Translate this page into:
Potent anticancer, antioxidant and antibacterial activities of isolated flavonoids from Asplenium nidus
⁎Corresponding author. lucki.chem09@gmail.com (Lakhveer Singh)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Bioactive flavonoids derived from Asplenium nidus (fern) possess potent tumoricidal, anti-bacterial, anti-oxidant activities that are responsible for their chemo-preventive potential against multidrug-resistant (MDR) pathogens, Proteus mirabilis, Proteus vulgaris and Pseudomonas aeruginosa. Fractionation and recognition of the flavonoids were attained through gas chromatography and mass spectrometry (GC/MS). Twelve known and three unknown compounds were revealed by fractions 1 & 3, representing 13.12% and 2.61% out of the total composition (15.12%). Gliricidin7-O-hexoside was found 3.83% followed by quercetin-7-O-rutinoside (3.09%) keampferol-3-O-rutinoside (0.19%) and myricetin-3-O-rhamnoside (1.10%). The anti-bacterial and anti-oxidant activities of present fractions along with pure bioactive constituents were tested against three MDR pathogens through microbroth dilution: MIC50 ranges for amoxicillin, gliricidin7-O-hexoside and quercetin 7-O-rutinoside were 0.0003–0.06 μg/mL, 0.004–0.06 μg/mL and 0.005–6.0 μg/mL, respectively. Gliricidin-7-O-hexoside and quercetin-7-O-rutinoside’s inhibitory activities were comparable to standard antibiotic, amoxicillin (p > 0.05). Herein we report for the first time a number of flavonoids that may act as the source of therapeutically useful compounds against MDR pathogens. In addition, the most extracted flavonoids (gliricidin-7-O-hexoside (78.1%) and quercetin-7-O-rutinoside (69.2%) showed a significant in vitro antioxidant activity i.e. DPPH radical scavenging activity. Both fractions of A. nidus showed obvious cytotoxic effects on human hepatoma HepG2 and human carcinoma HeLa cells. Moreover, the anti-cancer activity generally enhanced with ameliorating antioxidant and antibacterial potential of fern’s flavonoids. These findings illustrate the potential of this fern as a probable source of bioactive constituents and provide a scientific basis for its folklore or ethno-medicinal uses for infectious diseases and cancers.
Keywords
Asplenium nidus
Flavonoids
Anti-oxidant
Anti-cancer
Anti-bacterial
1 Introduction
Ferns are generally used in traditional medicine for the cure of many deadly diseases like skin problems, wounds, cough and reproductive problems as well as to make insect repellent (Nath et al., 2016; Bahadori et al., 2015). A wide range of medicine ferns like Adiantum capillus-veneris, Cheilanthes albomarginata including, A. nidus exist in Asia (Chang et al., 2005). A. nidus Linn is a member of Aspleniaceae which is commonly known to the locals as the ‘langsuyar’ or the bird’s nest fern (Fig. 1). It has a rosette-shaped basket consisting of long fronds (Ellwood et al., 2002). It has been reported that A. nidus not only is extensively used to treat elephantiasis and to, reduce fever but also showed remarkable antiviral activity toward Herpes simplex virus Type-1(HSV-1) (Hammami et al., 2016; Campos et al., 2011). A. nidus has been used locally in folk medicine for asthma, sores, weakness and halitosis (Duke, 2011).
Plant of Asplenium nidus.
In the plant kingdom, flavonoids are commonly found, with approximately 10,000 known structures (Babii et al., 2016; Wang et al., 2016; Bohin et al., 2012) and have attracted much enthusiasm for possessing potential bioactivities, just as neuro-protective (Wang et al., 2016; Hwang et al., 2012), tumoricidal, (Kurzawa-Zegota et al., 2012; Wei et al., 2012) and anti-glycative activities (Verzelloni et al., 2011). It is plausible because of their anti-oxidant properties (Wang et al., 2016; Xie et al., 2012).
The major identified constituents in A. nidus plants are phenols and flavonoids (Hammami et al., 2016) yet little information about flavonoids in A. nidus (Iwashina et al., 2000) is available. Likewise, little research has been performed on the phytochemical components of ferns including A. nidus and their bioactivities. The aim of our study was to assess the phytochemical investigations were carried out yielding in the isolation of new flavonol derivatives with antioxidant properties from A. nidus. Flavonoid derivatives of A. nidus were identified by gas chromatography and mass spectrometry (GC/MS). In addition the anti-oxidant, anti-cancer and anti-bacterial properties were investigated from the profiles of isolated flavonoids from A. nidus against MDR pathogens. To the best of our knowledge, this compound is being reported in A.nidus for the first time. It is worth mentioning that because of the excellent anti-oxidant, anti-bacterial and anti-cancer activities.
2 Experimental
2.1 Chemicals and reagents
All chemicals used were of the highest purity (⩾99.0%) and were purchased from Sigma Aldrich and Merck Malaysia, as follows: NaCl, HCl, FeCl2, ethanol, methanol, acetone, H2SO4, ferric chloride, KCl, Na2HPO4, chloroform, conc. H2SO4, acetic anhydride, KH2PO4, NaNO2, AlCl3, NaOH, H2O2, Na2CO3, ferrozine, FeSO4 and glacial acetic acid. Bacterial strains were bought by University Malaya (UM), Malaysia. The GC and GC/MS techniques were used on Hewlett-Packard (HP) 5973 with HP-6890 gas chromatograph having HP5 column of 30 m × 0.25 mm i.d with 0.25 μm of film thickness. Strains were cultivated in nutrient broth. The human carcinoma HeLa cells were provided by the University Malaya (UM) research laboratory. The Dulbecco’s Modified Eagle’s Medium (DMEM; Sigma Chemical Company, MO, Saint Louis, USA) with 10% heat-inactivated fetal bovine serum in a humidified atmosphere with 5% CO2 at 37 °C were used for cell maintenance.
2.2 Preparation of plant extract
The fresh fern/plant material (whole plant) was washed under running tap water, air-dried and homogenized into fine powder. The powered preparations were stored in air-tight glass vials. For preparing tissue extracts, 1.0 g of the homogenized powder was placed in 10 ml of methanol in a conical flask, covered with cotton cap and kept on a rotary shaker at 200 rpm for 48 h. The extract was filtered through Whatmann filter paper No.1, the resultant filtrate of which was centrifuged at 10,000 rpm at 4 °C for 15 min. The supernatant was collected and completely evaporated at room temperature. The left-over methanolic fern extract (MFE) residue was dissolved in 0.05 M Phosphate buffered saline (PBS), pH 7.2 to reach a final concentration and was sterilized by filtration (0.22 μm Millipore filter). The filter-sterilized preparation of MFEs thus obtained was stored in a freezer at −70° C in air-tight vials for further studies.
2.3 Flavonoid assays
The flavonoid assays were performed according to the reported procedure (Belmekki and Bendimerad, 2012). The determination of flavonoids was performed according to the colorimetric assay. Diluted ammonia (5 ml) solution was mixed with plant extract, followed by slow addition of concentrated H2SO4. A yellow coloration in the extract which diminished on standing indicated the presence of flavonoids.
2.4 Quantitative assay for flavonoids
The flavonoid assay was done according to the reported procedure. The aluminum chloride (AlCl3) colorimetric assay was used to determine the total flavonoid content in MFE (Marinova et al., 2005). 0.5 ml of sample (2000 μg/ml) was added to 2.8 ml of distilled water, 0.1 ml of 1 M potassium acetate, and 0.1 ml of 10% aluminum chloride. The content was mixed using a vortex and incubated at 37 °C for half an hour. The absorbance was measured at 510 nm with a micro plate reader (Molecular Devices).
2.5 Fractionation of crude extracts – using column chromatography
A column of 40 cm was filled up to 31 cm height having 2.5 cm diameter with slurry of silica gel 60 (Merck) (0.063–0.2 mm being the particle size 70–230 mesh). After equilibration of the column with 100% methanol for 30 min, 5 g of the plant extract along with 10 g gel was mixed in concentrated methanol slurry. Air-dried mixture was then decanted to the top of the packed column up to 7.5 cm in height. Firstly the column was eluted by methanol at 40 drops per minute followed by solvent combination of ethyl acetate/methanol/water (EMW) in the ratio 20:2.7:2.5 (Masoko et al., 2008). Each fraction was collected with a standard eluent volume of 200 mL. A total of 15 fractions thus collected were subjected to a rotary evaporator, concentrated until dryness and subsequently transferred to glass Petri dishes. Residual solvents were than gathered after air-drying the dishes for 48 Hours. To determine the number of phytocomponents in each fraction, primary phytochemical methods were used (Harborne, 1984; Kim et al., 2003).
2.6 Compound identification through gas chromatography & mass spectrometry (GC/MS)
A Hewlett-Packard (HP) mass spectrometer interfaced with an HP-6890 gas chromatograph with an HP5 column was used for GC and GC/MS analysis. In split mode, sample was added through the injector along with helium (carrier gas), with initial flow rate 0.7 ml/minute and a 32 cm/sec average linear velocity. The settings of the temperature were as described here in: injector temperature 220 °C; oven temperature 70 °C (initial), a 30 mL/min purge flow and 0.2 minute purge time, 5 °C/minute ramp, final temperature 240 °C, pressure of 8.20 psi. Mass spectra with pervious literature (Adams, 1995) were used as identifier for compounds. Peak areas of the total fraction composition were observed to record the percentage composition of every compound.
2.7 Anti-oxidant activity assay
Antioxidants react with 1, 1-diphenyl-2-picrylhydrazyl (DPPH) stable free radicals to produce its reduced form (DPPH-H). The DPPH-H formation in the reaction mixture is assayed (Xiao et al., 2011) at A517. The reduction of the DPPH radical to the DPPH-H form is indicated by the formation of a yellow color. A lower A517 of the reaction mixture indicated a higher free radical scavenging activity. DPPH solution (0.1 mM) in methanol was prepared and 3.0 ml of purified flavonoids at different concentrations was added to 1.0 ml of this solution and 30 minutes later the A517 values were recorded. A blank was prepared without adding flavonoids to serve as a control. Ascorbic acid (1–18 μg/ml) was used as a standard. The capability to scavenge the DPPH radicals was determined as follows:
2.8 Determination of antibacterial activity by inhibitory concentration (MIC50) of fractions
Micro-broth dilution method performed in 96-well plates to determine the MIC50 of test fractions. Test fractions were prepared at a concentration of 5.0 mg/mL, while gliricidin-7-O-hexoside and quercetin 7-O-rutinoside were prepared at a concentration of 2 μg/mL and 200 μg/mL respectively. For nutrient broth two-fold dilutions of every fraction were prepared in test wells. For such fractions, the ranges of the final concentration were 2–2500 μg/ml, 0.001–2 μg/ml and 0.003–200 μg/ml, gliricidin-7-O-hexoside and quercetin-7-O-rutinoside, respectively. Then, 20 μL of an 18-h-old broth culture of Gram-negative multidrug resistant suspension was mixed with 100 μL of the fraction having the culture medium. The preparation of control was done with the bacterial suspension, culture medium and broth only. Alongside each batch of tests at a concentration range of 0.0002–2 μg/mL, positive-control antibiotic was amoxicillin. To check absorbance of plates after and before three days of incubation at room temperature observed under micro-aerophilic situations, an automatic enzyme-linked immunosorbent assay (ELISA) microplate reader adjusted to 610 nm was used. To determine increase and decrease in bacterial growth, absorbencies were compared and were represented graphically against concentration. Least concentration of fraction resulting in inhibition of 50% bacterial growth was calculated as MIC50.
2.9 Cytotoxicity assay
Uniform volumes of HepG2 and HeLa cell suspension (200 μl/well; ∼2 × 104cell/ml) were poured into the selected wells of a 96-wells sterile tissue culture plate. The filter-sterilized fern extract (200 μg/ml stock) was dispensed into the wells of the microtiter plate to achieve final concentrations of 0–600 μg/ml in Dulbecco’s Modified Eagle’s Medium (DMEM; Sigma Chemical Company, MO, Saint Louis, USA). The cells treated with gliricidin-7-O-hexoside and quercetin-7-O-rutinoside (the flavonoids isolated in greatest quantities) were incubated in a CO2 incubator with 95% humidity at 37 °C for 16–18 h. The DMEM (100 μl) was removed from each of the wells and 20 μl of freshly prepared MTT (5 mg/ml) was mixed in these wells followed by incubation for 1 h at 37 °C in a CO2 incubator. Thereafter, the DMEM containing MTT was completely removed from the wells using a multi-channel auto-pipette and the purple colored product by the cells in each of the wells was extracted in DMSO (100 μl/well) and A570 values were recorded in an ELISA reader. Each of the MFE concentrations was tested in quadruplicate and mean values ± standard deviation (SD) were calculated after the MTT assay. The appropriate controls (containing an appropriate volume of methanol alone) were also considered as a negative control to determine the effect of methanol on the viability of the proliferating cells cultured in vitro. Each analysis was performed in triplicate and resulted as reproducible within experimental errors (RSD < 5.0%), as was nearly comparable to standard amoxicillin.
2.10 Statistical analysis
Results values were taken as mean ± S.D. of three independent and different experiments and were checked by a one-way analysis of variance (ANOVA). Remarkable differences were spotted at p < 0.05.
Gliricidin-7-O-hexoside, being the most abundant flavonoids (3.87%), followed by quercetin-7-O-rutinoside, (3.09%), keampferol-3-O-rutinoside and myricetin-3-O-rhamnoside were used against multidrug-resistant pathogens, Proteus mirabilis, Proteus vulgaris and Pseudomonas aeruginosa.
3 Results
3.1 Chemical composition and percentage composition of flavonoids in A.nidus
EAMW fractions 5 and 6 with the least number of components were based on GC/MS analysis. 12 flavonoids were recognized from present fractions, 3 were unknown flavonoids. EAMW two of the fractions (fractions 1 and 3) revealed the presence of compounds 3 and 12, respectively, representing 13.12% and 2.61% and the total composition was 15.12%. The flavonoids detected were gliricidin-7-O-hexoside (3.87%) and quercetin-7-O-rutinoside (3.09%) followed by keampferol-3-O-rutinoside (0.9%) and myricetin-3-O-rhamnoside (1.10%). Further information on the present phytochemical compound composition is outlined in Table 1. Rt, retention time; EMW, ethyl acetate/methanol/water.
Fraction
Compound
Chemical formula
Rt (min)
Area%
EAMW fraction 1
Globularin
C24H28O11
4.24
1.03
Unknown
–
4.83
0.3
Unknown
–
5.46
0.2
Gliricidin 7-O-hexoside
C21H20O11
7.93
3.83
Apigenin7-O-glucoside
C21H20O10
10.01
1.6
Unknown
–
16.20
0.4
Quercetin 7-O-rutinoside
C27H30O16
21.80
3.09
Keampferol 7-O-
C33H40O21
23.23
0.11
gentiobioside
Quercetin 7-O-galactoside
C27H30O17
25.99
1.22
Myricetin 3-O-rhamnoside
C21H20O12
28.57
1.10
Keampferol-3-O-rutinoside
C27H30O15
32.76
0.19
Linoleic acid dimer
C38H72N2O4
38.36
0.05
Total
13.12
Fraction 3
Keampferol 3-O-rhamnoside
C21H19O10
40.40
1.01
Keampferol-7-O-rutinoside
C27H30O15
43.34
0.7
Quercetin
C21H20O11
51.12
0.9
Total
2.61
3.2 Minimum inhibitory concentration (MIC50) of fractions
Out of 13 fractions collected, 12 showed remarkable anti-bacterial activity. The MIC50 values of the fractions ranged from 310 μg/ml to 2500 μg/ml; the ranges of amoxicillin, gliricidin-7-O-hexoside and quercetin-7-O-rutinoside were 0.0003–0.06 μg/ml, 0.004–0.06 μg/ml and 0.005–6.0 μg/mL, respectively, as shown in Table 2. Inhibition values for gliricidin-7-O-hexoside and quercetin-7-O-rutinoside were found to be comparable standard (amoxicillin) (p > 0.05). EAMW-ethyl acetate/methanol/water.
MIC 50(μg/mL)
Proteus mirabilis,
Proteus vulgaris
Pseudomonas aeruginosa
Fraction
EAMW1
310
630
1250
EAMW2
630
630
1250
EAMW3
630
310
630
EAMW4
630
310
310
EAMW5
630
310
310
EAMW6
310
630
630
EAMW7
630
630
630
EAMW8
630
–a
–
EAMW9
630
310
630
EAMW10
310
310
630
EAMW11
630
310
1250
EAMW12
310
310
310
EAMW13
–
–
–
Compound
Gliricidin7-O-hexoside
0.04
0.0005
0.02
Quercetin 7-O-rutinoside
6.0
0.005
3.1
Amoxicillin
0.03
0.0003
0.03
3.3 DPPH assay
The flavonoids from A. nidus exhibited notable DPPH radical scavenging activity, which increased with increasing concentrations. The scavenging activity of gliricidin-7-O-hexoside (78.1%) was higher than that of quercetin-7-O-rutinoside (69.2%) as presented in Fig. 2. The results indicate that the maximum yield extracted flavonoids by A. nidus had a remarkable DPPH radical-scavenging activity, underscoring the potential clinical utility of these flavonoids.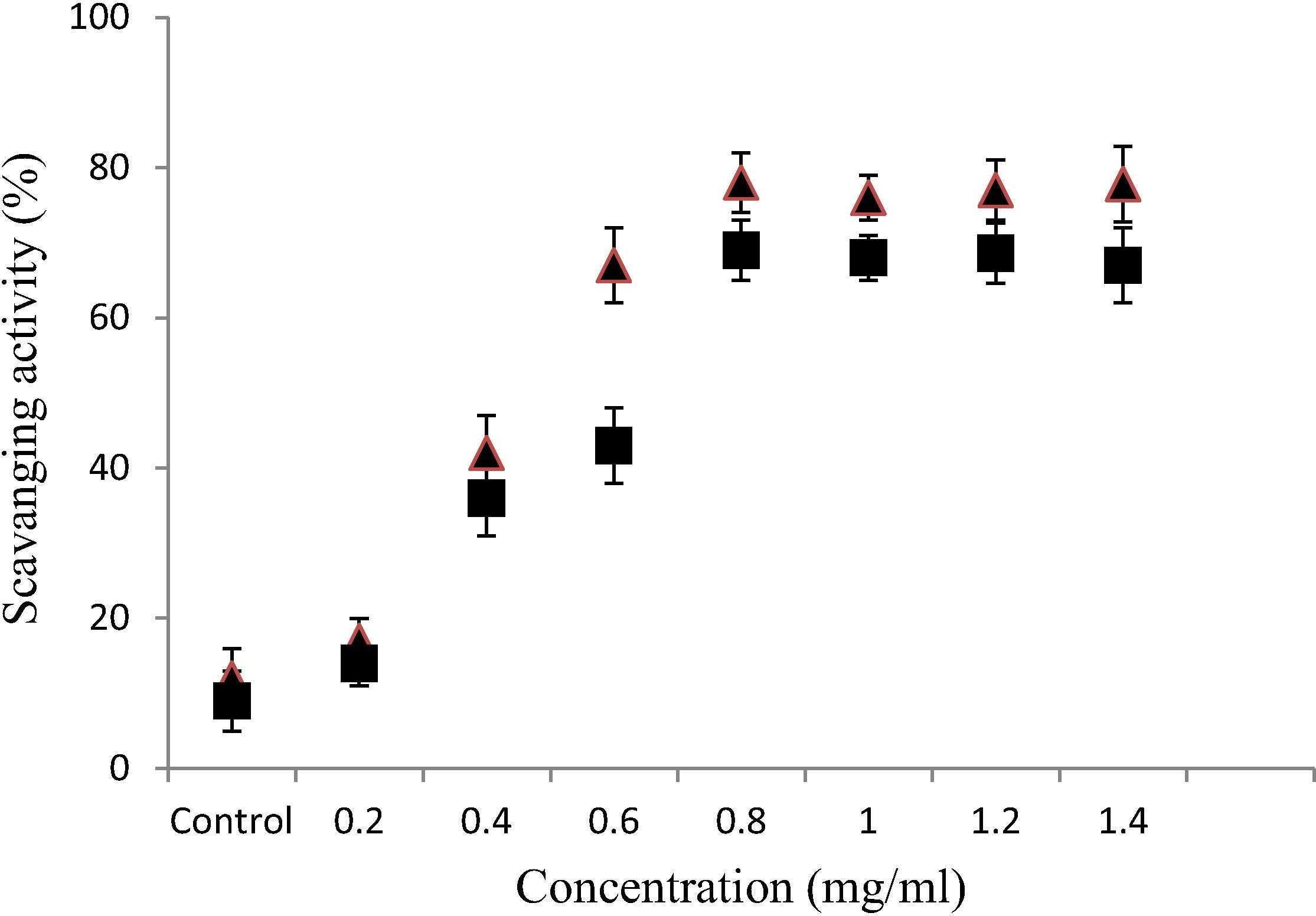
Anti-oxidant activity of flavonoids (gliricidin7-O-hexoside, and quercetin 7-O-rutinoside, from Asplenium nidus assessed by the DPPH radical-scavenging assay. Data represent means ± SD of three independent experiments. ■ – Gliricidin-7-O-hexoside. ▴ – Quercetin-7-O-rutinoside.
3.4 Cytotoxicity assay
The observed results of MTT assay indicated that both potent extracted flavonoids (gliricidin-7-O-hexoside and quercetin-7-O-rutinoside) at all the selected concentrations (240 to 600 μg/ml) exhibited marked cytotoxicity or inhibitory effect on the growth of HepG2 and HeLa cells (Table 3). The maximum viability of the mammalian cells was observed at 600 μg/ml of gliricidin-7-O-hexoside (HepaG2 76.15 ± 0.04) and HeLa (66.91 ± 1.93). At enhanced concentration of gliricidin-7-O-hexoside (600 μg/ml), a marked decline in the viability of the cells was noticed indicating the inhibitory effect of A.indus. These results indicate that flavonoids extracted by A. indus had a remarkable cytotoxic activity, with an IC50 value of 507 μg/ml. Sylvestre et al. (2007) showed that the bioactive compounds did not affect cell viability at concentrations <100 μg/mL, thus validating its safety within this range. It is interesting to note that in the current study, the compound was active at concentrations <1 μg/mL. The high IC50 of the two compounds indicate that they may be safe for human consumption.
Concentration (μg/mL)
Cytotoxicity %
Gliricidin7-O-hexoside
Quercetin 7-O-rutinoside
HepG2
HeLa
HepG2
HeLa
0
100
100
100
100
240
−30.64 ± 4.86
19.99 ± 3.33
−30.64 ± 4.86
19.99 ± 3.33
360
−5.67 ± 2.59
11.10 ± 2.10
−5.67 ± 2.59
11.10 ± 2.10
480
36.53 ± 5.58
37.77 ± 5.34
−5.67 ± 2.59
11.10 ± 2.10
600
76.15 ± 0.04
66.91 ± 1.93
−5.67 ± 2.59
11.10 ± 2.10
4 Discussion
Continuous usage of available drugs in the market for the cure of infections is severely hampered by the development of MDR strains of pathogens; this fuels the likelihood of treatment failure, warranting the quest for novel and inevitable therapeutic agents (Bohr and Malfertheiner, 2009). Study results (Hammami et al., 2016; Bouhlali et al., 2016) have indicated A. nidus (fern) as a promising, unexplored source of such agents. Here we screened anti-bacterial, anti-oxidant and anti-cancer inhibition activities against MDR pathogens of flavonoids extracted from A. nidus.
Anti-bacterial activities were demonstrated by most of the fractions as shown in Table 2, prime aim of fractionation was to purify active phytochemicals (0.0003–0.06 μg/ml 0.004–0.06 μg/ml & 0.005–6.0 μg/ml, respectively. As expected every fraction’s activity was similar to chemical composition of its components, present ratios and their interactions with examine bacteria, as referred to previously (Saidana et al., 2008). Present data suggest that gliricidin-7-O-hexoside and quercetin 7-O-rutinoside acted as major mediators for the antimicrobial activity observed in A. nidus. The inhibitory activity of gliricidin-7-O-hexoside was quite similar to amoxicillin (p > 0.05), an antibiotic commonly used in the clinical settings. This comparable inhibitory activity also enlightens that present compound can be utilized as a possible substitute in the formation of drug-resistant bacterial disease.
The activity of gliricidin-7-O-hexoside activity was significantly different from that of the EAMW fractions 4, 5, 1 and 12 (p < 0.05). Higher purification from these components will therefore improve the activities of these fractions. In the literature (Lai et al., 2009) also demonstrated anti-bacterial activity against E. coli and P. aeruginosa. A similar finding was reported for Dryopteris erythrosora (6.25 μg/ml) (Lee et al., 2009) by the micro-dilution method.
The present study provided evidence for the anti-cancer activity of gliricidin-7-O-hexoside on HepG2 and Hela cancer cells as reflected by the values of 76.15 ± 0.04, 66.91 ± 1.93, respectively, which were higher than the best value shown documented in previous studies. The EC50values of flavonoids on HepG2 and Hela cancer cells have been reported to be 69.50 and 22.64 l M (43.21 and 12.31 lg/mL), respectively (Alia et al., 2006) It is noteworthy that these flavonoids were active at concentration levels less than 1 μg/ml, as outlined in Table 3. The compounds did not affect cell viability at concentrations <100 μg/mL, thus validating its safety within this range. It is interesting to note that in the current study, the compound was active at concentrations <1 μg/mL (Sylvestre et al., 2007). The flavonol compounds display a remarkable spectrum of biological activities including those that might be able to influence processes that are dysregulated during cancer development. These include, for example, antiallergic, anti-inflammatory, antioxidant, antimutagenic, anticarcinogenic, and modulation of enzymatic activities (Craig, 1999; Middleton et al., 2000).
Both flavonoids with the greatest yield showed a significant anti-oxidative activity in vitro: the DPPH radical scavenging activity of gliricidin-7-O-hexoside (78.1%) was higher than the quercetin-7-O-rutinoside (69.2%).The gliricidin-7-O-hexoside has a higher number of hydroxyl groups that could be responsible for the higher scavenging activity. Previous reports have suggested that antioxidant activity of phenolics could be determined by the positions and number of hydroxyl groups and that ortho-dihydroxy and glycosylation groups were the most important structural features for their anti-oxidant activity (Zhang et al., 2012). O-dihydroxy structure in the B-ring (3′ 4′-OH), the 2, 3-double bonds in combination with a 4-oxo-group and the existence of both hydroxyl groups in positions 3 and 5 determine the antioxidant activity of any flavonoid. All these results illustrate the importance of the 3-OH group in the C ring. The gliricidin-7-O-hexoside has the highest and significant (p < 0.05) inhibitory potential than the quercetin-7-O-rutinoside. Plant acts as electron donors because of their content in phenolic compounds (Duthie and Dobson, 1999). This may justify the DPPH radical scavenging power noted in the tested falvonoids. This result corroborates with previous study which demonstrated that DPPH scavenging properties of plant extracts increase with the concentration of extracts (Gul et al., 2013; Kumar et al., 2014; Hilmi et al., 2014). The inhibitory potential of the extracts tested rise with the augmentation of concentration.
The percentage composition of gliricidin-7-O-hexoside (3.83%) in the present fern (A. nidus) in the present study is quite similar to the reports in Dryopteris erythrosora (2.1%) by Cao et al., 2013. At such high percentages this flavonoid is isolated from A. nidus for the first time to our knowledge. It is worth reporting that because of the meritorious anti-bacterial, anti-oxidant and anti-cancerous activity demonstrated by flavonoids (Mariya et al., 2015), they are being researched with a view that people having asymptomatic gastritis will be benefited from a nutritional approach. It may also be useful in curing infection & thereby reduce the chances of severe pathological conditions such as skin problems, wounds, fever, cough and reproductive problems.
Another compound isolated in abundance in the current study was quercetin-7-O-rutinoside (Table 1), which is used in the pharmaceutical industry as a strong basic derivative, especially as modifier anti-bacterial agents, (Cao et al., 2013; Nicoletta et al., 2007). In this regard, however, toxicological studies of flavonoids are warranted before therapeutic application. Our research refers to be cautious about ethnomedicinal uses of A. nidus. We have used stem, leaves, roots and barks of the present fern as remedies for many serious diseases and these phytochemicals are safe, but since their dosages are not standardized these may not be safe every time.
In addition, we also report, apigenin-7-O-glucoside, quercetinm-7-O-galactoside, kaempferol-7-O-gentiobioside, kaempferol-3-O-rutinoside, myricetin-3-O in the A. nidus (Table 1). However, flavonoid compositions are likely to be dependent upon the nutritional status of the plant, geographic location, soil composition, the parts of the plant subjected to analysis, age and weather conditions (Ndhlala et al., 2007). An important finding of the present study is that the pure flavonoids found in the fern A. nidus are responsible for anti-oxidant, anti-bacterial and anti-cancer activities.
These analyses suggest that A. nidus (fern) contains potentially health-protective phytochemical compounds with a potent source of natural anti-oxidants and anti-bacterial activities that may be clinically promising. Thus, it’s also adding new compounds to the ever increasing canvas of phenolic compounds acting as fountains of health.
5 Conclusion
The fern A. nidus is a rich source of anti-bacterial, anti-oxidant and anti-cancer phytochemical, with the major flavonoid constituents being gliricidin-7-O-hexoside and quercetin-7-O-rutinoside. Such potential properties of these two bioactive compounds present considerable appeal to utility in the pharmaceutical sector. The anti-bacterial activity demonstrated by these isolated compounds can potentially prove useful as novel agents against harmful pathogens and may serve as a benchmark for the chemical synthesis of active semi-synthetic analogs. Through further research on fern toxicity, in vivo potential and implementation against MDR pathogens will give better picture on their potent usage.
Acknowledgement
Authors wish to thank Faculty of Engineering Technology University of Malaysia Pahang for the financial support for this work. We are also thankful to Ministry of Higher Education Malaysia for Common wealth fellowship. Further, the authors declare that no conflict of interest exists amongst them or with the parent institution.
References
- Identification of Essential Oil Components by Gas Chromatography/Mass Spectroscopy. Carol Stream, IL: Allured Publishing; 1995. p. :69-351.
- Influence of quercetin and rutin on growth and antioxidant defense system of a human hepatoma cell line (HepG) Eur. J. Clin. Nutr.. 2006;45:19-28.
- [Google Scholar]
- Antibacterial activity and proposed action mechanism of a new class of synthetic tricyclic flavonoids. J. Appl. Microbiol.. 2016;120(3):630-637.
- [Google Scholar]
- Antibacterial evaluation and preliminary phytochemical screening of selected ferns from Iran. RPJ. 2015;2(2):53-59.
- [Google Scholar]
- Antioxidant activity and phenolic content in methanol crude extracts from three Lamiaceae grown in southwestern. Algeria J. Nat. Prod. Plant Resour.. 2012;2(1):175-181.
- [Google Scholar]
- Efficacy of food proteins as carriers for flavonoids. J. Agric. Food Chem.. 2012;60:4136-4143.
- [Google Scholar]
- Eradication of H. pylori infection: the challenge is on if standard therapy fails. Ther. Adv. Gastroenterol.. 2009;2:59-66.
- [Google Scholar]
- Evaluation of antioxidant, antihemolytic and antibacterial potential of six Moroccan date fruit (Phoenix dactyliferaL.) varieties. J. King Saud Univ Sci.. 2016;28:136-142.
- [Google Scholar]
- Chemo-protective activity and characterization of phenolic extracts from Corema album. Food Res. Int. 2011:3087-3093.
- [Google Scholar]
- Characterization of flavonoids from Dryopteris erythrosora and evaluation of their antioxidant, anticancer and acetylcholinesterase inhibition activities. Food Chem. Toxicol.. 2013;51:242-250.
- [Google Scholar]
- Study on the distribution and the total flavonoids content of medicial pteridophytes in Nanjing Zijin mountain. J. Northeast Agric.. 2005;36:320-323.
- [Google Scholar]
- Health-promoting properties of common herbs. Am. J. Clin. Nutr.. 1999;70:491S-499S.
- [Google Scholar]
- Duke, A.J., 2011. Phytochemical and Ethnobotanical Databases. Retrieved November 3.
- Dietary flavonoids protect human colonocyte DNA from oxidative attack in vitro. Eur. J. Nutr.. 1999;38:28-34.
- [Google Scholar]
- Canopy ferns in lowland dipterocarp forest support a prolific abundance of ants, termites, and other invertebrates. Biotropica. 2002;34(4):575-583.
- [Google Scholar]
- Antioxidant and antiproliferative activities of Abrus precatorius leaf extracts - an in vitro study. BMC Complement Altern. Med.. 2013;13(53):1-12.
- [Google Scholar]
- Essential Oil Constituents and Antioxidant Activity of Asplenium Ferns. J. Chromatogr. Sci. 2016 Epub ahead of print.
- [Google Scholar]
- Methods of Plant Analysis. Netherlands: Springer; 1984. p. :1-36.
- A study of antioxidant activity, enzymatic inhibition and in vitro toxicity of selected traditional sudanese plants with anti-diabetic potential. BMC Complement Altern. Med.. 2014;14(149):1-5.
- [Google Scholar]
- Neuroprotective effects of citrus flavonoids. J. Agric. Food Chem.. 2012;60:877-885.
- [Google Scholar]
- Flavone glycosides from Asplenium foreziense and its five related taxa and A. incisum. Biochem. Sys. Ecol.. 2000;28:665-671.
- [Google Scholar]
- Antioxidant capacity of phenolic phytochemicals from various cultivars of plums. Food Chem.. 2003;81(3):321-326.
- [Google Scholar]
- In vitro antioxidant, antibacterial, and cytotoxic activity and in vivo effect of syngonium podophyllum and eichhornia crassipes leaf extracts on isoniazid induced oxidative stress and hepatic markers. BioMed. Res. Int.. 2014;10:1-11.
- [Google Scholar]
- The protective effect of the flavonoids on food-mutagen-induced DNA damage in peripheral blood lymphocytes from colon cancer patients. Food Chem. Toxicol.. 2012;50:124-129.
- [Google Scholar]
- Antioxidative, tyrosinase inhibiting and antibacterial activities of leaf extracts from medicinal ferns. Biosci. Biotechnol. Biochem.. 2009;73(6):1362-1366.
- [Google Scholar]
- Antibacterial activity of two phloroglucinols, flavaspidic acids AB and PB, from Dryopteris crassirhizoma. Arch. Pharm. Res.. 2009;32:655-659.
- [Google Scholar]
- Total phenolics and total flavonoids in Bulgarian fruits and vegetables. J. Chem. Technol. Metall.. 2005;40:255-260.
- [Google Scholar]
- Antibacterial, cytotoxicity and antiviral activities of Asplenium. J. Chem. Pharm. Res.. 2015;7(7):440-444.
- [Google Scholar]
- In vitro evaluation of the antifungal activity of Sclerocarya birrea extracts against pathogenic yeasts. Afr. J. Biotechnol.. 2008;7:3521-3526.
- [Google Scholar]
- The effects of plant flavonoids on mammalian cells: Implications for inflammation, heart disease, and cancer. Pharmacol. Rev.. 2000;52:673-751.
- [Google Scholar]
- Antibacterial activity of rhizome extracts of four pteridophytes from Southern Assam. North East India. AJPCR. 2016;4(1):1-5.
- [Google Scholar]
- Phenolic composition of Flacourtia indica, Opuntia megacantha and Sclerocarya birrea. Food Chem.. 2007;103:82-87.
- [Google Scholar]
- Antimicrobial activity of aroma compounds against Saccharomyces cerevisiae and Improvement of microbiological stability of soft drinks as assessed by logistic regression. Appl. Environ. Microbiol.. 2007;8:5580-5586.
- [Google Scholar]
- Chemical composition and antimicrobial activity of volatile compounds of Tamarix boveana (Tamaricaceae) Microbiol. Res.. 2008;163:445-455.
- [Google Scholar]
- Composition and cytotoxic activity of the leaf essential oil of Comptonia peregrina(L.) Coulter. Phytother. Res.. 2007;21:536-540.
- [Google Scholar]
- Antiglycative and neuroprotective activity of colon-derived polyphenol catabolites. Mol. Nutr. Food Res.. 2011;55:S35-S43.
- [Google Scholar]
- Flavonoids, antioxidant potential, and acetylcholinesterase inhibition activity of the extracts from the gametophyte and archegoniophore of marchantia polymorpha L. Molecules. 2016;12:1-13.
- [Google Scholar]
- A novel non-aromatic bring flavonoid: isolation, structure elucidation and its induction of apoptosis in human colon HT-29 tumor cell via the reactive oxygen species-mitochondrial dysfunction and MAPK activation. Food Chem. Toxicol.. 2012;49:2445-2452.
- [Google Scholar]
- Chemical compositions and bioactivities of crude polysaccharides from tea leaves beyond their useful date. Int. J. Biol. Macromolec.. 2011;49:1143-1151.
- [Google Scholar]
- Glycation of plasma proteins in type II diabetes lowers the non-covalent interaction affinities for dietary polyphenols. Integr. Biol.. 2012;4:502-550.
- [Google Scholar]
- Flavonoids contents and free radical scavenging activity of extracts from leaves, stems, rachis and roots of Dryopteris erythrosora. Iran J. Pharm. Res.. 2012;11:991-995.
- [Google Scholar]







