Translate this page into:
Cytotoxicity and genotoxicity of gliotoxin on human lymphocytes in vitro
*Corresponding author
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Available online 31 December 2014
Peer review under responsibility of King Saud University.
Abstract
The cytotoxic effects on human lymphocytes of two gliotoxin samples (one pure sample produced in the laboratory for this study, and one sample purchased from a standard source) were assessed at four different concentrations (25, 50, 100 and 200 ng/ml) using the methylthiazol tetrazolium (MTT) bioassay. The results showed that growth was inhibited by 21, 39.10, 61.99 and 87.45% for each of the four concentrations of the pure sample, respectively, and by 17.89, 34.92, 58.34 and 85.22% respectively, in the case of the standard purchased sample. Deoxyribonucleic acid (DNA) was extracted from the lymphocytes and analysed by electrophoresis on a 1% agarose gel. Gliotoxin appeared to have the ability to degrade or damage the DNA. The present study showed that both the growth inhibition and DNA damage experienced by the human lymphocytes increased linearly with increasing concentrations of toxin.
Keywords
Cytotoxicity
Genotoxicity
Gliotoxin
MTT assay
1 Introduction
Mycotoxins are secondary fungal metabolites which pose a major risk to the health of humans, animals and crops (Wild and Gong, 2010). Amongst the mycotoxins, gliotoxin is one of the most abundant metabolites produced by Aspergillus fumigatus and some other types of fungi. Gliotoxin possesses a wide spectrum of biological activities, which include antibacterial and antiviral activities, inhibiting platelet function and is also a potent immunomodulating agent (Waring and Beaver, 1996) (Fig. 1).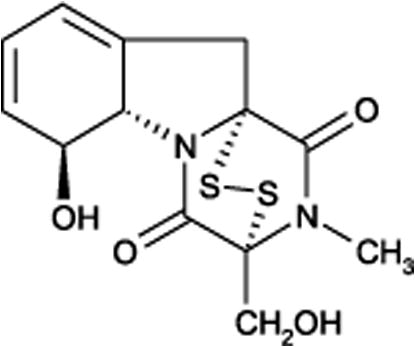
Chemical structure of gliotoxin.
In vitro, gliotoxin has been shown to be able to inhibit the activation of antigen mediated lymphocyte stimulation, cytotoxic-T-cell activation, and gamma interferon production by CD4 lymphocytes (Wichmann et al., 2002). It has also been shown to inhibit mitogenic stimulation of human lymphocytes (Richard et al., 1994). At higher concentrations, gliotoxin induces apoptosis in macrophages and lymphocytes by a mechanism which is distinct from its antiphagocytic effects. This toxicity against the immune system induces the apoptotic cell death of thymocytes, peripheral lymphocytes, macrophages, spleen cells and other characteristic changes caused by DNA fragmentation and adduct formation (Golden et al., 1998; Waring and Beaver, 1996). The toxicity of gliotoxin is thought to be mediated in at least two ways: firstly, through conjugation to proteins with susceptible thiol residues, and their subsequent inactivation; secondly, through generation of reactive oxygen species via redox cycling (Kwon-Chung and Sugui, 2009).
An important property of gliotoxin, which has been specifically identified in human lymphocyte cells following exposure to gliotoxin, is its ability to go through a redox cycle in the presence of an appropriate reducing agent (Waring and Beaver, 1996). Hydrogen peroxide, as well as other reactive oxygen species (ROS) which are produced by gliotoxin during the redox cycle in a cell-free system is known to damage plasmid directly and cellular DNA (Schnabl et al., 2002).
The objective of the present study is to assess the cytotoxic and genotoxic effects of gliotoxin purified from local A. fumigatus isolate and standard gliotoxin (purchased from Sigma–Aldrich for laboratory use) on human lymphocytes in vitro using an MTT assay.
2 Materials and methods
2.1 Extraction and purification of gliotoxin
Gliotoxin was produced by A. fumigatus under the following conditions: A. fumigatus was inoculated on a long grain rice medium for 10 days at 37 °C. The gliotoxin was extracted twice with an electric homogenizer using 50 ml chloroform and filtered through a Whatman No. 1 filter paper. The collected filtrate after extraction with chloroform was pooled and dried by evaporation and stored at 4 °C (Kosalec et al., 2005). The entire dried residue was combined, and dissolved in 2 ml methylene chloride before being filtered through a 0.45 μm Millipore filter. The filtrate was then placed on a silica gel Sep-Pak column and primed with 5 ml of methylene chloride. The Sep-Pak was eluted with 2 ml of hexane, ethyl acetate, chloroform, and methanol and was then evaporated to dryness and dissolved in 1 ml methylene chloride (Richard et al., 1989). After purification, the concentration was determined by Thin Layer Chromatography (TLC) and High Performance Liquid Chromatography (HPLC) and compared with standard gliotoxin, which had been purchased from Sigma–Aldrich PubChem Substance ID 24895384 (molecular weight of 326.39 and produced from Gliocladium fimbriatum).
The TLC plates were used for detection of the gliotoxin with a methylene chloride: methanol (97:3 v/v) solvent system as the mobile phase. The plates were air-dried and gliotoxin was visualized with a spray reagent of 5% silver nitrate in ethanol: water (90:5 v/v).
2.2 Preparation of toxin concentration
Both standard gliotoxin and gliotoxin extract were dissolved in methanol at 1 mg/ml and used to prepare concentrations of 25, 50, 100 and 200 ng/ml in complete culture media (RPMI-1640 medium supplemented with 10% fetal calf serum, containing a solution of penicillin 100 units/ml and streptomycin 100 μg/ml) (Freshney, 2012).
2.3 Collection and processing of human lymphocytes
Peripheral venous blood was taken from a healthy 26 year old male donor and lymphocytes were extracted following Rafael and Vaclav (2000). The suspension of cultured human lymphocytes was adjusted until the number of cells was about 1 × 104 cells/ml. 100 μl of the cell suspension was then dispensed into each of the 96 wells of a microtiter plate to give a final cell count of 1000 cells/well. The plates were then incubated at 37 °C in an incubator supplemented with 5% CO2 for 24 h (Wichmann et al., 2002). After incubation, gliotoxin was added to each well at different concentrations and incubated for 24 h (Rafael and Vaclav, 2000).
2.4 Cytotoxicity assay using the 3-[4,5-dimethylthiazoyl]-2, 5-diphenyltetrazolium bromide (MTT) test
This test was performed by dissolving 3-[4,5-dimethylthiazoyl]-2, 5-diphenyltetrazolium bromide in phosphate buffered saline (PBS) at 2 mg/ml, filtrated by a 0.22 μm millipore filter. 50 μl of the MTT dye was added to each of the microtiter plate wells containing human lymphocytes treated with different concentrations of gliotoxin for 24 h. The MTT-formazan crystals, which are formed only by live cells, were dissolved in 100 μl Dimethyl sulphoxide (DMSO), enabling the optical density of each well to be measured using an ELISA reader at a transmitting wavelength of 620 nm (Freshney, 1994). The inhibitory rate was measured according to Wang et al. (2003) as follows:
2.5 Genotoxicity assay
After the incubation period, all the treated human lymphocyte content of the wells was transferred from the well of the microtiter plate by micropipette before staining to a sterile Eppendorf tube for use in the genotoxicity assay. DNA was extracted from the cultured cells using a genomic DNA mini kit (Geneaid Company). The samples were analysed by electrophoresis on a 1% agarose gel, following Sambrook et al. (1989). Visual observations of DNA were recorded with UV camera system (Abraham et al., 2008). Statistical analysis was performed on all the concentrations and the control with mean ± standard error and differences between means being performed by One Way Analysis of Variance (ANOVA) according to SAS (2004).
3 Results and discussion
3.1 Detection of the gliotoxin by TLC and HPLC
Gliotoxin appeared brown in colour (under visible light) on the TLC plates before spraying with silver nitrate reagent, and yellow in colour under UV at 365 nm. After spraying with silver nitrate, it appeared dark brown in colour under UV at 365 nm (Fig. 2). Similar results were obtained by Abu-Seidah (2003). In the present study, different solvents were trialled for gliotoxin purification in a Sep-Pak column (hexane, ethyl acetate, chloroform and methanol). The results showed that, of these solvents, gliotoxin was only contained in the ethyl acetate fractions (Fig. 2). The use of TLC to separate compounds is based on the competition of the solute and the mobile phase for binding places on the stationary phase. The separation of components is measured by the retardation factor (Rf) value or by using fluorescent reagents that allow the visualization of spots under UV–Cabinet light.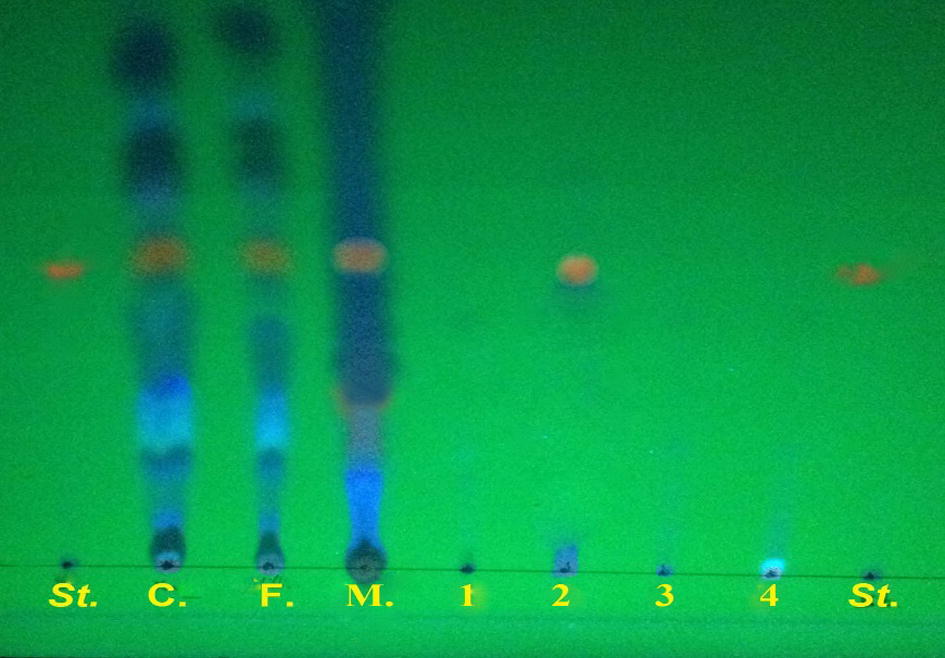
Detection of the gliotoxin by TLC plate, St: standard gliotoxin C: crude extract F: filtered extract by millipore filters 0.45 μm, M: methylene chloride 1: hexane 2: ethyl acetate 3: chloroform 4: methanol.
The sensitivity of TLC method is such that separations of less than microgram amounts of material can be achieved, while for large scale; the preparative. This technique is considered a good technique for extraction and purification in order to achieve a yield sufficient for use in different cytotoxic studies (Al-Atby, 2001).
The gliotoxin extract was analysed against the standard gliotoxin using HPLC analysis with a column with a length of 30 cm × 4.6 mm, and a solvent system composed of methanol/water at a ratio of 43:57 v/v. The gliotoxin extract was revealed to be very satisfactory because it gave a single peak at a retention time of 10.2 min, which was very similar to that of standard gliotoxin (Fig. 3).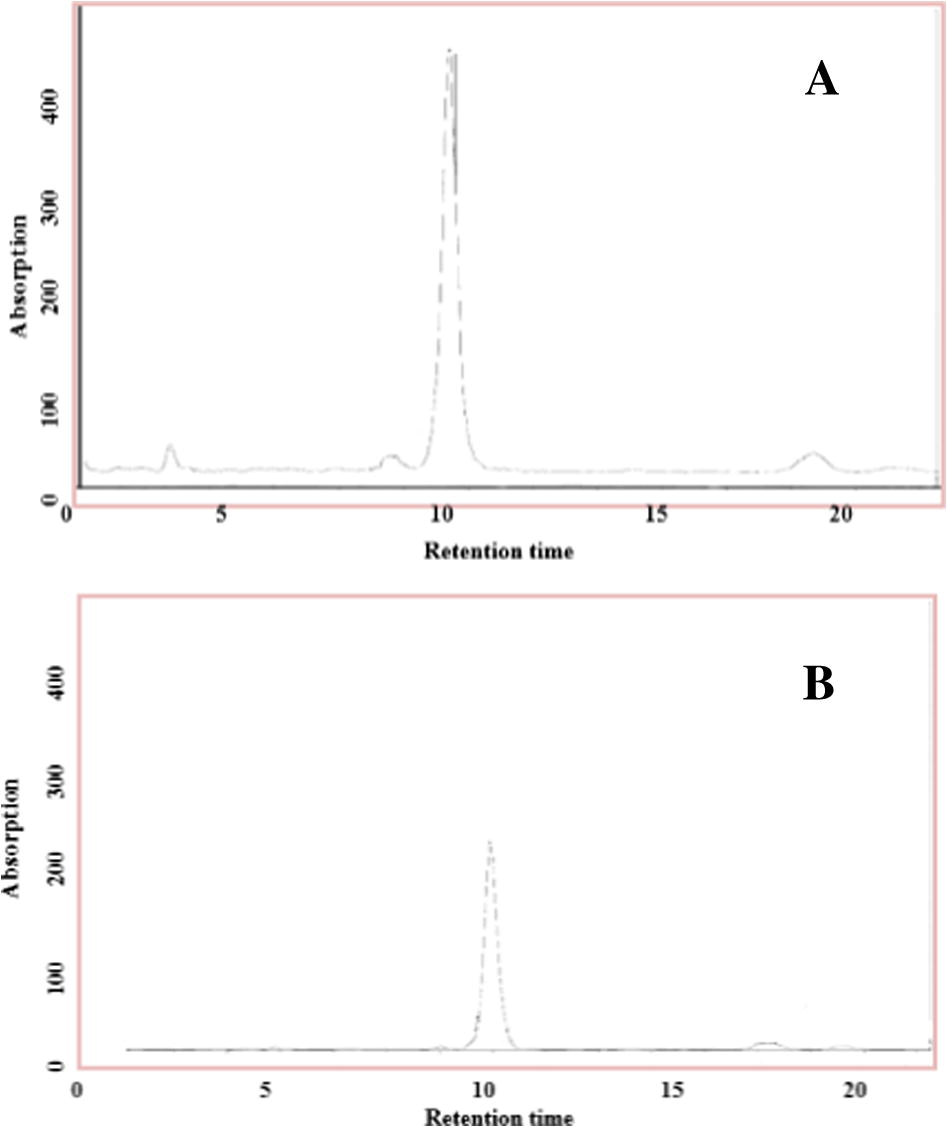
(A) Detection of standard Gliotoxin (B) Detection of cultured gliotoxin extract produced by A. fumigatus in rice medium conditions using HPLC analysis: gliotoxin retention time approximately 10.2 min, mobile phase methanol: water (43:57), wavelength 254 nm, flow rate was 2.0 ml/min, column length (30 cm × 4.6 mm).
Analysis of a wide variety of compounds has been achieved using HPLC, where the separation of a mixture into its components depends on the different degrees of retention of each one in the column. The retention time of 10.2 min observed here differs quite markedly from that reported by Richard et al. (1989) who used HPLC analysis with a column length of 10 cm × 4.6 mm to return a retention time of 4.8 min. This difference may be due to the difference in the length of the column that was used in the separation.
3.2 Cytotoxic effects of gliotoxin on human lymphocytes
A comparison between the cytotoxic effect of different concentrations of the sample gliotoxin extracted from A. fumigatus and the commercially available standard showed a significant difference P < 0.05, Fig. 4.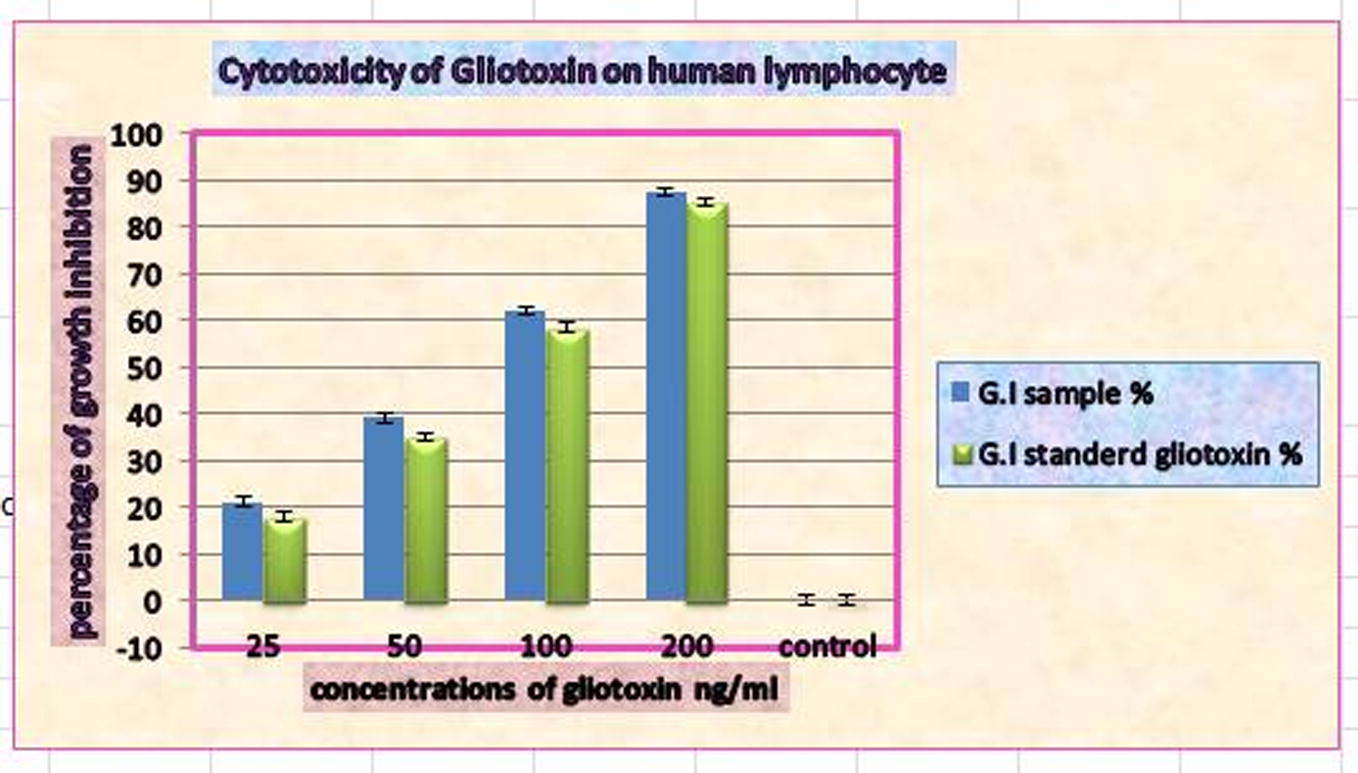
Cytotoxic effect of purified gliotoxin from Aspergillus fumigatus and standard gliotoxin on human lymphocytes after a 24 h incubation period.
The growth inhibition observed on the growth of lymphocytes incubated with concentrations of 200, 100, 50 and 25 ng/ml was 87.45%, 61.99%, 39.10%, 21%, respectively, for the sample gliotoxin and 85.22%, 58.34%, 34.92% and 17.89% for the standard gliotoxin, respectively. The results clearly indicate that the rate of inhibition of growth was concentration dependent. The inhibitory effect was increased when compared with the negative control (Fig. 4).
The active groups OH, CH3, CH2OH and an internal bridge of gliotoxin, or any chemical compound, are responsible for their biological activity. Gliotoxin therefore can inactivate proteins via a sulphide: thiol exchange and the cross linking with proteins occurs via cysteine residues, while the deleterious reactive oxygen species (ROS) observed between the reduced and oxidized form takes place through the redox cycling (Kwon-Chung and Sugui, 2009; Collins et al., 1997).
The colorimetric cytotoxic assay by MTT is widely used for the measurement of cell viability (Cetin and Bullerman, 2005). The assay is dependent on the fact that the MTT dye is reduced to a purple formazan product by live cells. The higher the number of live cells, therefore, the more MTT-formazan is produced. The quantity of MTT-formazan produced can be determined spectro-photometrically once it is solubilized in a suitable solvent (since it is insoluble in aqueous solution) (Abraham et al., 2008).
Gliotoxin induces apoptosis through the elevation of the cAMP concentration (Waring et al., 1997). Meanwhile, Dheeb et al. (2013) have described how gliotoxin causes morphological changes in normal cells due to a loss of adherence between the cells and their plastic container, which is inherently an apoptotic process. Nierman and Fedorova (2008) have gone on to describe this phenomenon in lymphocytes, spleen cells, thymocytes and macrophages.
Richard et al. (1994) investigated the cytotoxic and immunosuppressive effects of gliotoxin in turkey lymphocytes via a conversion of salt MTT to MTT formazan by the mitochondrial succinate dehydrogenase in living cells (Richard et al., 1994). They concluded that all lymphocyte cells appeared to be dead when they were exposed to gliotoxin at a concentration of 100 ng/ml, but that little or no response was evident below 100 ng/ml. The level of cytotoxicity in the lymphocyte proliferation assay was correlated with that demonstrated in the MTT test. McMinn et al. (1991), on the other hand, using a murine model, observed a reduction in the number of Langerhans cells as well as ultrastructural damage even when gliotoxin concentrations in comparison with control were lower than 100 ng/ml. Furthermore, they noted that, at lower concentrations, the toxin appeared to stimulate a proliferation response and increase the conversion of the salt to MTT formazan using mice and turkey lymphocytes.
Sutton et al. (1995) also used lymphocytes from mice to study the effect of gliotoxin. Their results showed that gliotoxin has potential clinical applications as an immunosuppressive agent in preventing allograft rejection. At low doses (<30 nM) gliotoxin displays co-mitogenic activity, and decreases intracellular cAMP levels, but at higher concentrations it induces apoptosis in cells and increases intracellular cAMP levels. The effects of gliotoxin on cell activation and the induction of apoptosis, therefore, may both be mediated by changed levels of cAMP.
Dheeb et al. (2013) studied the in vitro effects of different concentrations (0.12–125 ng/ml) of gliotoxin on the growth of the human hepatocellular carcinoma HepG2 cell line. Their results indicated that the rate of growth inhibition was concentration dependent. Ben-Ami et al. (2009) show that gliotoxin affects platelet function and inhibits angiogenesis in vitro at concentrations ranging from 30 to 3000 ng/ml.
Two of the properties of gliotoxin that might assist A. fumigatus in the colonization of the respiratory mucosa are that it causes damage to the ciliated respiratory epithelium and that it inhibits the oxidative burst of human neutrophils in vitro (Nierman and Fedorova, 2008).
3.3 Genotoxic effects of gliotoxin on human lymphocytes
DNA was extracted from lymphocytes treated with 200, 100, 50 and 25 ng/ml concentrations of gliotoxin and then analysed by electrophoresis on a 1% agarose gel. The gliotoxin appeared to have the ability to degrade or damage the DNA with the extent of damage increasing gradually as the concentration of gliotoxin increased (Fig. 5).
Agarose gel electrophoresis of the DNA extracted from human lymphocytes after being treated with different concentrations of standard gliotoxin and purified gliotoxin showing degradation of lymphocyte DNA when compared with control. M: marker, Nc: negative control, C: control, Yellow No.: concentrations of purified gliotoxin, White No.: concentrations of standard gliotoxin.
The known biological properties of gliotoxin include the capability to undergo redox cycling to generate oxygen free radicals that cause oxidative damage to isolated DNA in vitro (Eichner et al., 1988). This toxicity against the immune system induces apoptotic cell death of thymocytes, peripheral lymphocytes, macrophages, spleen cells and other characteristics, for instance by DNA fragmentation and adduct formation (Golden et al., 1998). Gliotoxin is involved with a number of proteins in the formation of disulphide bridges (Gardiner and Howlett, 2005). This toxicity is thought to be mediated in at least two ways. First, conjugation to proteins with susceptible thiol residues and subsequent inactivation, second, generation of reactive oxygen species via redox cycling (Kwon-Chung and Sugui, 2009). Also, the DNA-reactivity of gliotoxin has been investigated in some in vitro studies (Golden et al., 1998), which demonstrate that the reactive oxygen species produced by gliotoxin are capable of evoking DNA damage. This damage in DNA in cells treated with gliotoxin was observed at 24 h. Higher concentrations of gliotoxin (0.8–1.6 μg/ml), are toxic to the cells (Nieminen et al., 2002).
4 Conclusions
-
The sample gliotoxin shows a higher cytotoxic effect than standard gliotoxin.
-
The inhibition % in the growth of human lymphocytes increased with an increase in gliotoxin concentration.
-
Gliotoxin showed high genotoxic effects, even at low concentrations and DNA damage increased with an increase in gliotoxin concentration.
Acknowledgement
The authors wish to thank all the individuals and institutions who made this research possible.
References
- Application of a high-content multiparameter cytotoxicity assay to prioritize compounds based on toxicity potential in humans. J. Biomol. Screen.. 2008;13(6):527-537.
- [Google Scholar]
- Secondary metabolites as co-markers in the taxonomy of Aspergilli. Acta Microbiol. Pol.. 2003;52(1):15-23.
- [Google Scholar]
- Al-Atby, S.M.H., 2001. Effect of crude alcoholic extract of Withania Somnifera on growth of cancer cell line in vitro and on some physiological parameter in mice (Ph.D. thesis). College of Veterinary Medicine Univ. of Baghdad, Iraq.
- Aspergillus fumigatus inhibits angiogenesis through the production of gliotoxin and other secondary metabolites. Blood. 2009;114(26):5393-5399.
- [Google Scholar]
- Cytotoxicity of Fusarium mycotoxins to mammalian cell cultures as determined by the MTT bioassay. Food Chem. Toxicol.. 2005;43(5):755-764.
- [Google Scholar]
- Major DNA fragmentation is a late event in apoptosis. J. Histochem. Cytochem.. 1997;45(7):923-934.
- [Google Scholar]
- Cytotoxic effect of aflatoxin B1, gliotoxin, fumonisin B1, and zearalenone mycotoxins on HepG2 cell line in vitro. Int. J. Adv. Res.. 2013;1(8):355-363.
- [Google Scholar]
- Gliotoxin causes oxidative damage to plasmid and cellular DNA. J. Biol. Chem.. 1988;263(8):3772-3777.
- [Google Scholar]
- Culture of Animal Cell (sixth ed.). New York: Wiley-Liss; 2012.
- Culture of Animal Cells: A Manual for Basic Technique. New York: Wiley-Liss, A John Wiley and Sons, Inc., Publication; 1994.
- Bioinformatic and expression analysis of the putative gliotoxin biosynthetic gene cluster of Aspergillus fumigatus. FEMS. Microbiol. Lett.. 2005;248(2):241-248.
- [Google Scholar]
- DNA damage by gliotoxin from Aspergillus fumigatus. An occupational and environmental propagule: adduct detection as measured by 32P DNA radiolabelling and two-dimensional thin-layer chromatography. Mycoses. 1998;41:97-104.
- [Google Scholar]
- Influence of media and temperature on gliotoxin production in Aspergillus fumigatus strains. Arh. Hig. Rada. Toksikol.. 2005;56(3):269-273.
- [Google Scholar]
- What do we know about the role of gliotoxin in the pathobiology of Aspergillus fumigatus? Med. Mycol.. 2009;47(Suppl 1):S97-103.
- [Google Scholar]
- Langerhans cell depletion in gliotoxin-treated murine epidermis. Pathology. 1991;23(1):39-44.
- [Google Scholar]
- Genotoxicity of gliotoxin, a secondary metabolite of Aspergillus fumigatus, in a battery of short-term test systems. Mutat. Res.. 2002;520(1–2):161-170.
- [Google Scholar]
- Subtelomeric diversity as a major force in evolution of Aspergillus secondary metabolism and virulence pathways. In: 3rd Advances against Aspergillosis, January 16–19, 2008, MiamiBeach, Florida. San Diego, USA: University of California; 2008. p. :77.
- [Google Scholar]
- Rafael F., Vaclav V., eds. Advanced Methods in Cellular Immunology. Vol vol. 3. USA: CRC-Press, LLC; 2000.
- Gliotoxin inhibits transformation and is cytotoxic to turkey peripheral blood lymphocytes. Mycopathologia. 1994;126(2):109-114.
- [Google Scholar]
- Use of thin layer chromatography for detection and high performance liquid chromatography for quantitating gliotoxin from rice cultures of Aspergillus fumigatus fresenius. Mycopathologia. 1989;107(2):145-151.
- [Google Scholar]
- Molecular cloning: a laboratory manual (second ed.). Plainview, NY, USA: Cold Spring Harbor Laboratory Press; 1989.
- SAS users, 2004. Guide personal computer (ver.7), inst. Inc., Cary, NC, USA.
- Immortal activated human hepatic stellate cells generated by ectopic telomerase expression. Lab Invest.. 2002;82(3):323-333.
- [Google Scholar]
- Evidence that gliotoxin enhances lymphocyte activation and induces apoptosis by effects on cyclic AMP levels. Biochem. Pharmacol.. 1995;50(12):2009-2014.
- [Google Scholar]
- Evaluation of immunologic cross-reaction of antiasparaginase antibodies in acute lymphoblastic leukemia (ALL) and lymphoma patients. Leukemia. J.. 2003;17(8):1583-1588.
- [Google Scholar]
- Apoptosis induced by gliotoxin is preceded by phosphorylation of histone H3 and enhanced sensitivity of chromatin to nuclease digestion. J. Biol. Chem.. 1997;272(29):17929-17936.
- [Google Scholar]
- Gliotoxin and related epipolythiodioxopiperazines. Gen. Pharmacol.. 1996;17(8):1311-1316.
- [Google Scholar]
- The mycotoxins citrinin, gliotoxin, and patulin affect interferon-gamma rather than interleukin-4 production in human blood cells. Environ. Toxicol.. 2002;17(3):211-218.
- [Google Scholar]
- Mycotoxins and human disease: a largely ignored global health issue. Carcinogenesis. 2010;31(1):71-82.
- [Google Scholar]







