Translate this page into:
Biosynthesis of silver nanoparticle (AgNPs) using Lactobacillus and their effects on oxidative stress biomarkers in rats
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Biosynthesis method of nanoparticles acquires very important area due to their economic and ecofriendly benefits. The present study was aimed to the biosynthesis of silver nanoparticles (AgNPs) using Lactobacillus mixture and evaluating their antioxidant activity.
The characterization and biosynthesis AgNPs was achieved, using Ultra Violet (UV)–Visible spectrophotometry. Scanning electron microscope (SEM) was used to detect the size, shape and distribution of AgNPs. The occurrence of elemental silver was analyzed by Energy Dispersive-X-ray Spectroscopy (EDS) analysis. To evaluate the antioxidant activity of AgNPs and Lactobacillus in vivo, forty healthy adult rats were used and divided into eight equal groups, first group served as control, second group received LAB mix1 (1 ml/kg) and three groups were administrated with three concentration of AgNPs (5, 50 and 500 mg/kg AgNPs) respectively and other three groups were administrated with the same concentration of AgNPs along with LAB mix1 for two weeks.
The results revealed significant increased (p < 0.05) in total antioxidant capacity (TAC) of (LAB mix1 1 ml/kg, 5 mg/kg AgNPs, 5 mg/kg AgNPs + LAB mix1, 50 mg/kg AgNPs + LAB mix1 and 500 mg/kg AgNPs + LAB mix1) and significantly decreased (p < 0.05) in treatments of (50 and 500 mg/kg AgNPs).
The current study demonstrated that Lactobacillus afforded beneficial role by increasing the antioxidant activity with AgNPs and as ameliorative function for the effect of high dose of AgNPs.
Keywords
Silver nanoparticles
Oxidative stress
Scanning electron microscope (SEM)
1 Introduction
Nanotechnology is a modern field of science deals with synthesis and application of nanoparticles (NPs), it have a size of 1–100 nm. NPs have been studied extensively because of their unique physicochemical characteristics including antibacterial properties, catalytic activity, optical properties, electronic properties, and magnetic properties (Murugan and Shanmugasundaram, 2014)
Silver nanoparticles (AgNPs) have received significant attention because of their antimicrobial activity and prevention the biofilm formation, as well as their unique physical, chemical, biological properties, and their applicability in electronics, optics and medicine (Ansari et al., 2014). There are several number of physical, chemical, biological, and hybrid methods available for synthesizing different types of nanoparticles, physical and chemical methods which are more expensive, energy consuming and potentially toxic to the environment (Liu et al., 2011). Development of reliable, nontoxic, and eco-friendly methods for synthesis of nanoparticles are the most important to expand their biomedical applications. One of the options to achieve this goal is to use microorganisms to synthesize nanoparticles (Gade et al., 2008). The extracellular synthesis of silver nanoparticles using Lactobacillus species appears to be low cost effective and ecofriendly (Chaudhari et al., 2012) and to develop new effective antimicrobial agents that overcome the multiple antibiotics resistance of microorganisms (Franci et al., 2015). Nanoparticles exhibit new or improved properties based on specific characteristics such as size, distribution and morphology. New applications of nanoparticles and nanomaterial are increasing rapidly. Nanotechnology can be termed as the synthesis, design, manipulation of structure of particles with dimension smaller than 100 nm (Ahmad et al., 2003).
Reactive oxygen species (ROS) are produced by living organisms as a result of normal cellular metabolism and environmental factors .The human body is equipped with a variety of antioxidants that serve to counterbalance the effect of oxidants (Birben et al., 2012). Biosynthesizing AgNPs exhibited antioxidant and anti-inflammatory (El-Rafie and hamed, 2014).
Lactobacillus have probiotic functions, due to their antimicrobial and antioxidative properties, adjusting the balance of intestinal flora, reducing blood cholesterol, inhibiting and reducing the risk of tumors and cancer, stimulating the immune system, stimulation of vitamin C production and enhancement of digestion (Songisepp et al., 2004) (Fig. 1).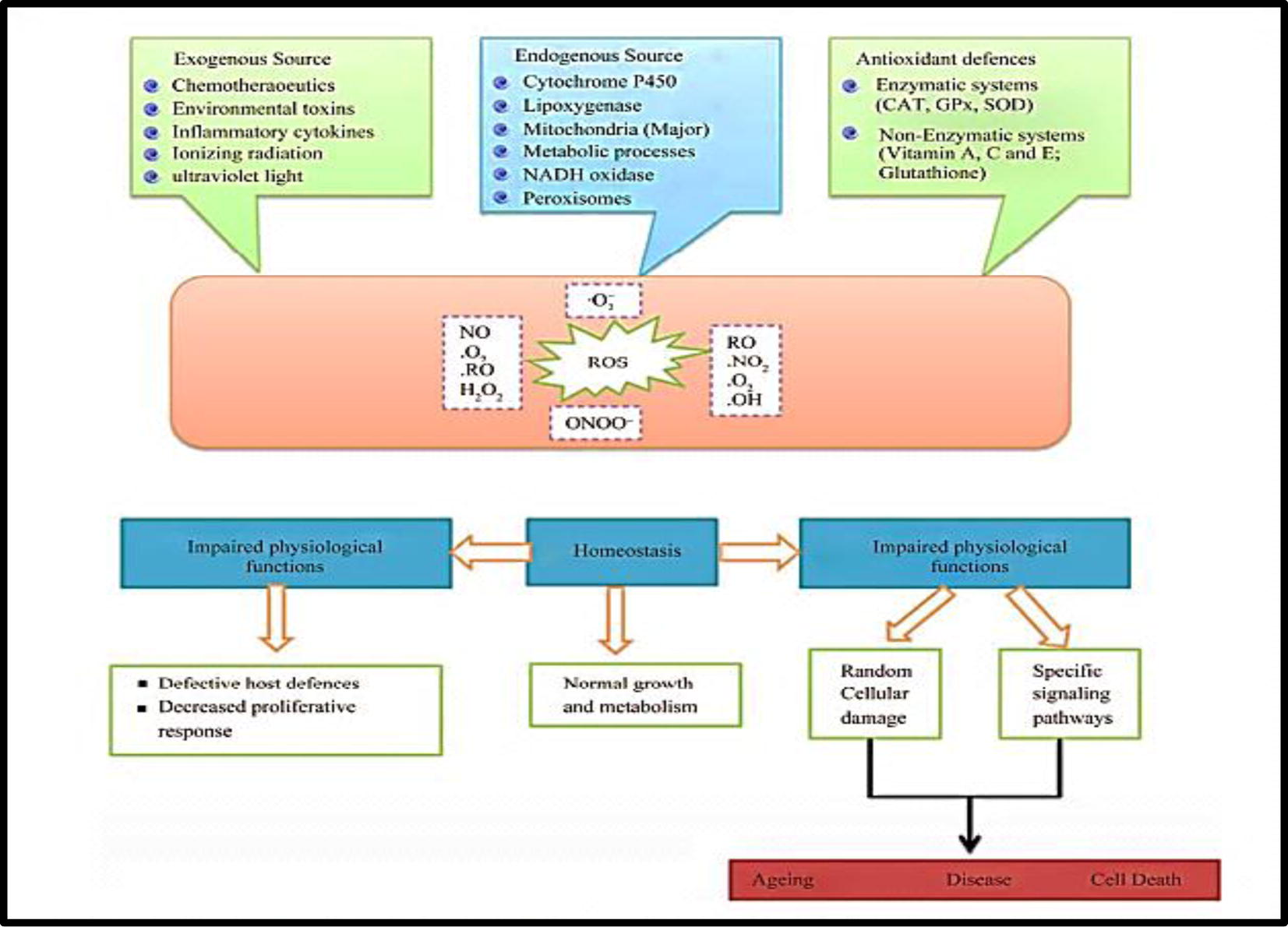
The sources and cellular responses to reactive oxygen (Rahman et al., 2012).
Therefore the present study has been designed to biosynthesis of silver nanoparticles using Lactobacillus mixtures and study antioxidant activity of nanoparticles and Lactobacillus mixture in vitro and in vivo in rats.
2 Materials and methods
2.1 Sources of Lactobacillus
The types of Lactobacillus mixture were obtained from pharmacy and there are three types identified (L. Acidophillus + L. plantarum), (L. acidophillus + L. bifidus) and (L. delbrueckii + L. fermentum), were used as a source of Lactobacillus and give symbols LAB mix1, LAB mix2 and LAB mix3, respectively. Identification of lactobacillus was depending on morphological and biochemical tests by the method used by Holt et al. (1994).
2.2 Biosynthesis of silver nanoparticles
2.2.1 Culture of Lactobacillus mixture
Each Lactobacillus mixtures were inoculated separately in prepared and autoclaved De Man, Rogasa and Sharpe broth (MRS broth, Sigma Aldrich, USA) and then cell free supernatant was prepared according to Chaudhari et al. (2012).
2.2.2 Biosynthesis of silver nanoparticles using cell free supernatant
Silver nitrate (AgNO3) was used as precursor for biosynthesis of silver nanoparticles by Lactobacillus. AgNO3 was added with a concentration of (1, 2, 3, 4 and 5 mM) to cell free supernatant of each Lactobacillus mixtures (LAB mix1, LAB mix2 and LAB mix3) which distributed in sterilized tubes and mixed well in ratio 1:1. The procedure was done according to Maria et al. (2015).
2.3 Characterization of silver nanoparticles
2.3.1 Ultraviolet (UV)–Visible spectrophotometer analysis
Silver nanoparticles were analyzed using UV–Vis spectrophotometer (Shimadzu, 1600, Japan). Color change of the reaction mixtures was monitored by measuring UV–visible spectrum of the reaction mixture after periodically diluting the reaction mixture with deionized distill water and subsequently measured by UV–Vis spectrophotometer (Caroling et al., 2013).
2.3.2 Analysis by Scanning electron microscope (SEM)
Scanning electron microscope (SEM) was used for characterization the morphological and size of nanoparticles. Preparation of slides by adding small drops of suspension of biosynthesis nanoparticles on slides, and left to dry and then analyzed by SEM (FEI, Netherland). The microscope operated at an accelerated voltage at 5–10 kV at different magnification, low vacuum, a spot size 4 and working distances 5–10 mm (Caroling et al., 2013).
2.3.3 Energy Dispersive-X-ray Spectroscopy (EDS) analysis
Elemental analysis of single particle was carried out using EDS (Braker, Netherland) attached with SEM in electron microscope unit, EDS performed for point analysis with accelerating voltage 10KV and spot size 5, working distances 10 mm, this analysis was used to detect presence of elements nanoparticles (Sarvamangala et al., 2013).
2.4 In vitro antioxidant activity of nanoparticles and Lactobacillus mixture
DPPH (1, 1-diphenyl-2-picrylhydrazyl) (Sigma–Aldrich, USA) free radical scavenging assay was used to evaluate the ability of the AgNPs and Lactobacillus mixture to annihilate the DPPH free radical. The method described by Harbone and Baxter (1995) was used.
2.5 In vivo antioxidant activity of AgNPs and Lactobacillus mixture
2.5.1 Preparation of AgNPs concentrations for animals treatment
To evaluate the antioxidant activity in vivo by AgNPs, three concentrations of AgNPs were used (5, 50 and 500 mg/kg) of body weight and the LD50 for silver nanoparticles was dependent according to Shayesteh et al. (2014).
2.5.2 Preparation of Lactobacillus suspension for animals treatment
LAB mix1 was activated at MRS broth (Sigma Aldrich, USA), after incubation, the optical density (O.D) of culture suspension of Lactobacillus mixture was measured by spectrophotometer (CECIL, England) at 600 nm and diluted by normal saline until it reached the optical density that is equivalent to 1 × 109 CFU/ml. 1 ml/kg of LAB mix1 culture suspension (1 × 109 CFU/ml) was administrated for each dose (Brown, 2010).
2.6 Experimental animals
Healthy adult female albino rats weighting between 240 and 266 g were obtained from Faculty of Veterinary Medicine/University of Wastte – Iraq. The animals were placed under standard environment condition (temperature 25–28 °C and 12 h. light-dark cycles). Standard pellets feed and water were provided to animals.
2.7 Experimental design
The study protocol was approved by the ethical committee of the Faculty of Medicine – University of Al-Qadisiyah. Forty mature female rats were distributed randomly into eight groups (5 rats for each group). One group served as control and second group received LAB mix1, three groups treated with AgNPs at concentration of (5, 50 and 500 mg/kg) of body weight and other three groups treated with same concentration of AgNPs along with 1 ml/kg of LAB mix1 (1 × 109 CFU/ml) for each animal using a medical syringe that was inserted orally, the dosing was daily for two weeks.
2.8 Sample collection
At the end of the experimental period, all rats were weighted. They were anesthetized, using a mixture of ketamine 0.2 ml and xylazine 0.1 ml (Denver, Netherlands) and then they were sacrificed (Schiller and McNamara, 1999). The recording of body weight of each animal was done initially and at the end of experiment. The blood sample was obtained from animal through heart puncture by using a disposable medical syringe, then placed in tubes without anticoagulant and centrifuged at 3000 rpm for 10 min. The blood serum was separated, transferred into 1.5 ml micro centrifuge tubes. All samples were kept in a refrigerator at −20 °C until the time of analysis.
2.9 Measurement of total antioxidant capacity
Measurement of total antioxidant capacity was performed through the immunosorbent assay (ELISA) (Biotek, USA) using TAC kit which was supplied by the manufacturing company (Elabsience, China) (Apak et al., 2008).
2.10 Statistical analysis
The data were analyzed by using windows software packages Graphpad prism V6. Data are expressed as (mean ± standard error). T-test was used for the statically comparison between control group and treated groups, P values of less than 0.05 were considered to be statistically significant (Motulsky, 2003).
3 Results
3.1 Morphological and microscopic properties of Lactobacillus mixtures
Morphological and microscopic examinations were used to confirm each type of probiotic mixture of Lactobacillus. Morphologically they were characterized through observing their small convex, smooth, glistening colonies and without pigments microscopically, after stained with gram stain (Himedia, India) the bacteria appeared gram positive, rod shaped, arranged singly, pairs or short chains (Fig. 2).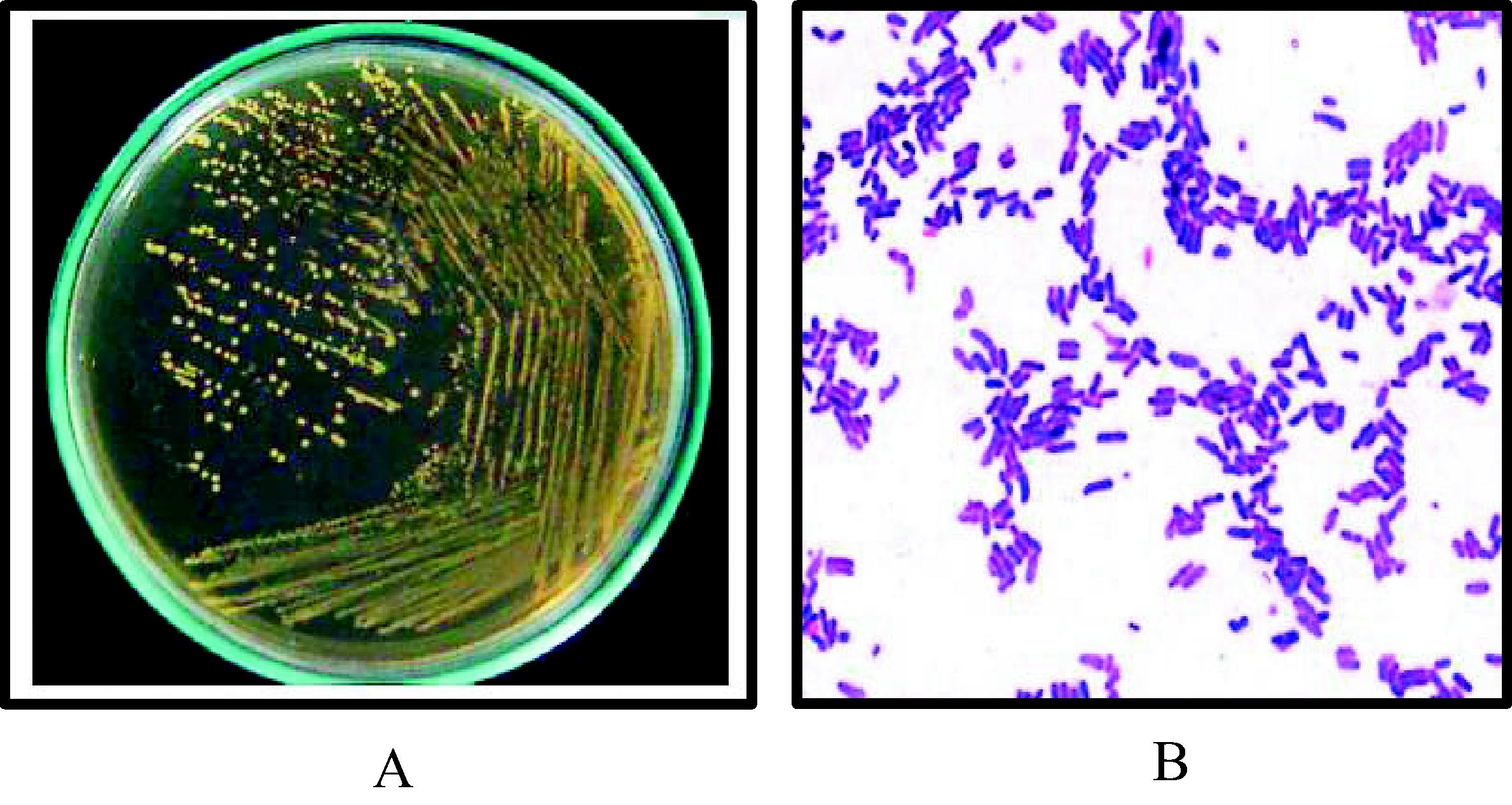
Morphological and microscopic properties of Lactobacillus mixtures. (A) Lactobacillus on MRS agar medium, 37 °C, 48 h, and anaerobic condition. (B) Lactobacillus stained with gram stain under light microscope (X1000).
3.2 Biosynthesis of AgNPs by supernatant of Lactobacillus mixtures
Lactobacilli showed their ability for extracellular biosynthesis of silver nanoparticles using cell free supernatant of each Lactobacillus mixture (LAB mix 1, LAB mix2 and LAB mix3) after adding silver nitrate (AgNO3) in a different concentrations (1, 2, 3, 4 and 5 mM) to cell free supernatant of each Lactobacillus mixture (see Section 2.2.2). Changing the color of reaction mixture from yellow to reddish brown after incubation of mixtures for 24 h at 37 °C in shaking incubator (150 rpm) (Labtech, USA) which represents as indicator for biosynthesis the AgNPs by Lactobacillus mixture.
3.3 Characterization of silver nanoparticles
3.3.1 UV–visible spectrophotometer analysis
The biosynthesis of nanoparticles can be confirmed by visual observation and measuring the surface Plasmon resonance (SPR) band using UV–visible spectroscopy. The absorption spectrum of nanoparticles formed in the reaction mixture has an absorption peak at 410 nm for AgNPs (Fig. 3) this evidence of the presence of surface Plasmon resonance (SPR) of nanoparticles and a single SPR band indicates that nanoparticles have spherical shape.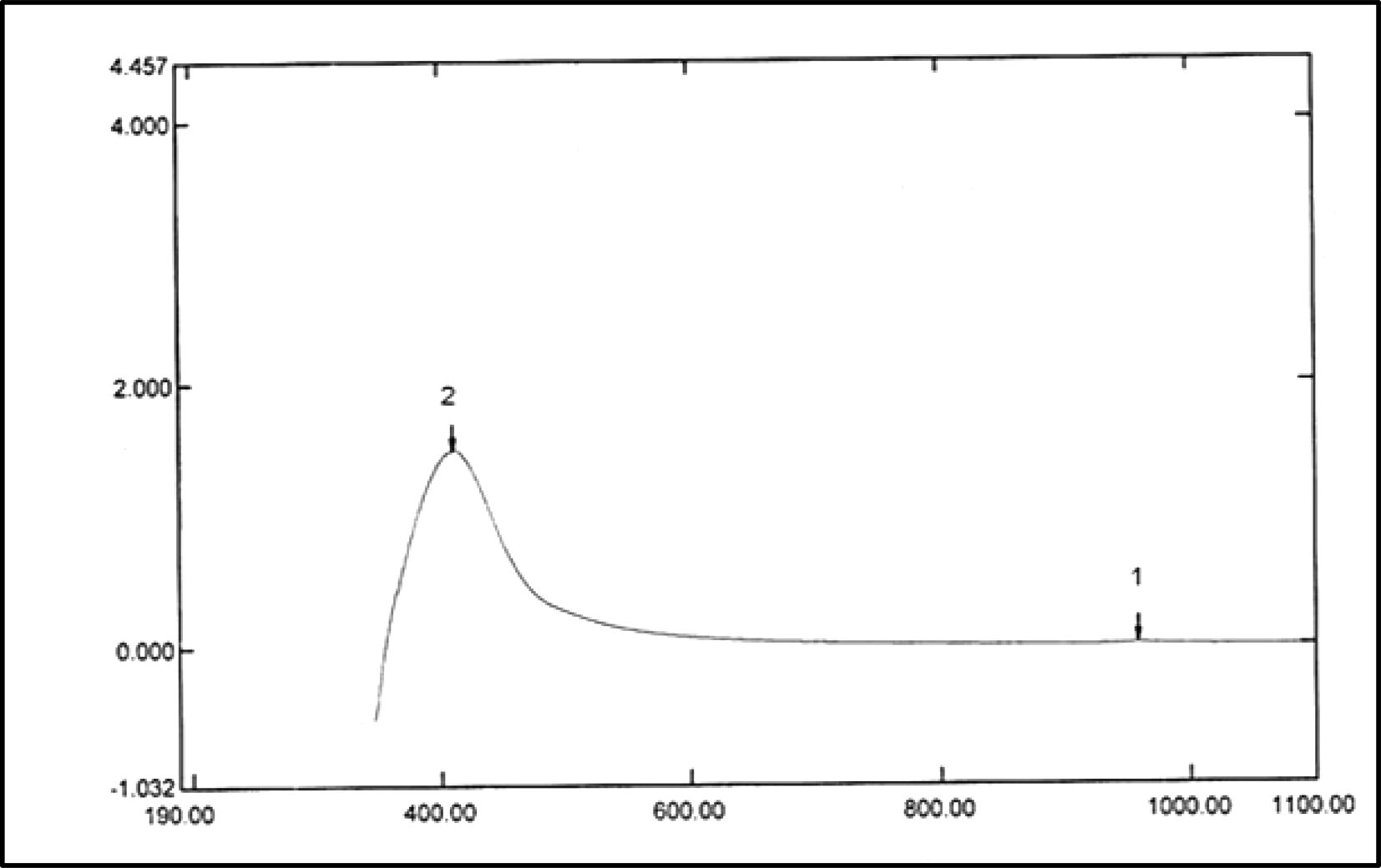
UV–visible absorption spectrum of AgNPs synthesized by LAB mix 1. The absorption spectrum of AgNPs exhibited a strong broad peak at 410 nm.
3.3.2 SEM analysis of nanoparticles
The results showed well-dispersed silver nanoparticles and homogenous with a diameter of (30–100 nm) with variable shapes most of them present in spherical form (Fig. 4). According to the results of characterization of nanoparticles by SEM it was observed difference in the ability of each Lactobacillus mixture for biosynthesis of silver. LAB mix1 and LAB mix2 showed their ability for biosynthesis AgNPs, while they were distinctive in their characteristics when formed by LAB mix1 through noticing the typical shape (spherical), size (30–100 nm) and homogenous distribution than their being formed by LAB mix2. LAB mix3 was less in its ability for synthesizing AgNPs. The concentration of AgNO3 which was added to the supernatant also have effect on characterization of formed nanoparticles, the best concentration in the biosynthesis of AgNPs was 4 mM AgNO3 in comparison with other concentrations.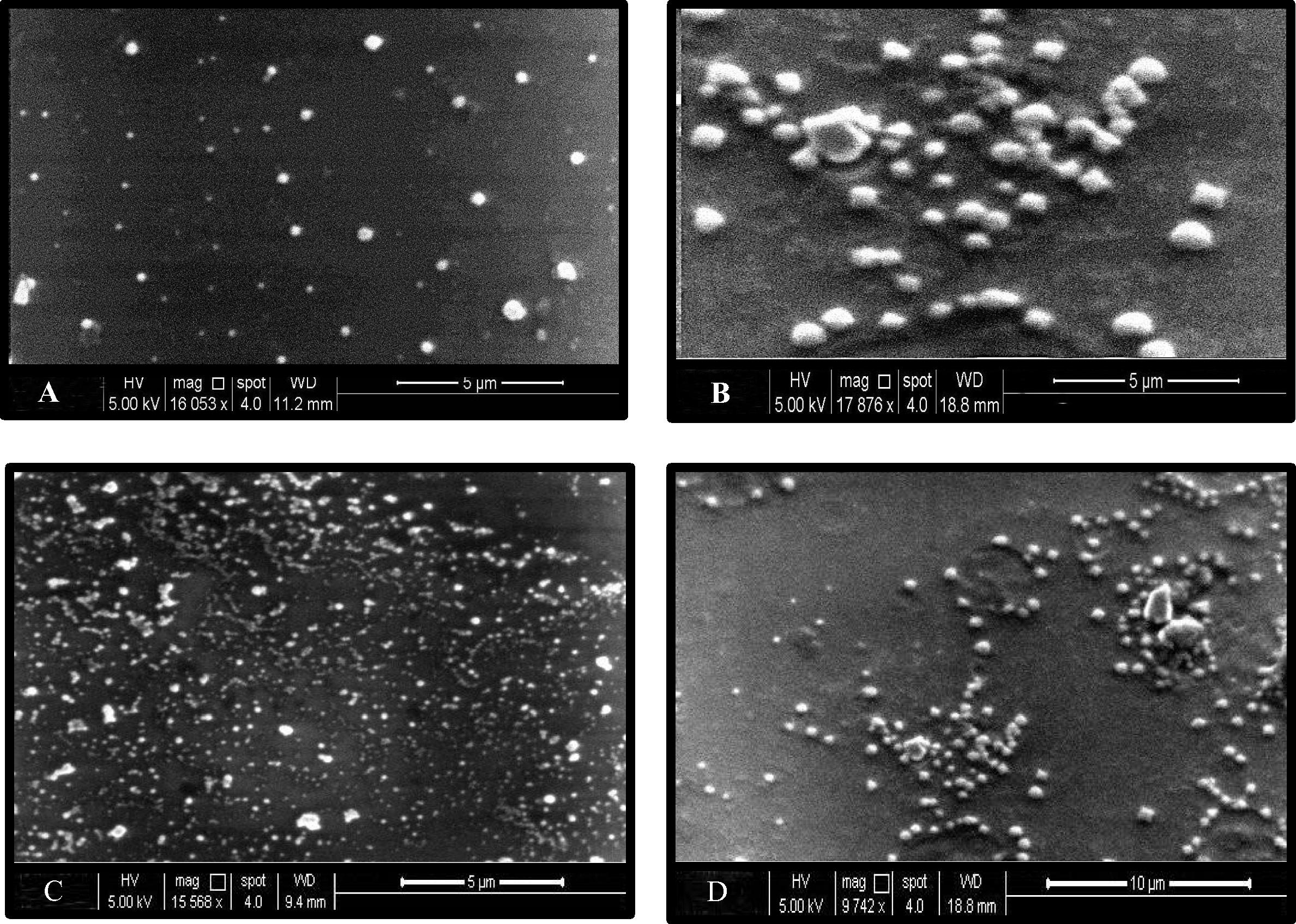
SEM of silver nanoparticles synthesized by LAB mix1.The shape of AgNPs was spherical and homogenous, size between (30–100 nm), magnification (16053×, 17876×, 15568×, 9742×), the voltage (5 kV) and spot size 4 for A, B, C and D respectively.
3.3.3 EDS analysis of nanoparticles
The presence of elemental silver is indicated the reduction of silver ion in the reaction mixture by Lactobacillus mixtures into silver metal. The EDS spectrum was recorded in the spot-profile mode, strong signals from the Ag atom are observed while medium signals from oxygen and weaker signals from other atoms. The weight percentage of elemental constituents for AgNPs that were formed by LAB mix1 was (67.35%) silver and (32.65%) oxygen (Fig. 5). The optical absorption peak of AgNPs was observed at 3 keV which is atypical absorption of metallic AgNPs.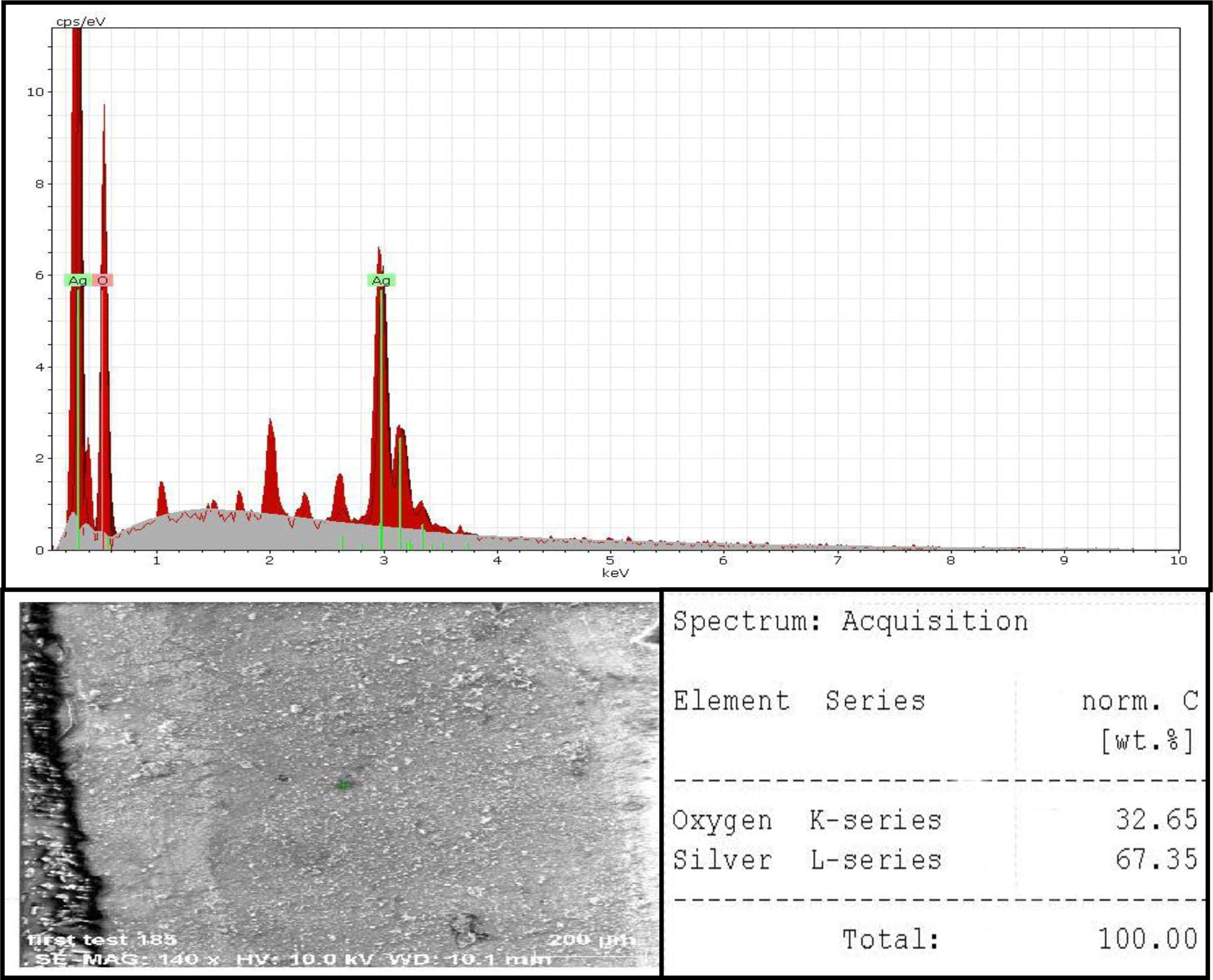
EDS analysis of AgNPs synthesized by LAB mix1illustrated strong signals from the Ag, medium signal from O2, the optical absorption peak of Ag was observed at 3 keV, the weight percentage of silver (67.35%) and oxygen (32.65%).
3.4 In vitro antioxidant activity of nanoparticles and Lactobacillus mixture
The results revealed ability of nanoparticles and LAB mix1 to scavenging DPPH free radicals that indicated by observing the color change from the original purple color of DPPH into yellow color. The inhibition proportion of DPPH scavenging free radicles by Lactobacillus mixture growth was (17%) and their supernatant was (10.3%). DPPH reducing activity of nanoparticles increased with increasing their concentration that indicated by the elevated percentage of inhibition of DPPH which increased with increase concentration of AgNPs. It was (92.4% in 1.5 mg/ml) while the inhibition percentage was 65.7% at 0.5 mg/ml (Fig. 6).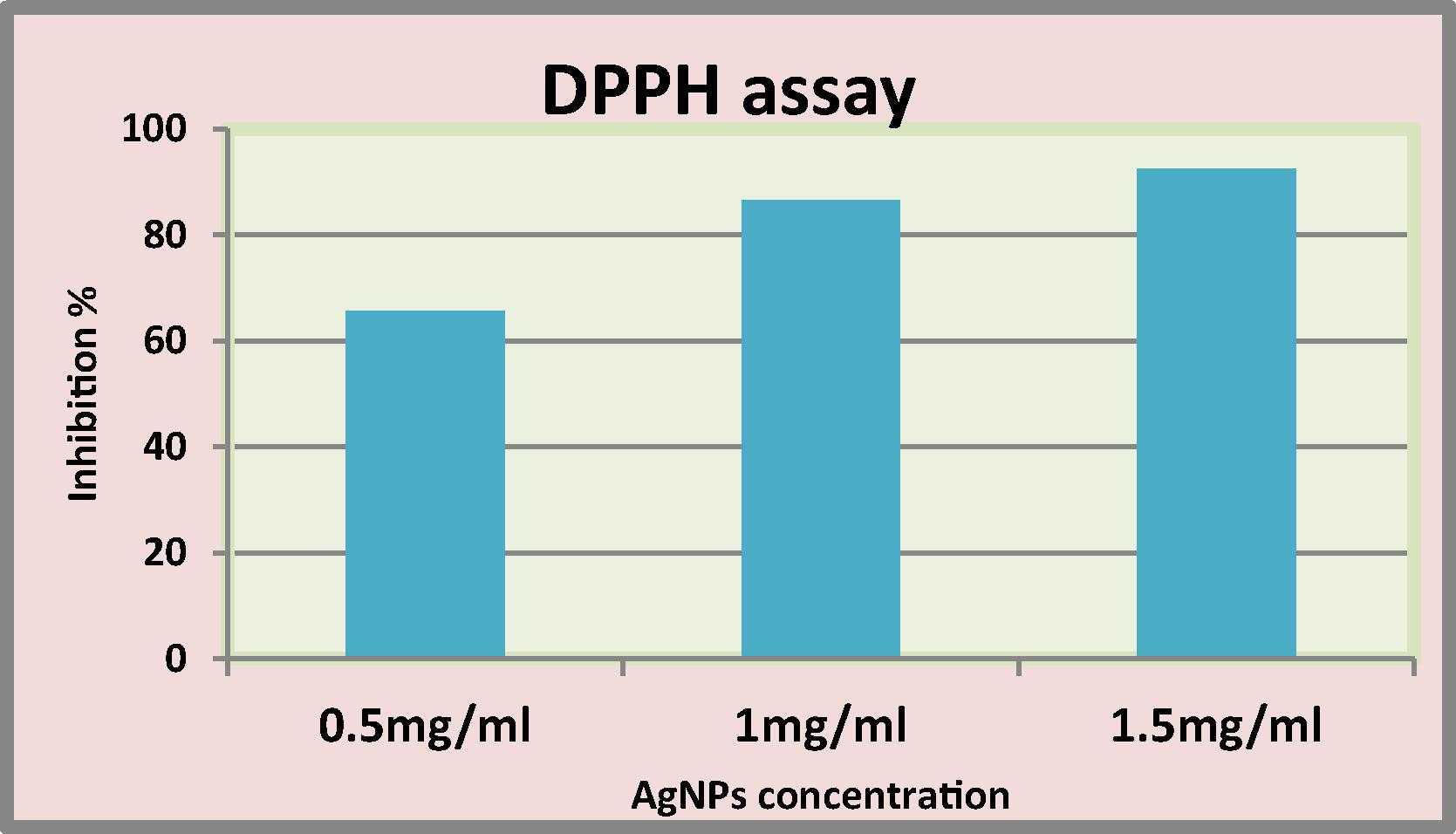
DPPH free radical scavenging ability of silver nanoparticles.
3.5 Effect of AgNPs and LAB mix1 on serum level of TAC activity
The results in Fig. 7 showed that serum total antioxidant capacity (TAC) was significant increased (p < 0.05) in LAB mix1 of 1 ml/kg and 5 mg/kg AgNPs treated groups respectively in comparison with control group. While observed significant decreased (p < 0.05) of TAC with increase of AgNPs concentrations in (50 and 500 mg/kg) treated groups respectively in comparison with control and 5 mg/kg AgNPs treated groups. Treatment of rats with (1 ml/kg) of LAB mix1 along with 5, 50 and 500 mg/kg AgNPs concentration respectively showed significant increased (p < 0.05) of TAC activity in 50 and 500 mg/kg AgNPs + LAB mix1 in comparison with AgNPs groups.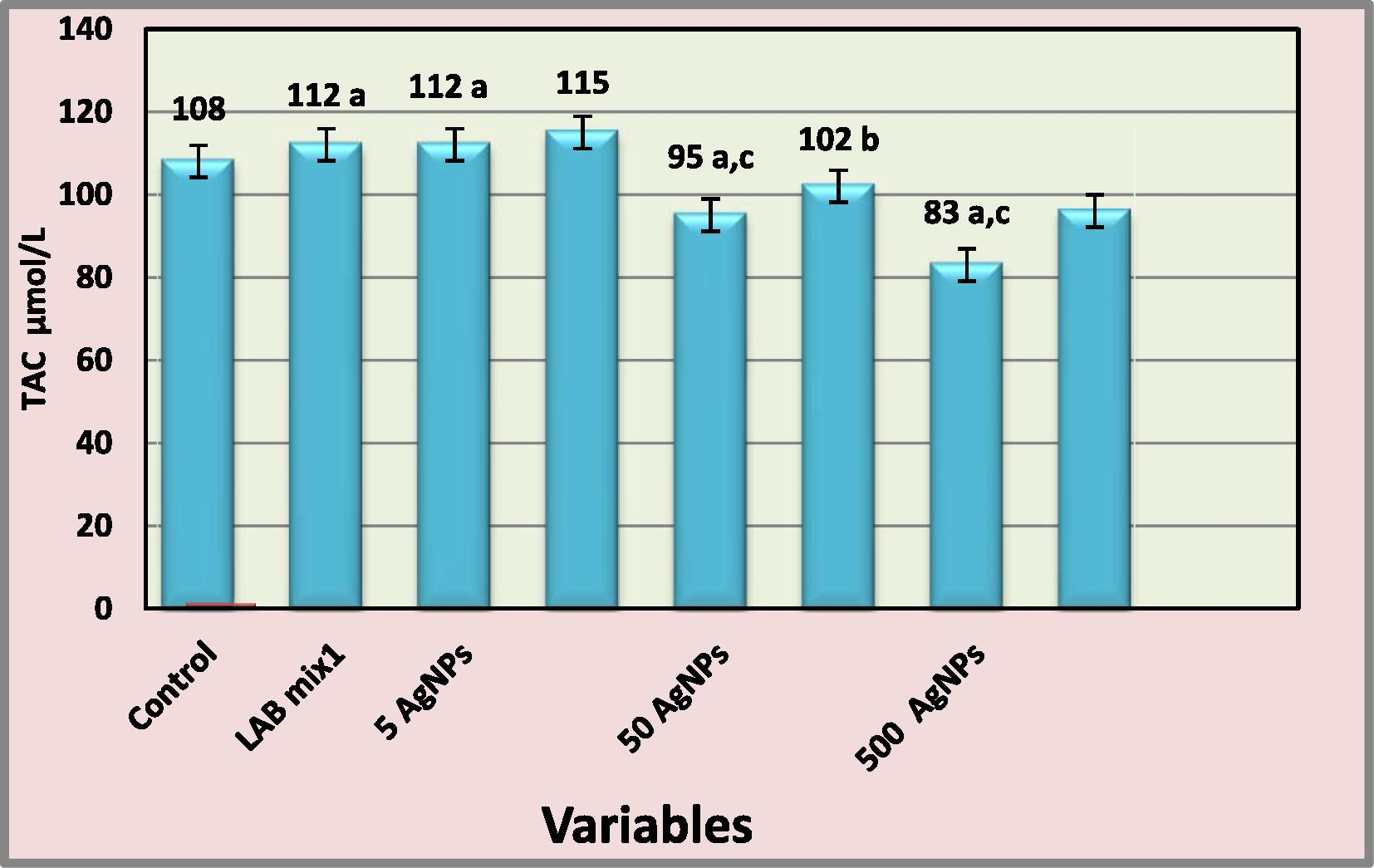
Effect of AgNPs and LAB mix1 on serum level TAC- a: represents significantly different from control at (p < 0.05) b: represents significantly different from AgNPs groups at (p < 0.05) c: represents significantly different from 5 mg/kg AgNPs group at (p < 0.05).
4 Discussion
It has been recently established that microorganisms have been explored as potential bio-factories for synthesis of metallic NPs (Mukherjee et al., 2002). In the present study an attempt was made to employ the potentials of three Lactobacillus mixtures to synthesize AgNPs. The appearance of a brown color in solution is a clear indication of the formation of silver nanoparticles in the reaction mixture due to reduction of Ag+ ions to Ag metal by the reducing agents in the cultural supernatants (Sreedevi et al., 2015). The exact reaction mechanism leading to the formation of silver nanoparticles by all organisms is yet to be elucidated. Extracts from microorganisms may act both as reducing and capping agents in AgNPs synthesis (Kalimuthu et al., 2008). The increase in intensity of the surface Plasmon resonance (SPR) peak could be due to increasing number of nanoparticles formed as a result of reduction of silver ions present in the aqueous solution. The surface Plasmon band in the silver nanoparticles solution remains close to 410 nm throughout the reaction period, indicating that the particles are dispersed in the aqueous solution with no evidence for aggregation (Ahmad et al., 2003). Experimental results showed well-dispersed nanoparticles with diameter of (30–100 nm) with variable shapes most of them present in spherical form, these results agreed with study achieved by Aldujaili et al. (2015). EDS analysis detect the presence of elemental silver, the optical absorption peak was observed at 3 keV which is a typical absorption of metallic AgNPs, these results are similar with study of Bhakya et al. (2015).
DPPH (1,1-diphenyl-2-picrylhydrazyl) scavenging activity of nanoparticles increased with increasing their concentration that indicated by the elevated percentage of inhibition of DPPH which increased with increase concentration of AgNPs that exhibited more inhibition (92.4% in 1.5 mg/ml) due to more an electron donated and accepts by DPPH (Xing et al., 2015). The mechanisms of antioxidants are not only by scavenging free radicals, but also by inhibiting production of free radicals (Ranjbar et al., 2014). The inhibition proportion by bacterial suspension of LAB mix1 was (17%) and cell free supernatant was (10.3%). The different mechanisms involved in the radical-antioxidant reactions may explain the different in scavenging potentials of the compounds (Niki, 2010; Xing et al., 2015).
The elevated and decreased of total antioxidant capacity (TAC) indicates that AgNPs with 5 mg/kg are more effective antioxidant than 50 mg/kg and 500 mg/kg which can induce the oxidative damage through a reactive oxygen species (ROS-mediated process), these results related with other study obtained by Schluesener and Schluesener (2013), they showed that AgNPs decrease the oxidative stress due to decreased superoxide dismutase (SOD) and glutathione peroxidase (GPx) activities and increase the TAC level in low dose of AgNPs, but the TAC level was decreased when SOD and GPx increased in high dose of AgNPs. These results also agreement with other study that indicated AgNPs with a concentration of (5 mg/kg) decrease the oxidative stress by observed increase TAC activity and a decreased catalase, but in 250 mg/kg and 500 mg/kg decrease TAC and increase catalase activity (Shayesteh et al., 2014). Ag-NPs concentrations that were used play very significant role to evaluate the antioxidants status, this implies that dose in addition to the size and chemical composition of nanoparticles are properties of great importance when appraising nontoxicity (Fabrega et al., 2009; El Badawy et al., 2010). However, it remains to be investigated whether Ag NPs induce free radicals directly or indirectly through depletion of antioxidant defense mechanisms depending dose by interactions with antioxidant systems (Schluesener and Schluesener, 2013). TAC was significantly increased (p < 0.05) after treatment of rats with suspension of LAB mix1 beside concentrations of AgNPs (5, 50 and 500 mg/kg AgNPs + LAB mix1) due to the antioxidant activity of Lactobacillus by using their antioxidant enzymes that play a critical role in defense against reactive oxygen species (ROS) such as superoxide dismutase (SOD) eliminates direct toxicity of superoxide anions and glutathione peroxidase (GPx) scavenges H2O2 and hydroxyl radicals or non-enzymatically by metal ion chelating (Persichetti et al., 2014). Lactobacillus have antioxidant effect similar to the effect of other antioxidants such as plant extracts or vitamins to present synergistic effect through increasing antioxidant activity and decrease the oxidative stress caused by high dose of nanoparticles (Diab et al., 2015).
5 Conclusions
Three test Lactobacillus mixtures were capable of synthesizing silver nanoparticles by reaction silver nitrate (AgNO3) for AgNPs supernatant. Comparatively, AgNPs synthesis was better in term of quality; minimum size and less polydispersity, with LAB mix1 (L. Acidophillus + L. plantarum). It has been also concluded that Lactobacillus afforded beneficial role by increasing the antioxidant activity with AgNPs and as ameliorative function for the effect of high dose of AgNPs.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Acknowledgements
The author wishes to thank the technical staff of Faculty of pharmacy and Science/University of Kufa/Iraq, for numerous assistances during this research, they contributed equally to the work, they also supported by characterization of nanoparticles.
References
- Extracellular biosynthesis of silver nanoparticles using the fungus Fusarium oxysporum. Colloids Surf. B: Biointerfaces. 2003;28:313-318.
- [Google Scholar]
- Biosynthesis and antibacterial activity of Titanium nanoparticles using Lactobacillus. Inter J. Recent Sci. Res.. 2015;6:7741-7751.
- [Google Scholar]
- Antibiofilm efficacy of silver nanoparticles against biofilm of extended spectrum B-lactamase isolates of Escherichia coli and Klebsiella pneumonia. Appl. Nanosci.. 2014;4:859-868.
- [Google Scholar]
- Mechanism of antioxidant capacity assays and the CUPRAC (cupric ion reducing antioxidant capacity) assay. Microchim. Acta. 2008;160:413-419.
- [Google Scholar]
- Biogenic synthesis of silver nanoparticles and their antioxidant and antibacterial activity. Appl. Nanosci.. 2015;6:755-766.
- [Google Scholar]
- Gene cloning and DNA analysis: an introduction (sixth ed.). UK: Wiley-Blackwell Publishing; 2010. p. :123-127.
- Biosynthesis of silver nanoparticles using aqueous broccoli extract-characterization and study of antimicrobial cytotoxic effects. Asian J. Pharm. Clin. Res.. 2013;6:165-172.
- [Google Scholar]
- Antimicrobial activity of extracellularly synthesized silver nanoparticles using Lactobacillus species obtained from vizylac capsule. J. Appl. Pharmaceut. Sci.. 2012;02:25-29.
- [Google Scholar]
- The impact of Moringa Oleifera extract and vitamin E against zinc oxide nanoparticles induced hepatotoxicity in male albino rats. J. Am. Sci.. 2015;11:33-41.
- [Google Scholar]
- Surface charge-dependent toxicity of silver nanoparticles. Environ. Sci. Technol.. 2010;45(1):283-287.
- [Google Scholar]
- Antioxidant and anti-inflammatory activities of silver nanoparticles biosynthesized from aqueous leaves extracts of four Terminalia species. Adv. Nat. Sci. Nanosci. Nanotechnol.. 2014;5:35-37.
- [Google Scholar]
- Silver nanoparticle impact on bacterial growth: effect of pH, concentration, and organic matter. Environ. Sci. Technol.. 2009;43(19):7285-7290.
- [Google Scholar]
- Silver nanoparticles as potential antibacterial agents. Molecules. 2015;20:8856-8874.
- [Google Scholar]
- Exploitation of Aspergillus niger for synthesis of silver nanoparticles. J. Biobased Mater. Bioenergy. 2008;2:243-247.
- [Google Scholar]
- Harbone, J.B., Baxter, H., 1995. Phytochemical Dictionary, A Handbook of Bioactive Compounds from Plants, second ed. Taylor and Francis, London. 143–55.
- Bergey's manual of determinative bacteriology (ninth ed.). USA: Williams & Wilkins; 1994. p. :532-551.
- Biosynthesis of silver nanocrystals by Bacillus licheniformis. Colloids Surf. B Biointerfaces. 2008;65:150-153.
- [Google Scholar]
- Magnetic nanocomposites with mesoporous structures: synthesis and applications. Small. 2011;7:425-443.
- [Google Scholar]
- Motulsky, H.J., 2003. Fitting Model to Biological Data using Linear and Nonlinear Regression. A Practical Guide to Curve Fitting. San Diego, CA, Graph Pad Software Inc.
- Extracellular synthesis of gold nanoparticles by the fungus Fusarium oxysporum. Chem. Bio. Chem.. 2002;3:461-463.
- [Google Scholar]
- Biosynthesis and characterization of silver nanoparticles using the aqueous of vitex Negundo Linn. World J. Pharm. Pharmaceut. Sci.. 2014;3:1385-1393.
- [Google Scholar]
- Assessment of antioxidant capacity in vitro and in vivo. Free Radic. Biol. Med.. 2010;49:503-515.
- [Google Scholar]
- Antioxidative capacity of Lactobacillus fermentum LF31 evaluated in vitro by oxygen radical absorbance capacity assay. Nutrition.. 2014;30:936-938.
- [Google Scholar]
- Effects of silver nanoparticle (Ag NP) on oxidative stress biomarkers in rat. Nanomed. J.. 2014;1:205-211.
- [Google Scholar]
- Synthesis characterization and antimicrobial studies of AgNP’s using probiotics. Inter Res. J. Pharm.. 2013;4:240-243.
- [Google Scholar]
- Balloon catheter vascular injury of the alloxan-induced diabetic rabbit: the role of insulin-like growth factor-1. Mol. Cell. Biochem.. 1999;202:159-167.
- [Google Scholar]
- Nanosilver: application and novel aspects of toxicology. Arch. Toxicol.. 2013;87:569-576.
- [Google Scholar]
- Effects of silver nanoparticle (Ag NP) on oxidative stress, liver function in rat: hepatotoxic or hepatoprotective? Issues Biol. Sci. Pharm. Res.. 2014;2:40-44.
- [Google Scholar]
- A new probiotic cheese with antioxidative and antimicrobial activity. J. Dairy Sci.. 2004;87:2017-2023.
- [Google Scholar]
- Synthesis, characterization and antibacterial studies of silver nanoparticles using Lactobacillus Plantarum. World J. Pharm. Res.. 2015;4:1757-1773.
- [Google Scholar]
- Synthesis of silver extracts nanoparticles using medicinal Zizyphus xylopyrus bark extract. Appl. Nanosci.. 2015;5:755-762.
- [Google Scholar]
- Determining antioxidant activities of Lactobacilli cell-free supernatants by cellular antioxidant assay: a comparison with traditional methods. PLoS ONE. 2015;10:3.
- [Google Scholar]







