Translate this page into:
Quantification of phenolic content from stem-bark and root of Hugonia mystax Linn. using RP-HPLC
⁎Corresponding author. Fax: +91 22 39486097. sandip_biochem@yahoo.com (Sandip S. Pawar)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Hugonia mystax Linn. a woody evergreen plant locally known as Modirakanni has been used in primary health care by tribals from Tiruvannamalai hills, Tamil Nadu, India. Ethnobotanically, the plant parts are used for rheumatism, skin diseases and inflammation. However, there is no data on active phytoconstituents in stem-bark and root mainly contributing to biological activities. In the present study an attempt has been made to quantify plant phenolics from aqueous, ethanolic and methanolic extracts of stem-bark and root of H. mystax. The extracts were also evaluated for their free radical scavenging potential. Quantitative determination of total phenolic content, total flavonoid content and DPPH free radical scavenging activity of plant extracts were carried out using colorimetric methods. Quantitative determination of individual phenolic compounds such as gallic acid, catechol, caffeic acid, vanillin, p-coumaric acid and ferulic acid in stem-bark and roots extracts were analyzed using RP-HPLC. The highest phenolic content was found in ethanol extract of root (262.2 ± 0.96 μg of gallic acid equivalent (GAE)/mg of dry plant material), whereas, the highest amount of flavonoids content was found in aqueous extract of root (18.06 ± 1.25 μg of quercetin equivalent (QE)/mg of dry plant material). Q. The highest amount of phenolic acid present was p-coumaric acid (3.775 mg/g of dry plant material) in methanol extract of stem-bark. All the solvent extracts of stem-bark and root have shown the presence of p-coumaric acid. Methanol extracts of stem-bark and root with IC50 values of 175.48 ± 2.14 μg/ml and 169.15 ± 1.10 μg/ml respectively, show potent free radical scavenging activity. In conclusion it can be said that, the plant is rich in phenolics and the major component p-coumaric acid may probably be responsible for the traditional claims of its biological activities. However, the mechanism of action of the active plant extracts needs to be investigated at the molecular level.
Keywords
Hugonia mystax
Reverse phase-high performance liquid chromatography
Total phenolic content
Total flavonoid content
- RP-HPLC reverse phase
-
high performance liquid chromatography
- TPC
-
total phenolic content
- TFC
-
total flavonoid content
Abbreviations
1 Introduction
According to the World Bank report, 80% of the South Asian population still uses traditional plant-based medicines to maintain and improve their health. The World Health Organization listed 21,000 plants used in traditional medicine around the world (WHO, 2002), whereas Schippmann and co-workers estimated this number to be 52,885 (Schippmann et al., 2002). Although there are conflicting claims on the number of plants, it is well established that medicinal plants are routinely used for primary health care in many parts of the world (Pan et al., 2014).
Plants have been integral part of herbal healing processes, which is deep rooted in the systematic classical system of Ayurveda, Siddha, and Unani, as an official health care system (Balakumar et al., 2011; Mohamed Saleem et al., 2011; Pour and Sasidharan, 2011). Folk medicine or oral tradition of health care, which exists among most tribal and rural communities also use medicinal plant knowledge of traditional healers, which is widely recognized as a valid alternative system of medicine (Al-Daihan et al., 2013; Alabri et al., 2014). In developing countries the efforts to recognize and promote the uncodified folk system of medicinal knowledge are still inadequate (Shukla and Gardner, 2006). Systematic scientific investigations are required to prove the traditional claims of effectiveness of these plants and the rationale behind their biological activities needs to be established.
Hugonia mystax Linn. a woody evergreen species, belongs to Linaceae family, which comprise about 40 species in the world; of which H. mystax L. was reported from India (Santapau and Henry, 1983; Pullaiah and Chennaiah, 1997). This plant locally known as Modirakanni plays a vital role in the primary health care of tribals from Tiruvannamalai hills, Tamil Nadu, India. The plant parts such as leaves, fruits, bark and roots are extensively used by traditional healers for the treatment of various ailments. Ethnobotanically, leaves and fruits have been used as an antihelmintics and for rheumatism (Sutha et al., 2009; Padel et al., 2010). Roots were used as antihelmintic and also used for dysentery, snake bite, fever, inflammation and rheumatism. Biological activities such as analgesic, anti-inflammatory and ulcerogenic were also reported (Balasubramaniam et al., 1997; Guha Bakshi et al., 2001; Rastogi et al., 2002). The decoction of bark in combination with Curcuma aromatica, is given with honey for inflammation in the stomach, vomiting, stomach pain, indigestion (Pushpangadan and Atal, 1984). Roots of H. mystax were evaluated for preliminary phytochemical screening and antimicrobial activity. Preliminary phytochemical screening showed the presence of various classes of secondary metabolites such as flavonoids, phenols, saponins, steroids, tannins and terpenoids. Antimicrobial activity of petroleum ether, chloroform, ethanol and aqueous extracts of root showed significant activity against various human pathogens (Vimalavady et al., 2012). This species was reported by Dalzell and Gibson in Bombay Flora in 1861. The species from this geographical location is yet to be explored for its phytochemical constituents and biological activity.
Considering the medicinal importance of the plant and previous findings in our laboratory, an attempt has been made to quantify plant phenolics from stem-bark and roots of the H. mystax, extracted with solvents of different polarity.
2 Materials and methods
2.1 Reagents and chemicals
Gallic acid, quercetin, cathechol, caffeic acid, vanillin, p-coumaric acid, ferulic acid and 1,1-diphenyl-2-picryl-hydrazyl (DPPH) were purchased from Sigma Chemical Co. (USA). HPLC grade ethanol (EtOH), methanol (MeOH), acetic acid, Folin Ciocalteu’s Phenol reagent, sodium carbonate and aluminum chloride were purchased from Merck (Germany).
2.2 Collection of plant material
The stem-bark and root of H. mystax (Fig. 1) were collected from Mochemad–Vengurla, Sindhudurg district, Maharashtra. The geographical location of the collection area was 15° 48. 040′ N, 073° 39. 283′ E. The plant specimen was authenticated from the Blatter Herbarium (BLAT), St. Xavier’s College, Mumbai. The submitted plant specimen matches with Blatter Herbarium specimen No. 22319 of H. Santapau.
Hugonia mystax plant used for the study.
2.3 Preparation of plant extracts
The air-dried stem-bark and roots of H. mystax were made into fine powder. 5 g of powder of each plant part were suspended in 50 ml of three different extracting solvent systems like distilled water, methanol and ethanol respectively for overnight extraction. Extracts were filtered using Whatman No. 1 paper and extracts were concentrated in vacuo using rotary vacuum evaporator. Percentage extractive value (w/w) of each extract was calculated. For HPLC analysis, stock solutions of extracts were filtered through the syringe filter 0.45 μm pore size (Millipore).
2.4 Determination of total phenolic (TPC) and total flavonoid content (TFP) content
2.4.1 Total phenolic content (TPC)
The total phenolic content (TPC) in H. mystax crude extracts was determined by using the Folin–Ciocalteu method (Chang et al., 2002). Standard solutions of gallic acid of concentration 1.56–100 μg/ml were prepared in water. 50 μl of extract (1 mg/ml) or standard solution were added to 50 μl of distilled water. 50 μl of 10% Follin–Cicocalteu’s (F–C) phenol reagent and 50 μl of 1 M sodium carbonate solution were added to the mixture in a 96-well plate. Distilled water was used as blank. Reactions were incubated for 60 min at room temperature and protected from light. The absorbance was measured at 750 nm with a Microplate Reader (Biotek, USA.). Total phenolic contents were expressed as μg Gallic Acid Equivalents (GAE) per mg of dry plant material.
2.4.2 Total flavonoid content
Total flavonoid content (TFC) was determined by the aluminum chloride colorimetric assay (Chatatikun and Chiabchalard, 2013). Standard solutions of quercetin of concentration 1.56–100 μg/ml were prepared in 80% ethanol. 50 μl of extracts (1 mg/ml) or standard solution was added to 10 μl of 10% the aluminum chloride solution and followed by 150 μl of 95% ethanol. 10 μl of 1 M sodium acetate was added to the mixture in a 96 well plate. 80% ethanol was used as reagent blank. All reagents were mixed and incubated for 40 min at room temperature protected from light. The absorbance was measured at 415 nm with a Microplate Reader (Biotek, USA.). Total flavonoid contents were expressed as μg Quercetin Equivalents (QE) per mg dry of plant material.
2.5 Antioxidant assay
2.5.1 Free radical scavenging activity assay
The 2,2-Diphenyl-1-picrylhydrazyl (DPPH) free radical scavenging activity was performed by the reported method (Yamasaki et al., 1994). 20 μl of extracts in respective solvents was added to 180 μl of DPPH reagent prepared in methanol in a 96 well plate. Absolute methanol was used as reagent blank. Reaction mixture was incubated for 30 min at room temperature, protected from light. The absorbance was measured at 517 nm with a Microplate Reader (Biotek, USA.). The percentages of the DPPH free radical scavenging activity were calculated as follows,
2.6 Quantitative analysis of phenolic acids using RP-HPLC
Quantitative analysis of individual phenolic compounds present in the different parts of plant extracts was performed on a Waters HPLC system (Model 2487), using Zorbax Eclipse ODS C18 reverse phase column (150 mm × 4.6 mm ID, 5 μm) (Agilent, Germany), 1% acetic acid in water: methanol (80:20) (v/v) used as mobile phase with 1 min/ml flow rate, UV detector set at 280 nm. Phenolic acids from each sample were identified by comparing their relative retention time with the standards of mixture chromatogram. All the chemicals and solvents used for analysis were of HPLC grade.
2.7 Statistical analysis
All the observations were taken in triplicate and the data were expressed in Mean ± Standard Error of Measurement (S.E.M). The data were analyzed using Analysis of Variance technique (ANOVA) (p < 0.05) and the means were separated by Bonferroni’s multiple comparison tests using software GraphPad Prism 5.
3 Results and discussion
3.1 Total phenolic content, flavonoid content
Aqueous (5.56%) and methanol (4.98%) had higher extractive values for extracts of stem-bark and root respectively. Though ethanol (3.02%) was recorded for lower extractive value, it was found to be the best solvent system for the extraction of phenolic acids. Ethanol extracts of plant parts have shown highest phenolic content in comparison to methanol and distilled water (Table 1). The total phenolic content in H. mystax was determined by using Folin–Ciocalteu reagent. The range of phenolic acid content was from 232.5 ± 0.44 to 262.2 ± 0.96 μg of GAE/mg of dry plant material. The highest phenolic acid content was found in ethanol extract of root viz. 262.2 ± 0.96 μg of GAE/mg of dry plant material. The total flavonoid content in H. mystax was determined using aluminum chloride method. The range of flavonoid content was from 1.753 ± 0.71 to 18.06 ± 1.25 μg of QE/mg of dry plant material. The highest amount of flavonoid content was found in aqueous extract of root viz. 18.06 ± 1.25 μg of QE/mg of dry plant material (Table 1). Phenolic compounds are important and widely distributed secondary metabolites of plant kingdom. Plant phenolics are mainly classified into phenolic acids and flavonoids. These compounds are involved in growth and reproduction of the plant, and also play an important role in defense against ultraviolet radiation and pathogens (Manach et al., 2004; Farah and Donangelo, 2006; Elzaawely et al., 2007; Piazzon et al., 2012). Therefore, quantification of phenolic compounds has paramount importance. There have been several studies that showed F–C reagent reacts with other phytoconstituents beside phenol (Prior et al., 2005; Ikawa et al., 2003; Everette et al., 2010), which could possibly show a high amount of phenolic content. Considering the limitations of the F–C assay (Amorati and Valgimigli, 2015), there are continuous efforts from the scientific community to resolve this problem by suggesting the different approaches to improve the specificity of F–C assay (Sanchez-Rangel et al., 2013; Berker et al., 2013). Values represent mean ± SEM (n = 3). ND – not detected.
Extracts
Solvent system
% Extractive value (w/w)
TPCa
TFCb
Stem-bark
D/W
5.56
232.5 ± 0.44
9.703 ± 1.79
Methanol
4.68
244.3 ± 5.74
17.98 ± 0.51
Ethanol
2.86
245.1 ± 2.09
1.753 ± 0.72
Root
D/W
4.2
250.1 ± 3.32
18.06 ± 1.25
Methanol
4.98
257.5 ± 1.37
14.19 ± 1.61
Ethanol
3.02
262.2 ± 0.96
ND
3.2 RP-HPLC analysis of individual phenolic acids and DPPH assay
Individual plant phenolics such as gallic acid, catechol, caffeic acid, vanillin, p-coumaric acid and ferulic acid in stem-bark and root of H. mystax were analyzed and quantified using RP-HPLC (Fig. 2). From the analysis it was observed that the plant parts showed the presence of all phenolic compounds except Ferulic acid. The phenolic compounds present in plant parts are, gallic acid (0.024–1.19 mg/g of dry plant material), catechol (0.067–0.292 mg/g of dry plant material), caffeic acid (0.047–0.401 mg/g of dry plant material), vanillin (0.037–0.117 mg/g of dry plant material) and p-coumaric acid (0.067–3.775 mg/g of dry plant material (Fig. 3). p-Coumaric acid was found to be the most predominant phytoconstituent present in both the plant parts selected for the study. The highest amount of phenolic acid present was p-coumaric acid (3.775 mg/g of dry plant material) in methanol extract of stem-bark (Fig. 4). Methanol extracts of stem-bark and root of H. mystax have shown potent free radical scavenging activity (Fig. 5) with the IC50 values, 175.48 ± 2.14 and 169.15 ± 1.10 μg/ml respectively.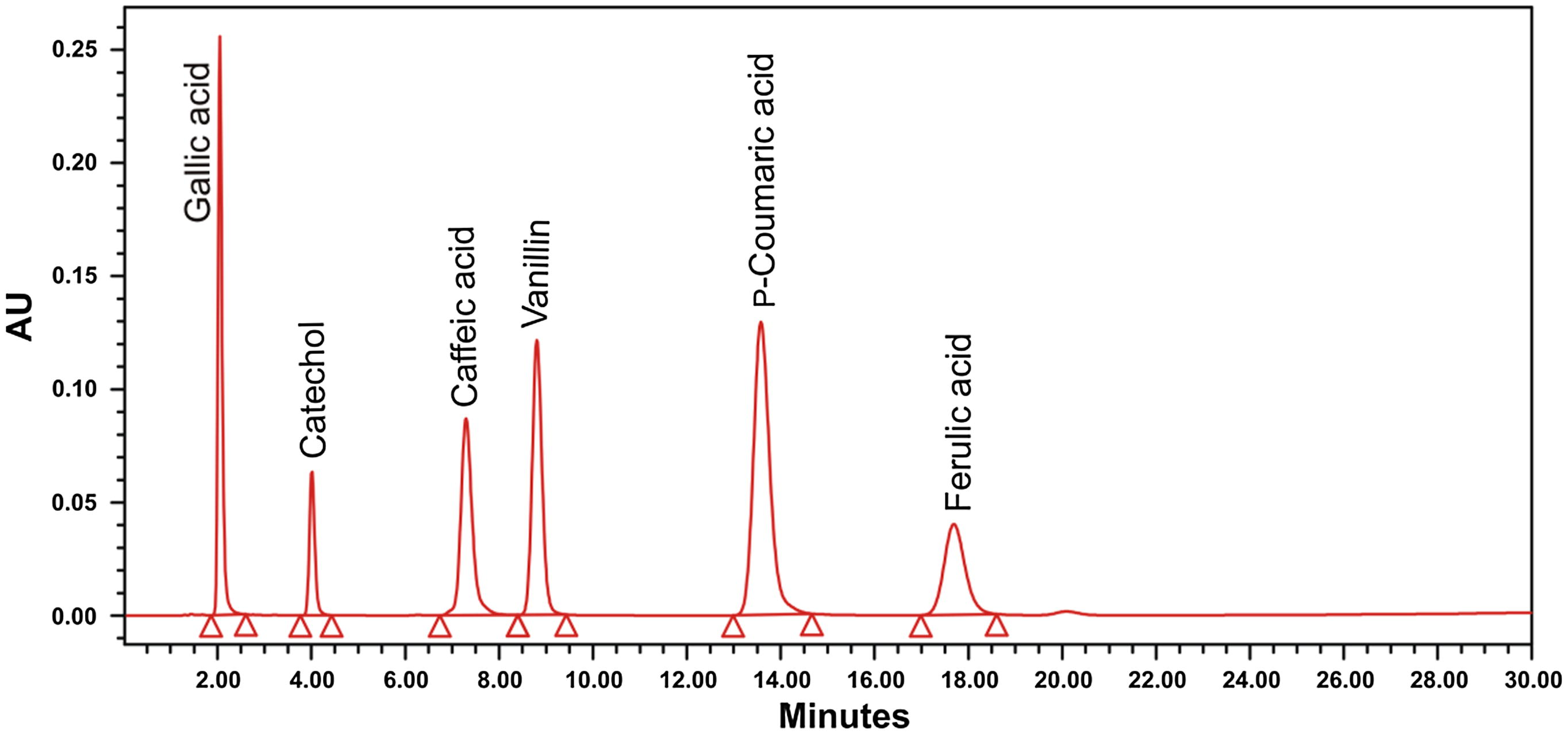
RP-HPLC chromatogram of standard phenolic compounds (20 ppm each): HPLC, Zorbax Eclipse ODS RP-C18 column ((150 mm × 4.6 mm ID, 5 μm), 1% (v/v) acetic acid in water:methanol (80:20) (v/v), 1 ml/min, absorbance at 280 nm (n = 2).
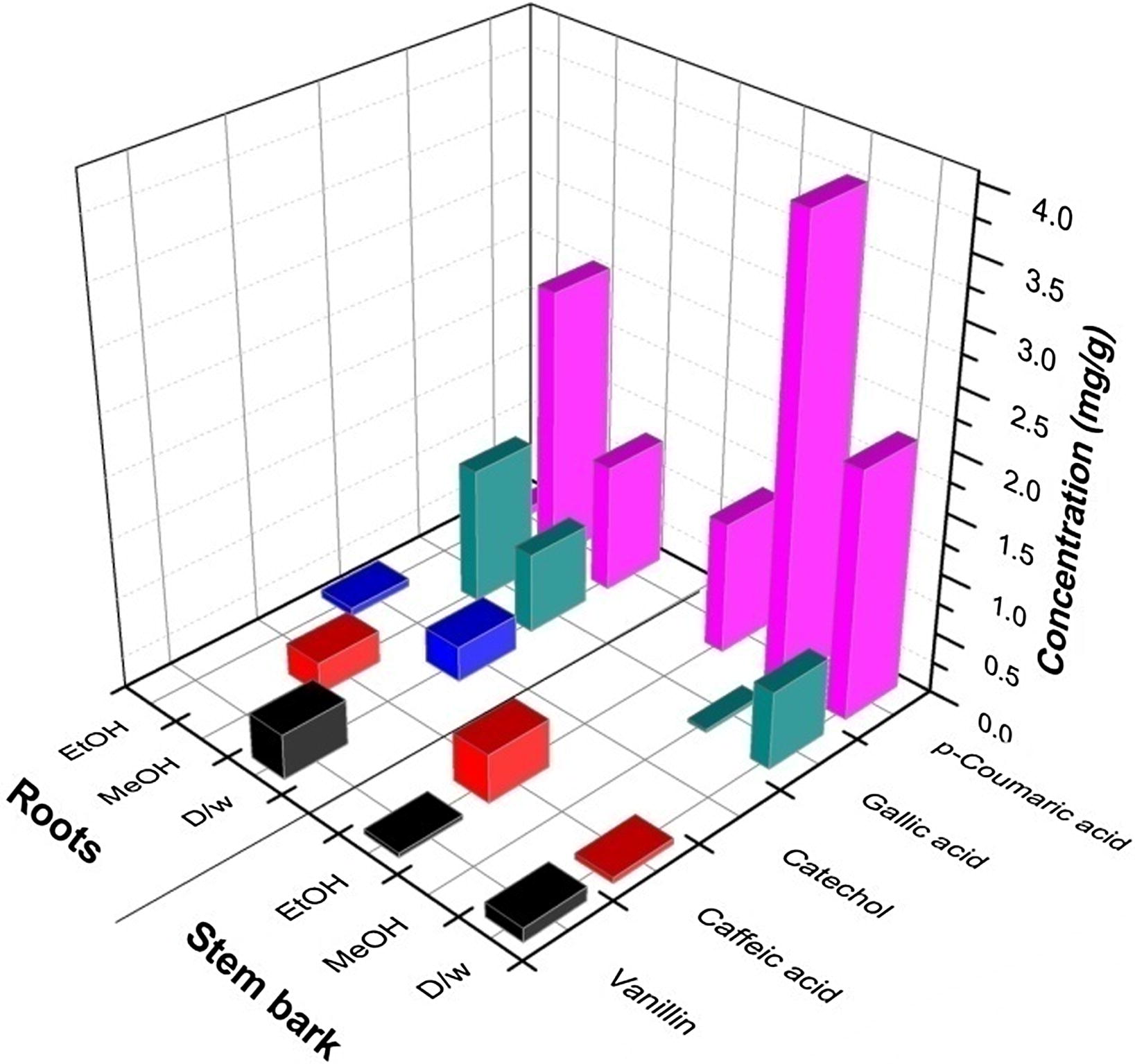
Quantification of individual phenolic compounds in plant parts of Hugonia mystax using HPLC (mg/g of dry plant material).
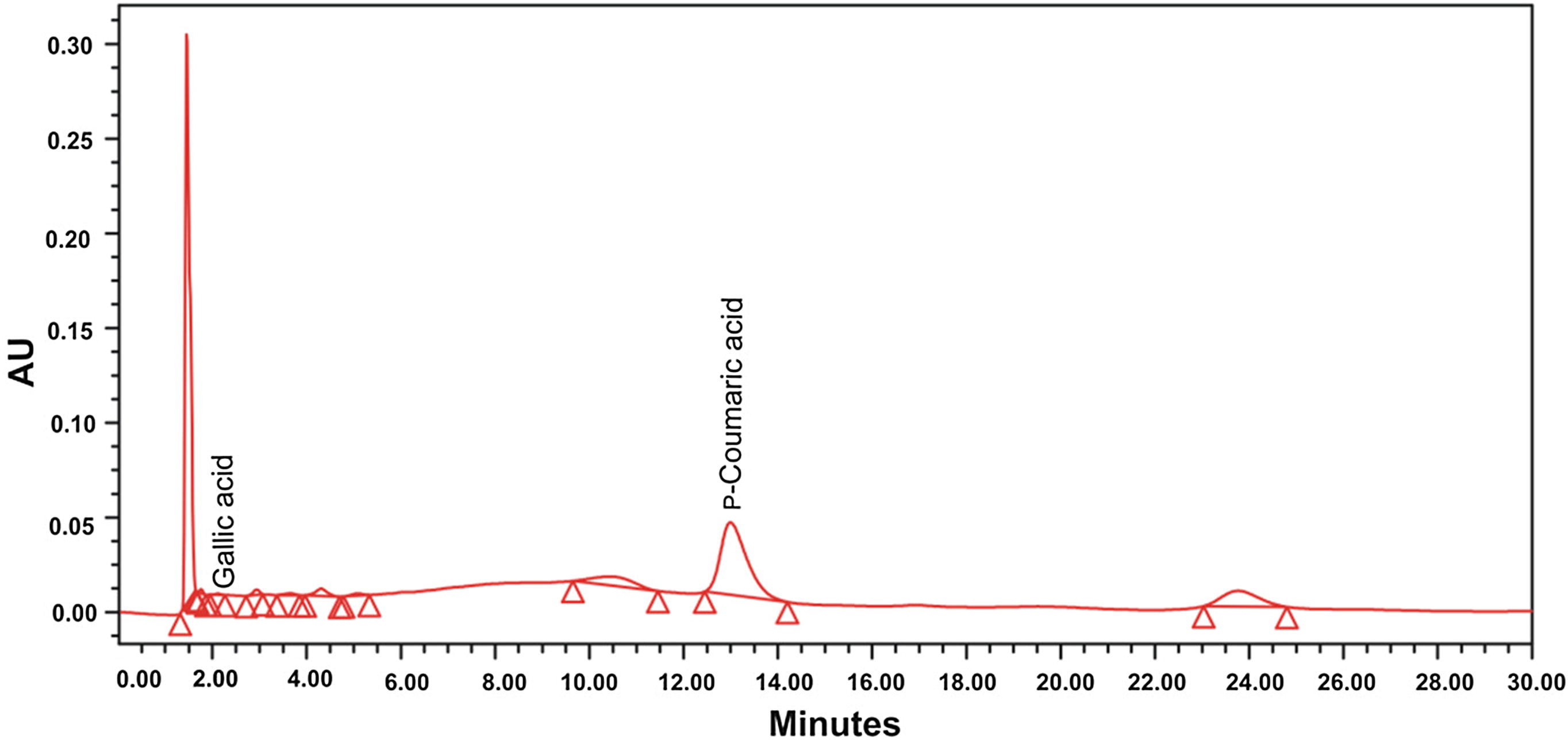
RP-HPLC chromatogram of methanolic extract of H. mystax stem-bark.
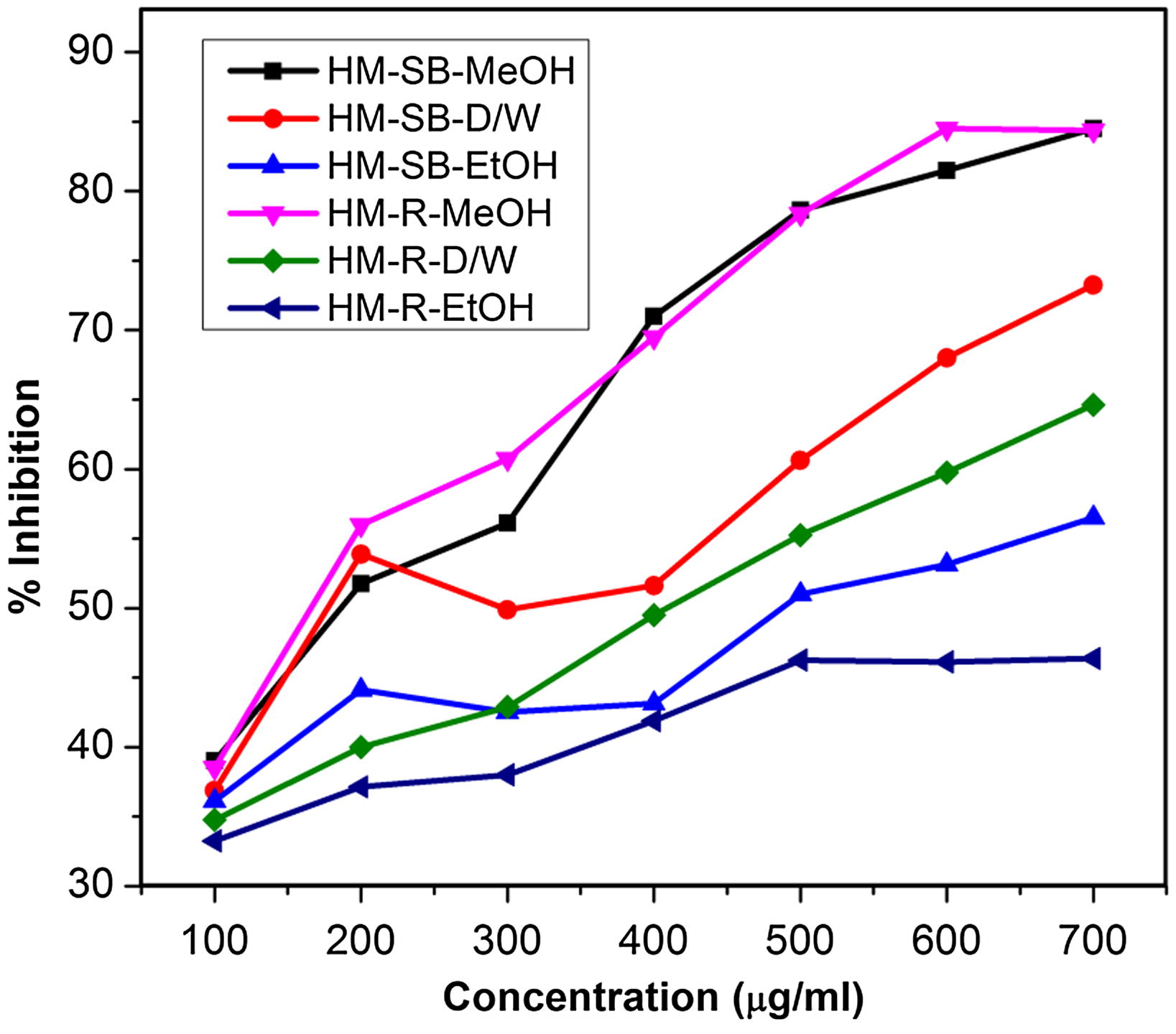
Free radical scavenging potential of various extracts of H. mystax plant parts (n = 3).
Biological processes generate reactive oxygen species (ROS) as a byproduct of various metabolic processes. These free radicals are highly unstable and damage various biomolecules such as proteins, lipids and nucleic acid. ROS has been implicated in the pathogenesis of several diseases including cardiovascular diseases, cancer and degenerative diseases (Saibabu et al., 2015; Cherubini et al., 2005; Nimse and Pal, 2015). The association between chronic inflammation and rheumatoid arthritis (RA) has already been well defined. In RA patients, level of antioxidant enzymes in serum and synovial fluid is found to be less compared to healthy individual; on the other side, the non-stop generation of free radicals in inflamed joints leads to the failure of endogenous antioxidant system (Kundu et al., 2011; Hold and El-Omar, 2008; Pattison and Winyard, 2008). Phenolic acids, a major class of phytochemicals have been attributed to act as antioxidants with various physiological actions in the living system. The experimental evidences revealed that the phenolic acids can be utilized for preventive and therapeutic purposes in oxidative stress related diseases (Fresco et al., 2006; Razzaghi-Asl et al., 2013). Therefore it is highly imperative to study the free radical scavenging potential of herbal extracts in correlation with their biological activity. DPPH is a purple colored nitrogen centered stable free radical with an absorbance at 517 nm. Reduction of DPPH• in the presence of antioxidants produces corresponding yellow colored hydrazine DPPH-H (Cai et al., 2003; Tirumani et al., 2016). The free radical scavenging potential of phenolic acids is well known and widely studied, but they also exhibit antioxidant activity by modulating the endogenous enzymes (Roy et al., 2013). Phenolic acids such as caffeic acid, gallic acid, p-coumaric acids and ferulic acid showed a remarkable antioxidant and antimicrobial activity (Badhani et al., 2015). Among various hydroxycinnamic acids, caffeic acid is found to be the most potent antioxidant compared to p-coumaric acid and ferulic acid (Gulcin, 2006). Statistical analysis revealed that, the total phenolic contents between the stem-bark and root extracts were not significantly different (p = 0.15). Whereas, DPPH radical scavenging activity was significantly different (p = 0.001). The correlation of the Total phenolic and flavonoid content with DPPH radical scavenging activity was analyzed and it was found that, the total flavonoid content of the stem-bark (R2 = 0.85) as well as root (R2 = 0.70) extracts exhibit stronger positive correlation with the DPPH radical scavenging activity as compared to the total phenolic content (Figs. 6 and 7). Recent observation suggests, DPPH assay is one of the most frequently used ones in vitro antioxidant evaluation. However, the assay needs several modifications to cover a wide range of applications (Alam et al., 2013; Kedare and Singh, 2011).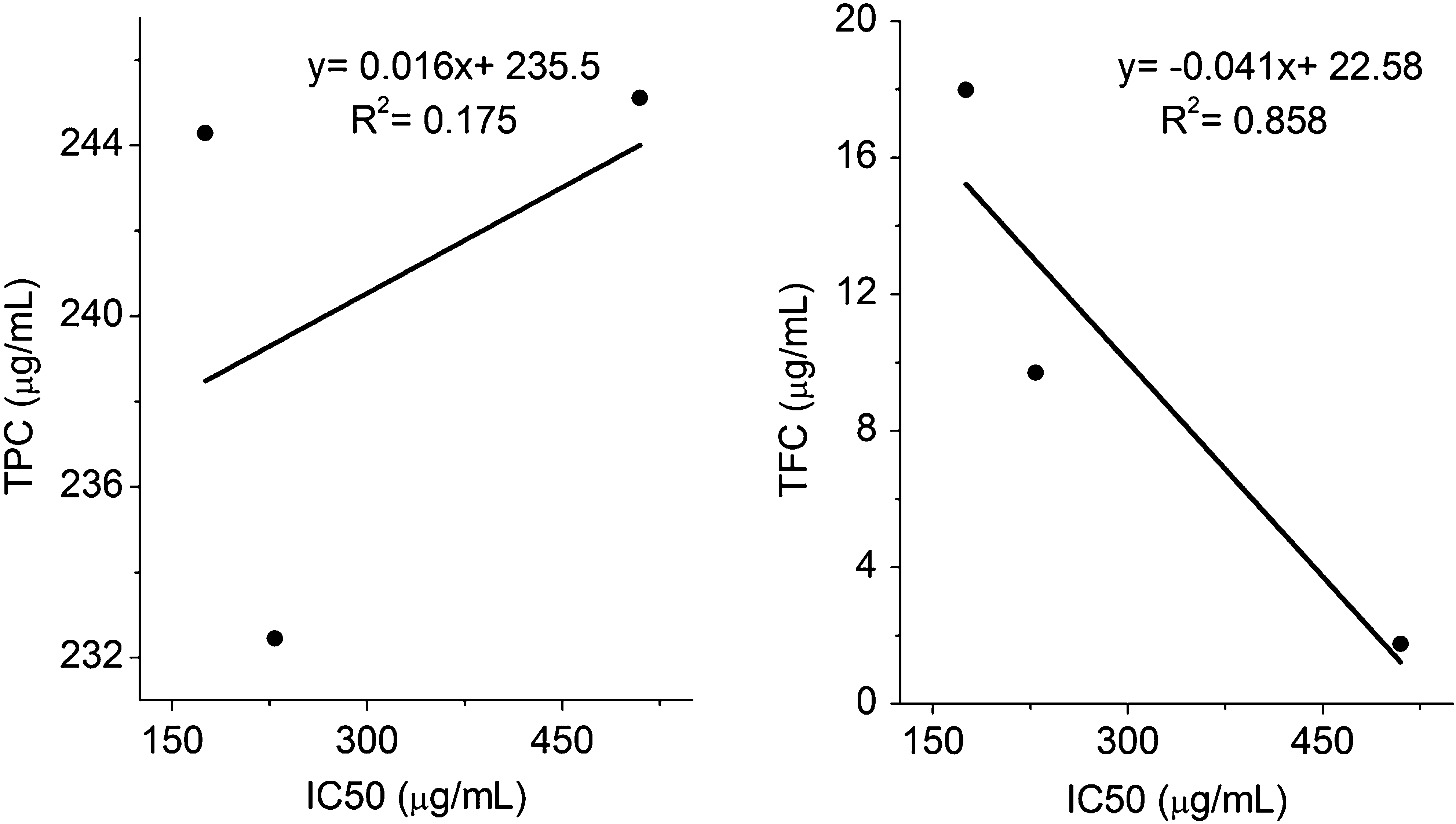
Correlation of total phenolic content and total flavonoid content with IC50 of DPPH free radical scavenging in stem-bark.
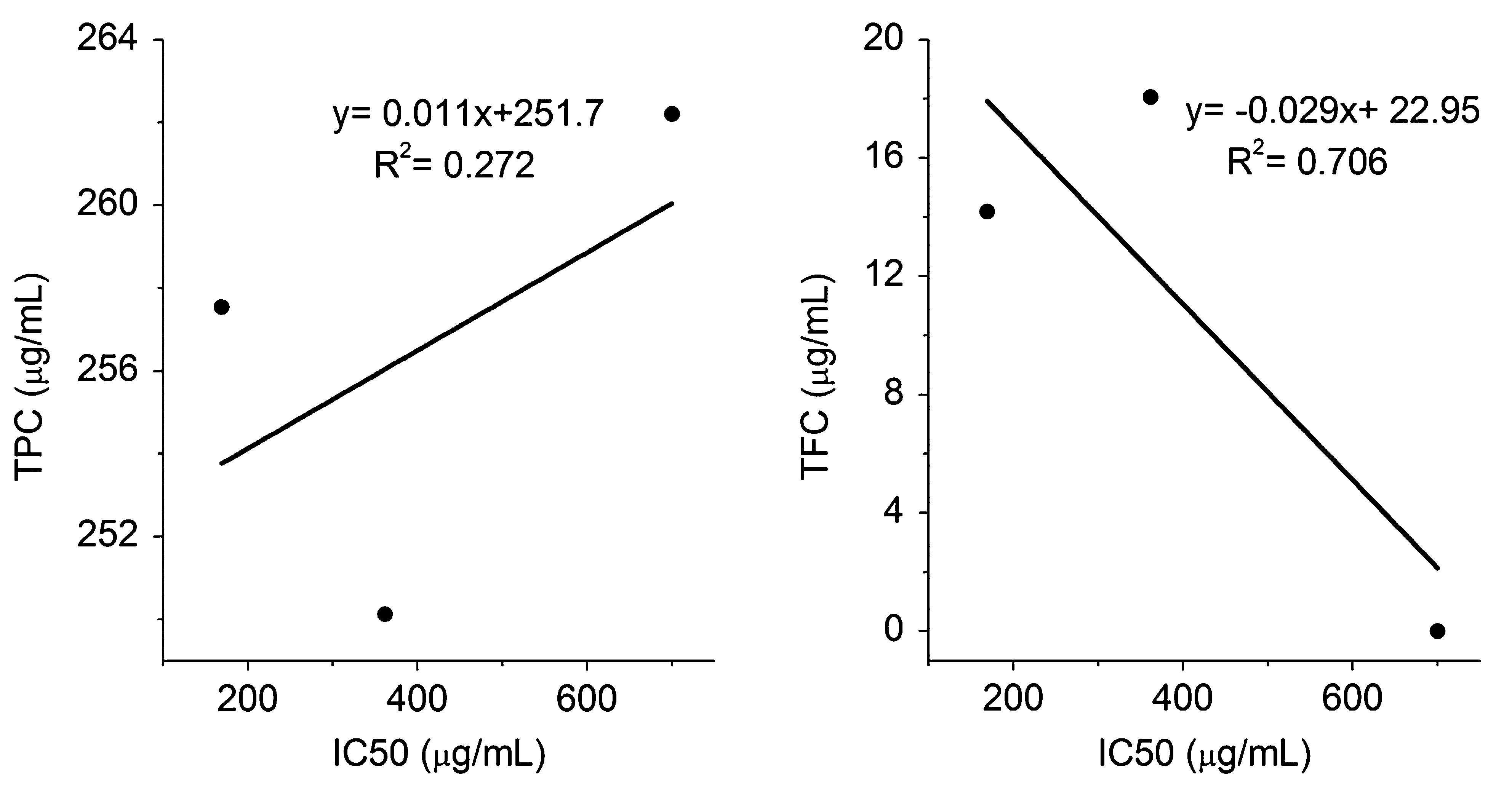
Correlation of total phenolic content and total flavonoid content with IC50 of DPPH free radical scavenging in root.
Recent studies on H. mystax were reviewed and it is found that, phytochemical analysis of leaf extracts (by Soxhlet method) of H. mystax showed the presence of secondary metabolites such as phenolic acids, flavonoids, tannins, terpenoids, steroids; whereas qualitative determination of individual phenolic compounds of extracts revealed the presence of caffeic acid, gallic acid, p-coumaric acid and vanillin (Pawar et al., 2014). Among the various solvent extracts tested for antioxidant potential, petroleum ether, methanol and ethanol extracts of leaf exhibit significant antioxidant activity in vitro (Rajeswari et al., 2014). In vivo anti-inflammatory activity of ethanol extracts of leaf and bark of H. mystax were investigated in albino rats by carrageenan induced rat paw edema method. It was observed that at the dose of 500 mg/kg of body weight of H. mystax extracts significantly inhibited the carrageenan induced paw edema (Rajeswari et al., 2013). However, the active phytoconstituents contributing to biological activity has not yet been identified. The molecular basis for biological effect is not yet understood. This investigation has identified p-coumaric acid as a major phytoconstituent predominantly present in plant parts of H. mystax L. It has been found that compounds rich in phenolic acids also significantly contribute to antirheumatic activity (Wang et al., 2012). In recent findings, p-coumaric acid significantly suppressed leukocyte infiltration and pro-inflammatory cytokine expression in ankle joints of adjuvant-induced arthritic rats model (Pragasam et al., 2013a,b). Zhao et al. (2016) revealed that p-coumaric acid may block NF-kB and MAPKs signaling pathways by inhibiting LPS induced inflammatory cytokines. Hence, p-coumaric acid has the potential of being used as immunosuppressive agent in treating autoimmune inflammatory diseases like rheumatoid arthritis.
4 Conclusion
Thus, it may be concluded from the present study that stem bark and root of H. mystax L. are a rich source of polyphenols, which possibly contribute to their biological activity. This is the very first study where individual phenolic compounds in stem bark and root of H. mystax L. have been quantified by the RP-HPLC method. Present research findings strongly support the traditional use of H. mystax L. plant parts by traditional healers. However, studies at molecular level are required to reveal the exact mechanism of action of active plant extracts exhibiting anti-inflammatory activity, which is under investigation.
Acknowledgments
Authors are thankful to Dr. Anilkumar Vaidya (Kolhapur) and Dr. B.G. Gavade (Kankavali) for sharing valuable information related to the plant selected for this study and are also thankful to Mr. Subhash Kudale, Research Associate, School of Biotechnology & Bioinformatics, DY Patil University, CBD – Belapur, Navi Mumbai, for his help in conducting HPLC study. The work was carried out by funding from the DY Patil University, Navi Mumbai for interdisciplinary research (CIDR/DYPU/Biotech/003).
References
- Antibacterial activity and phytochemical screening of some medicinal plants commonly used in Saudi Arabia against selected pathogenic microorganisms. J. King Saud Univ. Sci.. 2013;25(2):115-120.
- [Google Scholar]
- Comparative study of phytochemical screening, antioxidant and antimicrobial capacities of fresh and dry leaves crude plant extracts of Datura metel L. J. King Saud Univ. Sci.. 2014;26(3):237-243.
- [Google Scholar]
- Review on in vivo and in vitro methods evaluation of antioxidant activity. Saudi Pharm. J.. 2013;21:143-152.
- [Google Scholar]
- Advantages and limitations of common testing methods for antioxidants. Free Radic. Res.. 2015;49(5):633-649.
- [CrossRef] [Google Scholar]
- Gallic acid: a versatile antioxidant with promising therapeutic and industrial applications. RSC Adv.. 2015;5:27540-27557.
- [Google Scholar]
- Antifungal activity of Aegle marmelos (L.) Correa (Rutaceae) leaf extract on dermatophytes. Asian Pac. J. Trop. Biomed.. 2011;1(3):169-172.
- [Google Scholar]
- Modified Folin–Ciocalteu antioxidant capacity assay for measuring lipophilic antioxidants. J. Agric. Food Chem.. 2013;61:4783-4791.
- [Google Scholar]
- Antioxidant activity of betalains from plants of the Amaranthaceae. J. Agric. Food Chem.. 2003;51:2288-2294.
- [Google Scholar]
- Phytochemical screening and free radical scavenging activities of orange baby carrot and carrot (Daucus carota Linn.) root crude extracts. J. Chem. Pharm. Res.. 2013;5(4):97-102.
- [Google Scholar]
- Potential markers of oxidative stress in stroke. Free Radic. Biol. Med.. 2005;39(7):841-852.
- [Google Scholar]
- Essential oils, kava pyrones and phenolic compounds from leaves and rhizomes of Alpinia zerumbet and their antioxidant activity. Food Chem.. 2007;103(2):486-494.
- [Google Scholar]
- Thorough study of reactivity of various compound classes toward the Folin–Ciocalteu reagent. J. Agric. Food Chem.. 2010;58:8139-8144.
- [Google Scholar]
- New insights on the anticancer properties of dietary polyphenols. Med. Res. Rev.. 2006;26(6):747-766.
- [Google Scholar]
- Lexicon of Medicinal Plants in India. Vol vol. II. Calcutta, India: Naya Prokash Publishers; 2001.
- Antioxidant activity of caffeic acid (3,4-dihydroxycinnamic acid) Toxicology. 2006;217:213-220.
- [Google Scholar]
- Utilization of Folin–Ciocalteu reagent for the detection of certain nitrogen compounds. J. Agric. Food Chem.. 2003;51:1811-1815.
- [Google Scholar]
- Genesis and development of DPPH method of antioxidant assay. J. Food Sci. Technol.. 2011;48(4):412-422.
- [Google Scholar]
- Attenuation of oxidative stress by allylpyrocatechol in synovial cellular infiltrate of patients with Rheumatoid Arthritis. Free Radic. Res.. 2011;45(5):518-526.
- [Google Scholar]
- Polyphenols: food sources and bioavailability. Am. J. Clin. Nutr.. 2004;79:727-747.
- [Google Scholar]
- Anti-inflammatory activity of the leaf extracts of Gendarussa vulgaris Nees. Asian Pac. J. Trop. Biomed.. 2011;1(2):147-149.
- [Google Scholar]
- Free radicals, natural antioxidants, and their reaction mechanisms. RSC Adv.. 2015;5(35):27986-28006.
- [Google Scholar]
- Ethnomedicinal plants from Paderu Division of Visakapatnam District, A.P. India. J. Phytol.. 2010;2(8):70-91.
- [Google Scholar]
- Historical perspective of traditional indigenous medical practices: the current renaissance and conservation of herbal resources. Evid. Based Complement. Alternat. Med.. 2014;525340
- [CrossRef] [Google Scholar]
- Dietary antioxidants in inflammatory arthritis: do they have any role in etiology or therapy? Nat. Clin. Pract. Rheumatol.. 2008;4:590-596.
- [Google Scholar]
- Phytochemical evaluation and free radical scavenging potential of Hugonia mystax L. (Linaceae) leaf extracts. Bionanao Front.. 2014;7(2):3-6.
- [Google Scholar]
- In vivo toxicity study of Lantana camara. Asian Pac. J. Trop. Biomed.. 2011;1(3):189-191.
- [Google Scholar]
- Ameliorative effect of p-coumaric acid, a common dietary phenol, on adjuvant-induced arthritis in rats. Rheumatol. Int.. 2013;33:325-334.
- [Google Scholar]
- Immunomodulatory and anti-inflammatory effect of p-Coumaric Acid, a common dietary polyphenol on experimental inflammation in rats. Inflammation. 2013;36(1):169-176.
- [Google Scholar]
- Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem.. 2005;53:4290-4302.
- [Google Scholar]
- Antioxidant activity of phenolic acids and their metabolites: synthesis and antioxidant properties of the sulfate derivatives of ferulic and caffeic acids and of the acyl glucuronide of ferulic acid. J. Agric. Food Chem.. 2012;60(50):12312-12323.
- [Google Scholar]
- Flora of Andhra Pradesh (India).Ranaculace-Alangiaceae. Vol vol. 1. Jodhpur, India: Scientific Publishers; 1997.
- Ethno-medico-botanical investigations in Kerala I. Some primitive tribals of Western ghats and their herbal medicine. J. Ethnopharmacol.. 1984;11(1):59-77.
- [Google Scholar]
- Anti-inflammatory activity of leaf and bark of Hugonia mystax L. (Linaceae.) J. Harmo. Res. Pharm.. 2013;2(2):80-83.
- [Google Scholar]
- Total phenolic and flavonoid contents and in vitro antioxidant activity of Hugonia mystax Linn. leaf (Linaceae) Int. J. Preclin. Pharm. Res.. 2014;5(1):12-18.
- [Google Scholar]
- Compendium of Indian Medicinal Plants. Vol vol. 4. Central Drug Research Institute Lucknow; 2002. p. :386.
- Antioxidant properties of hydroxycinnamic acids: a review of structure–activity relationships. Curr. Med. Chem.. 2013;20(36):4436-4450.
- [Google Scholar]
- Treatment with ferulic acid to rats with streptozotocin induced diabetes: effects on oxidative stress, pro-inflammatory cytokines, and apoptosis in the pancreatic β cell. Endocrine. 2013;44(2):369-379.
- [Google Scholar]
- Therapeutic potential of dietary phenolic acids. Adv. Pharmacol. Sci. 2015:1-10. Article ID 823539
- [Google Scholar]
- The Folin–Ciocalteu assay revisited: improvement of its specificity for total phenolic content determination. Anal. Methods. 2013;5(21):5990-5999.
- [CrossRef] [Google Scholar]
- A Dictionary of flowering plants in India. New Delhi: Council of Scientific and Industrial Research; 1983.
- Impact of Cultivation and Gathering of Medicinal Plants on Biodiversity: Global Trends and Issues Inter-Department Working Group on Biology Diversity for Food and Agriculture. Rome, Italy: Food and Agriculture Organizations (FAO); 2002.
- Local knowledge in community-based approaches to medicinal plant conservation: lessons from India. J. Ethnobiol. Ethnomed.. 2006;2:20.
- [Google Scholar]
- Ethnomedicinal plants used by the tribals of Kalakad Mundanthurai Tiger Reserve (KMTR), Western Ghats, Tamil Nadu for the treatment of rheumatism. Ind. J. Tradit. Knowledge. 2009;9:502-509.
- [Google Scholar]
- Determination Phenolic Content and In vitro Antioxidant Activity of Leaves of Indian Lavender Plant Bursera Penicillata ENGL. Int. J. Pharm. Sci. Rev. Res.. 2016;37(1):125-129.
- [Google Scholar]
- Phytochemistry and antimicrobial activity of Hugonia mystax L. (Linaceae) Int. J. Pharm. Pharm. Sci.. 2012;4(1):381-384.
- [Google Scholar]
- Antirheumatoid arthritis activities and chemical compositions of phenolic compounds-rich fraction from Urtica atrichocaulis, an endemic plant to China. Evid. Based Complement. Alternat. Med. 2012:1-10.
- [Google Scholar]
- World Health Organization, 2002. Traditional Medicines Strategy 2002–2005 Geneva. <http://www.wpro.who.int/health_technology/book_who_traditional_medicine_strategy_2002_2005.pdf>.
- Electrochemical method for estimating the antioxidative effects of methanol extracts of crude drugs. Chem. Pharm. Bull.. 1994;42(8):1663-1665.
- [Google Scholar]
- Anti-inflammatory effects of p-coumaric acid in LPS-stimulated RAW264.7 cells: involvement of NF-κB and MAPKs pathways. Med. Chem. (Los Angeles). 2016;6:327-330.
- [Google Scholar]







