ac.uk/conts/ retrieving.html. Alternatively, you can contact the CCDC at their physical address: 12 Union Road, Cambridge CB2 1EZ, UK. You can also send a fax to +44-1223-336033 or an email to deposit@ccdc.cam.ac.uk. There is no charge for obtaining these copies. Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2024.103302.
Appendix A
Supplementary data
The following are the Supplementary data to this article:







