Translate this page into:
Phytochemical screening, total phenolics and antioxidant activities of bark and leaf extracts of Goniothalamus velutinus (Airy Shaw) from Brunei Darussalam
*Corresponding author. Tel.: +673 8748010; fax: +673 2461502 linda.lim@ubd.edu.bn (Linda B.L. Lim)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Available online 14 February 2015
Peer review under responsibility of King Saud University.
Abstract
Goniothalamus velutinus Airy Shaw belongs to the family Annonaceae which is known to have anticancer, antitumor and many other bioactivities. Natives of Sabah and Sarawak use root decoction of G. velutinus for the treatment of headache and food poisoning while the bark was used as a mosquito repellent. Bark and leaf extracts of this plant, obtained from Brunei Darussalam, were tested for phytochemical and antioxidant activities. Phytochemical screening of plant extracts revealed the presence of alkaloids, steroids, terpenoids and cardiac glycosides. Quantitative determination of total phenolics, total flavonoids, and various in vitro antioxidant activities (DPPH, ABTS and FRAP) of methanolic extract was carried out using colorimetric methods. The total phenolic content, expressed as mg of gallic acid equivalent (GAE) per gram of extract, was found to be 68 mg GAE/g and 78 mg GAE/g for bark and leaves respectively. The radical scavenging activity measurement, expressed in terms of EC50 (effective concentration of extract in μg/mL that reduces DPPH absorbance to 50% as compared to negative control), for leaf and bark extracts was found to be 155 μg/mL and 204 μg/mL respectively. Standards trolox and ascorbic acid show EC50 value of 5 μg/mL and 4 μg/mL respectively. Trolox equivalent antioxidant capacity (TEAC) was measured using the ABTS and FRAP method. Result for bark and leaf extracts was 79 mg and 106 mg trolox equivalent (TE)/g respectively for the ABTS method. For FRAP assay, results for bark and leaf extracts were 80 and 89 mg TE/g respectively.
Keywords
Goniothalamus velutinus
Phytochemical screening
Total phenolic content
Antioxidant activity
DPPH
EC50
1 Introduction
Plants such as vegetables, fruit, spices medicinal herbs, etc., have been used to cure many diseases since ancient time. Today in this modern world, even though synthetic drugs are readily available and highly effective in curing various diseases, there are people who still prefer using traditional folk medicines because of their less harmful effects. There is a wide diversity of compounds, especially secondary metabolites, found and isolated from plants and studies have shown that these compounds have anticancer, antibacterial, analgesic, anti-inflammatory, antitumor, antiviral and many other activities to a greater or lesser extent (Cai et al., 2004; Miliauskas et al., 2004). Distinguished examples of these phytochemical compounds include flavonoids, phenols and phenolic glycosides, saponins and cyanogenic glycosides, stilbenes, tannins, nitrogen compounds (alkaloids, amines, betalains), terpenoids and some other endogenous metabolites (Cai et al., 2004; Abdelwahab et al., 2010).
Antioxidants are significant regarding reducing oxidative stress which could affect and damage biological molecules (Farhat et al., 2013). Oxidative stress is the disproportion between oxidants and antioxidants in favor of oxidants potentially leading to damage. Reactive oxygen species (ROS) are a class of compounds that are formed from oxygen metabolism. These highly reactive molecules such as, hydroxyl radical (•OH), peroxide (ROO•) and superoxide radicals (O2•¯), can cause severe damage to cells and tissues during various diseases which are linked to heart disease, carcinogenesis and many other health issues. Synthetic antioxidants such as butylated hydroxyl anisole (BHA), propyl gallate (PG), butylated hydroxyl toluene (BHT) which have been used to prevent oxidation have been found to cause internal and external bleeding in rats and guinea pigs at high dose (Borneo et al., 2009; Lee et al., 2003). Attention is therefore turned to the use of natural antioxidants such as bioactive flavonoids which are of great importance due to their indigenous origin and strong efficiency to trap/scavenge free radicals. One such example is tea (black & green) which is frequently used as beverage all over the world and is a rich source of polyphenolic compounds (Lee and Shibamoto, 2000; Katalinic et al., 2006; Borneo et al., 2009).
Goniothalamus of the Annonaceae family grows in shady primary rainforest of tropical Asia and approximately 160 species of this genus have been discovered, of which phytochemically 22 species have so far been investigated (Wiart 2007). Goniothalamus spp. are widely distributed in the island of Borneo. About 40 species of Goniothalamus have been recorded in Borneo. This genus is widely used in traditional medicines by natives for skin diseases, fever, antidotes and especially for abortion and post-partum treatments. It is also known to have antioxidant, antimalarial, anti-inflammatory, anticancer and inhibitory effects on platelets activating factor properties (Abdelwahab et al., 2009a,b). For example, decoctions of Goniothalamus scortechinii and Goniothalamus macrophyllus are used as a post-partum protective remedy while the roots of Goniothalamus tapis and Goniothalamus giganteus are used for abortion during early months of pregnancy. Goniothalamus amuyon is used to treat scabies. Phytochemical investigations of Goniothalamus spp. have resulted in the isolation of acetogenins, styryl lactones and alkaloids with significant cytotoxic, insecticidal and antimicrobial activities (Wiart 2007; Fasihuddin et al., 2010). Some of the alkaloids isolated include goniothalactam, goniopedaline, aristololactam AII, aristololactam BII and velutinam.
This study focuses on Goniothalamus velutinus, locally known as ‘Limpanas hitam, Kayu hujan panas, talipanas hitam’ in Brunei Darussalam, which is one of the interesting Goniothalamus spp. found in Borneo. Its specific medicinal uses are not much described but people of Sabah and Sarawak use its root decoction for the treatment of headache and food poisoning while its stem bark, which has a strong smell, has been used as mosquito repellent. Some ethnics in Borneo use G. velutinus for treatment of tumors and research also showed that its cytotoxicity on various human cell lines and all the above mentioned alkaloids and styryl lactone goniothalamin have been isolated from this species(Omar et al., 1992; Fassihuddin, 2004; Fasihuddin et al.,2010).
The objective of this study was to carry out preliminary phytochemical screening and to determine the total phenolic, flavonoid, flavonol contents and antioxidant activities (by DPPH, ABTS and FRAP) of G. velutinus collected from the rainforest of Brunei Darussalam. To date there has been no study being conducted on G. velutinus collected from Brunei Darussalam. Further, to the best of our knowledge, there has been no previous work being published on the phytochemical screening and antioxidant activities of G. velutinus.
2 Material and methods
2.1 Plant material and sample preparation
Stems and leaves of G. velutinus were collected from Bukit Panjang in Kampung Kulapis Brunei Darussalam in February 2013. They were rinsed with tap water followed by distilled water to remove the dirt on the surface. Barks were removed from stems and cut into small pieces. They were then air dried for 2 days and then freeze dried until a constant mass was obtained. Dried samples were ground into fine powder and kept in desiccators until extracted. The extraction was carried out in a soxhlet apparatus for 10 h using absolute methanol. The solvent was then evaporated using rotary evaporator and the crude extracts were kept in desiccators.
2.2 Chemicals and reagents
All chemicals used were of analytical grade. 1,1-Diphenyl-2-picrylhydrazyl (DPPH), 6-hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid (trolox), Ascorbic acid, 2,4,6-tri(2-pyridyl)-s-triazine (TPTZ), ABTS•+ [(2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt], quercetin dehydrate, gallic acid, anhydrous sodium carbonate (Na2CO3), aluminum tri chloride, potassium acetate, sodium acetate, ferric chloride hexahydrate (FeCl3.6H2O), Folin–Ciocalteu reagent, Dragendorff’s reagent, mercuric chloride, potassium iodide, iodine were purchased from Sigma–Aldrich. Ethanol, methanol, hydrochloric acid (HCl), sulfuric acid (H2SO4), chloroform, ammonia, glacial acetic acid, sodium hydroxide (NaOH) were purchased from Merck and potassium peroxodisulfate from Fluka. All chemicals and reagents were used without further purification.
2.3 Phytochemical screening
The crude methanolic extracts of bark and leaves were tested for the presence of alkaloids, steroids, tannins, saponins and glycosides. The qualitative results are expressed as (+) for the presence and (−) for the absence of phytochemicals.
2.3.1 Test for alkaloids
Few mg (about 15 mg) of each extract (bark and leaf) was separately stirred with 1% HCl (6 mL) on a water bath for 5 min and filtered. These filtrates were divided into three equal parts.
-
Dragendorff’s test: To one portion of the filtrate, Dragendorff’s reagent (Potassium bismuth iodide solution) (1 mL) was added; an orange red precipitate shows the presence of alkaloids.
-
Mayer’s test: To one portion of filtrate, Mayer’s reagent (Potassium mercuric iodide solution) (1 mL) was added. Formation of cream colored precipitate gives an indication of the presence of alkaloids.
-
Wagner’s test: Potassium iodide (2 g) and iodine (1.27 g) were dissolved in distilled water (5 mL) and the solution was diluted to 100 mL with distilled water. Few drops of this solution were added to the filtrate; a brown colored precipitate indicates the presence of alkaloids. (Joshi et al., 2013; Abdullahi et al., 2013).
2.3.2 Tests for steroids and terpenoids
-
Salkowski test: The crude extract (about 100 mg) was separately shaken with chloroform (2 mL) followed by the addition of concentrated H2SO4 (2 mL) along the side of the test tube, a reddish brown coloration of the interface indicates the presence of terpenoid (Ayoola et al., 2008).
-
Liebermann-Burchard test: Each extract (100 mg) was shaken with chloroform in a test tube; few drops of acetic anhydride was added to the test tube and boiled in a water bath and rapidly cooled in iced water. Concentrated H2SO4 (2 mL) was added alongside of the test tube. Formation of a brown ring at the junction of two layers and turning the upper layer to green shows the presence of steroids while formation of deep red color indicates the presence of triterpenoids (Joshi et al., 2013).
2.3.3 Test for tannins
Extract (leaf and bark, 0.5 g each) was separately stirred with distilled water (10 mL) and then filtered. A few drops of 5% ferric chloride were then added. Black or blue-green coloration or precipitate was taken as positive result for the presence of tannins (Banso and Adeyemo, 2006).
2.3.4 Test for Saponins
Each of plant extracts (0.5 g) was separately shaken with distilled water (10 mL) in a test tube. The formation of frothing, which persists on warming in a water bath for 5 min, shows the presence of saponins (Banso and Adeyemo, 2006).
2.3.5 Tests for glycosides
-
Anthraquinone glycoside (Borntrager’s test): To the extract solution (1 mL), 5% H2SO4 (1 mL) was added. The mixture was boiled in a water bath and then filtered. Filtrate was then shaken with equal volume of chloroform and kept to stand for 5 min. Then lower layer of chloroform was shaken with half of its volume with dilute ammonia. The formation of rose pink to red color of the ammoniacal layer gives indication of anthraquinone glycosides (Joshi et al., 2013).
-
Cardiac glycoside (Keller-Killiani test): Extract (0.5 g) was shaken with distilled water (5 mL). To this, glacial acetic acid (2 mL) containing a few drops of ferric chloride was added, followed byH2SO4 (1 mL) along the side of the test tube. The formation of brown ring at the interface gives positive indication for cardiac glycoside and a violet ring may appear below the brown ring (Ayoola et al., 2008).
2.4 Determination of total phenolic (TPC), total flavonoid and total flavonol contents
2.4.1 Total phenolic content
Total phenolic content was analyzed using the Folin–Ciocalteu colorimetric method (Velioglu et al., 1998; Cai et al., 2004; Chlopicka et al., 2012) with some modifications. An aliquot of 0.3 mL of leaf or bark extract was mixed with Folin–Ciocalteu phenol reagent (2.25 mL). After 5 min, 6% sodium carbonate (2.25 mL) was added and the mixture was allowed to stand at room temperature for 90 min. The absorbance of the mixture was measured at 725 nm. Standard calibration curve for gallic acid in the range of 0–200 μg/mL was prepared in the same manner and results were expressed as mg gallic acid equivalent (GAE) per gram of extract.
2.4.2 Total flavonoid content (TFC)
Total flavonoid content was determined using the aluminum colorimetric method (Chang et al., 2002; Stankovic, 2011) with some modifications using quercetin as the standard. A calibration curve of quercetin was prepared in the range of 0–200 μg/mL. Briefly, extract (0.5 mL) and standard (0.5 mL) were placed in different test tubes and to each 10% aluminum chloride (0.1 mL), 1 M potassium acetate (0.1 mL), 80% methanol (1.5 mL) and distilled water (2.8 mL) were added and mixed. A blank was prepared in the same manner where 0.5 mL of distilled water was used instead of the sample or standard, and the amount of aluminum chloride was also replaced by distilled water. All tubes were incubated at room temperature for 30 min. The absorbance was taken at 415 nm. The concentration of flavonoid was expressed as mg quercetin equivalent (QE) per gram of extract.
2.4.3 Total flavonol content (TF)
Total flavonol content was analyzed using the aluminum chloride colorimetric method (Pattanayak et al., 2011; Kalita et al., 2013) with some modifications. In this method quercetin was used to make a standard calibration curve in the range of 0–100 μg/mL. In different test tubes, each extract (1 mL) and standard solutions (1 mL) were placed and then 2% aluminum chloride (1 mL), 5% sodium acetate (3 mL) were added and mixed well. The mixture was then centrifuged at 3000 rpm for 20 min to get a clear solution. The absorbance of standard and sample were taken at 440 nm. Results were expressed as mg quercetin equivalent (QE) per gram of extract.
2.5 Antioxidant assay
2.5.1 By ABTS method
In this method, the radical scavenging capacity was measured by using ABTS•+ solution radical cation. The assay was performed as according to the method described by Thaipong et al., (2006) and Gan et al. (2010). The stock solution of ABTS•+ was prepared by mixing 7.4 mM ABTS solution and 2.6 mM potassium per sulfate solution in the ratio of 1:1 and allowed to react for 12 h at room temperature in the dark. The ABTS•+ working solution was prepared by diluting the stock solution (3 mL stock solution in 100 mL volumetric flask, diluting it to the mark with methanol) to get the absorbance of 1.1 ± 0.05 unit at 734 nm using a UV–visible spectrophotometer. A series of standard were prepared in the range of 0–125 μg/mL.
Standard solutions (150 μL) and sample extract (150 μL) were placed in different test tubes then ABTS working solution (2850 μL) was added to each test tube. These tubes were kept in the dark for 30 min. After that their absorbance was taken at 734 nm. The % inhibition of both standard and samples were calculated and the concentration of ABTS content in the extract was reported as mg of trolox equivalent (TE)/g extract.
2.5.2 By ferric reducing antioxidant power (FRAP) assay
For FRAP assay, Fresh FRAP reagent was prepared by mixing 300 mM acetate buffer (100 mL), 10 mM TPTZ solution (10 mL) and 20 mM FeCl3.6H2O (10 mL) solution and kept warmed at 37 °C until used in experiment. 300 mM acetate buffer pH 3.6 was prepared by dissolving sodium acetate trihydrate (3.1 g) in distilled water (500 mL) then glacial acetic acid (16 mL) was added and made up to the mark of 1 L with distilled water and checked for its pH. 10 mM TPTZ solution was prepared in 40 mM HCl and 20 mM FeCl3.6H2O was prepared in distilled water. Trolox was used as the standard and a calibration curve in the range of 0–250 μg/mL was prepared (Thaipong et al., 2006; Gan et al., 2010).
Standard solution (150 μL) and sample extract (150 μL) were allowed to react with FRAP solution (2850 μL) in different test tubes for 30 min in the dark. Reading of the colored solution (ferrous tripyridyltriazine complex) of standard and sample was taken at 593 nm. The concentration of FRAP content in the extract was reported as mg trolox equivalent (TE)/g extract.
2.6 Radical scavenging activity by the DPPH method (EC50)
The free radical scavenging capacity of methanolic extract was determined by using DPPH assay according to the method described by Abdulwahab et al. (2011) with some modifications. The stock solution of 1 M DPPH was prepared in methanol and kept at −20 °C until analysis. Fresh 0.1 mM DPPH working solution was prepared by diluting 10 mL stock solution with 90 mL methanol. Trolox and ascorbic acid were used as standard and a series of standard and sample (leaf and bark extract) were prepared in the range of 1–12 μg/mL. Scavenging activity was expressed as EC50 (effective concentration in μg/mL of samples or positive control that reduces the absorbance of DPPH by 50% when compared with negative control).
Briefly, standard solutions (1.5 mL) and extracts (1.5 mL leaf or bark) were placed in different test tubes. To each of these tubes DPPH working solution (1.5 mL) was added. The tubes were kept in the dark for 30 min and their absorbance was measured at 517 nm. The % inhibition of both standard and samples was calculated for each concentration and graphs were plotted (% inhibition against concentration). From these graphs EC50 values were calculated for standard and extracts. The experiment was carried out in triplicate.
2.6.1 Assaying methods
All measurements of absorbance were carried out using a Shimadzu UV-1601 PC spectrophotometer. All experiments were done in triplicate unless otherwise stated.
The % inhibition for DPPH and ABTS assay was calculated according to the formula where AB is absorption of blank sample, AA is absorption of sample/standard extract. Calibration curve was obtained by plotting % inhibition against trolox standard concentration.
3 Results and discussion
3.1 Phytochemical screening
The phytochemical screening of crude methanolic extracts of leaf and bark samples of G. velutinus revealed the presence of some secondary metabolites such as alkaloids, steroids and cardiac glycosides as shown in Table 1. Tannins were detected in the leaf extract but not in the bark extract of G. velutinus. The phytochemical compounds detected are known to have medicinal importance. For example, alkaloids have been reported as powerful poison and many alkaloids derived from medicinal plants show biological activities like, anti-inflammatory (Augusto et al., 2011) antimalarial (Dua et al., 2013), antimicrobial (Benbott et al., 2012), cytotoxicity, antispasmodic and pharmacological effects (Ameyaw and Duker-Eshun, 2009; Thite et al., 2013). Similarly, steroids derived from plants are known to have cardiotonic effect and also possess antibacterial and insecticidal properties (Alexei et al., 2009). They are very often used in medicines due to their well-known biological activities. Tannins, according to research, are known to have antibacterial (Hisanori et al., 2001), antitumor and antiviral activities (Kumari and Jain, 2012). They work by precipitating microbial protein thus making nutritional protein unavailable for them. Other phytochemicals called cardiac glycosides have been used to treat congestive heart failure and cardiac arrhythmia (Vladimir and Ludmila, 2001). Their mode of action starts by inhibiting Na+/K+ pump which then increases the level of calcium ion, so more Ca+ would be available for the contraction of heart muscles which recover cardiac output and reduce the distension of heart (Banso and Adeyemo, 2006; Aiyelaagbe and Osamudiamen, 2009). These phytochemical compounds identified in the leaf and bark extracts may be responsible for the biological activities shown by G. velutinus and the reason for their use as a traditional medicine by the natives of Brunei Darussalam as well as throughout the Borneo Island. Phenanthrene lactam alkaloids have been isolated from G. velutinus bark and other species of the same genus. These alkaloids show bioactivity against Gram-positive bacteria and cytotoxicity against leukemia and HeLa cell lines (Omar et al., 1992). + = presence, − = absence.
Test
Interferences
Leaf
Bark
Alkaloids
(a) Dragendorff’s Test
+
+
(b) Mayer’s Test
+
+
(c) Wagner’s Test
+
+
Steroids/terpenoids
(a) Salkowski test
+
+
(b) Liebermann-Burchard Test
+
−
Tannins
+
−
Saponins
−
−
Glycosides
(a) Anthraquinone Glycoside (Borntrager’s Test)
−
−
(b) Cardiac Glycoside (Keller-Killiani Test)
+
+
3.2 Total phenolic, flavonoid and flavonol contents
The results (Table 2) revealed that the methanolic extract of G. velutinus leaf and bark has similar values with leaf being slightly higher in total phenolic content i.e., 77.7 mg while bark has 68.3 mg GAE/g of extract. Abdelwahab et al. (2009a,b) reported that the total phenolic content of Goniothalamus umbrosus was different when extracted with different solvents. Dichloromethane extract of G. umbrosus leaves has very low phenolic content and shows low antioxidant activity but a comparatively high value of total phenolics has been reported for methanolic and ethyl acetate extracts of G. umbrosus. GAE = Gallic acid equivalent, QE = Quercetin equivalent.
Plant material
Total phenolic content (mg GAE/g)
Total flavonoid content (mg QE/g)
Total flavonol content (mg QE/g)
Bark
68.33 ± 2.61
42.84 ± 2.38
22.79 ± 0.47
Leaves
77.74 ± 2.77
72.16 ± 1.63
7.73 ± 0.28
When total phenolic content of G. velutinus is compared with the data available for the same genus and for the same family, it is found that it has a lower value except for the dichloromethane extract of G. umbrosus. While in comparison to some other medicinal plants’ phenolic content, it clearly shows that G. velutinus extract has similar or a slightly higher value of phenolic content as shown in Table 3. Although this plant is not rich in phenolic compounds, as shown by the low total phenolic content, it may contain other phytochemicals. GAE = Gallic acid equivalent, MeOH = Methanol, CH2Cl2 = Dichloromethan, EtOAc = Ethyl acetate.
Plant material
Extracting solvent
Total phenolic content mg of GAE/g
Reference
G. velutinus bark
MeOH
68
This study
G. velutinus leaf
MeOH
78
This study
G. umbrosus leaf
CH2Cl2
1
Abdelwahab et al. (2009a,b)
G. umbrosus leaf
EtOAc
340
Abdelwahab et. al. (2010)
G. umbrosus leaf
MeOH
400
Abdelwahab et. al. (2010)
Anaxagorea dolichocarpa (Annonaceae family)
EtOH
57
Almeida et al. (2012)
Annona squamosal leaf (Annonaceae family)
MeOH
93
Mariod et al., 2012
Annona squamosal bark (Annonaceae family)
MeOH
167
Mariod et al., 2012
Duguetia chrysocarpa (Annonaceae family)
EtOH
191
Almeida et al. (2012)
Rumex dentatus
Pet. Ether
45
Nisa et al. (2013)
Lauris nobilis
MeOH
99
Skerget et al. (2005)
Dioscorea bulbifera L.
MeOH
59
Song et al. (2010)
Arctium lappa L.
MeOH
16
Song et al. (2010)
Citrus unshiu tissue
MeOH
66
Ghasemi et al. (2009)
Marrubium peregrinum
MeOH
49
Stankovic, 2011
Marrubium peregrinum
Acetone
48
Stankovic, 2011
Raw papino fruit
EtOAc
24
Sudha et al. (2011)
Plant polyphenols are the significant group of compounds acting as free radical scavenging or primary antioxidants; therefore, it is justifiable to determine phenolic content in plant extract. Polyphenolic compounds have an aromatic benzene ring with substituted hydroxyl groups, including their functional derivatives. These are able to absorb free radicals and can chelate metal ions that could catalyze formation of ROS which promotes lipid peroxidation. Among polyphenols, flavonoids are of great importance because they help human body to fight against diseases. The ability of flavonoids to act as potent antioxidants depends on their molecular structures, the position of the hydroxyl group and other features in its chemical structure. They are abundantly found in plants as their glycoside (Rajanandh and Kavitha, 2010). The most abundant flavonol which has a good antioxidant property is quercetin, as it has all the right structural features for free radical scavenging activity (Kalita et al., 2013).Our results for total flavonoid content for leaf and bark of G. velutinus are 71 and 43 mg QE/g, respectively (Table 3). These values are comparable or slightly higher than total flavonoid content of the same family and other medicinal plants. For example flavonoid contents of Ethiopian pepper [Xylopiaaethiopica (Annonaceae)], Melilotus officinalis, Adiantum capillusveneris, Plantago major, Urticadioica, Dendrophthoe falcata and Biophytum sensitivum are 3.5, 57, 78, 25, 43, 22 and 9 mg QE/g of extract, respectively (Pourmorad et al., 2006; Pattanayak et al., 2011; Adefegha and Oboh, 2012; Kalita et al., 2013). Therefore, it can be said that polyphenolic, and flavonoid may work together with other phytochemicals present in G. velutinus and make it medicinally important.
3.3 Antioxidant assay and radical scavenging ability (EC50)
The antioxidant ability and radical scavenging properties of plants are associated with its medicinal values. In this study, the antioxidant activity of G. velutinus was measured using three different assays, namely FRAP, DPPH and ABTS. Performing a single assay to evaluate the antioxidant properties would not give the correct result because antioxidant activity of plant extract is influenced by many factors, for example the test system and composition of extract. Therefore it is important to carry out more than one type of antioxidant capacity measurement to cover the various mechanisms of antioxidant action (Gan et al., 2010). Ferric reducing antioxidant power (FRAP) assay depends on the reduction of ferric ion into ferrous ion (Benzie and Strain, 1996). DPPH is nitrogen centered free radical having an odd electron which gives a strong absorption at 517 nm, its color changes from purple to yellow when DPPH• odd electron paired off in the presence of radical scavenger to form the reduced DPPH-H (Cai et al., 2003). ABTS assay depends on the antioxidant compound ability to scavenge ABTS radical. By this assay we can measure antioxidant capacity of lipophilic and hydrophilic compounds in the same sample. These three assays are very simple, inexpensive and usually employed methods for the determination of antioxidant activity and can give reproducible results. Fig. 1 shows the comparison of different antioxidant assays of G. velutinus.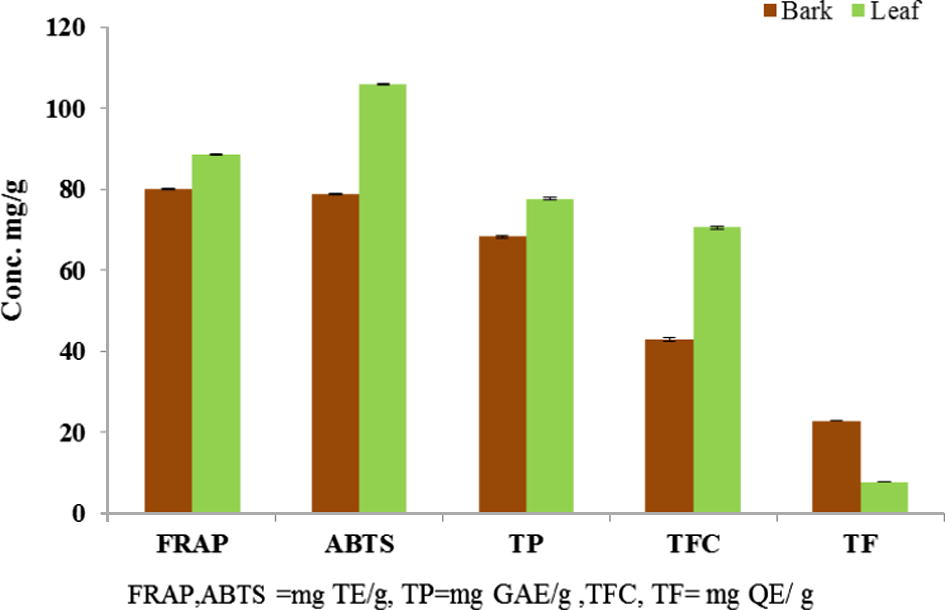
Comparison of different antioxidant assays of leaf and bark extracts of G. velutinus.
The results (Table 4) for FRAP and ABTS were calculated from calibration graphs which were linear over the calibration range with R2 value of 0.9997 and 0.9984 respectively. So far, no trolox equivalent antioxidant activity of Goniothalamus spp. have been reported, therefore, no comparison is possible with the same genus. However, when compared with other plants of the same family and with some Chinese medicinal plants, TEAC values of G. velutinus bark (316 μmol/g) and leaf (424 μmol/g) extracts are higher. For example, TEAC values of Ethiopian pepper [Xylopiaaethiopica (Dun.) (Annonaceae)], Bambusa breviflora (Poaceae), Chrysanthemum morifolium (Asteraceae), Ephedra sinica (Ephedraceae), Eriobotrya japonica (Rosaceae), Magnolia lilifora (Magnoliaceae), Mentha haplocalyx (Lamiaceae) are 2.07, 116, 149, 389, 437, 119 and 175 μmol TE/g respectively (Song et al., 2010; Adefegha and Oboh, 2012). Hence this shows that leaf and bark extracts of G. velutinus have good and promising antioxidant capacity in comparison to these known medicinal plants.
Plant material
FRAP (mg TE/g)
ABTS (mg TE/g)
Bark
80.11 ± 1.52
78.88 ± 0.56
Leaf
88.63 ± 0.67
106.03 ± 0.78
The free radical scavenging effect of crude methanolic extract of G. velutinus was determined using the DPPH method. The extracts of leaves and bark show EC50 values of 155 μg/mL and 204 μg/mL respectively, where the EC50 values of trolox and ascorbic acid are 5 μg/mL and 4 μg/mL. The results show that at 10 μg/mL concentration of trolox and ascorbic acid shows maximum inhibition of DPPH i.e., 91% and 96% respectively, while at the same concentration bark and leaf extracts show very low inhibition of DPPH. Methanolic leaf extract of G. umbrosus was reported to have promising antioxidant activity with EC50 value of 263 μg/mL (Abdulwahab et al., 2011). Compared to G. umbrosus, our results have much lower EC50 values than G. umbrosus especially the leaf extract with almost half the value. This may be because this plant has more antioxidant compounds than other species or other phytochemicals which is neutralizing the DPPH radical. Since the lower EC50 value means higher antioxidant activity, therefore both the leaf and bark of G. velutinus show potential antioxidant activities. The results for the DPPH assay for standards and samples (bark and leaf extracts) are shown in Fig. 2.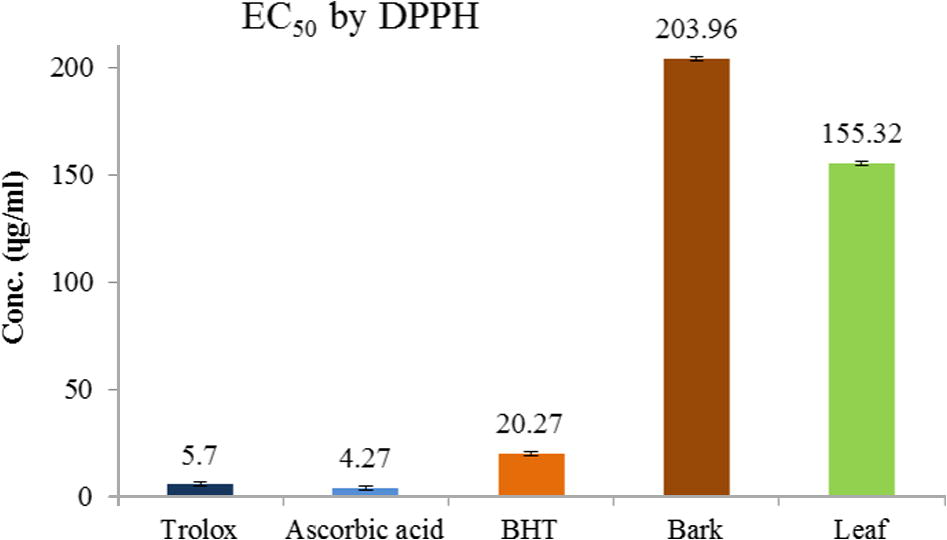
DPPH radical scavenging capacity (EC50) of bark and leaf methanolic extracts.
When comparing the EC50 values of bark and leaf extracts of G. velutinus with other medicinal plants (Table 5), it was observed that G. velutinus has lower values, once again confirming its greater potential antioxidant activity with other medicinal plants.
EC50 (DPPH) μg/mL
Plant material
Reference
204
Bark of G. velutinus
This study
155
Leaf of G. velutinus
This study
263
Leaf extract of G. umbrosus
Abdulwahab et al. (2011)
142
Anaxagorea dolichocarpa (Annonaceae)
Almeida et al. (2012)
79
Duguetia chrysocarpa (Annonaceae)
Almeida et al. (2012)
125
Bark extract Annona squamosal (Annonaceae)
Mariod et al., 2012
100
Medicago sativa
Rana et al. (2010)
198
Microliabum candidum
Borneo et al., 2009
2009
Thelesperma megapotamicum
Borneo et al., 2009
269
Baccharis stenophylla
Borneo et al., 2009
144
Piper nigrum
Khalaf et al. (2008)
100
Leaf extract Camellia sinensis
Povichit et al., 2010
187
Marrubium peregrinum
Stankovic, 2011
172
Leaf extract Melia azedarach
Kim et al., 2007
963
Leaf extract Pistia stratiotes
Nizamuddin Islam et al. (2013)
600
Leaf extract R. venulosa
Chauke et al. (2012)
5
Trolox (Std.)
This study
4
Ascorbic acid (Std.)
This study
20
BHT
This study
Based on antioxidant activities (FRAP, ABTS, DPPH), results are moderate for G. velutinus. As major plant compounds responsible for antioxidant activity are polyphenols, our result shows the phenolic content is similar to or slightly higher than some other medicinal plants. However, EC50 value is lower for G. velutinus than other species of Goniothalamus such as G. umbrosus (lowerEC50 means greater antioxidant activity) which means that G. velutinus has more potential antioxidant. The antioxidant activity of phenolic compounds are due to their redox property which plays an important role in absorbing and neutralizing free radicals, quenching singlet and triplet oxygen and a metal chelation potential (Abdelwahab et al., 2009a,b).
It was found that the anticancer activity of Annonaceous plant species is not because of direct antioxidant activity due to polyphenols but is due to the presence of goniothalamin and other styryl lactones which trigger inhibition of superoxide dismutase activity in malignant cells that cause free radical mediated damage to mitochondrial membrane, ultimately apoptosis of cells. Furthermore, goniothalamin at its non-apoptic concentration inhibits TNF-α-induced nuclear factor (NF)-κB activation without inducing toxicity toward healthy blood cells (Abdulwahab et al., 2011; Choo et al., 2014).
4 Conclusion
The result of this study shows the presence of some phytochemicals such as alkaloids, steroids, tannins and cardiac glycosides in bark and leaf methanolic extracts of G. velutinus. Alkaloids are already known to have spasmolytic, antifungal, antimicrobial and antitumor activities. The result also shows both extracts have phenolic content greater than other Goniothalamus species but lower than other medicinal plant. Generally, phenolic compounds can capture free radicals and neutralize them thus preventing our cells from aging process. Further, high phenolic content in plants generally shows some anticancer activities. In the case of Goniothalamus species low content of phenolic compounds has been reported but literature shows its anticancer activities against many cancer cell lines which may be due to the presence of other phytochemicals isolated from the species like goniothalamin and some alkaloids. This study also revealed that the bark and leaf extracts of G. velutinus have DPPH radical scavenging capacity greater than other species of Goniothalamus and showed slightly higher value of antioxidant activity in comparison with some other medicinal plants. Thus, it is concluded from the above study that its medicinal properties might be due to the presence of some phenolic compounds and other phytochemicals present in this plant and as it shows radical scavenging activity greater than other known medicinal plants therefore, it could be used as a source of antioxidant. This is an ongoing study and further work is being carried to investigate its biological activities.
Acknowledgements
The authors would like to thank the Government of Negara Brunei Darussalam and the Universiti Brunei Darussalam for their financial support.
References
- Antimicrobial and free radical scavenging activities of dichloromethane extract of Goniothalamus umbrosus. Int. J. Trop. Med.. 2009;4:32-36.
- [Google Scholar]
- Biological and phytochemical investigations of Goniothalamus umbrosus leaves hexane extract. J. Med. Plants Res.. 2009;3:2009.
- [Google Scholar]
- Phenolic Content and antioxidant activities of Goniothalamus umbrosus extracts. Int. J. Nat. Prod. Pharm. Sci.. 2010;1:1-6.
- [Google Scholar]
- Evaluation of phytochemical screening and analgesic activity of aqueous extract of the leaves of Microtrichia perotitii dc (Asteraceae) in mice using hotplate method. Med. Plant Res.. 2013;3:37-43.
- [Google Scholar]
- Antioxidant, antibacterial and antiviral properties of Goniothalamus umbrosus leaves methanolic extract. Afr. J. Micro. Res.. 2011;5:3138-3143.
- [Google Scholar]
- Effect of diets supplemented with Ethiopian pepper [Xylopia aethiopica (Dun.) A. Rich (Annonaceae)] and Ashanti pepper [Piper guineense Schumach. et Thonn (Piperaceae)] on some biochemical parameters in normal rats. Asian Pac. J. Trop. Biomed. 2012:S559-S567.
- [Google Scholar]
- Phytochemical screening for active compounds in Mangifera indica leaves from Ibadan, Oyo State. Plant Sci. Res.. 2009;2:11-13.
- [Google Scholar]
- Endogenous cardiotonic steroids: physiology, pharmacology and novel therapeutic targets. Pharmacol. Rev.. 2009;61:9-38.
- [Google Scholar]
- Phenolic quantification and antioxidant activity of Anaxagorea dolichocarpa and Duguetia chrysocarpa (Annonaceae) Int. J. Pharm. Biol. Sci.. 2012;2:367-374.
- [Google Scholar]
- The alkaloid contents of the ethno-plant organs of three antimalarial medicinal plant species in the eastern region of Ghana. Int. J. Chem. Sci.. 2009;7:48-58.
- [Google Scholar]
- Anti-inflammatory activity of alkaloids: an update from 2000 to 2010. Molecules. 2011;16:8515-8534.
- [Google Scholar]
- Phytochemical screening and antioxidant activities of some selected medicinal plants used for malaria therapy in southwestern Nigeria. Trop. J. Pharm. Res.. 2008;7:1019-1024.
- [Google Scholar]
- Phytochemical screening and antimalarial assessment of Abutilon mauritianum, Bacopa monnifera and Datura stramonium. Biokemistri. 2006;18:39-44.
- [Google Scholar]
- Assessment of the antibacterial activity of crude alkaloids extracted from seeds and roots of the plant Peganum harmala L. J. Nat. Prod. Plant Resour.. 2012;2:568-573.
- [Google Scholar]
- The Ferric Reducing Ability of Plasma (FRAP) as a measure of ‘‘Antioxidant Power’’: the FRAP assay. Anal. Biochem.. 1996;239:70-76.
- [Google Scholar]
- Antioxidant capacity of medicinal plants from the Province of Cordoba (Argentina) and their in vitro testing in a model food system. Food Chem.. 2009;112:664-670.
- [Google Scholar]
- Antioxidant activity of betalains from plants of the Amaranthaceae. J. Agric. Food Chem.. 2003;51:2288-2294.
- [Google Scholar]
- Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer. Life Sci.. 2004;74:2157-2184.
- [Google Scholar]
- Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J. Food Drug Anal.. 2002;10:178-182.
- [Google Scholar]
- Radical scavenging activity of selected medicinal plants from Limpopo province of South Africa. Afr. J. Tradit. Complement Altern. Med.. 2012;9:426-430.
- [Google Scholar]
- Total phenolic and total flavonoid content, antioxidant activity and sensory evaluation of pseudocereal breads. LWT – Food Sci. Tech.. 2012;46:548-555.
- [Google Scholar]
- Cytotoxic activity and mechanism of action of metabolites from the Goniothamus genus. Phytochem. Rev. 2014
- [CrossRef] [Google Scholar]
- Anti-malarial property of steroidal alkaloid conessine isolated from the bark of Holarrhena antidysenterica. Malaria J.. 2013;12:1-6.
- [Google Scholar]
- Characterization and quantification of phenolic compounds and antioxidant properties of Salvia species growing in different habitats. Indus. Crops Prod.. 2013;49:904-914.
- [Google Scholar]
- Phytochemical and biological studies on Goniothalamus spp. in Borneo. Iran. J. Pharm. R.. 2004;3:13-14.
- [Google Scholar]
- Chemical constituents and antiviral study of Goniothalamus velutinus. J. Fundam. Sci.. 2010;6:72-75.
- [Google Scholar]
- Antioxidant activity and total phenolic content of medicinal plants associated with prevention and treatment of cardiovascular and cerebrovascular diseases. J. Med. Plants Res.. 2010;4:2438-2444.
- [Google Scholar]
- Antioxidant activity, phenol and flavonoid contents of 13 citrus species peels and tissues. Pak. J. Pharm. Sci.. 2009;22:277-281.
- [Google Scholar]
- Antibacterial action of several tannins against Staphylococcus aureus. J. Antimicrob. Chemother.. 2001;48:487-491.
- [Google Scholar]
- Phytochemical investigation of the roots of Grewia microcos Linn. J. Chem. Pharm. Res.. 2013;5:80-87.
- [Google Scholar]
- Estimation of total flavonoids content (TFC) and antioxidant activities of methanolic whole plant extract of Biophytum sensitivum linn. J. Drug Delivery Ther.. 2013;3:33-37.
- [Google Scholar]
- Screening of 70 medicinal plant extracts for antioxidant capacity and total phenols. Food Chem.. 2006;94:550-557.
- [Google Scholar]
- In vitro screening of Jeju medicinal plants for cosmeceutical materials. J. Appl. Biol. Chem.. 2007;50:215-220.
- [Google Scholar]
- Review paper, Tannins: an antinutrient with positive effect to manage diabetes. Res. J. Recent Sci.. 2012;1:70-73.
- [Google Scholar]
- Antioxidant properties of aroma compounds isolated from soybeans and mung beans. J. Agric. Food Chem.. 2000;48:4290-4293.
- [Google Scholar]
- Screening of medicinal plant extracts for antioxidant activity. Life Sci.. 2003;73:167-179.
- [Google Scholar]
- Antioxidant activity of different parts from Annonasquamosa, and Catunaregam nilotica methanolic extract. Acta Sci. Pol. Technol. Aliment.. 2012;11(3):249-257.
- [Google Scholar]
- Screening of radical scavenging activity of some medicinal and aromatic plant extracts. Food Chem.. 2004;85:231-237.
- [Google Scholar]
- Phytochemical screening, antimicrobial and antioxidant efficacy of different extracts of Rumex dentatus L. - A locally used medicinal herb of Kashmir Himalaya. Asian Pac. J. Trop. Dis.. 2013;3(6):434-440.
- [Google Scholar]
- DPPH scavenging assay of eighty four Bangladeshi medicinal plants. J. Pharm. Biol. Sci.. 2013;6:66-73.
- [Google Scholar]
- Total phenolic content, flavonoid content and in vitro antioxidant activities of Dendrophthoe falcata (L.f.) Ettingsh. Int. J. Pharm Tech Res.. 2011;3:1392-1406.
- [Google Scholar]
- Antioxidant activity, phenol and flavonoid contents of some selected Iranian medicinal plants. Afr. J. Biotechnol.. 2006;5:1142-1145.
- [Google Scholar]
- Phenolic content and in vitro inhibitory effects on oxidation and protein glycation of some Thai medicinal plants. Pak. J. Pharm. Sci.. 2010;23:403-408.
- [Google Scholar]
- Quantitative estimation of β-sitosterol, total phenolic and flavonoid compounds in the leaves of Moringa oleifera. Int. J. Pharm Tech Res.. 2010;2:1409-1414.
- [Google Scholar]
- In vitro antioxidant and free radical scavenging studies of alcoholic extract of Medicago sativa L. Rom. J. Biol. – Plant Biol.. 2010;55:15-22.
- [Google Scholar]
- Phenols, proanthocyanidins, flavones and flavonols in some plant materials and their antioxidant activities. Food Chem.. 2005;89:191-198.
- [Google Scholar]
- Total phenolic contents and antioxidant capacities of selected chinese medicinal plants. Int. J. Mol. Sci.. 2010;11(6):2362-2372.
- [Google Scholar]
- Total phenolic content, flavonoid concentration and antioxidant activity of Marrubium peregrinum L. extracts. Kragujevac J. Sci.. 2011;33:63-72.
- [Google Scholar]
- In vitro free radical scavenging activity of raw pepino fruit (Solanum muricatum aiton) Int. J. Curr. Pharm. Res.. 2011;3:137-140.
- [Google Scholar]
- Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J. Food Comp. Analysis. 2006;19:669-675.
- [Google Scholar]
- Preliminary phytochemical screening of some medicinal plants. Int. J. Pharm. Chem. Biol. Sci.. 2013;3:87-90.
- [Google Scholar]
- Antioxidant activity and total phenolics in selected fruits, vegetables, and grain products. J. Agric. Food Chem.. 1998;46:4113-4117.
- [Google Scholar]
- Glycosides in medicine: the role of glycosidic residue in biological activity. Curr. Med. Chem.. 2001;8:1303-1328.
- [Google Scholar]
- Review-Goniothalamus species: a source of drugs for the treatment of cancers and bacterial infections? eCAM. 2007;4:299-311.
- [Google Scholar]







