Translate this page into:
Microbial evaluation and occurrence of antidrug multi-resistant organisms among the indigenous Clarias species in River Oluwa, Nigeria
⁎Corresponding author. Tel.: +234 7032511675. dahunsi.olatunde@lmu.edu.ng (S.O. Dahunsi)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Fish may harbor pathogens on or inside its body when in contaminated environment. Clarias gariepinus and Clarias buthopogon were analyzed to evaluate the likely impact of pollution on the antidrug resistance pattern of their microbial isolates. Different bacterial and fungal counts were observed on the fish organs (skin, muscles and gills). The highest bacterial count was 1,040,000 Cfu/mL while the lowest was 101 Cfu/mL. The highest fungal count obtained was 344,000 Cfu/mL while the lowest was 65 Cfu/mL. Bacterial isolates belonging to genera Bacillus, Clostridium, Alcaligenes, Flavobacterium, Enterobacter and Corynebacterium were obtained from the organs. Also, fungal isolates belonging to the genera Penicillium, Aspergillus, Rhizopus, Monila and Fusarium were isolated. The resistance of isolates from C. gariepinus to drugs was between 50% and 90% with Bacillus species showing the highest resistance. For isolates from C. buthopogon, 40–90% resistance was observed with Alcaligenes faecalis showing highest resistance. Five patterns of multiple drug resistance were observed among the bacterial isolates with antibiotics ranging from 4 to 9. Also, result of fungal isolates showed susceptibility to ketoconazole and resistant to fluconazole and griseofulvin. The public health implications of consuming these fishes are discussed.
Keywords
Bacteria
Bitumen
Contamination
Drugs
Microbial evaluation
Pollution
1 Introduction
The aquatic ecosystem is usually a reservoir of toxic substances which may be introduced deliberately or accidentally. When such pollutants are present, they impair the quality of water and make it unsuitable for aquatic life (Ayandiran and Dahunsi, 2016; Dahunsi et al., 2011). In Nigeria, pollution of the surface waters by oil and solid wastes is widespread over the last decades and is rendering most of them unsuitable for human use (Bakare et al., 2003). Such toxic substances discharged into water bodies are not only accumulated through the food chain (Dahunsi et al., 2012a), but may also either limit the number of species or lead to high microbial load being formed (Okafor, 1985). It is known that contamination of a particular water body can always be linked to industrial processes or sewage/effluent disposal as reported (Sathware et al., 2007).
In the past, it was thought that fish in open waters (marine and fresh) were generally safe due to lack or paucity of epidemiological evidence of fish-borne diseases. However, recent reports from studies in the areas of environmental pollution, fish and water preservation/management suggest otherwise (Obasohan et al., 2010). Fish are usually found at the top of the food chain and as such can accumulate large quantities of pollutants in their muscle and organs (Dahunsi et al., 2012b). Pathogenic microbes are known to cause many diseases in both wild and cultured fish and they may range from primary to opportunistic pathogens (Inglis et al., 1994). Fish may also harbor pathogens on or inside its body after exposure to contaminated water or food but the study of diseases of fish is hindered by the lack of knowledge of numerous interactions existing between pathogens and their fish hosts (Nyaku et al., 2007). Several researchers have reported the isolation of bacteria belonging to different genera from fish in different polluted water bodies (Adewoye and Lateef, 2004; Al-Harbi, 2003; Hamed et al., 2013; Kolawole et al., 2011).
Bacterial resistance in the environment is a growing phenomenon among environmentalists and several reports have failed to establish a reliable relationship between anthropogenic activities and antibiotic resistance in bacteria as many believe that resistance elements are naturally innate in the microbial genome (Baltz, 2008; Brown and Balkwill, 2009; Bhullar et al., 2012; Cox and Wright, 2013; D’Costa et al., 2011; Thaller et al., 2010; Toth et al., 2010; Wright, 2007, 2010). However, other reports have documented increased antibiotic resistance in bacteria and also established tangible relationship between the transfer of resistance elements and anthropogenic activities (Ayandiran et al., 2014; Bhullar et al., 2012; Knapp et al., 2010). To this effect, bacterial resistance to antibiotics has been considered a global public health menace. Besides the human health risks posed by the presence of antibiotic resistant organisms (bacteria and fungi) in the environment, and the unwanted presence of antibiotics in water bodies, concern for the ecological fate and environmental risk of antibiotics in the aquatic environment is on the increase Kümmerer, 2009.
Ondo State constitutes an economically significant part of South-Western Nigeria and has one of the largest fresh and coastal water areas in the country (Ayandiran and Dahunsi, 2016). It is located in the coordinates 6°35′19N, 4°50′3E and altitude 61 m. This is where bitumen was first spotted in Nigeria in 1910 and two bitumen observatory wells were dug in the State in the 60 s during the early explorative activity of Nigerian natural bitumen. The seepage of the bitumen material exists especially during the dry season when temperature is above 37 °C during which it occurs as a free flowing liquid. Oluwa is a major river of economic, agricultural and environmental significance flowing through many communities within the State. The major pollutant of the river is bitumen runoff (Olajire et al., 2007) besides other domestic and agricultural activities carried out along it course and from its many tributaries thereby contributing to its pollution.
Clarias species constitutes the major fauna population of River Oluwa (Ayandiran and Dahunsi, 2016), and is usually found in abundance especially during the rainy season. It is also a major source of livelihood for the populace who are majorly farmers and fishermen. Prior to this research, the microbiological quality and antibiotic resistance pattern of the microbial species in the fish from this river is yet to be established and so, their public health impact is not ascertained. The present work is therefore necessary to bridge the lack of understanding about the relationship between anthropogenic activities such as the presence of bitumen seepage and other domestic and agricultural pollutants and emergence of antibiotic resistance pattern in different bacteria inhabiting the fish species in the Oluwa River.
2 Materials and methods
2.1 Sample collection
Adult sizes between 4 and 5 month old, (weight 0.6 ± 0.2 kg; length 24 ± 2 cm) of Clarias gariepinus and Clarias buthopogon were collected twice during the same rainy season (early and peak) of a year from the different polluted portions (up and down streams) of River Oluwa while control samples were collected from the unpolluted portion of the river and this was done from year 2011 to 2013. In all, one hundred and eighty (180) fish samples (60 each from upstream, downstream and control sites) were collected for each of C. gariepinus and C. buthopogon. Fishing was done during late night with the help of professional local fishermen. Gill nets about 12.192 m long and 1.828 m wide with a cork line at the top rope and metal line with the ground rope made locally of nylon were used for fishing. Two fishermen with the help of a boat helped in the collection of fish samples into sterilized plastic bucket and were aseptically transported to the laboratory.
2.2 Sample preparation
After arrival at the laboratory, fish samples were dissected and the various organs separated. Twenty (20) gram of each part was homogenized separately in 250 mL of 0.1% (w/v) peptone water before serial dilution using the method of Odoli (2006). Sterilized mortar and pestle and other glass wares were used for the homogenization.
2.3 Isolation, characterization and identification of bacterial isolates
Microbial isolation was carried out on the samples (skin, muscles and gills) via serial dilution and pour plate method already described (Adewoye and Lateef, 2004) using nutrient agar, MacConkey agar, Mannitol salt agar and Potato Dextrose agar (Oxoid). One milliliter from each prepared fish organ was serially diluted, and then plated in duplicate on nutrient agar, MacConkey agar, Mannitol salt agar and Salmonella–Shigella agar. All plates for aerobic organisms were incubated appropriately at 37 °C for 24–48 h. For the isolation of Clostridium spp., samples were first cultured on Reinforced Clostridia Medium (RCM) and then sub-cultured on blood agar incubated in an anaerobic jar (Oxoid) containing a moistened pack of gas generating kit (Bio-oxoid) at 37 °C for 7 days. Colonies which developed on the plates were counted and recorded using colony forming unit per gram (Cfu/g) of the sample (Ayandiran et al., 2014; Guo et al., 2013; Hussain et al., 2013; Lateef et al., 2005). Sub-culturing was carried out on distinct colonies until pure cultures were obtained and were transferred onto slant bottles containing freshly prepared media. The presumptive colonies were confirmed by standard morphological and biochemical techniques and using respective rapid API kits (20 NE 20050, 20 E 20160, Coryne 20900, 20 A 20300, and LyfoCults VT2 GP Comprehensive QC Set 410584 BioMerieux) (Odjadjare et al., 2012).
2.4 Determination of antibiotic sensitivity
In order to evaluate the bacterial resistance to the 17 clinically relevant antibiotics used in this study, disk diffusion assay as previously described (Kovacevic et al., 2012) was employed. Bacterial cultures were grown at 35 °C for 18 ± 2 h in tryptic soy broth (TSB; Difco), diluted to 1 × 107 Cfu/mL in tempered 0.75% agar (45 °C; Difco), mixed gently, and poured onto Mueller-Hinton agar (MHA; Difco). After solidification, antimicrobial susceptibility test disks (BBL™ Sensi-Disc™, BD Diagnostics, Sparks, MD, USA) were applied and plates were incubated at 35 °C for 24 h after which the zones of inhibition around each antibiotic was observed and read. The added antibiotic concentrations for antibiotic resistant bacteria (ARB) in polluted water have previously been defined as the maximum value of the minimum inhibitory concentrations (MICs) for Enterobacter, Enterococcus and Staphylococcus spp. resistant to that antibiotic (Huang et al., 2011), since these three are the most common species related to human health in polluted water. The antibiotics used are from ten (10) different drug families and are shown as follows with their respective dosages according to manufacturer’s description: Macrolides (Erythromycin, Ery, 15 μg); Quinolones (Ciprofloxacin, Cip, 5 μg; Norfloxacin, Nox, 30 μg; Ofloxacin, Ofl, 30 μg); Sulfonamides (Cotrimoxazole, Cot, 10 μg); Aminoglycosides (Gentamicin, Gen, 10 μg; Kanamycin, Kan, 10 μg); Cephalosporins (Ceftriaxone, Cef, 5 μg; Cephalexin, Cep, 10 μg; Cefuroxime, Cef, 10 μg); Chloramphenicol (Chloramphenicol, Chl, 30 μg); Penicillins/Aminopenicillins (Ampicillin, Amp, 25 μg; Amoxicillin, Amx, 25 μg; Augmentin, Aug, 30 μg); Nitrofurans (Nitrofurantoin, Nit, 25 μg); Tetracyclines (Tetracycline, Tet, 30 μg) and Lincosamides (Clindamycin, Cli, 10 μg). All antibiotic disks used were supplied by Oxoid Ltd. (Basingstoke, Hampshire, England). The Multi-drug Antibiotics Resistance (MAR) index was calculated using the method earlier described (Blasco et al., 2008; Odjadjare et al., 2012) as follows: where
a = No. of antibiotics to which the isolate was resistant;
b = total No. of antibiotics against which individual isolate was tested.
2.5 Fungal susceptibility testing
Three antifungal drugs (fluconazole, ketoconazole and griseofulvin) were used in this study. They were obtained from different manufacturers as follows: fluconazole from Hydex Chemicals Pvt. Ltd., ketoconazole from Jansen Pharmaceuticals, and griseofulvin from Glaxo Laboratories. All drugs were prepared according to the standard protocol of CLSI by dissolving in 100% dimethyl sulfoxide (Gibco) and were prepared in stock solutions of 1000 μg/ml and fluconazole in sterile distilled water. They were then kept till usage when they were freshly prepared as stock solution and serial two fold dilutions were carried out. The final concentrations of 100 and 200 μmg/mL were used for fluconazole and ketoconazole while it was 250, 500 and 1000 μmg/mL for griseofulvin.
2.6 Fungal identification and inoculum preparation
Antifungal evaluation was carried out using a broth microdilution method (Rambali et al., 2001; Indira, 2014) and based on CLSI M38-A standard recommendation (CLSI, 2002). Species identification for the isolated fungi was based on morphological and biochemical characteristics (Chander, 2002). Fungal stock inocula were prepared from 7 to 14 day cultures grown on Sabouraud’s dextrose agar (SDA) plus chloramphenicol to prevent bacterial growth. After sufficient growth appearance, the fungal colonies were covered with 5 mL of sterile saline (0.9%), after which the surface was gently probed with the tip of a sterile Pasteur pipette in order to make suspensions. The resulting suspended mixture was withdrawn and transferred to a sterile tube where the heavy particles were allowed to settle for 15 min at room temperature and the upper homogenous suspension was then used for further testing.
2.7 Turbidity standard for inoculum preparation
A BaSO4 turbidity standard which is the equivalent of a 0.5 McFarland standard or its optical equivalent is usually used to standardize the inoculum density for a susceptibility test. The inoculum’s size in this study was adjusted by microscopic enumeration to be within the range of 1,000,000 and 5,000,000 spores/mL with the aid of a cell counting hemocytometer (Neubauer chamber). The fungi did not produce the needed conidia in some cases and for such, small portion of the mycelial was harvested and gently homogenized in 2 mL of sterile saline using a tissue grinder and the resulting suspensions were adjusted to the opacity of 0.5 McFarland standards with sterile saline. The inocula were quantified by counting microconidia in a hematocytometer and by further plating 0.01 mL of the suspensions on SDA. The plates were then incubated at 28 °C and were examined daily to check the presence of fungal colonies before fungal viability testing (Indira, 2014).
2.8 Test procedure
The tests were performed using polystyrene microtiter plates with flat bottom wells in which a multichannel pipette was used to inoculate the aliquots of 100 μl of two fold drug dilutions into each well. The microtiter plates were stored at -50 °C in a deep freezer prior usage when they were inoculated with 100 μl fungal inoculum in order to maintain the dilutions from 5,000 to 50,000 spores ml−1. The plates were incubated at 28 °C for 7 days (Santos and Hamdan, 2005) for growth of the fungi. Furthermore, growth and sterility control wells were maintained for each assay and all tests were performed in duplicate and the highest dilution of the drug which inhibited the fungal growth was taken as the MIC (Indira, 2014).
3 Results
The heterotrophic bacterial and fungal counts of the organs (muscles, skin surfaces and gills) of the C. gariepinus are shown in Table 1. During the early raining season, the bacterial count ranged from 110 to 160 Cfu/mL for the control samples while the fungal count for control ranged from 90 to 108 Cfu/mL. The bacterial count for the polluted samples ranged between 480,000 and 760,000 Cfu/mL while the fungal count for the polluted samples ranged from 210,000 to 321,000 Cfu/mL. During the peak of rainfall, the bacterial count for the control samples ranged between 220 and 330 Cfu/mL while the fungal counts ranged from 102 to 141 Cfu/mL. In the polluted group, the bacterial count ranged between 660,000 and 1,020,000 Cfu/mL while the fungal count was between 201,000 and 344,000 Cfu/mL.
Muscles
Skin
Gills
Clarias gariepinus (Cfu/mL)
Early raining season
⁎Control
110 ± 0.50
120 ± 0.21
160 ± 0.12
⁎Polluted
480,000 ± 5.13
760,000 ± 1.31
570,000 ± 1.61
#Control
108 ± 0.03
90 ± 0.06
102 ± 0.11
#Polluted
230,000 ± 0.04
321,000 ± 0.12
210,000 ± 2.02
Peak of raining season
⁎Control
220 ± 0.05
240 ± 0.04
330 ± 0.04
⁎Polluted
660,000 ± 1.21
1,020,000 ± 2.21
710,000 ± 1.21
#Control
120 ± 0.01
141 ± 0.12
102 ± 0.01
#Polluted
310,000 ± 0.02
344,000 ± 0.22
201,000 ± 0.02
Clarias buthopogon (Cfu/mL)
Early rainy season
⁎Control
102 ± 0.11
109 ± 0.01
118 ± 0.11
⁎Polluted
871,000 ± 1.30
741,000 ± 1.31
490,000 ± 0.20
#Control
121 ± 0.01
142 ± 0.02
111 ± 0.01
#Polluted
210,000 ± 0.02
301,000 ± 0.02
165,000 ± 0.01
Peak of raining season
⁎Control
101 ± 0.01
201 ± 0.01
160 ± 0.05
⁎Polluted
204,000 ± 0.05
104,000 ± 2.02
120,000 ± 0.01
#Control
101 ± 0.01
121 ± 0.01
065 ± 0.01
#Polluted
200,000 ± 0.01
280,000 ± 0.02
200,000 ± 0.01
The table also shows the total microbial counts in the C. buthopogon species collected during different times in the rainy season. During the early raining period, bacterial counts from the control samples ranged between 102 and 118 Cfu/mL while the fungal counts ranged between 111 and 142 Cfu/mL. From the polluted group, the bacterial count was from 490,000 to 871,000 Cfu/mL while the fungal count ranged from 165,000 to 301,000 Cfu/mL. During the peak of raining season, bacterial count for the control group ranged between 101 and 201 Cfu/mL while the fungal count ranged between 65 and 121 Cfu/mL. In the polluted groups, bacterial count ranged between 120,000 and 1,040,000 Cfu/mL while fungal count was between 200,000 and 280,000 Cfu/mL. In all, the highest bacterial count (1,040,000 Cfu/mL) was recorded for the skin sample of C. buthopogon while the lowest count (101 Cfu/mL) recorded for the muscle sample of C. buthopogon. In the same vein, the highest fungal count (344,000 Cfu/mL) was recorded for the skin sample of C. gariepinus while the lowest count (65 Cfu/mL) was from the gill sample of C. buthopogon.
3.1 Percentage occurrence of isolates
Organisms belonging to five each of bacterial and fungal genera were isolated from the different organs of C. gariepinus among which were six different species of Bacillus and three of Clostridium. For C. buthopogon, organisms belonging to four bacterial and five fungal genera were isolated among which were three different species of Bacillus. Among the bacteria isolates from both fish species, Bacillus had the highest percentage occurrence while Aspergillus species was the highest among the fungal isolates in both fish species (Table 2). Table 3 shows the antibiotic resistance of organisms isolated from the different organs of C. gariepinus. All isolates from skin showed 100% resistance to Erythromycin, Ciprofloxacin, Gentamicin, Cephalexin and Ceftriaxone and were 100% susceptible to Ampicillin. Both Bacillus and Clostridium isolates show 60% and 80% resistance to antibiotics respectively. All isolates from muscles showed a 100% resistance to Erythromycin, Gentamicin, Ceftriaxone and Ampicillin, and showed zones of inhibition around Ofloxacin, Ciprofloxacin, Cephalexin, Cotrimoxazole, Clindamycin and Augmentin. Bacillus and Clostridium isolates from the muscle shows between 70% to 90% resistance to the antibiotics used. In the same vein, all isolates from the gills showed a 100% resistance to Erythromycin, Ciprofloxacin, Gentamicin and Ceftriaxone and showed total susceptibility to Augmentin. Zones of inhibition were also recorded around other antibiotics. Bacillus and Clostridium species showed between 60% and 70% resistance to the all antibiotics. +Positive/susceptible; −negative/resistant.
Isolates
% occurrence
Isolates
% occurrence
Bacteria from Clarias gariepinus
Bacteria from Clarias buthopogon
Bacillus species
55.56 ± 0.12
Bacillus species
55.55 ± 1.22
Clostridium species
17.67 ± 1.02
Clostridium species
22.22 ± 0.12
Alcaligenes faecalis
13.41 ± 1.24
Alcaligenes faecalis
11.11 ± 0.14
Flavobacterium aquatile
6.70 ± 0.12
Corynebacterium striatum
11.11 ± 1.20
Enterobacter dissolvens
6.66 ± 0.11
Fungi from Clarias gariepinus
Fungi from Clarias buthopogon
Penicillium species
27.78 ± 0.10
Penicillium species
27.27 ± 1.22
Aspergillus species
44.44 ± 0.02
Aspergillus species
46.38 ± 1.11
Rhizopus species
11.11 ± 0.10
Fusarium oxysporum
9.09 ± 0.22
Monilia sitophilia
11.11 ± 0.02
Monilia sitophilia
9.09 ± 1.13
Fusarium oxysporium
5.56 ± 1.11
Rhizopus species
9.09 ± 0.22
Organisms
Ofl
Ery
Cip
Gen
Cep
Cot
Cez
Amp
Cli
Aug
⁎⁎(%)
Gram positive isolates
Skin isolates
⁎Bacillus circulans
+
−
−
−
−
+
−
+
−
+
60
Clostridium perfringens
−
−
−
−
−
−
−
+
+
−
80
Bacillus megaterium
+
−
−
−
−
−
−
+
+
+
60
#Sub-total (%)
33.33
100
100
100
100
66.66
100
0
33.33
66.66
Muscle isolates
⁎Bacillus circulans
−
−
−
−
−
−
−
−
+
−
90
Bacillus subtilis
−
−
−
−
−
−
−
−
+
+
80
Clostridium sporogenes
+
−
−
−
−
−
−
−
+
+
70
Bacillus sphaericus
−
−
+
−
−
−
−
−
−
-
90
Bacillus megaterium
+
−
−
−
−
−
−
−
−
−
90
#Sub-total (%)
50
100
83.33
100
83.33
83.33
100
100
33.33
50
Gills isolates
Bacillus cereus
+
−
−
−
−
−
−
+
+
+
60
Clostridium sporogenes
+
−
−
−
−
−
−
+
+
+
60
Bacillus polymyxa
+
−
−
−
−
−
−
+
−
+
70
Clostridium septicum
−
−
−
−
+
+
−
−
+
+
60
#Sub-total (%)
25
100
100
100
75
75
100
25
25
0
$Grand total (%)
36.11
100
94.44
100
86.11
74.99
100
41.66
30.55
38.88
Gram negative isolates
Organisms
Ofl
Chl
Cef
Amx
Gen
Nit
Cip
Tet
Nox
Kan
⁎⁎(%)
Skin isolates
Alcaligenes faecalis
+
−
−
−
−
−
+
−
−
−
80
Flavobacterium aquatile
+
+
−
−
+
−
−
−
−
+
60
#Sub-total (%)
0
50
100
100
50
100
50
100
100
50
Muscle isolates
Enterobacter dissolvens
+
−
−
−
+
−
+
+
+
−
50
#Sub−total (%)
0
100
100
100
0
100
0
0
0
100
$Grand total (%)
0
75
100
100
25
100
25
50
50
75
The Gram negative isolates of the skin showed total resistance to Cefuroxime, Kanamycin, Nitrofurantoin, Tetracycline and Norfloxacin. They showed total susceptibility to Ofloxacin and different degrees of susceptibility to Ciprofloxacin, Chloramphenicol, Gentamicin and Amoxicillin. Alcaligenes faecalis and Flavobacterium aquatile from the C. gariepinus skin were 80% and 60% each resistant to antibiotics. Enterobacter dissolvens in the muscle showed 100% resistance to Chloramphenicol, Cefuroxime, Amoxicillin, Nitrofurantoin and Norfloxacin while showing total susceptibility to Ofloxacin, Gentamicin, Ciprofloxacin, Tetracycline and Norfloxacin. The bacteria showed 50% overall resistance to antibiotics used.
Table 4 shows the antibiotic resistance of organisms isolated from the organs of C. buthopogon. The skin isolate showed 100% resistance to Erythromycin, Ceftriaxone, Ampicillin, and Augmentin while showing total susceptibility to Ciprofloxacin, Cotrimoxazole and Clindamycin. Different degrees of resistance were shown to Ofloxacin, Gentamicin and Cephalexin. In all, the two Bacillus species from skin showed 70% and 40% resistance to antibiotics respectively. The isolates from C. buthopogon’s muscle exhibits 100% resistance to Erythromycin, Gentamicin, Cotrimoxazole, Ceftriaxone, Ampicillin and Augmentin. Total susceptibility was shown to Cephalexin and Clindamycin while 50% susceptibility was recorded for Ofloxacin and Ciprofloxacin. Bacillus and Clostridium isolates showed 80% and 60% resistance to antibiotics respectively. In the gills, Corynebacterium striatum showed total susceptibility to Ofloxacin, Cotrimoxazole, Ceftriaxone and Clindamycin while showing 100% resistance to Erythromycin, Ciprofloxacin, Gentamicin, Cephalexin, Ampicillin and Augmentin. The Gram negative bacteria; A. faecalis was resistant to all antibiotics used except Ciprofloxacin. +Positive/susceptible; −negative/resistant.
Organisms
Ofl
Ery
Cip
Gen
Cep
Cot
Cez
Amp
Cli
Aug
⁎⁎(%)
Gram positive isolates
Skin isolates
Bacillus circulans
−
−
+
−
−
+
−
−
+
−
70
⁎Bacillus megaterium
+
−
+
+
+
+
−
−
+
−
40
#Sub-total (%)
50
100
0
50
50
0
100
100
0
100
Muscle isolates
Bacillus subtilis
−
−
−
−
+
−
−
−
+
−
80
Clostridium sporogenes
+
−
+
−
+
−
−
−
+
−
60
#Sub-total (%)
50
100
50
100
0
100
100
100
0
100
Gill isolates
Corynebacterium striatum
+
−
−
−
−
+
+
−
+
−
60
#Sub-total (%)
0
100
100
100
100
0
0
100
0
100
$Grand total (%)
33.33
100
75
83.33
75
33.33
66.66
100
0
100
Gram negative isolates
Organisms
Ofl
Chl
Cef
Amx
Gen
Nit
Cip
Tet
Nox
Kan
⁎⁎(%)
Muscle isolates
Alcaligenes faecalis
−
−
−
−
−
−
+
−
−
−
90
#Sub-total (%)
100
100
100
100
100
100
0
100
100
100
$Grand total (%)
100
100
100
100
100
100
0
100
100
100
3.2 Antimicrobial Sensitivity Pattern
The antibiotics resistant patterns of C. gariepinus and C. buthopogon are shown in Table 5. For C. gariepinus, five different resistance patterns were shown which range between five and nine antibiotics while for C. buthopogon, five different resistance patterns were also shown ranging between four and nine antibiotics. The highest level of multiple antibiotic resistances (MAR) was found in the fish muscle isolates from both fish species with resistance to nine (9) antibiotics tested for in each case. Organisms showing these resistances are Bacillus circulans, Bacillus megaterium and Bacillus sphaericus from C. gariepinus and A. faecalis from C. buthopogon. The level of multiple resistances in the gills and the skin ranged from four (4) to eight (8) in the antibiotics used for testing. Among the ten antibiotics family used, members of eight families (Macrolides, Aminoglycosides, Cephalosporins, Penicillins, Nitrofurans, Quinolones, Chloramphenicols and Tetracycline) recorded the highest (100%) level of resistance against the organisms isolated while members of the Aminoglycosides and Quinolones recorded the lowest (25%) resistance against organisms. The MAR index obtained varied from 0.40 to 0.90 and 0.40 for the control strain. The modal MAR index for the isolates was 0.60.
Clarias gariepinus
No. of drugs
Resistance pattern
No. of isolates
Organisms
MAR index
5
Chl, Cef, Amx, Nit, Kan
1
Enterobacter dissolvens
0.50
6
Ery, Cip, Gen, Cep, Cez, Cli
1
Bacillus circulans
0.60
Ery, Cip, Gen, Cep, Cot, Cez
3
Bacillus megaterium; Bacillus polymyxa; Clostridium sporogenes
0.60
Ofl, Ery, Cip, Gen, Cez, Amp
1
Clostridium septicum
0.60
Cef, Amx, Nit, Cip, Tet, Nox
1
Flavobacterium aquatile
0.60
7
Ery, Cip, Gen, Cep, Cot, Cez, Amp
1
Clostridium sporogenes
0.70
Ery, Cip, Gen, Cep, Cot, Cez, Cli
1
Bacillus polymyxa
0.70
8
Ofl, Ery, Cip, Gen, Cep, Cot, Cez, Aug
1
Clostridium perfringens
0.80
Ofl, Ery, Cip, Gen, Cep, Cot, Cez, Amp
1
Bacillus subtilis
0.80
Chl, Cef, Amx, Gen, Nit, Tet, Nox, Kan
1
Alcaligenes faecalis
0.80
9
Ofl, Ery, Cip, Gen, Cep, Cot, Cez, Amp, Aug
1
Bacillus circulans
0.90
Ery, Cip, Gen, Cep, Cot, Cez, Amp, Cli, Aug
1
Bacillus megaterium
0.90
Ofl, Ery, Gen, Cep, Cot, Cez, Amp, Cli, Aug
1
Bacillus sphaericus
0.90
Clarias buthopogon
4
Ery, Cez, Amp, Aug
1
Bacillus megaterium
0.40
6
Ery, Gen, Cot, Cez, Amp, Aug
1
Clostridium sporogenes
0.60
Ery, Cip, Gen, Cep, Amp, Aug
1
Corynebacterium striatum
0.60
7
Ofl, Ery, Gen, Cep, Cez, Amp, Aug
1
Bacillus circulans
0.70
8
Ofl, Ery, Cip, Gen, Cot, Cez, Amp, Aug
1
Bacillus subtilis
0.80
9
Ofl, Chl, Cef, Amx, Gen, Nit, Tet, Nox, Kan
1
Alcaligenes faecalis
0.90
3.3 Susceptibility result of fungal isolates
The results of fungal susceptibility testing showed that the isolates were susceptible to ketoconazole at the two (2) concentrations with susceptibility ranging from 10 mm to 19 mm for 200 μmg/mL and 11 mm to 20 mm for 400 μmg/mL. Rhizopus arrhizus showed the highest susceptibility range of 19–20 mm for 200 μmg/mL and 400 μmg/mL respectively and Aspergillus niger showed the least susceptibility to ketoconazole with a range of 10–11 mm at 200 μmg/mL and 400 μmg/mL respectively. All fungal isolates were resistant to fluconazole and griseofulvin at all concentrations.
4 Discussion
The quality of a water body is neither a static condition, nor the measurement of a single parameter since there are lots of chemical, physical and biological factors affecting its status (Dahunsi et al., 2014; UNEPGEM, 2000). The microbial counts obtained in this study are similar to those of Adewoye and Lateef (2003) who reported counts in the order of 100,000 Cfu/mL for bacterial populations in some Nigerian polluted water bodies exposed to industrial, human and agricultural wastes. In the same vein, Lateef (2004) obtained bacterial count of 215,000 Cfu/mL from a pharmaceutical effluent in Nigeria.
The high occurrence of Bacillus and Clostridium species in the fish organs could be due to their high adaptability to such environments. Both are Gram positive, spore forming bacteria found in water, soil or gut of many animals. Bacterial flora of fish would always reveal the bacteriological conditions of the water where fish inhabit (Blanco et al., 2000). Bacillus was also isolated in the gills and muscle of the Clarias species indicating its abundance in the environment which may have been made possible by its microaerophilic nature. This result validates the report of Adewoye and Lateef (2003) that Bacillus species are commonly found in polluted waters.
The higher occurrence of Penicillium species may be due to the presence of conidiospores (which is the main dispersal route of the fungi). These spores can withstand both favorable and unfavorable environmental conditions, can be carried and found anywhere ranging from soil, air, water, clothing materials etc. hence its high occurrence in the samples. This supports the findings of Kolawole et al. (2011) who observed a dense population of microorganisms in the downstream Asa Dam, Nigeria. In another previous study, Lateef (2004) reported high bacterial and fungal counts in polluted rivers in Nigeria.
The prevalence of multi-resistance to common antimicrobial agents has been documented by various researchers (Baltz, 2008; Brown and Balkwill, 2009; Bhullar et al., 2012; Cox and Wright, 2013; D’Costa et al., 2011; Thaller et al., 2010; Toth et al., 2010; Wright, 2007). It has been reported that bacteria can acquire antimicrobial resistance by horizontal gene transfer and the uncontrolled usage of antibiotic or heavy presence of pollutants are known to trigger existing resistance mechanisms (Stokes and Gillings, 2011). Also, the abuse of antibiotic and pollutant accumulation is a veritable way of building an environmental reservoir of AR bacteria (Murphy, 2012; Seyfried and Newton, 2010). As shown in this study, anthropogenic activities i.e. the presence of bitumen and other pollutants (agricultural and domestic) may have contributed to the acquisition of drug resistance by the isolates. Evidence abound that increased antibiotic resistance in microorganisms and the transfer/acquisition of resistance elements are a modern phenomenon having a strong link with anthropogenic activities (Bhullar et al., 2012; Knapp et al., 2010).
The fact that bitumen exploration coupled with other pollution activities have been ongoing in the river for decades may also suggest that previously reported antibiotics resistance in water from Oluwa river (Ayandiran et al., 2014) and in inhabiting fish species (this study) has been a long time phenomenon which was not brought to limelight prior to this research. The possibility is high that the polluted nature of the river has tremendous effects on these organisms by redirecting/manipulating their metabolic pathways in ensuring survival in that condition over a long period. Thus, such change in physiological mechanism could be the cause of the resistance found in the organisms. This supports the report of Bhullar et al. (2012) that AR bacteria have the tendency to persist and then make up a huge reserve in the environment. Similar antimicrobial resistance profiles were also reported in studies involving isolates from coastal environments and farmed fish (Yano et al., 2014).
Moreover, as shown in this study, the high antibiotic resistance shown by most Gram positive bacteria correlates with the findings of Puah et al. (2013) who reported the highest rate of multiple resistance to antibiotics by Gram positive bacteria. Other researchers Lateef (2004), Odjadjare et al. (2012) and Paul et al. (1997) have all obtained different levels of resistance for bacterial isolates. Species of the same genera isolated from different sources may show varying levels of resistance to drugs as a physiological mechanism of survival as recorded in the present study. Shakibaie et al. (2009) had earlier reported different rates of AR for two fish species from different sampling points and periods. Resistances to tetracycline, gentamycin, clindamycin, cephalexin ciprofloxacin and others have been earlier reported (Guo et al., 2012, 2013).
Two important intrinsic mechanisms have been reported to be responsible for the conferment of resistance to multiple antimicrobial drug classes and these are: mutations in outer membrane porins resulting in reduced permeability to antimicrobials and over expression of multidrug efflux pumps, which usually pump out antibiotics before they act on their target site (Ashish et al., 2011; Navon-Venezia et al., 2005). Furthermore, this phenomenon (MAR) may also arise due to unrelated mechanisms accumulating sequentially in an organism (Navon-Venezia et al., 2005). Also, the level of resistance recorded by fungal isolates to fluconazole and griseofulvin is in agreement with previous reports (Indira, 2014; Pakshir et al., 2009). All the MAR indices obtained in this study were higher than the 0.20 limit (Table 5). This could indicate that the isolates were from highly contaminated source where antibiotics usage is likely to be high as reported by Paul et al. (1997). One of the potent sources of pollution of River Oluwa is animal wastes since the inhabitants are majorly farmers with good population of livestock (see Table 6). R = Resistance.
Fungi species
Fluconazole
Ketoconazole
Griseofulvin
200 μmg/mL
400 μmg/mL
200 μmg/mL
400 μmg/mL
250 μmg/mL
500 μmg/mL
1000 μmg/mL
Penicillium notatum
R
R
16
17
R
R
R
Fusarium oxysporium
R
R
15
16
R
R
R
Monilia sitophilia
R
R
17
19
R
R
R
Aspergillus terreus
R
R
16
16
R
R
R
Penicillium chrysogenum
R
R
14
15
R
R
R
Aspergillus niger
R
R
10
11
R
R
R
Rhizopus stolonifer
R
R
16
17
R
R
R
Aspergillus glaucus
R
R
14
15
R
R
R
Rhizopus arrhizus
R
R
19
20
R
R
R
The result of this study is a signal that the spread of antibiotic resistant microorganisms in the environment is an important public health issue because handlers and consumers of this fishes are on the risk of contracting serious diseases caused by the organisms either from the water or the fish themselves. Multidrug resistance is a serious public health issue which has been previously associated with the outbreak of major diseases the world over (Fattahi et al., 2013; Prescott et al., 1999). The current results also agree with the findings of other authors on the need to protect water bodies from pollution thus avoiding spread of AR bacteria to the surrounding aquatic environment (Nardelli et al., 2012).
Although the bacterial and fungal species found in the present study did not cause mortality to the fishes probably because the latter have strong host defense response yet the species are both opportunistic and pathogenic which could be involved in causing fish diseases. In addition, these organisms could also be involved in the transmission of diseases to human beings. Both locally and internationally, the problem of antibiotic resistance is becoming more and more pronounced and this can be attributed chiefly to the abuse of antibiotics both by humans and in animal husbandry. This study has afforded us the opportunity to know the genera and species of microorganisms majorly found in the respective organs (skin, muscle, and gills) of the fish species found in this polluted river. Therefore, rapid and accurate identification of microorganisms will have a significant impact on fish health management programs. Since this is a preliminary investigation, studies are ongoing on the use of molecular biology techniques for confirming the identified organisms and to detect the antibiotic-resistance genes in the various isolates from this study. Stakeholders concerned with environmental policy making should make frantic effort to stop the introduction of pollutants into the river as the aftermath is fatal and/or irreversible (see Fig. 1).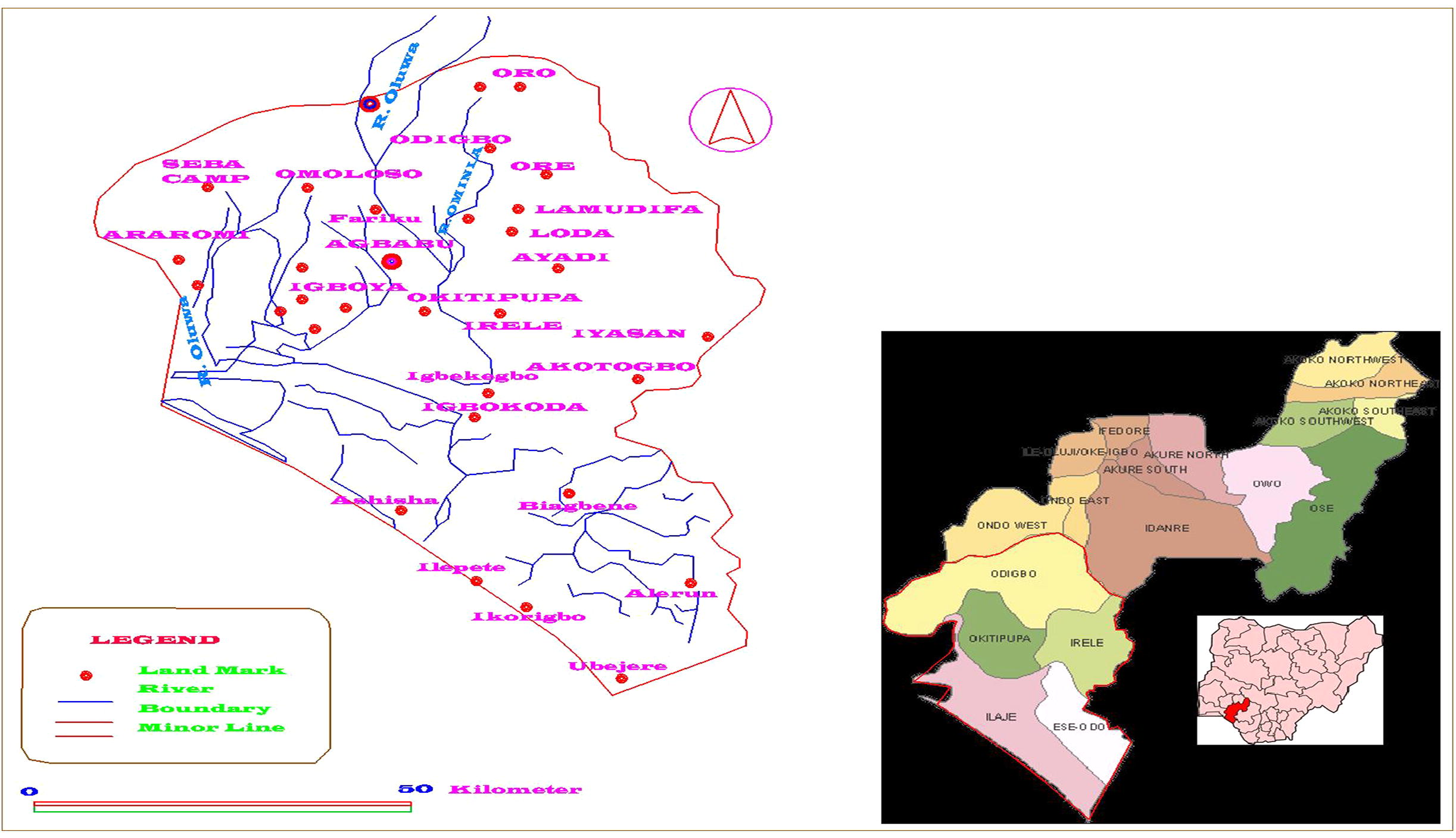
This figure shows the geographical position and description of River Oluwa in order to validate our claims on carrying out this study on the River.
Acknowledgement
The authors wish to thank the technical staff members of the Department of Pure and Applied Biology, Ladoke Akintola University of Technology, Nigeria for their numerous assistances during this research. We also appreciate the efforts of our final year students.
References
- Faecal coliforms in pond water, sediments and hybrid tilapia Oreochromis niloticus x Oreochromis aureus in Saudi Arabia. Aquat. Res.. 2003;34:517-524.
- [Google Scholar]
- Evaluation of the microbiological characteristics of Oyun River-a polluted River in North-central Nigeria. Pollut. Res.. 2003;22:457-461.
- [Google Scholar]
- Assessment of the microbiological quality of Clarias gariepinus exposed to an industrial effluent in Nigeria. Environment. 2004;24:249-254.
- [Google Scholar]
- Isolation and characterization of Pseudomonas species from Godavari River sample. Asiat. J. Biotechnol. Res.. 2011;2:862-866.
- [Google Scholar]
- Microbial assessment and prevalence of antibiotic resistance in polluted Oluwa River, Nigeria. Egypt. J. Aquat. Res.. 2014;40:291-299.
- [Google Scholar]
- Toxicological assessment of fish (Clarias gariepinus) from bitumen-polluted River Oluwa, Nigeria. Environ. Monit. Assess.. 2016;188:1-18.
- [Google Scholar]
- Renaissance in antibacterial discovery from actinomycetes. Curr. Opin. Pharmacol.. 2008;8:557-563.
- [Google Scholar]
- Antibiotic resistance is prevalent in an isolated cave microbiome. PLoS One. 2012;7:34953.
- [Google Scholar]
- Multiresistant waterborne pathogens isolated from water reservoirs and cooling systems. J. Appl. Microbiol.. 2008;105:469-475.
- [Google Scholar]
- Antibiotic resistance in bacteria isolated from the deep terrestrial subsurface. Microb. Ecol.. 2009;57:484-493.
- [Google Scholar]
- Influence of fish health management: bases, procedures and economic implications. Cah. Options Méditer.. 2000;51:45-49.
- [Google Scholar]
- Text Book of Medical Mycology (Second ed.). New Delhi: Mehta Publishers; 2002.
- Reference method for broth dilution antifungal susceptibility testing of filamentous fungi. In: Wayne P.A., ed. Approved Standard M38-A. Clinical and Laboratory Standards Institute; 2002.
- [Google Scholar]
- Intrinsic antibiotic resistance: mechanisms, origin, challenges and solutions. Int. J. Med. Microbiol.. 2013;303:287-292.
- [Google Scholar]
- Biochemical profile of Clarias gariepinus exposed to sub-lethal concentrations of chemical additives effluent. Int. J. Res. Environ. Sci. Technol.. 2011;1(4):52-58.
- [Google Scholar]
- Bioaccumulation pattern of cadmium and lead in the head capsule and body muscle of Clarias gariepinus [Burchell, 1822] exposed to paint emulsion effluent. Res. J. Environ. Earth Sci.. 2012;4(2):166-170.
- [Google Scholar]
- Differential bioaccumulation of heavy metals in selected biomarkers of Clarias gariepinus (Burchell, 1822) exposed to chemical additives effluent. J. Res. Environ. Sci. Toxicol.. 2012;1(5):100-106.
- [Google Scholar]
- Drinking water quality and public health of selected communities in South Western Nigeria. Water Qual. Exp. Health. 2014;6:143-153.
- [Google Scholar]
- Microbial selectivity of UV treatment on antibiotic resistant heterotrophic bacteria in secondary effluents of a municipal wastewater treatment plant. Water Res. 2013
- [CrossRef] [Google Scholar]
- UV inactivation and characteristics after photoreactivation of Escherichia coli with plasmid: health safety concern about UV disinfection. Water Res.. 2012;46:4031-4036.
- [Google Scholar]
- Assessment of heavy metals pollution and microbial contamination in water, sediments and fish of Lake Manzala. Egypt. Life Sci. J.. 2013;10(1):86-99.
- [Google Scholar]
- Inactivation and reactivation of antibiotic-resistant bacteria by chlorination in secondary effluents of a municipal wastewater treatment plant. Water Res.. 2011;45(9):2775-2781.
- [Google Scholar]
- Biochemical characteristics and identification of bacterial strains from drinking water sources of Kohat, Pakistan. Afr. J. Microbiol. Res.. 2013;7(16):1579-1590.
- [Google Scholar]
- In Vitro antifungal susceptibility testing of 5 antifungal agents against dermatophytic species by CLSI (M38-A) micro dilution method. Clin. Microbiol.. 2014;3:145.
- [CrossRef] [Google Scholar]
- Bacteriological Diseases of Fish. Cambridge, UK: Blackwell Science Ltd., University Press; 1994.
- Evidence of increasing antibiotic resistance gene abundances in archived soils since 1940. Environ. Sci. Technol.. 2010;44:580-587.
- [Google Scholar]
- Assessment of water quality in Asa River (Nigeria) and its indigenous Clarias gariepinus fish. Int. J. Environ. Res. Public Health. 2011;8(11):4332-4352.
- [Google Scholar]
- Occurrence and characterization of Listeria spp. in ready-to-eat retail foods from Vancouver, British Columbia. Food Microbiol.. 2012;30:372-378.
- [Google Scholar]
- The microbiology of a pharmaceutical effluent and its public health implications. World J. Microbiol. Biotechnol.. 2004;22:167-171.
- [Google Scholar]
- Prevalence of bacterial resistance in clinical, food, water and some environmental samples in Southwest Nigeria. Environ. Monit. Assess.. 2005;100(1–3):59-69.
- [Google Scholar]
- Class 1 integrons in environments with different degrees of urbanization. PLoS One. 2012;7:e39223. (Epub. 22.06.12)
- [Google Scholar]
- Update on Pseudomonas aeruginosa and Acinetobacter baumannii infections in the healthcare setting. Curr. Opin. Infect. Dis.. 2005;18:306-313.
- [Google Scholar]
- Nyaku, R.E., Okayi, R.G., Ataguba, G.A., Mohammed, A., 2007 Diseases associated with livestock integrated fish farming in Nigeria: a review. In: FISON Conference Proceedings, Kebbi State, Nigeria, 12–16 Nov., 2007.
- Water pollution: a review of microbial quality and health concerns of water, sediment and fish in the aquatic ecosystem. Afr. J. Biotechnol.. 2010;9(4):423-427.
- [Google Scholar]
- The effect of temperature abuse at the beginning of storage on the quality and shelf life of fresh water arctic charr (Salvelinus alpinus) Fish. Training Program. 2006:1-46.
- [Google Scholar]
- Prevalence of Multiple Antibiotics Resistant (MAR) Pseudomonas species in the final effluents of three municipal wastewater treatment facilities in South Africa. Int. J. Environ. Res. Public Health. 2012;9:2092-2107.
- [Google Scholar]
- Aquatic and Waste Microbiology (fourth ed.). Enugu: Dimension Publishing Co., Ltd.; 1985. p. :169.
- Distribution of polycyclic aromatic hydrocarbons in surface soils and water from the vicinity of Agbabu bitumen field of South Western Nigeria. Environ. Sci. Health Part A. 2007;42:1043.
- [Google Scholar]
- In vitro activity of six antifungal drugs against clinically important dermatophytes. Jundishapur J. Microbiol.. 2009;2(4):158-163.
- [Google Scholar]
- Multiple antibiotic resistance index and its reversion in Pseudomonas aeruginosa. Lett. Appl. Microbiol.. 1997;24:169-171.
- [Google Scholar]
- Microbiology (fourth ed.). New York: McGraw Hill; 1999. p. :678-697. ISBN 0-35439-3
- Aeromonas aquariorum clinical isolates: antimicrobial profiles, plasmids and genetic determinants. Int. J. Antimicrob. Agents. 2013;41:281-284.
- [Google Scholar]
- Susceptibility testing of pathogenic fungi with Itraconazole: a process analysis of test variables. J. Antimicrob. Chemother.. 2001;48:163-177.
- [Google Scholar]
- Evaluation of broth microdilution antifungal susceptibility testing conditions for Trichophyton rubrum. J. Clin. Microbiol.. 2005;43:1917-1920.
- [Google Scholar]
- Chromium exposure study in chemical based industry. J. Environ. Biol.. 2007;28:405-408.
- [Google Scholar]
- Occurrence of tetracycline resistance genes in aquaculture facilities with varying use of oxytetracycline. Microbiol. Ecol.. 2010;59:799-807.
- [Google Scholar]
- Horizontal transfer of antibiotic resistance genes among Gram negative bacteria in sewage and lake water and influence of some physico-chemical parameters of water on conjugation process. J. Environ. Biol.. 2009;30:45-49.
- [Google Scholar]
- Gene flow, mobile genetic elements and the recruitment of antibiotic resistance genes into gram-negative pathogens. FEMS Microbiol. Rev.. 2011;35:790-819.
- [Google Scholar]
- Tracking acquired antibiotic resistance in commensal bacteria of Galapagos land iguanas: no man, no resistance. PLoS One. 2010;5:e8989.
- [Google Scholar]
- An antibiotic resistance enzyme from a deep-sea bacterium. J. Am. Chem. Soc.. 2010;132:816-823.
- [Google Scholar]
- United Nations Environment Programme Global Environment Monitoring System/Water Programme. Water Quality for Ecosystem and Human Health. Burlington, ON, Canada: National Water Research Institute; 2000.
- The antibiotic resistome: the nexus of chemical and genetic diversity. Nat. Rev. Microbiol.. 2007;5:175-186.
- [Google Scholar]
- Prevalence and antimicrobial susceptibility of Vibrio species related to food safety isolated from shrimp cultured at inland ponds in Thailand. Food Control. 2014;38:30-36.
- [Google Scholar]







