Translate this page into:
Effect of selected baculoviruses on oviposition preference by Trichogramma chilonis (Trichogrammatidae: Hymenoptera)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Microbial insecticides are effective, environmental friendly and are widely used worldwide to control insect pests. Nucleopolyhedroviruses and granuloviruses belonging to family Baculoviridae are widely used for control of caterpillar pests on wide varieties of crops and vegetables. The selected baculoviruses (BVs) were evaluated for oviposition preference by Trichogramma chilonis (Ishii) of virus treated and untreated (water: control) host eggs (Sitotroga cerealella Olivier), which revealed no significant difference among the used concentrations regarding oviposition preference. All the used concentrations of Helicoverpa armigera nucleopolyhedrovirus (HaNPV), Spodoptera exigua nucleopolyhedrovirus (SeMNPV) and Cydia pomonella granulovirus (CpGV) including 12.5×, 6.25×, 2.5×, 1.25× and 0.625× were harmless (E > 30%) for parasitism by T. chilonis as comparison of virus treated and untreated control eggs showed similar parasitism i.e., ⩽15% reduction over control in parasitism. Thus it was concluded that all three types of baculoviruses were compatible with the parasitism by T. chilonis at all treated concentrations.
Keywords
Baculoviruses
Oviposition preference
T. chilonis
Compatible
1 Introduction
Biological control is globally preferred over synthetic pesticides for its effective role to suppress the population of insect pests (Omkar and Kumar, 2016). Determination of adverse impacts of pesticide on beneficials is required to find chemicals not compatible with natural enemies, in order to effectively integrate both chemical and biological controls (Croft, 1990; Ruberson et al., 1998; Stark et al., 2007; Shoeb, 2010; Khan et al., 2014; 2015a,b). Integrated pest management and sensible use of pesticides are needed to keep the losses caused by pests under economic threshold levels (Karuppuchamy and Venugopal, 2016).
Insecticides resistance to the broad-spectrum pesticides led to limit the effectiveness of many such chemicals and this resulted in intensive efforts to find out alternate methods of control (Nathan et al., 2004; Sagheer et al., 2008). Selective insecticides may be valuable to effectively overcome increasing pesticide resistance (Nabil and El-Wakeil, 2013). Thus use of selective insecticides to manage pests contributes to the conservation of natural enemies associated with crops (Thomson et al., 2000).
Biopesticides including botanical insecticides and microbial pesticides are safe, ecologically acceptable, and are highly effective against target pest but are relatively safe to natural enemies (Sagheer et al., 2008). The microbial insecticides can be equally as effective as synthetic chemicals to control insect pests (Khan et al., 2014).
Nucleopolyhedroviruses (NPVs) and granuloviruses (GVs) are microbial insecticides belonging to the major group of viruses known as baculoviruses. They are widely used as natural enemies of insect pests (Moscardi, 1999; Khan et al., 2014), and have been used since the early 1890s (Huber, 1986; Khan et al., 2014). They are obligate pathogens and are commonly used to control Lepidoptera and Hymenoptera (Mazzone, 1985; Khan et al., 2014). They are host specific (Federici, 1997; Khan et al., 2014), and replicate in the host cells. They usually infect their larval hosts following ingestion (Andreadis, 1987; Khan et al., 2014).
Trichogramma species are the most widely used among the parasitoids for pest management worldwide (Jalali et al., 2016). They have been extensively used as natural enemies (Shoeb, 2010), and have achieved appreciable pest control success in many crop ecosystems, while their role in the biological control programs of pest management is well understood (Smith, 1996; Sorokina, 1999; Hussain et al., 2010). They were recognized as biological control agents in the 1900s, which led to their mass rearing, aiming to use them in pest control programs (Smith, 1996; Bastos et al., 2006). They control pests particularly among the Lepidoptera (Khan et al., 2015a). Around 18 different species of Trichogramma are being mass reared in 16 countries to control insect pest on 18 million of hectares (Hassan, 1994).
Trichogramma chilonis (Ishii) is widely distributed throughout the Indian subcontinent and has been effectively used to control caterpillar pests in the field (Manjunath et al., 1985; Khan et al., 2014). They control common pests in Pakistan including sugarcane borer (Chilo sacchariphagus) in sugar cane, diamondback moth (Plutella xylostella) in cabbage and other vegetables, and cotton bollworms (Helicoverpa armigera) in cotton and corn.
The baculoviruses used in this work are: HaNPV (HELICOVEX), SeMNPV (SPEXIT) and CpGV (MADEX), two of them are NPVs and the other is a GV. They were evaluated for their effect on the oviposition preference by T. chilonis.
2 Materials and methods
2.1 Rearing of Sitotroga cerealella
The young larvae of grain moth S. cerealella hatched and infested the wheat grain within a week host eggs were sprinkled on sterilized grains in a plastic/metal tray (30 × 18 cm) in the laboratory of Entomology Division, NIFA, Peshawar, (Pakistan). The infested wheat was then shifted to plastic rearing jars (15 × 20 cm), and their openings were subsequently covered with a piece of cotton cloth, and were maintained in the laboratory at average conditions of 24 ± 6 °C, 65 ± 10% relative humidity (RH) and 16:8 (L:D) until adults’ emergence after 20–25 days.
Regular collections of emerged moths from the rearing jars every 24 h were carried out by an electric suction apparatus in the oviposition jar (10 × 15 cm) covered at bottom by mesh (mesh No. 30–40 pore size). The jar containing adult moths was placed over the corn flour in a metal/plastic tray, and was given a single turn to adhere the flour to the jar mesh at the bottom for egg laying. The jar was then carefully placed on metal/plastic tray until next day (24 h) allowing the moths to lay eggs in the flour. Next day, the host eggs were collected by sieving the flour and the eggs were used in the experimental work as well as for maintenance of S. cerealella culture in the laboratory.
2.2 Rearing of Trichogramma chilonis
Approximately 1000–1300 eggs of S. cerealella (less than 24 h old) were glued onto a hard paper card (5 × 8 cm). Several cards were prepared and dried for one h. and each card was subsequently exposed for parasitism in glass jar (5 × 12 cm) for 24 h containing approximately 30–40 adults (mixed-gender) of T. chilonis. The opening of the glass jars was tightly covered with muslin cloth to prevent escape of the adults. Droplets of honey were scattered on the inner surface of the glass jar walls as food for the parasitoid. The jar was placed in the lamp light in order to obtain good parasitism by the tiny wasp. Subsequently, the parasitized card was removed and was transferred to another glass jar of the same size, and the jar was incubated at the 23 ± 3 °C, 70 ± 10% RH and 14:10 (L:D) conditions until adult emergence. Stock culture of T. chilonis was produced for use in the experimental work.
2.3 Preparation of different concentrations of pesticides solution
Commercially available three types of BVs (Table 1) including HaNPV, SeNPV and CpGV were diluted with tap water to prepare their respective stock solutions. The stock solution was diluted (serial dilutions) and 5 different concentrations (12.5×, 6.25×, 2.5×, 1.25× and 0.625×) of insecticides were prepared for use in the experiments by the formula: C1V1 = C2V2, where C1 and V1 are the concentration and volume of commercial pesticides/stock solution, respectively, while C2 and V2 are the concentration and volume of the required pesticide solutions (diluted), respectively.
Virus and formulation
Active ingredient
Conc
Trade name
Manufacturer/supplier
Condition of storage
FRC
HaNPV (suspension concentrate)
Helicoverpa armigera nucleopolyhedrovirus
7.5 × 1012 NPV/liter
HELICOVEX
Andermatt Biocontrol (Switzerland)
−10–37 °C, RH (70 ± 10%) (protected from light)
50–200 ml
SeNPV (suspension concentrate)
Spodoptera exigua nucleopolyhedrovirus
3.75 × 1012 NPV/liter
SPEXIT
Andermatt Biocontrol (Switzerland)
Same as above
100–200 ml
CpGV (suspension concentrate)
Cydia pomonella granulovirus
3 × 1013 GV/liter
MADEX
Andermatt Biocontrol (Switzerland)
Same as above
100 ml
2.4 Testing for oviposition preference by Trichogramma chilonis
Approximately 60–65 fresh S. cerealella eggs were glued on the hard paper card (5 × 8 cm). The card was dried for 1–2 h and was subsequently cut into six card strips (0.9 × 8 cm each) each containing 10 host eggs. Card strips were treated by dipping for 1–2 s in the different solution of each type of BVs or control in the laboratory. Each card was dried at the aforementioned laboratory conditions, and subsequently one card containing virus treated host eggs and the other containing water treated (control) host eggs were exposed to a pair of T. chilonis (<24 h old) in the glass vial (1 × 10 cm) to evaluate their effect on oviposition preference in choice design. The vial was exposed to light for 3 h for completion of parasitization. The trial consisted of six replications for each concentration and treatment. The exposed parasitizing female was removed after 3 h from each vial and all the vials were incubated at aforementioned conditions until pupae formation. The data were recorded by counting darkened eggs (pupae) 7 days after exposure to the parasitoids separately for each card, and data were compared for both virus treated and control to determine oviposition preference by the parasitoid.
2.5 Data analysis
The data were analyzed using GLM (Statistix 9) on average parasitization. Tukey HSD test (p = 0.05) were used for mean separation. Reduction in parasitism (%) over controls were evaluated by toxicity categories of International Organization for Biological Control (IOBC)/West Palaearctic Regional Section (WPRS) (Hassan et al., 1994; Sterk et al., 1999): 1 = harmless (E < 30%); 2 = slight harmful (30 ⩽ E ⩽ 79%); 3 = moderately harmful (79 < E ⩽ 99%); 4 = harmful (>99%), where “E” stand for effect of the pesticide on the biological control agent measured as the reduction in percentage of parasitism over control.
3 Results and discussion
All three types of BVs including HaNPV, SeMNPV and CpGV were evaluated for oviposition preference of host eggs (S. cerealella) by T. chilonis. Their active ingredient, supplier of such products and the conditions used for their storage are given in Table 1. The analysis of variance revealed (Table 2) no significant difference for parasitism among the used concentrations regarding both viruses treated and untreated (control:water) host eggs (p > 0.05). All means sharing same letter “a” within a column/among columns, are not significantly different (Tukey’s HSD, p > 0.05). T stands for virus treatment and C stands for control.
Type of BVs
Concentration (mean ± SE); virus treatments and control
12.5×
6.25×
2.5×
1.25×
0.625×
T
C
T
C
T
C
T
C
T
C
HaNPV
8.83 ± 0.75a
9.00 ± 0.63a
8.83 ± 0.48a
9.17 ± 0.48a
8.17 ± 0.91a
9.17 ± 0.40a
8.17 ± 0.60a
8.50 ± 0.43a
7.83 ± 1.60a
8.00 ± 0.45a
SeMNPV
8.67 ± 0.80a
9.00 ± 0.26a
7.50 ± 1.63a
7.50 ± 0.72a
8.00 ± 0.89a
8.00 ± 0.93a
8.17 ± 0.98a
8.17 ± 0.48a
7.50 ± 0.89a
8.83 ± 0.31a
CpGV
9.17 ± 0.40a
9.50 ± 0.34a
9.83 ± 0.17a
9.83 ± 0.17a
9.83 ± 0.17a
10.0 ± 0.00a
7.83 ± 0.95a
9.17 ± 0.65a
9.17 ± 0.48a
9.50 ± 0.22a
ANOVA results for HaNPV
Concentration
df
f
p
Remarks
T
C
T
C
T
C
4
4
0.22
1.10
0.9246
0.3775
Not significant
ANOVA results for SeMNPV
Concentration
df
f
p
Remarks
T
C
T
C
T
C
4
4
0.21
1.07
0.9321
0.3905
Not significant
ANOVA results for CpGV
Concentration
df
f
p
Remarks
T
C
T
C
T
C
4
4
2.49
0.85
0.0690
0.5081
Not significant
All the used concentrations of selected BVs were found harmless for parasitism by T. chilonis based on the comparison of virus treated and control host eggs which showed ⩽15% reduction over control in parasitism (Fig. 1). Furthermore, both types of host eggs were similarly preferred for parasitism by tiny parasitoid, and all used concentrations of BVs showed statistically at par with control (Table 2). Therefore, results demonstrated that both virus treated and untreated host eggs observed similar parasitism.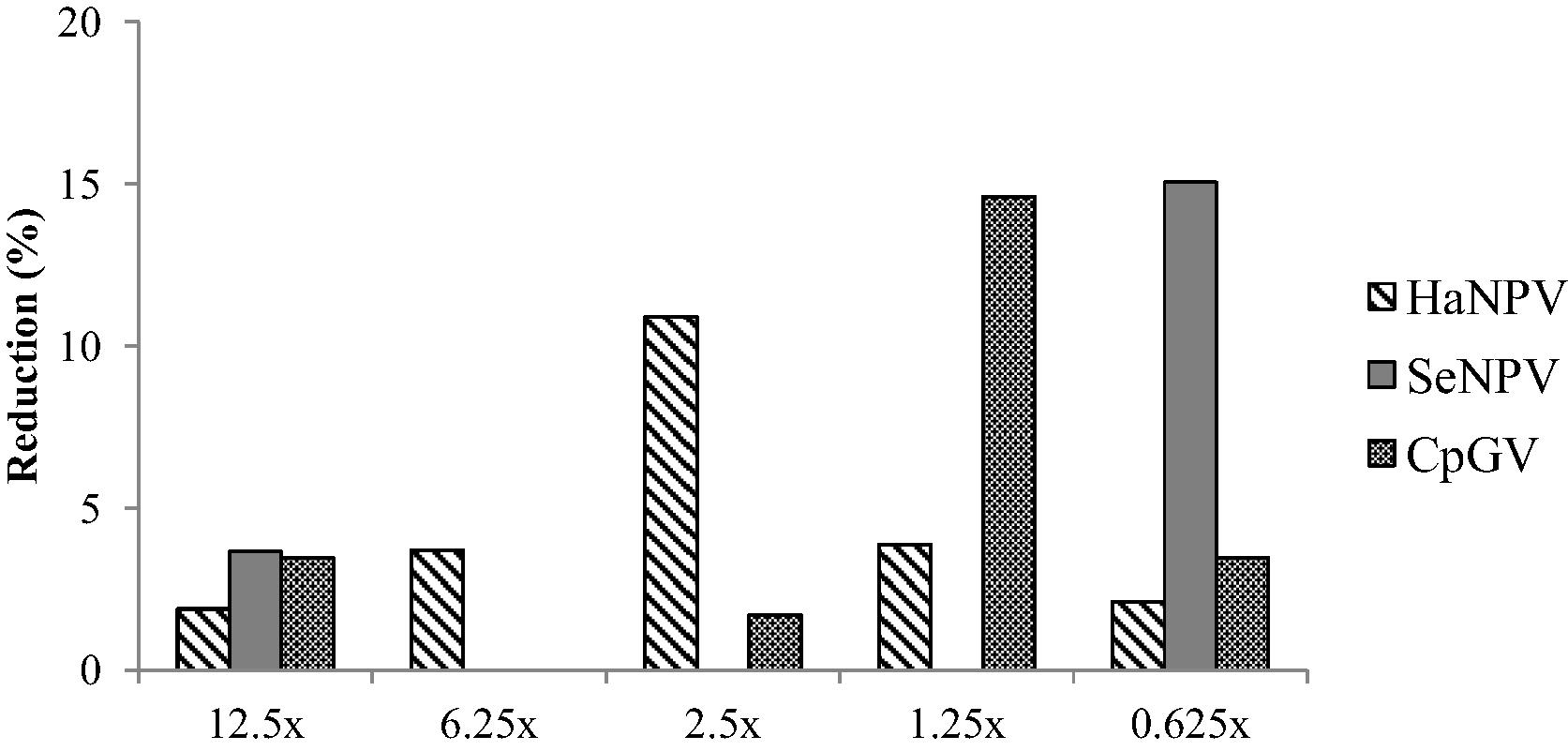
Percent reduction in parasitism over control by T. chilonis in oviposition preference test (choice design).
Sufficient published studies are not available on effects of the pesticides on Trichogramma spp. However, few experiments were conducted by various scientists assessing toxicity of microbial insecticides to beneficials including Trichogramma (Khan et al., 2014). The compatibility of BVs with T. chilonis in the current study were supported by Moscardi (1999) and Khan et al. (2014), who described viral insecticides as not harmful to humans and are compatible with natural enemies of target pests. Similarly, Khan et al. (2014) described HaNPV as very safe microbial insecticide for emergence of as well as parasitism by T. chilonis and can effectively manage the target pests. For example, Ramteke and Gangurde (2011) described that both fresh HaNPV (2 × 109 POBs/ml @ 250 ml/ha and 1 × 109 POBs/ml @ 500 ml/ha), and stored HaNPV formulations (stored for 1 year (2 × 109 POBs/ml @ 250 ml/ha and 1 × 109 POBs/ml @ 500 ml/ha) led to effectively reduced larval populations of H. armigera and led to higher yields of pigeon pea. Similarly, treatment of bacterium Pseudomonas fluorescens did not exhibit adverse impacts on the parasitism and emergence success of the T. chilonis (Gandhi et al., 2005; Khan et al., 2014). Sagheer et al. (2008) reported that integration of bioinsecticides (neem and Bacillus thuringiensis-Bt) and Trichogramma spp. can enhance effectiveness of the parasitic wasps against rice leaf folder Cnaphalocrocis medinalis.
Plant extracts and microbial formulations may effectively replace conventional synthetic insecticides (Khan et al., 2014). Biopesticides replaced synthetic pesticides based on their generally low environmental pollution, low toxicity to humans, and other benefits (DeBach and Rosen, 1991; Qi et al., 2001; Gandhi et al., 2005), and are effective under field conditions when integrated with biological control (Huffaker, 1974; Beddington et al., 1978; Barclay, 1982; DeBach and Rosen, 1991; Van Driesche and Bellows, 1996; Qi et al., 2001; Gandhi et al., 2005).
Viruses belonging to family Baculoviridae have been used as pesticides for biological control of pests (Copping and Menn, 2000; Souza de et al., 2007). They have narrow specificity, and are harmless to people and wildlife and have been used in many countries around the world (Souza de et al., 2007). More than 600 species of baculoviruses attack pests belonging to order Lepidoptera, Hymenoptera and Diptera (Souza de et al., 2007). Successful pest controls rely on the use of chemicals and several viruses (Prasad and Srivastava, 2016). According to modern classification based on ICTV (International Committee on Taxonomy of Viruses): the family Baculoviridae have been divided into four genera: (1) Alphabaculovirus (lepidopteran-specific NPV), (2) Betabaculovirus (lepidopteran-specific Granuloviruses), (3) Gammabaculovirus (hymenopteran-specific NPV) and (4) Deltabaculovirus (dipteran-specific NPV) (Jehle et al., 2006).
Nucleopolyhedrovirus belonging to baculoviruses in the family Baculoviridae, consists of large rod-shaped nucleocapsids with circular double-stranded DNA (Bilimoria, 1986; Khan et al., 2014). The outer lipoprotein envelope surrounds the nucleocapsid (Khan et al., 2014). The virions are invisible by light microscope, however, large occlusion bodies (OB) produced in the host cell, range from 1 to 15 μm, are visible in a compound microscope, and occlude many virions protecting them to some degree during host-to-host transfer (Benz, 1987; Ignoffo et al., 1989; Khan et al., 2014). They are commonly associated with the Lepidoptera and Hymenoptera (Mazzone, 1985) including cotton bollworm, beat armyworm and codling moth. They are very effective to manage a variety of pest insects, although some insects survive and show only sublethal effects ranging from deformed pupae (Peng et al., 1997) to slower development, lower weight, reduced reproduction and shorter life span (Rothman and Myers, 1996). Attempts at controlling insect populations with nucleopolyhedroviruses (NPVs) date to at least the early 1890s (Huber, 1986). Cydia pomonella granulosis virus (CpGV) is a granulovirus with double-stranded DNA and forms small bodies called granules containing a single virion. CpGV is a biological control agent of Codling moth C. pomonella, and kills its host in the same instar as infection.
H. armigera nucleopolyhedrovirus or HaNPV is a microbial pesticide, marketed as “HELICOVEX” in the world including Pakistan, and effectively control caterpillar pests including, H. armigera (Hubner) in cotton, tomato, pea, tobacco, maize, sweet corn and lettuce. Pulses, sunflower, wheat, lucerne, potato and other crops are hosts of H. armigera larvae in Pakistan (Ahmed et al., 1992; Khan et al., 2014). The virus kills young instars (L1–L3) and infects older larvae. It is well suited for organic and integrated pest management strategies and resistance management programs (Andermatt and Andermatt, 2015).
Spodoptera exigua nucleopolyhedrovirus available as SPEXIT worldwide including Pakistan is a highly specific/selective insecticide which effectively controls beet armyworms Spodoptera exigua (Hübner) on various greenhouse and open field crops including corn, cotton, soybean, alfalfa, sweet pepper, tomato, melon, cucurbit, strawberry, sugar-beet, bean, cabbage, citrus, garlic, groundnut, lettuce, maize, onion, potato, pea, rice and tobacco in many parts of the world. The use of SPEXIT significantly reduces crop damage and pest population (Andermatt and Andermatt, 2015).
MADEX, a bioinsecticide containing C. pomonella Granulovirus (CpGV) as active ingredient, is a highly specific/selective insecticide against the codling moth (C. pomonella (Linnaeus) (Andermatt and Andermatt, 2015). They kill the larvae in their early instars before causing damage to the plants (Andermatt and Andermatt, 2015). At a lower dosage, the larvae will be killed at a later instar providing excellent population control. It can be used by organic growers, but is also an effective product for use in IPM and conventional control programs against codling moths in apple, pear, walnuts, quinces, apricots, peaches, almonds, kakis, medlars, oranges and others (Andermatt and Andermatt, 2015).
4 Conclusion
Selected baculoviruses were assessed for their effect on the oviposition preference of virus treated and untreated host eggs of Sitotroga cerealella by Trichogramma chilonis. All the three types of baculoviruses including HaNPV, SeNPV and CpGV tested at concentrations including 12.5x, 6.25x, 2.5x, 1.25x and 0.625x against T. chilonis did not demonstrated oviposition preference of untreated host eggs compared to virus treated eggs. Therefore, it was concluded that all the three types of BV are compatible with parasitism by parasitoids at the used concentrations (under the choice).
Acknowledgements
Higher Education Commission of Pakistan is highly acknowledged for their financial support under 5000 indigenous PhD program. I am also very thankful to the administration of Nuclear Institute of Food and Agriculture (NIFA), Peshawar (Pakistan) for the provision of laboratory facilities to conduct the research. Thanks also to the Dr. Hizbullah Khan of the University of Peshawar for his contribution in obtaining permission to work in the NIFA.
References
- Heliothis Management in Pakistan. Govt. Punjab: Agriculture Department; 1992.
- Product Portfolio. Stahlermatten 6, 6146 Grossdietwil, Switzerland: Andermatt Biocontrol AG; 2015.
- Transmission. In: Fuxa J.R., Tanada Y., eds. Epizootiology of Insect Diseases. New York: Wiley; 1987. p. :159-176.
- [Google Scholar]
- Models for pest control using predator release, habitat management and pesticide release in combination. J. Appl. Ecol.. 1982;19:337-348.
- [Google Scholar]
- Selectivity of pesticides used on cotton (Gossypium hirsutum) to Trichogramma pretiosum, reared on two laboratory-reared hosts. Manage. Sci.. 2006;62:91-98.
- [Google Scholar]
- Characteristics of successful natural enemies in models of biological control of insect pests. Nature. 1978;273:513-519.
- [Google Scholar]
- Environment. In: Fuxa J.R., Tanada Y., eds. Epizootiology of Insect Diseases. New York: Wiley; 1987. p. :177-214.
- [Google Scholar]
- Taxonomy and identification of baculoviruses. In: Granados R.R., Federici B.A., eds. The Biology of Baculoviruses. Vol. I. Biological Properties and Molecular. Boca Raton, FL: CRC Press; 1986. p. :37-59.
- [Google Scholar]
- Biopesticides: a review of their action, application and efficacy. Pest. Manage. Sci.. 2000;56:651-676.
- [Google Scholar]
- Arthropod Biological Control Agents and Pesticides. New York: Wiley; 1990.
- Biological Control by Natural Enemies. Melbourne, Australia: Cambridge University Press; 1991.
- Baculovirus pathogenesis. In: Miller L.K., ed. The Baculoviruses. New York: Plenum Press; 1997. p. :33-59.
- [Google Scholar]
- Laboratory Evaluation of Relative Toxicities of Some Insecticides against Trichogramma chilonis (Hymenoptera: Trichogrammatidae) and Chrysoperla carnea (Neuroptera Chrysopidae) J. Asia-Pac. Entomol.. 2005;8(4):381-386.
- [Google Scholar]
- Comparison of three different laboratory methods and one semi-field test method to assess the side effects of pesticides on Trichogramma cacoeciae. IOBC/WPRS Bull. 1994;17:133-141.
- [Google Scholar]
- Results of the sixth joint pesticide testing programme of the IOBC/WPRS-working group “Pesticides and Beneficial Organisms”. Entomophaga. 1994;39(1):107-119.
- [Google Scholar]
- Use of baculoviruses in pest management programs. In: Granados R.R., Federici B.A., eds. The Biology of Baculoviruses. Vol. II. Practical Application for Insect Control. Boca Raton, FL: CRC Press; 1986. p. :181-202.
- [Google Scholar]
- Biological Control. New York: Plenum; 1974.
- Effect of insecticides on Trichogramma chilonis Ishii. (Hymenoptera: Trichogrammatidae) immature and adult survival. J. Agric. Res.. 2010;48:531-537.
- [Google Scholar]
- Inactivation of nonoccluded and occluded baculoviruses and baculovirus-DNA exposed to simulated sunlight. Environ. Entomol.. 1989;18:177-183.
- [Google Scholar]
- On the classification and nomenclature of baculoviruses: a proposal for revision. Arch. Virol.. 2006;151(7):1257-1266.
- [CrossRef] [Google Scholar]
- Integrated pest management. Ecofriendly Pest Management for Food Security 2016:651-684.
- [Google Scholar]
- Assessment of the lethal and parasitism effects of Helicoverpa armigera Nucleopolyhedrovirus (HaNPV) on Trichogramma chilonis (Ishii) (Hymenoptera: Trichogrammatidae) Sarhad J. Agric.. 2014;30(4):425-432.
- [Google Scholar]
- Evaluation of Toxicity of some Novel Pesticides to Parasitism by Trichogramma chilonis (Hymenoptera: Trichogramm-atidae) J. Agric. Res.. 2015;53(1):63-73.
- [Google Scholar]
- Lethal and behavioral effects of selected novel pesticides on adults of Trichogramma pretiosum (Hymenoptera: Trichogrammatidae) Pest. Manage. Sci. 2015 (Wiley Online Library)
- [CrossRef] [Google Scholar]
- Economic importance of Heliothis spp. in India and an assessment of their natural enemi-es and host plants. In: Proc. Workshop on Biol. Control of Heliothis, New Delhi, India. 1985. p. :197-228.
- [Google Scholar]
- Pathology associated with baculovirus infection. In: Maramorosch K., Sherman K.E., eds. Viral Insecticides for Biological Control. Orlando, FL: Academic Press; 1985. p. :81-120.
- [Google Scholar]
- Assessment of the application of baculoviruses for control of Lepidoptera. Annu. Rev. Entomol.. 1999;44:257-289.
- [Google Scholar]
- Effect of botanicals and bacterial toxin on the gut enzyme of Cnaphalocrocis medinalis. Phytoparasitica. 2004;32:433-443.
- [Google Scholar]
- Biocontrol of insect pests. In: Ecofriendly Pest Management for Food Security. 2016. p. :25-61.
- [Google Scholar]
- Susceptibility of Anticarsia gemmatalis (Lepidoptera: Noctuidae), reared on four host plants, to a nuclear polyhedrosis virus. Environ. Entomol.. 1997;26:973-977.
- [Google Scholar]
- Effects of neem-fed prey on the predacious insects Harmonia conformis (Boisduval) (Coleoptera: Coccinellidae) and Mallada signatus (Schneider) (Neuroptera: Chrysopidae) Biol. Control. 2001;22:185-190.
- [Google Scholar]
- Evaluation of fresh and stored HaNPV formulations on Helicoverpa armigera (Hubner) larval population and production of Cajanascajan (L. Mill) J. Biopesticides. 2011;4(1):49-52.
- [Google Scholar]
- Debilitating effects of viral diseases on host Lepidoptera. J. Invertebr. Pathol.. 1996;67:1-10.
- [Google Scholar]
- Pesticides and conservation of natural enemies in pest management. In: Barbosa P., ed. Conservation Biological Control. New York: Academic Press; 1998. p. :207-220.
- [Google Scholar]
- Integration of some biopesticides and Trichogramma chilonis for the sustainable management of rice leaf folder Cnaphalocrocis medinalis (Guenee) (Lepidoptera: Pyralidae) Pak. J. Agric. Sci.. 2008;45(1):69-74.
- [Google Scholar]
- Effect of some insecticides on the immature stages of the egg parasitoid Trichogramma evanescens West. (Hym., Trichogrammatidae) Egypt. Acad. J. Biol. Sci.. 2010;3(1):31-38.
- [Google Scholar]
- Biological control with Trichogramma advances, successes, and potential of their use. Annu. Rev. Entomol.. 1996;41:375-406.
- [Google Scholar]
- Trophic links of species of the genus Trichogramma West. (Hym. Trichogrammatidae) of the world fauna. Ent. Rev.. 1999;79:125-132.
- [Google Scholar]
- Incorporating ecologically relevant measures of pesticide effect for estimating the compatibility of pesticides and biocontrol agents. J. Econ. Entomol.. 2007;100:1027-1032.
- [Google Scholar]
- Results of the seventh joint pesticide testing programme carried out by the IOBC/WPRS-Working Group ‘‘Pesticides and Beneficial Organisms’’. Biocontrol. 1999;44:99-117.
- [Google Scholar]
- Effects of sulfur on Trichogramma egg parasitoids in vineyards: measuring toxic effects and establishing release windows. Aust. J. Exp. Agric.. 2000;40:1165-1171.
- [Google Scholar]
- Biological Control. New York: Chapman and Hall; 1996. p. :539.







