Translate this page into:
Two new flavonoids from Adenium obesum grown in Oman
⁎Corresponding author. Tel.: +968 99708496; fax: +968 25446236. hossainabi@gmail.com (Mohammad Amzad Hossain)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
In this study, two new antimicrobial flavonoid compounds were isolated and characterized, and their biological activities were assayed. The pure antimicrobial compounds were isolated and characterized from the ethyl acetate extract using different chromatographic techniques. The antimicrobial activity of the isolated pure compounds was assessed using disk diffusion method. Phytochemical investigation on the ethyl acetate crude extract of stem of Adenium obesum (A. obesum) resulted in the isolation of two pure new flavonoids 5,7,3′,4′-tetrahydroxy flavone 1 and 3,5,7,3′,4′,5′-hexahydroxyflavone 2 with several other minor compounds. Their structures were deduced on the basis of 1H NMR, 13C NMR, DEPT 90 and 135, COXY, HMBC, and MS. The pure antimicrobial flavonoid compounds showed significant antibacterial activities against Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa, and Proteus vulgaris in the disk diffusion assay. Inhibition zones were 9–14 mm. The maximum inhibition was shown by compound 2 at concentration 200 μg/ml against P. vulgaris (IZ = 14 mm) when compared with the standard amoxicillin. The results showed that the isolated pure antimicrobial flavonoid compounds have significant antimicrobial activity, which can be used as antibiotics. This is the first report on isolation of these secondary metabolites from A. obesum.
Keywords
Adenium obesum
Apocynaceae
Flavonoids
Antimicrobial activity
Oman
1 Introduction
Medicinal plants are traditionally used along with the modern medicine in developed and developing countries in the world. They play an important role in promoting a primary health care system (World Health Organisation, 2001). About 60% of the medicines used for the treatment of chronic and incurable diseases were prepared from medicinal plants. The beneficial medicinal effects of plant materials typically result from the combinations of secondary products present in the plant. The medicinal actions of plants are unique to particular plant species or groups and are consistent with the concept that the combination of secondary products in a particular plant is taxonomically distinct (Abdulbasit, 2014). Plant based natural constituents can be derived from any part of the plant like bark, leaves, flowers, roots, fruits, seeds, etc (Gordon and David, 2001) i.e. any part of the plant may contain active components. Arid and semi-arid plants are good sources for the production of various types of secondary metabolites which make them resistant to various environmental stress e.g. scarcity of water, salinity, pathogens, etc. These compounds include alkaloids, flavonoids, steroids, phenolics, terpenes, and volatile oils. Man has been exploiting these natural plant products for use in medicines, cosmetics, dyes, flavors and foods.
The phytochemical flavonoids are a group of bioactive compound that are produced by the plants in high quantities (Hodek et al., 2002). Since ancient times, several flavonoid structures have been identified and characterized by scientists (Hakim et al., 2002; Dewick et al., 1992). Naturally occurring flavonoids are usually glycosides; however, their structures are more complex. Plants and animals’ growth and development depend on flavonoids. They can protect plants and animals against different microorganisms and pests serving as means of plant–animal warfare (Masry et al., 2002).
Adenium obesum is a wild flowering medicinal plant belonging to the family Apocynaceae found in Oman and other Gulf countries (Codd, 2011). A. obesum and other species belonging to this family produce a milky sap which is toxic and can cause skin irritation (Rowley, 1983). It grows up to four meters in height. This plant is pale grayish-green, gray, brown, smooth, with sticky, clear or white latex; branchlets glabrescent, pubescent at the apex. The leaves are arranged spirally, clustered at the end of branchlets (Dimmitt and Hanson, 1991; Watt and Breyer-brandwijk, 2013; Neuwinger, 2011).
A. Obesum is used as a medicinal plant in several countries all over the world as well as a poison on arrows (Dimmitt and Hanson, 1991). In Oman, this species is used for the treatment of different diseases especially venereal diseases. The extract from the root and bark is used to prepare a lotion for the treatment of different skin diseases and to kill lice (Neuwinger, 2011; Watt and Breyer-brandwijk, 2013). The latex is used as a medicine for the recovery of decaying teeth and septic wounds (Tijjani et al., 2011), and the Somalian people are using it as nasal drops (Tijjani et al., 2011). A. obesum bark is also used as an abortifacient (Naji et al., 2013; Wang et al., 2013). Therapeutically, the Nigerian people are using the whole plant for its antiplasmodial, anti-trypanosomal and anti-leishmanial activities (Malebo et al., 2009). Almost all groups of phytochemicals such as alkaloids, steroids, saponins, glycosides, anthraquinones, tannins and flavonoids are present in this plant (Tijjani et al., 2011; Codd, 2011; Malebo et al., 2009). This paper describes the isolation and antibacterial activities of 5,7,3′,4′-tetrahydroxyflavone (1) and 3,5,7,3′,4′,5′-hexahydroxyflavone (2, Fig. 1).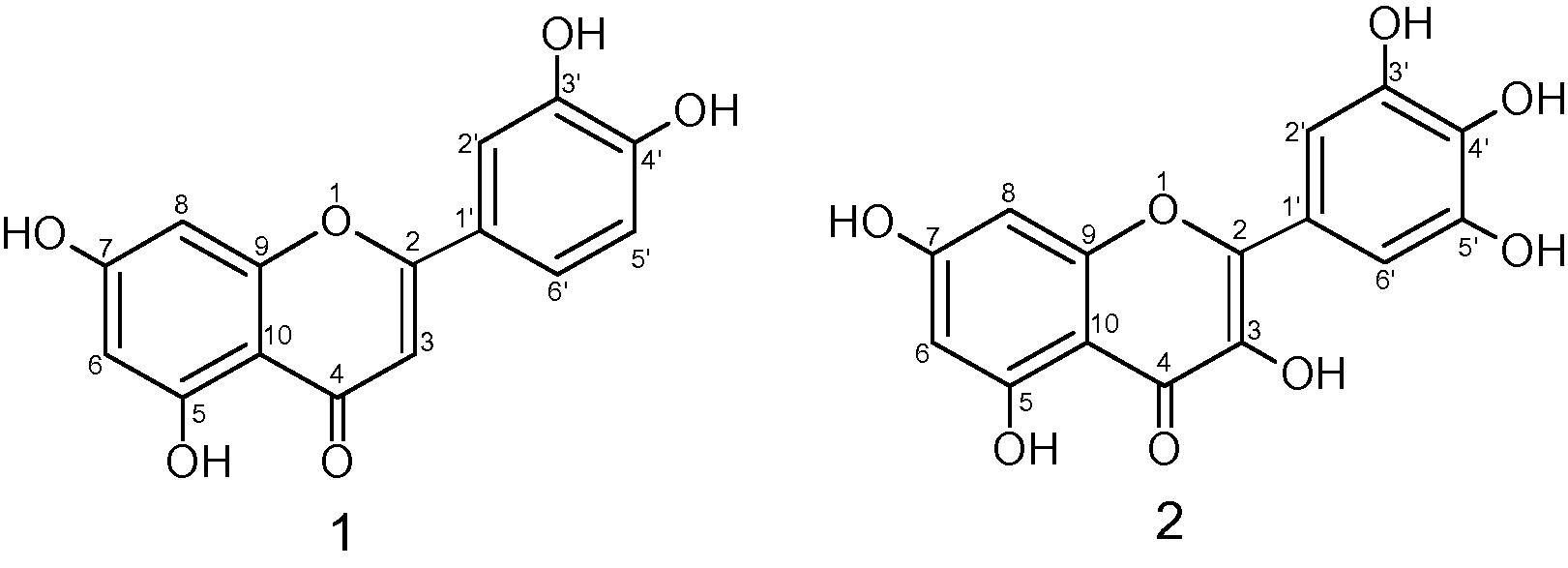
Structure of compound 1 and compound 2.
2 Experimental
2.1 General
All the solvents and reagents used in this study were of analytical grade and were used without further purification. The melting points were determined on an electrochemical micro-melting point apparatus (Gallenkamp) and were uncorrected. 1H NMR spectra were recorded on a Bruker (600 MHz) instrument in CD3OD (purity 99.99%) with TMS (99.999%) as an internal standard (chemical shifts in δ ppm). Mass spectra were recorded on Waters Quattro Premier XE Tandem Quadrupole system (Waters Inc. USA). Electron multiplier (ESI+) voltage was obtained from Autotune. All data were obtained by collecting the full-scan mass spectra within the scan range 50–500 amu. TLC was performed using silica gel GF254 (E. Merck, Germany). Dimethyl sulfoxide (DMSO, purity 99%) and amoxicillin antibiotic (purity 99.9%) were obtained from Sigma, St. Louis, USA. The remaining mentioned chemicals and solvents used in this experiment were obtained from Sigma–Aldrich Company, UK.
2.2 Microorganism
Bacterial strains, Staphylococcus aureus (S. aureus), Escherichia coli (E. coli), Pseudomonas aeruginosa (P. aeruginosa), and Proteus vulgaris (P. vulgaris) were obtained from Nizwa hospital, Nizwa, Sultanate of Oman. The collected organisms were subcultures in nutrient agar plates and kept at 4 °C until needed for use.
2.3 Plant materials
The stem samples were collected from Al Mughsayl, Salalah, Sultanate of Oman during the month of November 2013. The plant morphological identification was done by Engineer Mr. Ismail Al-Rashdi, Horticulture Senior Specialist, Ministry of Agriculture, Oman. The plant species were photographed for documentation and further taxonomic identification at the Natural Products Laboratory, School of Pharmacy, University of Nizwa, Sultanate of Oman. The collected samples were transported to the Natural Products Laboratory in the University of Nizwa for further processing.
2.4 Preparation of crude extract
The collected stems samples were washed thoroughly with water and cut into small pieces. The samples were dried under shade for five days and ground into a coarse powder by a mechanical mill. The shade dried, powdered samples (100 g) were extracted for 72 h with methanol (1 L) by a hot extraction method (Hossain et al., 2013). The methanol was evaporated from the methanol crude extract using a rotary evaporator at 22 °C under reduced pressure to get the crude extract (11.23 g). The methanol free crude extract (10 g) was suspended in water and fractionated successively with hexane, ethyl acetate, chloroform and butanol to give hexane (2.43 g, yield 24.3%), ethyl acetate (1.29 g, yield 12.9%), chloroform (2.96 g, yield 29.6), and butanol (0.876 g, yield 8.76%), respectively; the whole process being repeated twice. The crude extracts were combined and concentrated using a rotary evaporator.
2.5 Antimicrobial activity
The antimicrobial activity of pure isolated flavonoid compounds from the stem of A. obesum was estimated using the agar disk diffusion method against one Gram positive (S. aureus) and three Gram-negative bacteria (E. coli, P. aeruginosa and P. vulgaris) cultured at different concentration as described by Tahiya et al. (2014). Two concentrations, 100 and 200 μg/ml of each pure isolated compound were prepared with dimethyl sulfoxide (DMSO) in this experiment. DMSO was used as a negative control and 300 μg/ml of amoxicillin antibiotic (Sigma, St. Louis, USA) was used as a positive control for this assay. Sterile filter paper disks of 5 mm in diameter were impregnated with each pure compound of A. obesum and placed on the inoculated agar. The plates were incubated micro aerobically at 37 °C for 24 h. The antibacterial activity was measured by the diameter of the zone of inhibition against the tested bacteria. Each method in this experiment was replicated three times.
2.6 Isolation of pure compounds
Gravitational elution of ethyl acetate extract in silica gel column with petroleum ether in increasing amounts of ethyl acetate gave six fractions; fractions 2 and were 4 obtained by eluting the column with 20% and 90% ethyl acetate/petroleum ether respectively, giving yellow gums each containing one major compound. Repeated column chromatography on these gums produced impure compounds 1 and 2 respectively (Fig. 1).
2.7 Compound 1 (5,7,3′,4′-tetrahydroxyflavone)
Obtained from column was further purified by preparative TLC over silica gel GF254 using methanol-hexane (95:5) as a developing solvent. It was recrystallized from hexane to give an yellow crystals (9.5 mg); m.p. 319 °C (Fairouz et al., 2010; m.p. 321 °C); (M+, 286.93); 1H NMR (δ ppm, CD3OD): 6.19 (d, 1H, J = 2.1 Hz, H-6), 6.42 (d, 1H, J = 2.1 Hz, H-8), 6.53 (s, 1H, H-3), 6.88 (d, 1H, 8.5 Hz, H-5′), 7.37 (dd, 1H, J = 8.5 and 2.2 Hz, H-6′), 7.38 (d, 1H, J = 2.2 Hz, H-2′). 13C NMR (δ ppm, CD3OD): 95.01 (C-8), 100.13 (C-6), 103.85 (C-3), 105.287 (C-10), 114.14 (C-2′), 116.77 (C-5′), 120.29 (C-6′), 123.68 (C-1′), 147.06 (C-3′), 151.01 (C-4′), 159.43 (C-9), 163.22 (C-5), 166.12 (C-2), 166.36 (C-7), 183.68 (C-4).
2.8 Compound 2 (3,5,7,3′,4′,5′-hexahydroxyflavone)
It was further purified by preparative TLC over silica gel GF254 using methanol-hexane (95:5) as a developing solvent. It was recrystallized from petroleum ether to give an yellow crystals (11.9 mg); m.p. 299 °C (Koo Hui et al., 2001; m.p. 301 °C); (M+, 318.91); 1H NMR (δ ppm, CD3OD): 4.56 (s, 1H, –OH-3), 6.17 (d, 1H, J = 2.1 Hz, H-6), 6.36 (d, 1H, J = 2.04 Hz, H-8), 7.33 (s, 2H, H-2′ and H-6′); 13C NMR (δ ppm, CD3OD): 92.94 (C-8), 97.78 (C-6), 103.06 (C-10), 107.07 (C-2′ and C-6′), 121.65 (C-1′), 135.51 (C-4′), 135.94 (C-3), 145.30 (C-3′ and C-5′), 146.58 (C-2), 156.77 (C-9), 161.08 (C-5), 164.16 (C-7), 175.87 (C-4).
3 Results
The dry powdered stem samples were extracted with methanol. The methanol-free crude extract was suspended in water and successively extracted with solvents of different polarities in increasing polarities to give hexane, chloroform, ethyl acetate, butanol and remaining water crude extract respectively.
3.1 Compound 1 (5,7,3′,4′-tetrahydroxyflavone)
The pure compound 1 was obtained as yellow crystals. It had a melting point of 319 °C (Lit. m.p 321 °C Fairouz et al., 2010). High-resolution mass spectroscopy of compound (1) indicated the molecular formula C15H10O6 (M+, 286.93). Compound 1 was identified on the basis of 1D and 2D NMR (Figs. 2 and 3).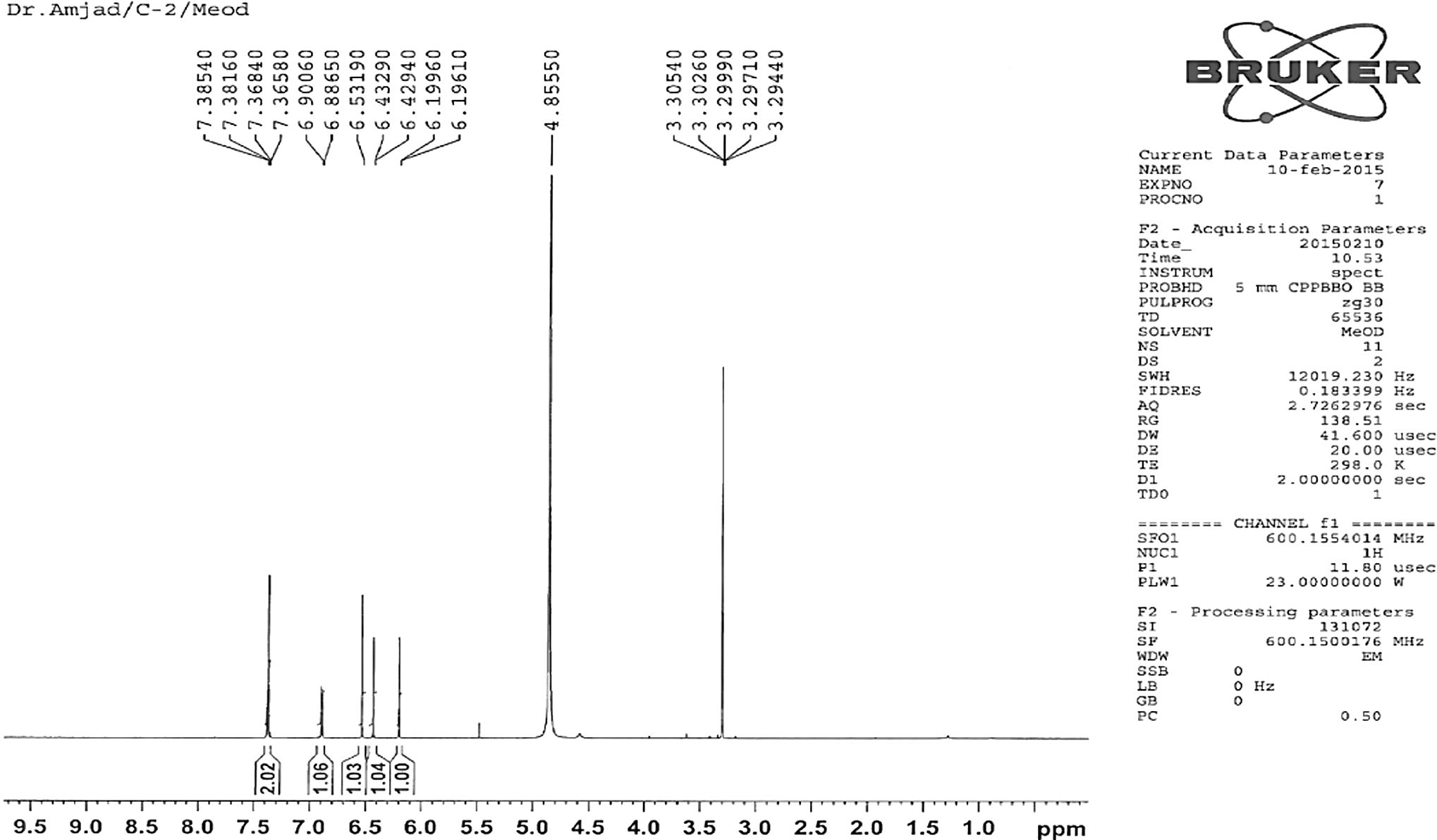
1H NMR spectra of compound 1.
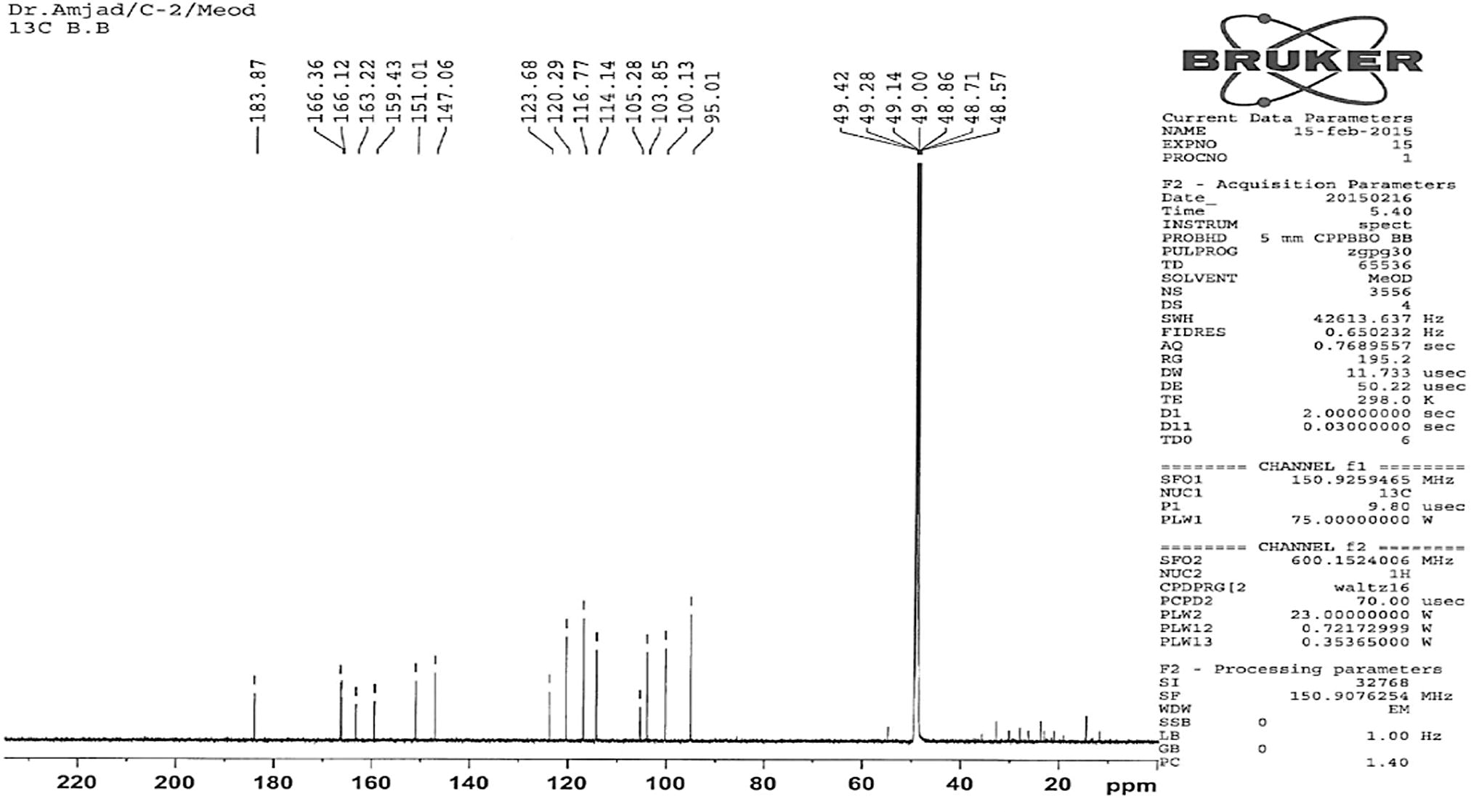
13C NMR spectra of compound 1.
3.2 Compound 2 (3,5,7,3′,4′,5′-hexahydroxyflavone)
Compound 2 was also obtained as light yellow crystals. It had a melting point 299 °C (Lit., m.p. 301 °C Koo Hui et al., 2001). The peak at 318.91 corresponds to the M+ ion of the isolated compound 2; the compound gave a fragmentation peaks at [M+H]+ = 319.91 or M+ = 318.91, [M+H-H2O] = 300.96, [M+H-H2O-CO]+ = 272.90, [M+H-CO]+ = 290.90, [M+H-2CO]+ = 263. These correspond to the molecular formula C15H10O8. Compound 2 was identified on the basis of 1D and 2D NMR (Figs. 4 and 5).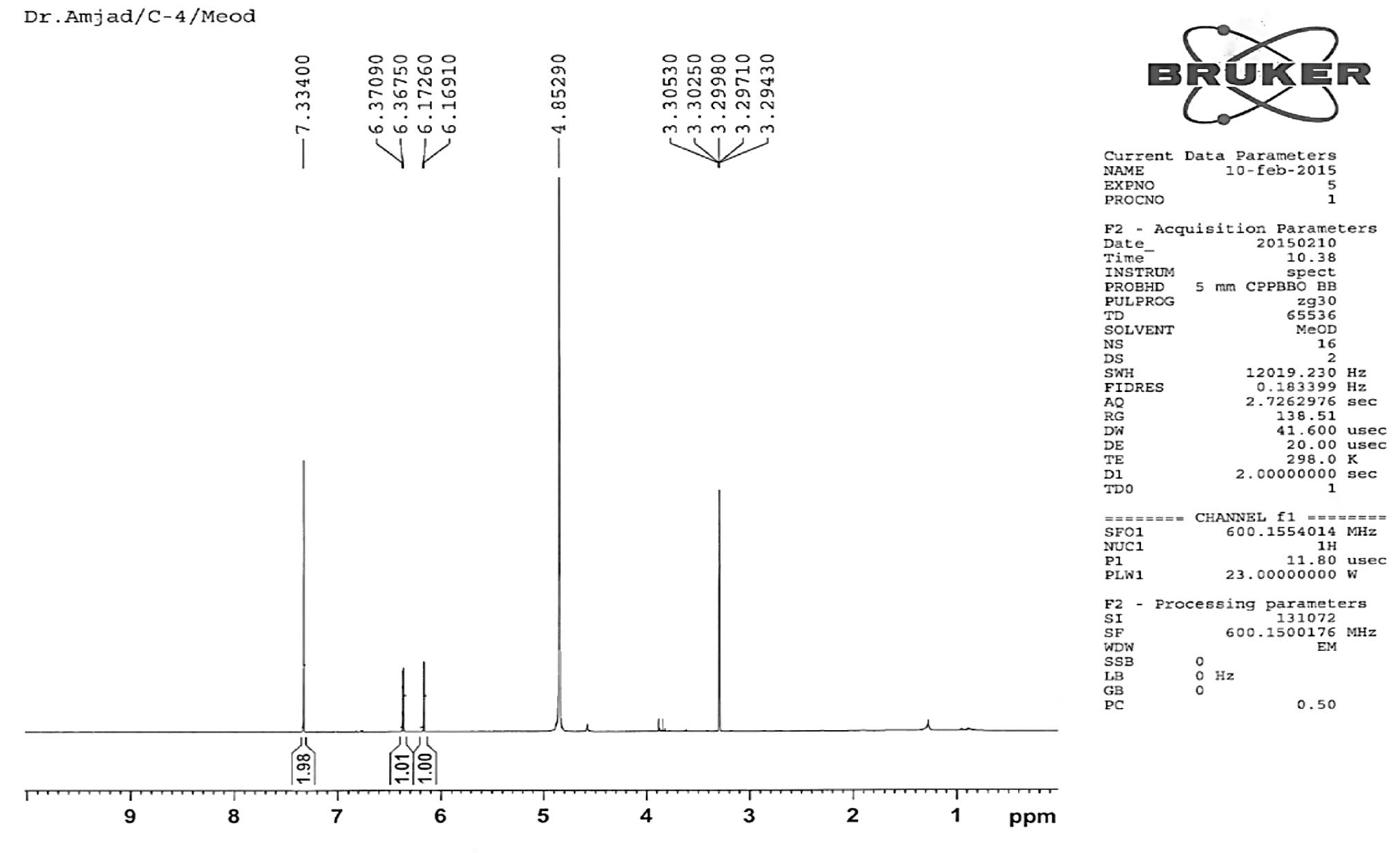
1H NMR spectra of compound 2.
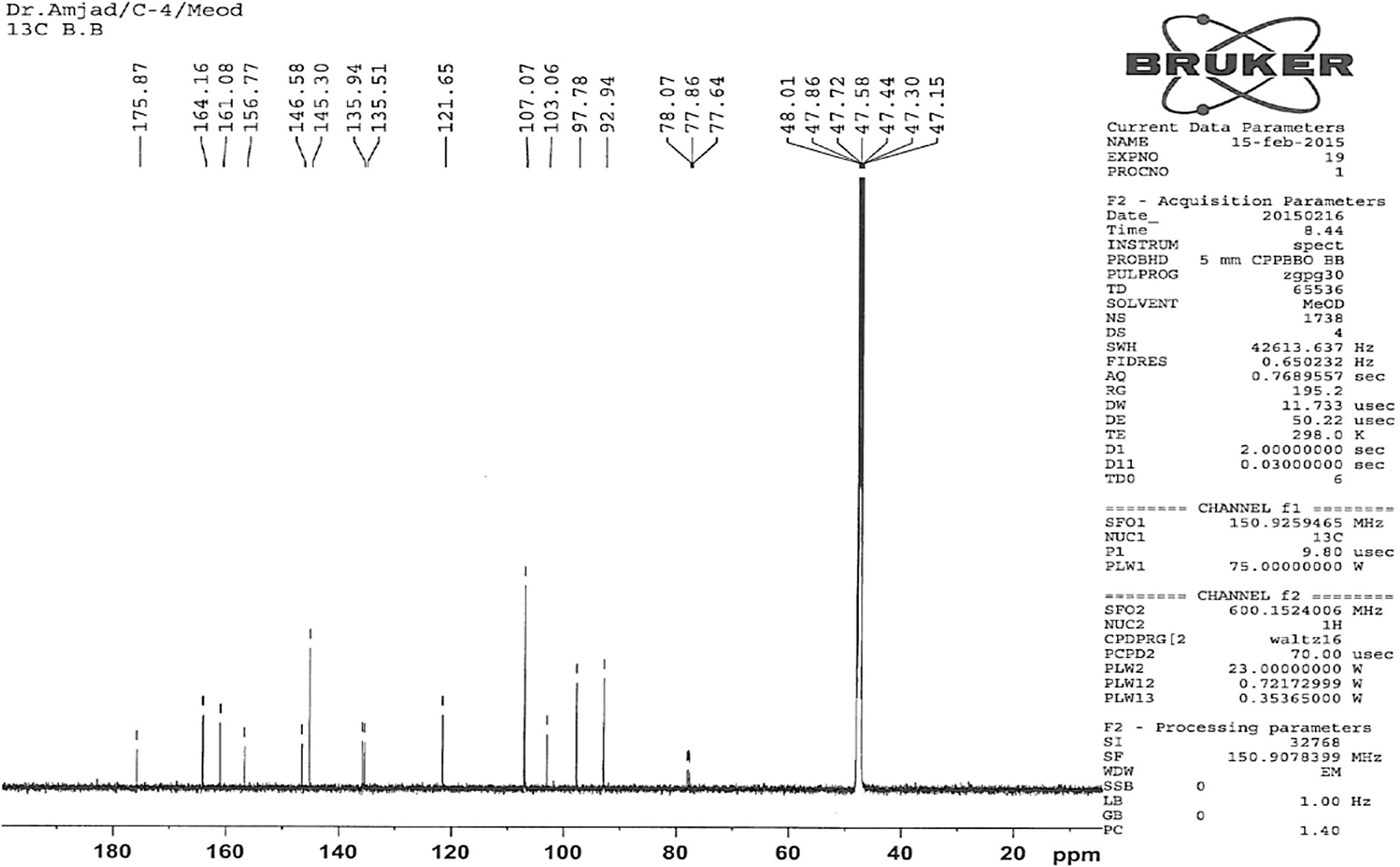
13C NMR spectra of compound 2.
3.3 Antibacterial activity
The results of antimicrobial activity of the isolated pure flavonoid compounds 1 and 2 against S. aureus, E. coli, P. aeruginosa and P. vulgaris are presented in Table 1. All tested bacteria were susceptible at all concentrations of the two pure flavonoid compounds. Inhibition zones were in the range 9–4 mm. The maximum inhibition was shown by compound 2 against P. vulgaris (IZ = 14 mm) in comparison with the standard amoxicillin.
Pure Compounds
Conc (μg/ml)
E. coli
P. aeruginosa
S. aureus
P. vulgaris
Compound 1
100
11 ± 0.12
10 ± 0.09
13 ± 0.13
13 ± 0.17
50
9 ± 0.42
9 ± 0.19
11 ± 0.22
12 ± 0.65
Standard
28 ± 0.09
21 ± 0.24
25 ± 0.15
29 ± 0.32
Compound 2
100
13 ± 0.33
12 ± 0.29
11 ± 0.54
14 ± 0.12
50
9 ± 0.15
9 ± 0.42
12 ± 0.16
12 ± 0.14
Standard
27 ± 0.67
31 ± 0.55
26 ± 0.17
28 ± 0.13
4 Discussion
The dry powdered stem samples were extracted with methanol. The methanol-free crude extract was suspended in water and successively extracted with solvents of different polarities in increasing polarities to give hexane, chloroform, ethyl acetate, butanol and water crude extracts, respectively. So far, 50 chemical constituents were isolated from different parts of this plant. Its crude extracts and isolated compounds have been reported to have diverse biological features, such as antitumor, antimicrobial, anti-influenza, molluscicidal, locusticidal, and antiviral activities, in addition to potential immunomodulatory and cardiotonic activities (Tijjani et al., 2011; Codd, 2011; Malebo et al., 2009). It was noted that much of the chemical and biological investigations on this plant had focused on its moderately polar constituents (Codd, 2011). The nonpolar petroleum ether and highly polar aqueous fractions of the crude extracts received little or no attention, but many of the highly nonpolar and polar constituents are still undiscovered. Phytochemical investigation on this plant revealed that it is rich in cardiac glycosides which have cytotoxic and antitumor activities; however, no systematic study was attempted regarding the cardiovascular properties of the extracts, fractions, and pure compounds (Tijjani and Sallau, 2011; Tijjani et al., 2011; Codd, 2011; Malebo et al., 2009). This report shows that various parts of AO possess promising biological activities while no in-depth phytochemical and pharmacological studies have been carried out. Still, no work has been carried out on the antimicrobial compounds of any part of this plant. Therefore, the aim of this study was to isolate and evaluate its chemical constituents systematically, employing bioassay-guided fractionation procedures for the isolation of active principles, and to understand mechanisms of active components.
4.1 Compound 1 (5,7,3′,4′-tetrahydroxyflavone)
The active ethyl acetate crude extract was used for the separation of pure compounds using different chromatographic techniques with petroleum ether (40–60 °C) followed by a mixture of petroleum ether in an increasing amount of ethyl acetate, and finally with methanol. Yellow crystals were precipitated out at the bottom of the test tube. The isolated major pure compound was further purified by column chromatography over silica gel using methanol-hexane (95:5) as a developing solvent. TLC examination of the isolated pure flavonoid compound from the ethyl acetate crude extract of A. obesum showed a single bright orange spot upon exposure to iodine chamber. A single bright yellow spot was also observed on the TLC plate when the vanilla-sulfuric acid reagents were sprayed followed by heating at 110 °C for about 10 min. The pure compound 1 (Fig. 1) was obtained as yellow crystals. It had a melting point 319 °C (Lit. m.p. 321 °C; Fairouz et al., 2010). High-resolution mass spectroscopy of compound (1) indicated the molecular formula C15H10O6 (M+, 286.93). In the 1H NMR spectrum, one singlet at δ 6.53 indicated the presence of a H-3 proton. Three doublets at δ 6.19, 6.42 and 6.88 indicated the presence of H-6, H-8, and H-5′. Another doublet of doublet δ 7.36 indicated the presence of two protons at H-2′ and H-6′ on the B ring (Fig. 2). A 1H NMR signal at δ 6.53 correlated with carbon signals resonating at δ 105.28 (C-10), 123.68 (C-1′) and 183.68 (C-4) in the HMBC spectrum, and it was hence assigned to H-3 (Figs. 3 and 6). Another connectivity was deduced on the basis of 1D and 2D NMR as indicated in Fig. 6. The 13C NMR spectra showed the presence of twelve aromatic carbons; eight quaternary carbons, six methine carbons, and an unsaturated carbonyl carbon (Fig. 3). The 1H and 13C NMR values for all the carbons were assigned on the basis of HSQC and HMBC correlations (Fig. 6). The structure was further supported by the COSY and HMBC correlations as shown in Fig. 6. 5,7,3′,4′-Tetrahydroxyflavone (Fig. 1) was isolated from this plant for the first time in our laboratory.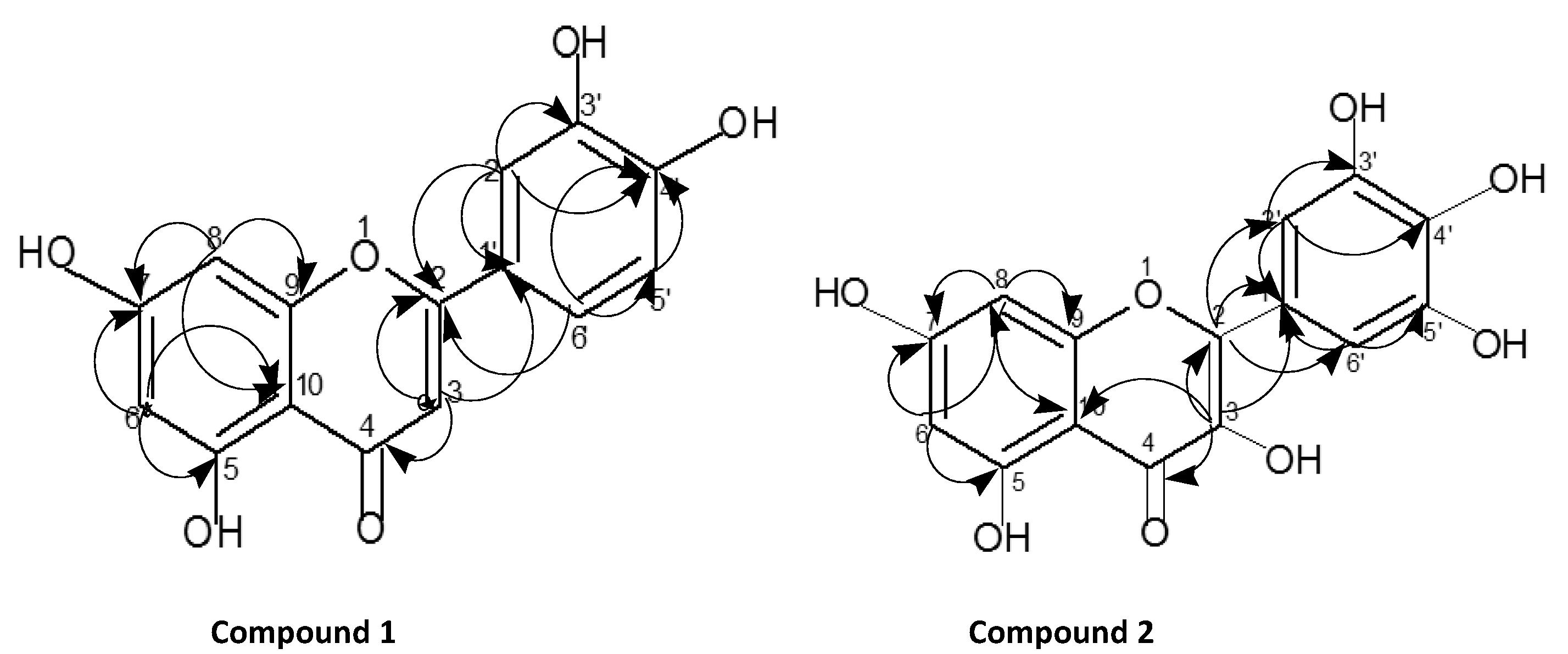
COSY and HMBC correlation of compound 1 and compound 2.
4.2 Compound 2 (3,5,7,3′,4′,5′-hexahydroxyflavone)
Compound 2 (Fig. 1) was also obtained as light yellow crystals. It had a melting point of 299 °C (Lit. m.p. 301 °C; Koo Hui et al., 2001). The peak at 318.91 corresponds to the M+ ion of the isolated compound 2; the compound gave fragmentation peaks at [M+H]+ = 319.91 or M+ = 318.91, [M+H-H2O] = 300.96, [M+H-H2O-CO]+ = 272.90, [M+H-CO]+ = 290.90, [M+H-2CO]+ = 263. This corresponds to the molecular formula C15H10O8. The presence of M-152.92 base peak (Mabry et al., 1997; Le et al., 2011; Shweta and Padma, 2012; Markhman, 1992; Harborne, 1986) in compound 2 in high abundance in the MS indicated the presence of a flavor skeleton in compound 2. Its structure was assigned, based on its 1D and 2D NMR spectral data as depicted in Fig. 1. It was completely soluble in chloroform, methanol, and other organic solvents. The 1H NMR spectrum of the isolated pure compound 2 showed two sharp singlets at δ 4.56 and 7.33, indicating the presence of 3-OH and H-2′ and H-6′ protons. Two doublets at δ 6.17 and 6.36 indicated the presence of H-8 and H-6 protons, confirming that compound 2 is a substituted flavone (Fig. 7). The 1H and 13C NMR values for all the protons and carbons for the compound 2 were assigned on the basis of COSY, HMQC, and HMBC correlations and are presented in Fig. 4 and Fig. 5. The key HMBC correlations confirmed the placement of all the six hydroxyl groups at 3, 5, 7, 3′, 4′, 5′ positions as shown in Figs. 1 and 6. On the basis of above 1D and 2D NMR spectroscopic data, the structure of compound 2 was determined as 3,5,7,3′,4′,5′-hexahydroxyflavone (2, Fig. 1). To the best of our knowledge, this flavonoid compound (2) has not been previously isolated or reported from the selected plant.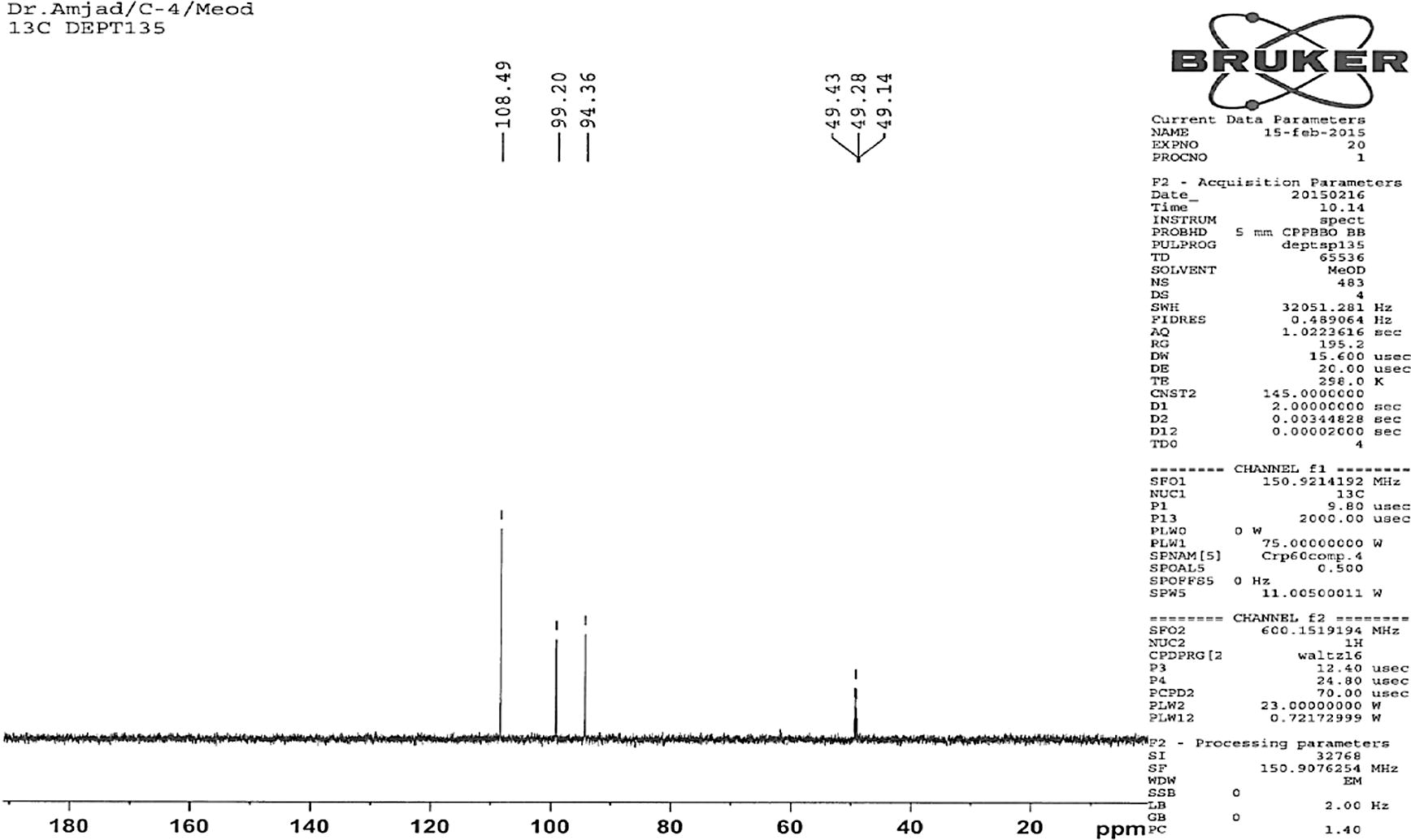
DEPT 135 spectra of compound 2.
4.3 Antibacterial activity
The maximum inhibition was shown by compound 2 at a concentration of 200 μg/ml against P. vulgaris in comparison with the standard amoxicillin (Table 1). The results of antimicrobial activity of the isolated pure compounds 1 and 2 against S. aureus, E. coli, P. aeruginosa and P. vulgaris are presented in Table 1. All tested bacteria were susceptible at all the concentrations of the two compounds. The inhibition zones were in the range of 9–14 mm. The maximum inhibition was shown by compound 2 against P. vulgaris (IZ = 14 mm) in comparison with the standard amoxicillin.
5 Conclusion
We herewith report the isolation and characterization of two antibacterial flavonoids of the ethyl acetate extract of the stems of A. obesum, whose structure has been characterized as 5,7,3′,4′-tetrahydroxyflavone (1) and 3,5,7,3′,4′,5′-hexahydroxyflavone (2) on the basis of extensive 1D (1H and 13C) and 2D (COSY, HMQC and HMBC) NMR as well as high resolution mass spectral (HRMS) data. This is the first report on isolation of flavonoid compounds from A. obesum collected in Oman. The presence of antibacterial metabolites might justify the present ethno-medical practices on this species. However, further biomedical studies are required to standardize its use in herbal remedies.
Acknowledgements
The authors wish to thank the University of Nizwa, Sultanate of Oman for providing financial support (Grant Ref. No. A/13-14-UoN/04/CPN/IF) for the completion of the present study. Thanks to Sommya Saif Said Al Riyani, Lab Technicians, Natural Product Lab, University of Nizwa for her continuous help during the experiment. Authors are thankful to Dr. Ahmed Ibrahim Yagi, Associate Professor, School of Arts and Science, University of Nizwa, Oman for editing the manuscript. We are also thankful to Dr. Ali Elyassi DARIS Centre for Scientific Research and Technology Development, University of Nizwa for MS spectra.
References
- Total phenolic, total flavonoid contents and radical scavenging activities of 10 Arabian herbs and spices. Unique J. Pharm. Biol. Sci.. 2014;2(3):5-11.
- [Google Scholar]
- Adenium obesum. The flowering plants of Africa. South African National Biodiversity Institute (SANBI) Pretoria South African. 2011;49:1953-1960.
- [Google Scholar]
- The Flavonoids. Advances in Research. London (fifth ed.). USA: Chapman and Hall; 1992.
- The genus Adenium in cultivation. Diplorhynchus Welw Fic & Hiern (Apocynaceae), Mededelingen. Cactus Success J.. 1991;63:223-225.
- [Google Scholar]
- A new prenylated flavone with cytotoxic activity from Artocarpus lanceifolius. Fitoterapia. 2002;73:668-672.
- [Google Scholar]
- Nature, distribution and function of plant flavonoids, in plant flavonoids in biology and medicine. In: Cody V., Middleton E., Harborne J.B., Alan R., eds. Biochemical, Pharmacological and Structure-Activity Relationships. New York: Liss Inc; 1986. p. :15-24.
- [Google Scholar]
- Flavonoids-potent and versatile biologically active compounds interacting with cytochromes P450. Chem. Biol. Int.. 2002;139:1-12.
- [Google Scholar]
- Effect of temperature and extraction process on antioxidant activity of various leaves crude extracts of Thymus vulgaris. J. Coastal Life Med.. 2013;1(2):118-122.
- [Google Scholar]
- Flavonoids (myricetin, quercetin, luteolin and apigenin) content of edible tropical plants. J. Agric. Food Chem.. 2001;49:3106-3112.
- [Google Scholar]
- Apigenin isolated from the medicinal plant Elsholtzia rugulosa prevents β-amyloid 25–35-induces toxicity in rat cerebral microvascular endothelial cells. Molecules. 2011;16:4005-4019.
- [Google Scholar]
- The Systematic Identification of Flavonoids. Berlin, Germany: Springer; 1997.
- Antiplasmodial, anti-trypanosomal, anti-leishmanial and cytotoxicity activity of selected Tanzanian medicinal plants. Tanzanian J. Health Res.. 2009;11:226-234.
- [Google Scholar]
- In Techniques of Flavonoid Identification. New York, USA: Academic Press; 1992. p. :39-88.
- Prenylated flavonoids of Erythrina lysistemon grown in Egypt. Phytochemistry. 2002;60:783-792.
- [Google Scholar]
- Free radical scavenging activity and anthocyanin in flower of Adenium obesum collected from Yemen. J. Pharm. Physiother.. 2013;1:5-7.
- [Google Scholar]
- African Traditional Medicine: A Dictionary of Plant Use and Applications. Stuttgart, Germany: Medicinal Pharmaceutical Sciences; 2011. p. :589-594.
- The Adenium and Pachypodium Handbook. Brackley, United Kingdom: Smart & Co Ltd; 1983. p. :95-105.
- Production, isolation and identification of flavonoids from aerial parts of Hiptage benghalensis. Int. J. Life Sci. Pharm. Res.. 2012;2(3):1-5.
- [Google Scholar]
- Comparative study of phytochemical screening, antioxidant and antimicrobial capacities of fresh and dry leaves crude plant extracts of Datura metel L. J. King Saud Univ. Sci.. 2014;26:237-243.
- [Google Scholar]
- Synergistic activity of methanolic extract of Adenium obesum (Apocynaceae) stem-bark and oxytetracycline against some clinical bacterial isolates. Bayero J. Pure Appl. Sci.. 2011;4(1):79-82.
- [Google Scholar]
- Studies on antibacterial activity of Adenium obesum (Apocynaceae) stem-bark. Cont. J. Microbiol.. 2011;5(1):12-17.
- [Google Scholar]
- Antioxidant and antiinflammatory activities of anthocyanins and their aglycon, cyanidin, from tart cherries. J. Nat. Prod.. 2013;62:294-296.
- [Google Scholar]
- The Medicinal and Poisonous Plants of Southern and Eastern Africa (Second ed.). Edinburgh and London: E & S Livingstone Ltd; 2013. p. :941-946.
- Geneva Legal Status of Traditional Medicine and Complementary/Alternative Medicine. A Worldwide Review; 2001. p. :129-134.







