Translate this page into:
Nanoparticles for mosquito control: Challenges and constraints
⁎Corresponding author at: Insect Behavior Group, Department of Agriculture, Food and Environment, University of Pisa, via del Borghetto 80, 56124 Pisa, Italy. Fax: +39 0502216087. benelli.giovanni@gmail.com (Giovanni Benelli) g.benelli@sssup.it (Giovanni Benelli)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Mosquito control programs are facing important and timely challenges, including the recent outbreaks of novel arbovirus, the development of resistance in several Culicidae species, and the rapid spreading of highly invasive mosquitoes worldwide. Current control tools mainly rely on the employment of (i) synthetic or microbial pesticides, (ii) insecticide-treated bed nets, (iii) adult repellents, (iv) biological control agents against mosquito young instars (mainly fishes, amphibians and copepods) (v) Sterile Insect Technique (SIT), (vi) “boosted SIT”, (vii) symbiont-based methods and (viii) transgenic mosquitoes. Currently, none of these single strategies is fully successful. Novel eco-friendly strategies to manage mosquito vectors are urgently needed. The plant-mediated fabrication of nanoparticles is advantageous over chemical and physical methods, since it is cheap, single-step, and does not require high pressure, energy, temperature, or the use of highly toxic chemicals. In the latest years, a growing number of plant-borne compounds have been proposed for efficient and rapid extracellular synthesis of metal nanoparticles effective against mosquitoes at very low doses (i.e. 1–30 ppm). In this review, we focused on the promising potential of green-fabricated nanoparticles as toxic agents against mosquito young instars, and as adult oviposition deterrents. Furthermore, we analyzed current evidences about non-target effects of these nanocomposites used for mosquito control, pointing out their moderate acute toxicity for non-target aquatic organisms, absence of genotoxicity at the doses tested against mosquitoes, and the possibility to boost the predation rates of biological control agents against mosquitoes treating the aquatic environment with ultra-low doses (e.g. 1–3 ppm) of green-synthesized nanoparticles, which reduce the motility of mosquito larvae. Challenges for future research should shed light on (i) the precise mechanism(s) of action of green-fabricated metal nanoparticles, (ii) their fate in the aquatic environment, and (iii) the possible toxicity of residual silver ions in the aquatic ecosystems, (iv) the standardization of chemical composition of botanical products used as sources of reducing and capping metabolites, (v) the optimization of the green nanosynthetic routes, in order to develop large-scale production of eco-friendly nanomosquitocides.
Keywords
Aedes
Anopheles
Dengue
Malaria
Green nanosynthesis
Zika virus
1 Introduction
Neglected tropical diseases are the most common infections of the poorest people in the world. They include ancient scourges such as hookworm and other soil-transmitted helminth infections, Chagas disease, amoebiasis, schistosomiasis, leishmaniasis, and dengue. Together, neglected tropical diseases produce a burden of disease that in certain regions even exceeds HIV/AIDS, while simultaneously trapping “bottom billion” in poverty through their deleterious effects on child physical and intellectual development, pregnancy outcome, and worker productivity (WHO, 2016a).
Arthropods are extremely dangerous vectors of pathogens and parasites, which may hit as epidemics or pandemics in the increasing world population of humans and animals (Bonizzoni et al., 2013; Mehlhorn, 2015; Mehlhorn et al., 2012; Benelli et al., 2016a). Among them, mosquitoes (Diptera: Culicidae) represent a huge threat for millions of people worldwide, vectoring important diseases, including malaria, dengue, yellow fever, filariasis, Japanese encephalitis and Zika virus (Jensen and Mehlhorn, 2009; Benelli and Mehlhorn, 2016; Pastula et al., 2016; Saxena et al., 2016). Furthermore, Culicidae transmit key pathogens and parasites that dogs and horses are very susceptible to, including dog heartworm, West Nile virus, and Eastern equine encephalitis (WHO, 2012; Mehlhorn, 2015). Unfortunately, no treatment is available for most of the arboviruses vectored by mosquitoes, with special reference to dengue. In addition, even for other mosquito-borne diseases, such as malaria, there are significant challenges that still preclude their successful management (see Benelli and Mehlhorn, 2016).
Malaria is caused by Plasmodium parasites, which are vectored to people and animals through the bites of infected Anopheles mosquitoes (Fig. 1a). 2015 was an extraordinary year for malaria control, due to three hot news: the Nobel Prize to Y. Tu for the discovery of artemisinin, the development of the first vaccine against Plasmodium falciparum malaria [i.e. RTS, S/AS01 (RTS,S)], and the fall of malaria infection rates worldwide, with special reference to sub-Saharan Africa (White, 2015; WHO, 2015a; Benelli and Mehlhorn, 2016). However, there are major challenges that still deserve attention, in order to boost malaria prevention and control. Indeed, parasite strains resistant to artemisinin have been detected (WHO, 2015b), the RTS,S vaccine does not offer protection against Plasmodium vivax malaria, which predominates in many countries outside of Africa, and a number of malaria prevention and control tools currently available are quite expensive, thus not readily available for poor and marginalized populations in tropical and sub-tropical areas worldwide (Benelli and Mehlhorn, 2016).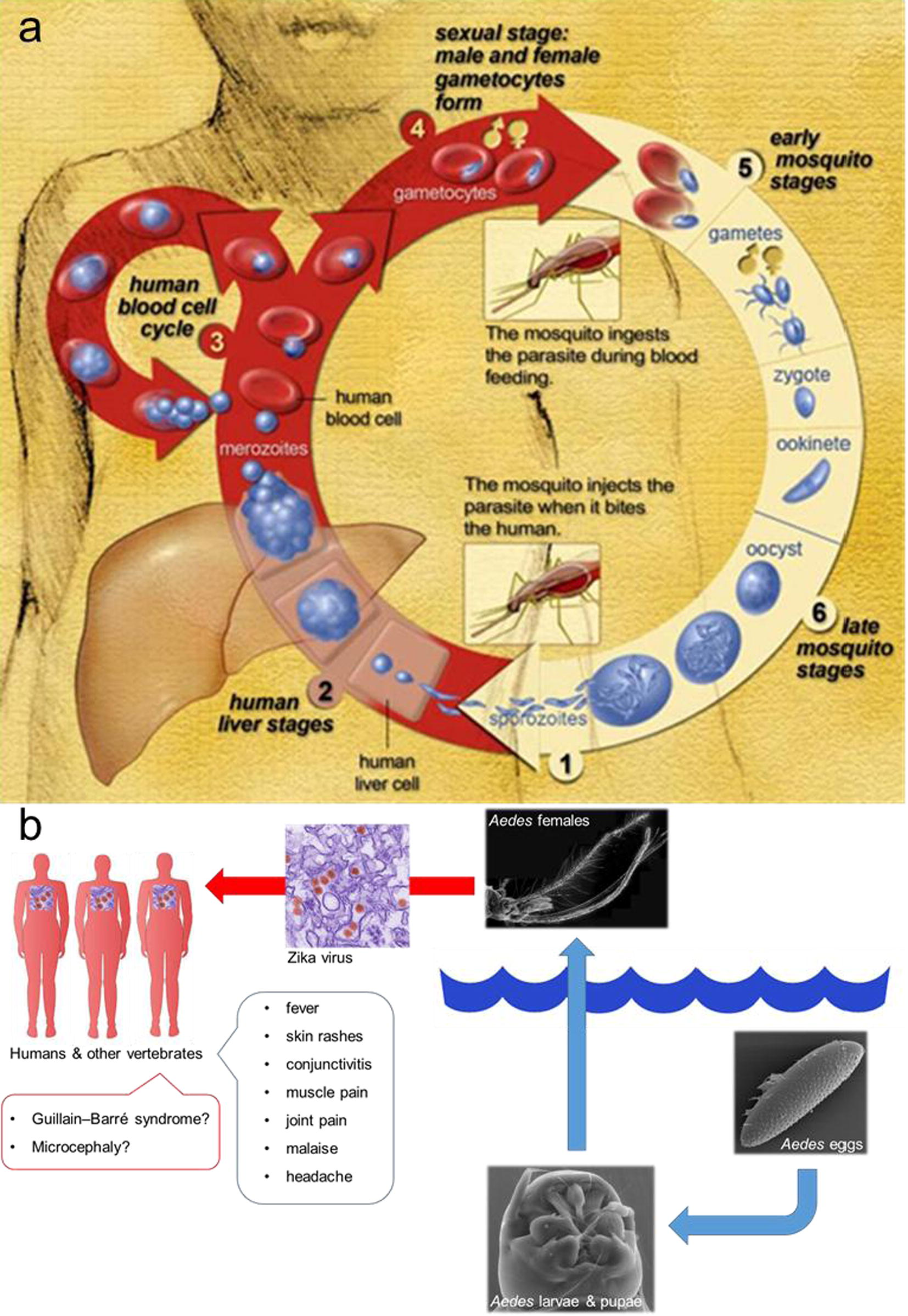
(a) Life cycle of Plasmodium parasites, differentiating between the parasites development inside the Anopheles mosquito vector, and development inside the human host, specifically inside the liver hepatocytes, and red blood cells circulating in the blood (modified from National Institute of Allergy and Infectious Diseases, Centers for Disease Control and Prevention - PHIL). (b) Zika virus is spread to humans through Aedes mosquito bites. Aedes young instars live in aquatic environments, including water reservoirs in urban and peri-urban areas. Aedes females vectored the Zika virus during blood feeding. On the red arrow (indicating blood feeding), a digitally colorized transmission electron micrograph of Zika virus (Flaviviridae). The virus particles (colored in red), are 40 nm in diameter, with an outer envelope, and an inner dense core (modified from Benelli et al., 2016b).
Filariasis is caused by nematodes that in vertebrate hosts act as parasites of blood or the lymphatic system, muscles, and connective tissue. Main species include Wuchereria bancrofti, Brugia malayi and Brugia timori. They are mainly transmitted by the mosquito Culex quinquefasciatus. The adult nematodes obstruct the flow in lymphatic system causing firstly the inflammation of lymphatic vessel, and elephantasis. Filariasis is concentrated in Africa and India, but can be also be found in China, Japan, Sri Lanka and various Pacific islands. WHO has launched its “Global Programme to Eliminate Lymphatic Filariasis” in 2000. In 2012, the WHO neglected tropical diseases roadmap reconfirmed the target date for achieving elimination by 2020. In this framework, besides preventive chemotherapy and morbidity management, vector control in select settings contributed to the elimination of lymphatic filariasis (WHO, 2014).
Among arboviruses, a major role is played by dengue, a viral infection that slyly arrived in the Western Hemisphere over decades and became more aggressive in the 1990s, becoming a major international public health concern. Dengue is mainly vectored by Aedes mosquitoes (i.e. Aedes aegypti and, to a lesser extent, Aedes albopictus). The actual numbers of dengue cases are underreported and many cases are misclassified. 3900 million people, in 128 countries, are at risk of infection with dengue viruses (Becker et al., 2012; Brady et al., 2012; Bhatt et al., 2013). Four distinct, but closely related, serotypes of the virus cause dengue (DEN-1, DEN-2, DEN-3, and DEN-4). Recovery from infection by one provides lifelong immunity against that particular serotype. However, cross-immunity to the other serotypes after recovery is only partial and temporary. Currently, there is no specific treatment for dengue (WHO, 2015c; Sujitha et al., 2015).
Yellow fever is an acute viral hemorrhagic disease endemic in tropical areas of Africa and Central and South America (WHO, 2016b). It is transmitted by infected mosquitoes belonging to the genus Aedes, with special reference to A. aegypti. Symptoms of yellow fever include fever, headache, jaundice, muscle pain, nausea, vomiting and fatigue. A small proportion of patients who contract the virus develop severe symptoms and approximately half of those die within 7–10 days. Yellow fever is prevented by an extremely effective vaccine, which is safe and affordable. A single dose of yellow fever vaccine is sufficient to confer sustained immunity and life-long protection against yellow fever disease. The vaccine provides effective immunity within 30 days for 99% of persons vaccinated. There is currently no specific anti-viral drug for yellow fever treatment (WHO, 2016b).
The recent outbreaks of Zika virus infections, occurring in South America, Central America, and the Caribbean, represent the most recent of four arrivals of important arboviruses in the Western Hemisphere, over the last 20 years (Fauci and Morens, 2016). Zika virus follows dengue, West Nile virus (emerged in 1999), and chikungunya (emerged in 2013) (Attar, 2016; Benelli and Mehlhorn, 2016; WHO, 2016c). Even if Zika symptoms last only a few days in adult persons and are similar to other arbovirus infections, such as dengue (fever, skin rashes, conjunctivitis, muscle and joint pain, malaise, and headache) (Fig. 1b), the surveys conducted on the high numbers of cases of Zika virus infections in French Polynesia (year 2013) and Brazil (year 2015) highlighted potential neurological and autoimmune complications. Indeed, during the Zika virus outbreaks in French Polynesia, a concomitant epidemic of 73 cases of Guillain–Barré syndrome and other neurologic conditions was observed in a population of about 270,000 people (Oehler et al., 2014). In northeast Brazil, during 2015, the increase in Zika virus infections has been reported in close concurrence of an increase in babies born with microcephaly. However, further studies are needed to shed light on the relationship between these potential complications and Zika virus infections (Benelli and Mehlhorn, 2016; Benelli et al., 2016b).
2 Current mosquito control tools
The scenarios reported above for malaria, filariasis and arboviruses vectored by mosquitoes highlighted the key role of effective Culicidae control strategies (Benelli, 2015a). Main mosquito control tools currently employed are outlined in the following subparagraphs, with the exception of behavior-based control tools (i.e. sound traps, swarming manipulation and lure and kill, see Benelli and Mehlhorn, 2016 for a recent dedicated review). Currently, none of these single strategies is fully successful. Therefore, novel and safer eco-friendly strategies to manage mosquito vectors are urgently needed.
2.1 Mosquito repellents
Nowadays, major prevention tools are represented by the employment of mosquito repellents [e.g. N,N-diethyl-meta-toluamide (DEET), dimethyl phthalate (DMP), N,N-diethyl mandelic acid amide (DEM), as well as plant-borne molecules] (Mehlhorn, 2015), light-colored clothes covering as much of the body as possible, and sleeping under mosquito nets (Benelli, 2015a). People living in regions with endemic mosquito borne diseases should synergize these strategies with the reduction or removal of Culicidae breeding sites, as well as with mosquitocidal treatments using chemical or microbiological ovicides, larvicides and pupicides (Semmler et al., 2009; Benelli, 2015b).
2.2 Synthetic, microbial and plant-borne mosquitocides
Concerning the employment of synthetic pesticides, particular attention should be given to the development of mosquito resistant strains, as well as to environmental concerns (Hemingway and Ranson, 2000; Naqqash et al., 2016). Indeed, in the past, Culicidae young instars (Fig. 2) have been massively targeted using organophosphates, carbamates and pyrethroids, with important negative effects on human health and the environment (Naqqash et al., 2016). Later on, insect growth regulators and microbial control agents have been introduced, and Bacillus thuringiensis var. israelensis is currently the most common mosquito larvicide employed in European countries. Its insecticidal activity was due to a parasporal crystal produced in the stationary phase of the bacterium growth cycle (Schnepf et al., 1998; Lacey, 2007). The crystal proteins (mainly composed by δ-endotoxin) dissolve in the midgut of the mosquito larval stage, where it is converted into toxic core fragments. The midgut epithelial cells rapidly swell and burst, causing the death of insect (Hofte and Whitely, 1989). Furthermore, the δ-endotoxins cause no harm to humans and non-target organisms. However, recent studies demonstrated that several pests, including mosquito, manifested resistance toward crystal proteins of B. thuringiensis var. israelensis (Tabashnik, 1994; Lacey, 2007; Naqqash et al., 2016).
Mosquito young instars as usually targeted with synthetic and microbial pesticides, this often leads to the rapid development of resistance and environmental concerns. Here the larvae of three important mosquito species: the malaria vector Anopheles stephensi (a), the West Nile vector Aedes triseriatus (b), and the filaraisis vector Culex quinquefasciatus (c). Among biological control agents, in the past a prominent role has been played by the mosquitofish Gambusia affinis (d), which has been lately recognized as an important predator also for young instars of endangered endemic amphibians and fishes. Lastly, the process of teaching to local people how to recognize mosquito larvae is crucial for the success of any mosquito-borne disease control program (d): here Dr. D. Hoel, Ph.D., U.S. Navy is teaching children how to recognize mosquito larvae in Northern Uganda (photo courtesy of Centers for Disease Control and Prevention – PHIL).
Due to the constraints highlighted above, a growing number of studies focused on the potential of plant-borne products are mosquito ovicides, larvicides and adulticides, as well as on their repellent and ovideterrent potential (e.g. Amer and Mehlhorn, 2006a,b,c,d; see Benelli, 2015b; Pavela, 2015 and Pavela and Benelli, 2016 for reviews). In particular, Pavela (2015) evaluated the current research relying on essential oils as potential mosquito larvicides, showing that the most common families used for extraction of effective mosquitocidal essential oils are Lamiaceae, Cupressaceae, Rutaceae, Apiaceae, and Myrtaceae. Of 122 surveyed species, 77 showed LC50 < 50 ppm. Only seven essential oils (Blumea densiflora, Auxemma glazioviana, Callitris glaucophylla, Cinnamomum microphyllum, Cinnamomum mollissimum, Cinnamomum rhyncophyllum and Zanthoxylum oxyphyllum) showed LC50 < 10 ppm (Pavela, 2015), highlighting the promising potential of these essential oils and the related main constituents for the development of newer and effective control tools against mosquito young instars.
2.3 Biological control agents
Among biological control agents employed against mosquito young instars, fishes received most of the research attention. Indeed, a number of fishes take advantage of the larval stage of mosquitoes as a source of food. The most popular are mosquitofishes, Gambusia sp. (Cyprinodontiformes: Poeciliidae) (Fig. 2). Gambusia sp. were released for biocontrol purposes in several areas all over the world because of their great predation effectiveness against mosquito larvae. However, very soon these fishes were considered as invasive species (Mischke et al., 2016). In fact, Gambusia spp. are great predators not only of mosquito larvae, but also of native species of fishes and reptiles such as tadpoles. In 20’s Gambusia holbrooki was introduced in Australia for mosquito control (Wilson, 1960; Walton et al., 2012), with serious consequences for Australian endemic aquatic species. G. holbrooki became an exotic plague and the mosquitoes control failed (Arthington et al., 1983; Keane and Neira, 2004). In this scenario, Griffin (2014) investigated the predatory behavior of four species of fishes in laboratory conditions. Three of them were native from Australia, Pseudomugil signifer (Atheriniformes: Pseudomugilidae) (Kner), Hypseleotris galii (Perciformes: Eleotridae) (Ogilby) and Pseudogobius sp. (Perciformes: Gobiidae), and the exotic G. holbrooki (Griffin, 2014). All fish species were good predators of mosquito larvae, but the best results were achieved by G. holbrooki and P. signifer, which demonstrated high predation on both second and fourth instar larvae (Griffin, 2014). However, the high environmental impact of G. holbrooki disqualifies it as a biological control agent. Therefore, the native species P. signifer seems to be a viable alternative for mosquito biocontrol.
Gambusia affinis is also one of the more troubling species accused of amphibian decline. Indeed, G. affinis feed also on amphibian eggs and tadpoles, as in the case of the Pacific treefrog Hyla regilla (Anura: Hylidae) (Goodsell and Kats, 1999). H. regilla tadpoles evolved a lot of behavior strategies to escape from predators attacks (Goodsell and Kats, 1999), but these methods are not efficient toward G. affinis. Of 36 G. affinis analyzed, 65% of them had in their stomach H. regilla tadpoles, and 56% mosquito larvae. Thus, G. affinis did not show preference between these two preys eating tadpoles and mosquito larvae as well. Thus, the generalist predaceous behavior of G. affinis makes it not suitable as a biological control agent (Goodsell and Kats, 1999).
However, Gambusia sp. is not the only fish genus that prey on mosquito larvae. Han et al. (2015) evaluated different larvivorous fishes as biological control agents against the dengue mosquitoes Aedes spp., including Poecilia reticulata (Cyprinodontiformes: Poeciliidae), Tilapia mossambica (Perciformes: Cichlidae), Sarotherodon niloticus (Perciformes: Cichlidae), Astyanax fasciatus (Characiformes: Characidae), Betta splendens (Perciformes: Osphronemidae), Oreochromis mossambicus (Perciformes: Ciichlidae), Lepisosteus tropicus (Lepisosteiformes: Lepisosteidae), Brycon guatemalensis (Characiformes: Characidae), Ictalurus meridionalis (Siluriformes: Ictaluridae) and Cyprinus carassius (Cypriniformes: Cyprinidae). All of them were considered as effective mosquito predators in laboratory conditions. Combining the use of predator fishes with other methods of control can reduce significantly infestation of the immature mosquito instars (Han et al., 2015). P. reticulata, also known as guppy, is one of the few mosquito control agents proven in field (Ekaneyeka et al., 2007). Anogwih et al. (2015) investigated the potential combining P. reticulata and larvicides for the control of mosquito larvae. The larvicides in the experiment were spinosad and two organophosphates, pirimiphos methyl and chlorpyrifos. More Culex larvae were predated in spinosad treatments than in synthetic ones (Anogwih et al., 2015). This indicated good level of compatibility of spinosad and predatory fishes, if compared to the treatments with synthetic pesticides. The combination of guppies and low doses of larvicides increased the rate of predated larvae, to a range of 43–90%, and the best results have been attributed to spinosad treatments (Anogwih et al., 2015). Thus, the integration of fish and ultra-low doses of mosquito larvicides seem promising, pending detailed non-target evaluations in the field.
Currently, the studies focusing on amphibians as mosquito control agents are limited (Raghavendra et al., 2008). However, the role of tadpoles in biological control is of potential interest. Indeed, Bowatte et al. (2013) tested five frog genera as dengue mosquito predators in laboratory conditions, i.e. Polypedates (Anura: Rhacophoridae), Bufo (Anura: Bufonidae), Ramanella (Anura: Microhylidae), Euphlyctis (Anura: Dicroglossidae) and Hoplobatrachus (Anura: Dicroglossidae). Results showed effective egg predation by the five frog genera (Bowatte et al., 2013). In tadpoles’ gut, researchers find crushed or entire A. aegypti eggs. The role of amphibians in biological control programs should be considered further, also because the phenomenon recorded by this experiment could be more widespread (Bowatte et al., 2013). Later on, Weterings (2015) affirmed that the majority of tadpoles are strictly herbivorous, thus they are not attracted by mosquito eggs. Although it is scientifically demonstrated that some species of tadpoles presented predator traits against mosquito eggs, Weterings (2015) affirmed that the only limiting factor for larval mosquito population could be the competition, no predation, since both mosquito larvae and tadpoles feed on detritus (Blaustein and Margalit, 1994; Mokany and Shine, 2003). Furthermore, Weterings (2015) reported that some mosquito species detect with their antennal and tarsal receptors the presence or the absence of tadpoles in the place of oviposition. In this way, they are able to accept the microhabitat as an oviposition site or to move away, if the presence of potential predators is detected (Weterings, 2015). Overall, further research is needed to elucidate the effective potential of tadpoles in biocontrol programs against mosquito vectors.
Furthermore, a number of omnivorous copepods, which are small aquatic cyclopoid crustaceans, can prey on immature mosquitoes, especially first-instar larvae, but rarely on later stages (Hurlbut, 1938; Marten et al., 1989; Williamson, 1999; Kumar and Hwang, 2006). Several species of copepods, such as Cyclops vernalis, Megacyclops formosanus, Mesocyclops aspericornis, Mesocyclops edax, Mesocyclops guangxiensis, Mesocyclops longisetus and Mesocyclops thermocyclopoides have been reported as active predators of mosquito young instars (Rawlins et al., 1991; Manrique-Saide et al., 1998; Schaper, 1999; Schreiber et al., 1993; Mahesh Kumar et al., 2012, 2016; Murugan et al., 2015b,c; Anbu et al., 2016). From a practical point of view, the use of predatory copepods against mosquitoes in urban and semi-urban habitats is not expensive and requires little labor for colony maintenance, pointing out their easy and cheap potential as mass-reared biological control agents (Soumare and Cilek, 2011; Chitra et al., 2013). In addition, among insects, besides well-known mosquito predators, such as water bugs (e.g. Diplonychus indicus, which usually predate also a wide number of non-target organisms, such as tadpoles), a noteworthy option is the mosquito genus Toxorhynchites, also called “elephant mosquito” or “mosquito eater”. The genus includes the largest known species of mosquito, and it is among the few kinds of mosquito that do not consume blood. While the adults feed on sugar-rich materials, such as honeydew, or saps and juices from damaged plants, fruit, and nectar, the larvae prey on the larvae of other mosquitoes as well as other nektonic preys (Mahesh Kumar et al., 2016).
2.4 Sterile Insect Technique and “boosted SIT”
The Sterile Insect Technique (SIT) is an eco-friendly method that can be used for the control of disease vectors (Knipling, 1959). In recent years, the interest for this technique was renewed (Lees et al., 2014, 2015). The SIT is a species-specific control method, based on the mass rearing of target species, the utilization of ionizing radiation to make sterile males and their consequential release in a target area. The amount of released sterile males need to be high, allowing the sterile males to compete with wild males for mating. If a sterile male mates with a wild female, no progeny will be originated. The result is a drastic reduction or, potentially, even eradication of the target population (Bourtzis et al., 2016). The success of this control method is mainly linked with the competition ability of the sterile males over wild males (Benelli, 2015a).
To the best of our knowledge, one of the most interesting evidences about SIT against mosquitoes concerns A. albopictus control. Sterilized male pupae were separated from females by a specific sieve, and then they were released in a target area. The egg-hatching rate demonstrated that, in the target population, the sterility percentage ranged from 18% to 68%. For population suppression, the sterility rate should be maintained over 81% (Bellini et al., 2013). SIT is very promising to control mosquito populations. Furthermore, this technique takes advantage of the fact that it is largely immune to resistance phenomenon in view that irradiation produces a high number of dominant lethal mutations. In contrast, there is the disadvantage of the continuous production and release of sterile insects (Bourtzis et al., 2016).
Furthermore, SIT has been recently combined with auto-dissemination (i.e., adult females contaminated with dissemination stations of juvenile hormone to treat breeding habitats), a technique recently proved very efficient to control Aedes species but that cannot be used in large scale. This has led to formulate a new control concept, named “boosted SIT” that might enable the area-wide eradication of mosquitoes and other vectors of medical and veterinary importance (Bouyer and Lefrançois, 2014).
2.5 Symbiont-based methods
The exploitation of cytoplasmic incompatibility (CI) can be an advantageous mosquito control method (Lees et al., 2015; Bourtzis et al., 2016), particularly for Aedes control (Bourtzis et al., 2016). CI can be induced by the widespread bacterium symbiont Wolbachia (Lees et al., 2015; Bourtzis et al., 2016; Yakob and Walker, 2016). This method of control can be used for both the population replacement as well as for population suppression. In the last case, the control method is defined as Incompatible Insect Technique (IIT) (Lees et al., 2015). The CI is expressed as embryonic death after mating between males infected by Wolbachia and Wolbachia-free females (suppression), or females infected with a different Wolbachia strains (replacement) (Lees et al., 2015; Bourtzis et al., 2016).
The combination of SIT and IIT can be a successful tool for mosquito control. In both the strategies, there is the prerequisite of efficient sex-separation methods. The combination of these two techniques is based on the sterilization of any females, which required lower doses for sterilization than males. Thus, the risk of accidental releases of fertile females is passed away, and the combination of Wolbachia-induced CI and irradiation ensure male sterility (Arunachalam and Curtis, 1985; Brelsfoard et al., 2009; Calvitti et al., 2015). Overall, the use of Wolbachia is a promising tool for mosquito control either alone or associated with SIT (Yakob and Walker, 2016).
2.6 Transgenic mosquitoes
Another recent method for mosquito control relied to transgenic approaches. Through this tool is possible to introduce novel genes into the genome of several mosquito vector species, using transposable elements (Bourtzis et al., 2016). This strategy can be used for population suppression, and is based on the continuous release of males carrying a genetic system which can be repressed during rearing, but which is lethal for the progeny without a repressor (Thomas et al., 2000). Several researches demonstrated that A. aegypti may be controlled using this control strategy. Recently, a version that killed both males and females was evaluated; results were promising and the suppression rate was about 70–80% (Harris et al., 2012; Carvalho et al., 2015).
3 Green-fabricated nanoparticles: effectiveness against mosquito vectors
Nanobiotechnologies have the potential to revolutionize a wide array of applications, including drug delivery, imaging, gene delivery, tissue engineering, parasitology and pest management (Rai et al., 2009; Heng et al., 2013; Benelli, 2016a; Mehlhorn, 2016). The plant-mediated fabrication of nanoparticles is advantageous over chemical and physical methods, since it is cheap, single-step, and does not require high pressure, energy, temperature, or the use of highly toxic chemicals (Goodsell, 2004; Kumar et al., 2015). Currently, a growing number of plant-borne compounds have been proposed for efficient and rapid extracellular synthesis of metal nanoparticles (see Rajan et al., 2015 for a recent review), which showed excellent antiplasmodial potential, as well as mosquitocidal properties, even in field conditions (Amerasan et al., 2016; Benelli, 2016a,b,c; Govindarajan and Benelli, 2016a,b; Murugan et al., 2016a,b,c,d). Indeed, more than 100 studies highlighted that one-pot plant-fabricated polydisperse metal nanoparticles showed highly effective mosquitocidal toxicity (see Benelli, 2016a for a dedicated review). The majority of the studies focused on mosquito larvicidal, pupicidal and adulticidal toxicity, and extremely low LC50 were calculated (e.g. Marimuthu et al., 2011; Raman et al., 2012; Rawani et al., 2013; Dinesh et al., 2015; Ramanibai and Velayutham, 2015; Murugan et al., 2015a,b,c; Sujitha et al., 2015; Suresh et al., 2015; Benelli, 2016a; Subramaniam et al., 2015, 2016). Most of them fall within 1–30 mg/L. For example, silver-(protein-lipid) nanoparticles (Ag-PL NPs) (core–shell) fabricated using the seed extract from wild Indian almond tree, Sterculia foetida showed LC50 values lower than 4.5 ppm against larvae of Anopheles stephensi, A. aegypti and C. quinquefasciatus (Rajasekharreddy and Rani, 2014).
Interestingly, the employment of different botanicals as reducing and stabilizing agents lead to metal nanoparticles with different size, shape and toxic properties against mosquito vectors. For instance, neem-synthesized silver nanoparticles are mostly spherical (Murugan et al., 2015a), while silver nanoparticles fabricated using Carissa spinarum leaves are cubical in shape (Govindarajan et al., 2016a).
Besides botanicals, also invertebrates can be easily exploited for one-pot fabrication of effective mosquito larvicides and pupicides. Recently, earthworms (Eudrilus eugeniae)-synthesized silver nanoparticles were studied for their acute toxicity on young instars of the malaria vector A. stephensi. LC50 were 4.8 ppm (larva I), 5.8 ppm (larva II), 6.9 ppm (larva III), 8.5 ppm (larva IV), and 15.5 ppm (pupa) (Jaganathan et al., 2016). In addition to botanical-mediated synthesis, TiO2 nanoparticles fabricated using the hydrothermal method were also highly effective against A. aegypti young instars, showing LC50 values ranging from 4.02 ppm (larva I) to 7.527 ppm (pupa) (Murugan et al., 2016b).
Furthermore, nanosynthesis of mosquito ovicides, adulticides and oviposition deterrents has been attempted (Benelli, 2016a). In experiments conducted with A. stephensi, A. aegypti, and C. quinquefasciatus, egg hatchability was reduced by 100 % after a single exposure to 30 ppm of Sargassum muticum-synthesized silver nanoparticles (Madhiyazhagan et al., 2015). Little knowledge is also available on the impact of metal nanoparticles on oviposition behavior of mosquito vectors. Barik et al. (2012) investigated the oviposition behavior of three mosquito species in the presence of different types of nanosilica. Complete ovideterrence activity of hydrophobic nanosilica was observed at 112.5 ppm in A. aegypti, A. stephensi and C. quinquefasciatus, while there was no effect of lipophilic nanosilica on oviposition behavior of the three vectors (Barik et al., 2012). Later on, Madhiyazhagan et al. (2015) showed that 10 ppm of silver nanoparticles synthesized using S. muticum reduced oviposition rates of more than 70 % in A. aegypti, A. stephensi, and C. quinquefasciatus (OAI = −0.61, −0.63, and −0.58, respectively). Unfortunately, the mechanism(s) leading to egg mortality and oviposition deterrence post-treatment with green-synthesized nanoparticles are currently unknown. Further research on this issue is encouraged.
As a general trend, little efforts have been done to shed light on the toxicity mechanism(s) leading to larval and pupal death in mosquito larvae and pupae exposed to green-synthesized nanoparticles (Benelli, 2016b, but see also Foldbjerg et al., 2015). It has been hypothesized that the biotoxicity against mosquito young instars may be related to the ability of nanoparticles to penetrate through the exoskeleton. In the intracellular space, nanoparticles can bind to sulfur from proteins or to phosphorus from DNA, leading to the rapid denaturation of organelles and enzymes. Subsequently, the decrease in membrane permeability and disturbance in proton motive force may cause loss of cellular function and cell death (Benelli, 2016a,b,c; Subramaniam et al., 2015, 2016).
4 Green-fabricated nanoparticles: non-target effects
To the best of our knowledge, only limited evidences of toxicity of green-fabricated mosquitocidal nanoparticles have been reported on non-target aquatic organisms (Benelli, 2016a,b; Govindarajan et al., 2016a,b). Moderate information is available about the acute toxicity toward aquatic non-target species. Plumeria rubra- and Pergularia daemia-synthesized silver nanoparticles did not exhibit any evident toxicity effect against P. reticulata fishes after 48 h of exposure to LC50 and LC90 values calculated on IV instar larvae of A. aegypti and A. stephensi (Patil et al., 2012a,b). Subarani et al. (2013) did not report toxicity effects of Vinca rosea-synthesized silver nanoparticles against P. reticulata, after 72 h of exposure to dosages toxic against A. stephensi and C. quinquefasciatus. Similarly, Haldar et al. (2013) did not detect toxicity of silver nanoparticles produced using dried green fruits of Drypetes roxburghii against P. reticulata, after 48 h exposure to LC50 of fourth instar larvae of A. stephensi and C. quinquefasciatus. Rawani et al. (2013) showed that mosquitocidal silver nanoparticles synthesized using Solanum nigrum berry extracts were not toxic against two mosquito predators, Toxorhynchites larvae and Diplonychus annulatum, and Chironomus circumdatus larvae, exposed to lethal concentrations of dry nanoparticles calculated on A. stephensi and C. quinquefasciatus larvae (see also Mahesh Kumar et al., 2016). Silver nanoparticles fabricated using the 2,7.bis[2-[diethylamino]-ethoxy]fluorence isolate from the Melia azedarach leaves did not show acute toxicity against Mesocyclops pehpeiensis copepods (Ramanibai and Velayutham, 2015). Later on, Govindarajan et al. (2016a) assessed the biotoxicity of C. spinarum-synthesized silver nanoparticles on the non-target aquatic organisms Anisops bouvieri, D. indicus and G. affinis. Toxicity testing revealed minimal toxicity, obtaining LC50 values in the range of 424 to 6402 μg/mL. Similarly, Govindarajan et al. (2016b) reported that the Malva sylvestris-synthesized silver nanoparticles exhibited minimal biotoxicity against non-target organisms D. indicus and G. affinis, as with LC50 values ranging from 813 to 10,459 μg/mL. B. cristata-fabricated silver nanoparticles tested on the non-target organisms A. bouvieri, D. indicus and G. affinis, showed LC50 values ranging from 633 to 8595 μg/mL (Govindarajan and Benelli, 2016a).
Genotoxicity experiments testing neem-cake synthesized silver nanoparticles on Carassius auratus erythrocytes showed no significant damages at doses below 12 ppm (Chandramohan et al., 2016), while when carbon nanoparticles were tested, C. auratus erythrocytes showed no significant damages at doses below 25 ppm (Murugan et al., 2016e).
Notably, sub-lethal doses of mangrove-fabricated silver nanoparticles did not reduced the predation efficiency of mosquito natural enemies, such as Carassius auratus, on A. aegypti mosquito larvae (Murugan et al., 2015a). In addition, in an aquatic environment contaminated by green-fabricated gold nanoparticles (i.e. 1 ppm), the M. aspericornis predation efficiency against A. stephensi was 45.6% (larva I) and 26.7% (larva II), while against A. aegypti was 77.3% (I) and 51.6% (II). Both values were higher if compared to the standard predation rates of this copepod species, since the standard predatory efficiency of the cyclopoid crustacean against A. stephensi larvae was 26.8% (larva I) and 17% (larva II), while against A. aegypti was 56% (I) and 35.1% (II). Predation against late-instar larvae was minimal (Murugan et al., 2015b). Similarly, little non-target effects of earthworms-synthesized silver nanoparticles against mosquito natural enemies were found. The predation efficiency of the mosquitofish G. affinis toward the II and II instar larvae of A. stephensi was 68.50% (II) and 47.00% (III), respectively, while in nanoparticle-contaminated environments, predation was boosted to 89.25% (II) and 70.75% (III), respectively (Jaganathan et al., 2016). Overall, extremely low doses of gold and silver nanoparticles may help to boost the control of Anopheles, Aedes and Culex larval populations in copepod-, tadpole- and fish- based control programs (Benelli, 2016a).
5 Conclusions and insights for future research
Nowadays, parasitology is facing a number of crucial challenges, which mostly deal with the paucity of effective preventive and/or curative tools against malaria and arboviruses, with special reference to recent dengue, chikungunya and Zika virus outbreaks (Benelli, 2015a; Benelli and Mehlhorn, 2016). In this scenario, the employment of botanicals and invertebrates as reducing, stabilizing and capping agents for the green-synthesis of nanoparticles is advantageous over chemical and physical methods, since it is cheap, single-step, and does not require high pressure, energy, temperature, and the use of highly toxic chemicals (Goodsell, 2004; Heng et al., 2013). Besides radiation, transgenic and symbiont-based mosquito control approaches (Bourtzis et al., 2016), an effective option may be the employment of biological control agents of mosquito young instars, in the presence of ultra-low quantities of bio-fabricated nanoparticles, which boost their predation rates in the aquatic environment (Murugan et al., 2015a,b,c; Benelli, 2016a). Indeed, from an entomological point of view, green nanoparticles have been successfully employed to reduce mosquito young instar populations in the field, as well as to induce egg mortality and oviposition deterrence (Benelli, 2016a,b). Notably, little non-target effects have been reported (Benelli, 2016c), with no acute toxicity or genotoxicity on aquatic organisms at the doses lethal to mosquito young instars. To our mind, further research efforts to shed light on (i) the precise mechanism(s) of action of green-fabricated metal nanoparticles (Foldbjerg et al., 2015; Murugan et al., 2016e), (ii) their fate in the aquatic environment, and (iii) the possible toxicity of residual silver ions in the aquatic ecosystems (even if this seems to exert negligible toxicity on mosquito young instars, see Marimuthu et al., 2011, as well as Govindarajan and Benelli, 2016b) are urgently needed. In addition, renewed efforts to (iv) standardize the chemical composition of botanical products used as sources of reducing and capping metabolites, as well as (v) to optimize the green nanosynthetic routes are required, in order to develop large-scale production of eco-friendly nanomosquitocides.
Funding
G. Benelli is sponsored by PROAPI (PRAF 2015) and University of Pisa, Department of Agriculture, Food and Environment (Grant ID: COFIN2015_22). Funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Acknowledgements
The authors are grateful to Prof. Ahmad H. Al Ghamdi for inviting this review on Journal of King Saud University – Science.
Giovanni Benelli is an Associate Editor of Journal of King Saud University – Science. This does not alter the authors’ adherence to all the Journal of King Saud University – Science policies on sharing data and materials.
References
- Repellency effect of forty-one essential oils against Aedes, Anopheles and Culex mosquitoes. Parasitol. Res.. 2006;99:478-490.
- [Google Scholar]
- The sensilla of Aedes and Anopheles mosquitoes and their importance in repellency. Parasitol. Res.. 2006;99:491-499.
- [Google Scholar]
- Larvicidal effects of various essential oils against Aedes, Anopheles, and Culex larvae (Diptera, Culicidae) Parasitol. Res.. 2006;99:466-472.
- [Google Scholar]
- Persistency of larvicidal effects of plant oil extracts under different storage conditions. Parasitol. Res.. 2006;99:473-477.
- [Google Scholar]
- Myco-synthesis of silver nanoparticles using Metarhizium anisopliae against the rural malaria vector Anopheles culicifacies Giles (Diptera: Culicidae) J. Pest. Sci.. 2016;89:249-256.
- [Google Scholar]
- Green-synthesised nanoparticles from Melia azedarach seeds and the cyclopoid crustacean Cyclops vernalis: an eco-friendly route to control the malaria vector Anopheles stephensi? Nat. Prod. Res. 2016
- [CrossRef] [Google Scholar]
- Potential for integrated control of Culex quinquefasciatus (Diptera: Culicidae) using larvicides and guppies. Biol. Control. 2015;81:31-36.
- [Google Scholar]
- Effects of urban development and habitat alterations on the distribution and abundance of native and exotic freshwater fish in the Brisbane region, Queensland. Aust. J. Ecol.. 1983;8:87-101.
- [Google Scholar]
- Integration of radiation with cytoplasmic incompatibility for genetic control in the Culex pipiens complex (Diptera: Culicidae) J. Med. Entomol.. 1985;22:648-653.
- [Google Scholar]
- Silica nanoparticle: a potential new insecticide for mosquito vector control. Parasitol. Res.. 2012;111:1075-1083.
- [Google Scholar]
- Exotic mosquitoes conquer the world. In: Mehlhorn H., ed. Arthropods as Vectors of Emerging Diseases. Parasitol Res Monographs. Vol 3. 2012. p. :31-60.
- [Google Scholar]
- Mating competitiveness of Aedes albopictus radio-sterilized males in large enclosures exposed to natural conditions. J. Med. Entomol.. 2013;50:94-102.
- [Google Scholar]
- Research in mosquito control: current challenges for a brighter future. Parasitol. Res.. 2015;114:2801-2805.
- [Google Scholar]
- Plant-borne ovicides in the fight against mosquito vectors of medical and veterinary importance: a systematic review. Parasitol. Res.. 2015;114:3201-3212.
- [Google Scholar]
- Plant-mediated biosynthesis of nanoparticles as an emerging tool against mosquitoes of medical and veterinary importance: a review. Parasitol. Res.. 2016;115:23-34.
- [Google Scholar]
- Plant-mediated synthesis of nanoparticles: a newer and safer tool against mosquito-borne diseases? Asia Pac. J. Trop. Biomed.. 2016;6:353-354.
- [Google Scholar]
- Green synthesized nanoparticles in the fight against mosquito-borne diseases and cancer – a brief review. Enzyme Microbial. Technol. 2016
- [CrossRef] [Google Scholar]
- Declining malaria, rising dengue and Zika virus: insights for mosquito vector control. Parasitol. Res.. 2016;115:1747-1754.
- [Google Scholar]
- Mosquito vectors and the spread of cancer: an overlooked connection? Parasitol. Res.. 2016;115:2131-2137.
- [Google Scholar]
- The outbreaks of Zika virus: mosquito control faces a further challenge. Asia Pac. J. Trop. Dis.. 2016;6:253-258.
- [Google Scholar]
- Mosquito larvae (Culiseta longiareolata) prey upon and compete with toad tadpoles (Bufo virdis) J. Anim. Ecol.. 1994;63:841-850.
- [Google Scholar]
- The invasive mosquito species Aedes albopictus: current knowledge and future perspectives. Trends Parasitol.. 2013;29:460-468.
- [Google Scholar]
- More than one rabbit out of the hat: radiation, transgenic and symbiont-based approaches for sustainable management of mosquito and tsetse fly populations. Acta Trop.. 2016;157:115-130.
- [Google Scholar]
- Boosting the sterile insect technique to control mosquitoes. Trends Parasitol.. 2014;30:271-273.
- [Google Scholar]
- Tadpoles as dengue mosquito (Aedes aegypti) egg predators. Biol. Control. 2013;67:469-474.
- [Google Scholar]
- Refining the global spatial limits of dengue virus transmission by evidence-based consensus. PLoS Negl. Trop. Dis.. 2012;6:e1760.
- [Google Scholar]
- Integration of irradiation with cytoplasmic incompatibility to facilitate a lymphatic filariasis vector elimination approach. Parasites Vectors. 2009;2:38.
- [Google Scholar]
- Wolbachia density and cytoplasmic incompatibility in Aedes albopictus: concerns with using artificial Wolbachia infection as a vector suppression tool. PLoS One. 2015;10:e0121813.
- [Google Scholar]
- Suppression of a field population of Aedes aegypti in Brazil by sustained release of transgenic male mosquitoes. PLoS Negl. Trop. Dis.. 2015;9:e0003864.
- [Google Scholar]
- Characterization and mosquitocidal potential of neem cake-synthesized silver nanoparticles: genotoxicity and impact on predation efficiency of mosquito natural enemies. Parasitol. Res.. 2016;115:1015-1025.
- [Google Scholar]
- Laboratory and field efficacy of Pedalium murex and predatory copepod Mesocyclops longisetus on rural malaria vector Anopheles culicifacies. Asia Pac. J. Trop. Dis.. 2013;3:111-118.
- [Google Scholar]
- Mosquitocidal and antibacterial activity of green-synthesized silver nanoparticles from Aloe vera extracts: towards an effective tool against the malaria vector Anopheles stephensi? Parasitol. Res.. 2015;114:1519-1529.
- [Google Scholar]
- Potential of some selected larvivorous fish species in Aedes mosquito control. In: Proceedings, Peradeniya University Research Sessions, 30 November, Peradeniya, Sri Lanka. Vol 12:1. 2007.
- [Google Scholar]
- Zika virus in the Americas — yet another arbovirus threat. N. Engl. J. Med.. 2016;374:601-604.
- [Google Scholar]
- Bionanotechnology: Lessons from Nature. Hoboken: Wiley; 2004.
- Effect of introduced mosquitofish on Pacific Tree frogs and the role of alternative prey. Conserv. Biol.. 1999;13:921-924.
- [Google Scholar]
- Facile biosynthesis of silver nanoparticles using Barleria cristata: mosquitocidal potential and biotoxicity on three non-target aquatic organisms. Parasitol. Res. 2016
- [CrossRef] [Google Scholar]
- One-pot green synthesis of silver nanocrystals using Hymenodictyon orixense: a cheap and effective tool against malaria, chikungunya and Japanese encephalitis mosquito vectors? RSC Adv.. 2016;6:59021-59029.
- [Google Scholar]
- Bio-physical characterization of poly-dispersed silver nanocrystals fabricated using Carissa spinarum: a potent tool against mosquito vectors. J. Cluster Sci.. 2016;27:745-761.
- [Google Scholar]
- One-step synthesis of polydispersed silver nanocrystals using Malva sylvestris: an eco-friendly mosquito larvicide with negligible impact on non-target aquatic organisms. Parasitol. Res. 2016
- [CrossRef] [Google Scholar]
- Laboratory evaluation of predation on mosquito larvae by Australian mangrove fish. J. Vect. Ecol.. 2014;39:197-203.
- [Google Scholar]
- Fabrication, characterization and mosquito larvicidal bioassay of silver nanoparticles synthesized from aqueous fruit extract of putranjiva, Drypetes roxburghii (Wall.) Parasitol. Res.. 2013;112:1451-1459.
- [Google Scholar]
- Efficacy and community effectiveness of larvivorous fish for dengue vector control. Trop. Med. Int. Health. 2015;20:1239-1256.
- [Google Scholar]
- Successful suppression of a field mosquito population by sustained release of engineered male mosquitoes. Nat. Biotechnol.. 2012;30:828-830.
- [Google Scholar]
- Insecticide resistance in insect vectors of human disease. Annu. Rev. Entomol.. 2000;45:371-391.
- [Google Scholar]
- Emerging green technologies for the chemical standardization of botanicals and herbal preparations. Trends Anal. Chem.. 2013;50:1-10.
- [Google Scholar]
- Insecticidal crystal proteins of Bacillus thuringiensis. Microb. Rev.. 1989;53:242-255.
- [Google Scholar]
- Copepod observed preying on first instar larva of Anopheles quadrimaculatus. J. Parasitol.. 1938;24:281.
- [Google Scholar]
- Earthworm-mediated synthesis of silver nanoparticles: a potent tool against hepatocellular carcinoma, pathogenic bacteria, Plasmodium parasites and malaria mosquitoes. Parasitol. Int.. 2016;65:276-284.
- [Google Scholar]
- Seventy-five years of Resochin® in the fight against malaria. Parasitol. Res.. 2009;105:609-627.
- [Google Scholar]
- First record of mosquitofish, Gambusia holbrooki, in Tasmania, Australia: stock structure and reproductive biology. N. Z. J. Mar. Freshwater Res.. 2004;38:857-867.
- [Google Scholar]
- Larvicidal efficiency of aquatic predators: a perspective for mosquito biocontrol. Zool. Stud.. 2006;45(4):447-466.
- [Google Scholar]
- Green synthesis of therapeutic nanoparticles: an expanding horizon. Nanomedicine. 2015;10:2451-2471.
- [Google Scholar]
- Bacillus thuringiensis serovariety israelensis and Bacillus sphaericus for mosquito control. J. Am. Mosq. Control Assoc.. 2007;23:133-163.
- [Google Scholar]
- Review: improving our knowledge of male mosquito biology in relation to genetic control programmes. Acta Trop.. 2014;132S:S2-S11.
- [Google Scholar]
- Back to the future: the sterile insect technique against mosquito disease vectors. Curr. Opin. Insect Sci.. 2015;10:156-162.
- [Google Scholar]
- Sargassum muticum-synthesized silver nanoparticles: an effective control tool against mosquito vectors and bacterial pathogens. Parasitol. Res.. 2015;114:4305-4317.
- [Google Scholar]
- Mosquitocidal activity of Solanum xanthocarpum fruit extract and copepod Mesocyclops thermocyclopoides for the control of dengue vector Aedes aegypti. Parasitol. Res.. 2012;111:609-618.
- [Google Scholar]
- Biosynthesis, characterization and acute toxicity of Berberis tinctoria-fabricated silver nanoparticles against the Asian tiger mosquito, Aedes albopictus, and the mosquito predators Toxorhynchites splendens and Mesocyclops thermocyclopoides. Parasitol. Res.. 2016;115:751-759.
- [Google Scholar]
- Mesocyclops longisetus effects on survivorship of Aedes aegypti immature stages in car tyres. Med. Vet. Entomol.. 1998;12:386-390.
- [Google Scholar]
- Evaluation of green synthesized silver nanoparticles against parasites. Parasitol. Res.. 2011;10:2212-2224.
- [Google Scholar]
- Natural control of larval Anopheles albuminus (Diptera: Culicidae) by the predator Mesocyclops (Copepoda: Cyclopoida) J. Med. Entomol.. 1989;26:624-627.
- [Google Scholar]
- Mehlhorn H., ed. Encyclopedia of Parasitology (fourth ed.). New York: Springer; 2015. p. :893.
- Mehlhorn, H. (Ed), 2016. Nanoparticles in the fight against parasites. Parasitol. Res. Monographs vol. 8, Springer, Berlin, New York.
- Research and increase of expertise in arachno-entomology are urgently needed. Parasitol. Res.. 2012;110:259-265.
- [Google Scholar]
- Effect of mosquitofish, Gambusia affinis, on channel catfish Ictalurus punctatus, production ponds. J. World Aquacult. Soc.. 2016;44:288-292.
- [Google Scholar]
- Nanoparticles in the fight against mosquito-borne diseases: bioactivity of Bruguiera cylindrica-synthesized nanoparticles against dengue virus DEN-2 (in vitro) and its mosquito vector Aedes aegypti (Diptera: Culicidae) Parasitol. Res.. 2015;114:4349-4361.
- [Google Scholar]
- Toxicity of seaweed-synthesized silver nanoparticles against the filariasis vector Culex quinquefasciatus and its impact on predation efficiency of the cyclopoid crustacean Mesocyclops longisetus. Parasitol. Res.. 2015;114:2243-2253.
- [Google Scholar]
- Cymbopogon citratus-synthesized gold nanoparticles boost the predation efficiency of copepod Mesocyclops aspericornis against malaria and dengue mosquitoes. Exp. Parasitol.. 2015;153:129-138.
- [Google Scholar]
- In vivo and in vitro effectiveness of Azadirachta indica-synthesized silver nanocrystals against Plasmodium berghei and Plasmodium falciparum, and their potential against malaria mosquitoes. Res. Vet. Sci.. 2016;106:14-22.
- [Google Scholar]
- Hydrothermal synthesis of titanium dioxide nanoparticles: mosquitocidal potential and anticancer activity on human breast cancer cells (MCF-7) Parasitol. Res.. 2016;115:1085-1096.
- [Google Scholar]
- Fighting arboviral diseases: low toxicity on mammalian cells, dengue growth inhibition (in vitro) and mosquitocidal activity of Centroceras clavulatum-synthesized silver nanoparticles. Parasitol. Res.. 2016;115:651-662.
- [Google Scholar]
- Eco-friendly drugs from the marine environment: spongeweed-synthesized silver nanoparticles are highly effective on Plasmodium falciparum and its vector Anopheles stephensi, with little non-target effects on predatory copepods. Environ. Sci. Pollut. Res. 2016
- [CrossRef] [Google Scholar]
- Carbon and silver nanoparticles in the fight against the filariasis vector Culex quinquefasciatus: genotoxicity and impact on behavioral traits of non-target aquatic organisms. Parasitol. Res.. 2016;115:1071-1083.
- [Google Scholar]
- Insecticide resistance and its molecular basis in urban insect pests. Parasitol. Res.. 2016;115:1363-1373.
- [Google Scholar]
- Zika virus infection complicated by Guillain-Barré syndrome – case report, French Polynesia. Euro Surveill.. 2014;19(9) pii = 20720
- [Google Scholar]
- Four emerging arboviral diseases in North America: Jamestown Canyon, Powassan, chikungunya and Zika virus diseases. J. Neurovirol. 2016
- [CrossRef] [Google Scholar]
- Larvicidal activity of silver nanoparticles synthesized using Plumeria rubra plant latex against Aedes aegypti and Anopheles stephensi. Parasitol. Res.. 2012;110:1815-1822.
- [Google Scholar]
- Larvicidal activity of silver nanoparticles synthesized using Pergularia daemia plant latex against Aedes aegypti and Anopheles stephensi and nontarget fish Poecilia reticulata. Parasitol. Res.. 2012;111:555-562.
- [Google Scholar]
- Essential oils for the development of eco-friendly mosquito larvicides: a review. Ind. Crops Prod.. 2015;76:174-187.
- [Google Scholar]
- Ethnobotanical knowledge on botanical repellents employed in the African region against mosquito vectors – a review. Exp. Parasitol.. 2016;167C:103-108.
- [Google Scholar]
- Biological control of mosquito populations through frogs: opportunities and constrains. Indian J. Med. Res.. 2008;128:22-25.
- [Google Scholar]
- Silver nanoparticles as a new generation of antimicrobials. Biotechnol. Adv.. 2009;27:76-83.
- [Google Scholar]
- Plant extract synthesized nanoparticles: an ongoing source of novel biocompatible materials. Ind. Crops Prod.. 2015;70:356-373.
- [Google Scholar]
- Biofabrication of Ag nanoparticles using Sterculia foetida L. seed extract and their toxic potential against mosquito vectors and HeLa cancer cells. Mater. Sci. Eng. C Mater. Biol. Appl.. 2014;39:203-212.
- [Google Scholar]
- Pithecellobium dulce mediated extra-cellular green synthesis of larvicidal silver nanoparticles. Spectrochim. Acta A Mol. Biomol. Spectrosc.. 2012;96:1031-1037.
- [Google Scholar]
- Bioactive compound synthesis of Ag nanoparticles from leaves of Melia azedarach and its control for mosquito larvae. Res. Vet. Sci.. 2015;98:82-88.
- [Google Scholar]
- Mosquito larvicidal and antimicrobial activity of synthesized nano-crystalline silver particles using leaves and green berry extract of Solanum nigrum L. (Solanaceae: Solanales) Acta Trop.. 2013;128:613-622.
- [Google Scholar]
- Effects of single introduction of Toxorhynchites moctezuma upon Aedes aegypti on a Caribbean island. J. Am. Mosq. Control Assoc.. 1991;7:7-10.
- [Google Scholar]
- Zika virus outbreak: an overview of the experimental therapeutics and treatment. Virus Dis. 2016
- [CrossRef] [Google Scholar]
- Evaluation of Costa Rican copepods (Crustacea: Eudecapoda) for larval Aedes aegypti control with special reference to Mesocyclops thermocyclopoides. J. Am. Mosq. Control Assoc.. 1999;15:510-519.
- [Google Scholar]
- Bacillus thuringiensis and its pesticidal crystal proteins. Microbiol. Mol. Rev.. 1998;62:775-806.
- [Google Scholar]
- Evaluation of two cyclopoid copepods for Aedes albopictus control in tires in the panhandle of Florida at low introduction rates. J. Fla. Mosq. Control Assoc.. 1993;64:7317.
- [Google Scholar]
- Nature helps: from research to products against blood sucking arthropods. Parasitol. Res.. 2009;105:1483-1487.
- [Google Scholar]
- The effectiveness of Mesocyclops longisetus (Copepoda) for the control of container-inhabiting mosquitoes in residential environments. J. Am. Mosq. Control Assoc.. 2011;27:376-383.
- [Google Scholar]
- Studies on the impact of biosynthesized silver nanoparticles (AgNPs) in relation to malaria and filariasis vector control against Anopheles stephensi Liston and Culex quinquefasciatus Say (Diptera: Culicidae) Parasitol. Res.. 2013;112:487-499.
- [Google Scholar]
- Eco-friendly control of malaria and arbovirus vectors using the mosquitofish Gambusia affinis and ultra-low dosages of Mimusops elengi-synthesized silver nanoparticles: towards an integrative approach? Environ. Sci. Pollut. Res. Int.. 2015;22:20067-20083.
- [Google Scholar]
- Multipurpose effectiveness of Couroupita guianensis-synthesized gold nanoparticles: high antiplasmodial potential, field efficacy against malaria vectors and synergy with Aplocheilus lineatus predators. Environ. Sci. Pollut. Res.. 2016;23:7543-7558.
- [Google Scholar]
- Green synthesized silver nanoparticles as a novel control tool against dengue virus (DEN-2) and its primary vector Aedes aegypti. Parasitol. Res.. 2015;114:3315-3325.
- [Google Scholar]
- Tackling the growing threat of dengue: Phyllanthus niruri-mediated synthesis of silver nanoparticles and their mosquitocidal properties against the dengue vector Aedes aegypti (Diptera: Culicidae) Parasitol. Res.. 2015;114:1551-1562.
- [Google Scholar]
- Evolution of resistance to Bacillus thuringiensis. Annu. Rev. Entomol.. 1994;39:47-79.
- [Google Scholar]
- Insect population control using a dominant, repressible, lethal genetic system. Science. 2000;287:2474-2476.
- [Google Scholar]
- Gambusia affinis (Baird and Girard) and Gambusia holbrooki Girard (Mosquitofish) In: Francis R.A., ed. A Handbook of Global Freshwater Invasive Species. London: Earthscan; 2012. p. :261-273.
- [Google Scholar]
- Tadpoles of three common anuran species from Thailand do not prey on mosquito larvae. J. Vectors Ecol.. 2015;40:230-232.
- [Google Scholar]
- Declining malaria transmission and pregnancy outcomes in Southern Mozambique. N. Engl. J. Med.. 2015;373:1670-1671.
- [Google Scholar]
- Handbook for Integrated Vector Management. Geneva: World Health Organization; 2012.
- Lymphatic Filariasis. Fact Sheet N°102. Geneva: World Health Organization; 2014.
- WHO, 2015a. Fact Sheet: World Malaria Report 2015. Updated 9 December 2015.
- WHO, 2015b. WHO updates on artemisinin resistance. <http://www.who.int/malaria/areas/drug_resistance/updates/en/>.
- WHO, 2015c. Dengue and severe dengue. Fact sheet N°117. Updated May 2015.
- WHO, 2016a. Neglected Tropical Diseases. Geneva (accessed July 2016) <http://www.who.int/neglected_diseases/diseases/en/>.
- WHO, 2016b. Yellow fever. Factsheet. Updated May 2016.
- WHO, 2016c. Zika virus. Fact sheet N°1. Updated January 2016.
- Ecology and Classification of North American Freshwater Invertebrates. San Diego: Academic; 1999. p. :787-822.
- A Review of the Biological Control of Insects and Weeds in Australia and Australian New Guinea. Vol 1. Commonwealth Institute of Biological Control Technical Communication; 1960. p. :80-91.
- Zika virus outbreak in the Americas: the need for novel mosquito control methods. Lancet Global Health. 2016;4:148-149.
- [Google Scholar]







