Translate this page into:
Preliminary phytochemical screening and in vitro antibacterial activity of Anamirta cocculus (Linn.) seeds
*Corresponding author. Tel.: +91 9443046081 drissacpaul@gmail.com (V.I. Paul)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Available online 23 April 2014
Peer review under responsibility of King Saud University.

Abstract
The present work is aimed at primary screening of the phytochemical contents of seven solvents (petroleum ether, benzene, chloroform, acetone, methanol, ethanol and a mixture of methanol and ethyl acetate (1:3)) extracts of the seeds of Anamirta cocculus (Linn.) and to evaluate the antibacterial activity of these extracts against five species of pathogenic bacteria. The powdered seeds of A. cocculus were extracted with seven solvents (polar and non-polar) with Soxhlet apparatus and the extracts were subjected to preliminary phytochemical screening by standard biochemical tests. The antibacterial activity of various extracts was evaluated against five bacterial species (Staphylococcus aureus, Proteus vulgaris, Escherichia coli, Salmonella typhi and Klebsiella pneumoniae). The zone of inhibitions and minimum inhibitory concentration (MIC) of the various extracts were determined by running the experiments in triplicates. The extracts of the seeds of A. cocculus contained various pharmaceutically active substances viz., aldehydes, alkaloids, phenolic compounds, flavonoids, saponins, carbohydrates, proteins, lipids, glycosides, phytosterols, volatile oils, gums and mucilage and other minor phytochemicals. All the extracts showed significant levels of antibacterial activity. Methanol extract was the most active one with remarkable antibacterial activity on the various species tested. MICs of the extracts revealed methanol extract as the most potent one with the lowest inhibitory concentration of 3 μg/mL on E. coli. The findings of the present study indicated that the seeds of A. cocculus possess various secondary metabolites having the potential for developing pharmaceutical drugs, especially antimicrobial ones.
Keywords
Anamirta cocculus
Phytochemical screening
Minimum inhibitory concentration
Antibacterial activity
1 Introduction
Several plants and herbs that are used traditionally have potential antimicrobial and antiviral properties and such reports have raised the optimism about phyto-antimicrobial agents (Das et al., 1999). During the last decade, reports of prevalence of resistance among microbes have increased astronomically but the development of antimicrobial drugs has not maintained the pace with the rate of development of drug resistance. A probable way to overcome this trend is the identification of more plant extracts with potential antimicrobial activities. Anamirta cocculus (Linn.) is a wild woody climber belonging to the family Menispermaceae, distributed throughout India as well as South-East Asia. It is primarily a piscicidal plant which produces various primary and secondary metabolites (Satya and Paridhavi, 2012). Its seeds are known as Indian fish berry or crow killer and are being exploited by humans for hunting and fishing (Jothivel and Paul, 2008a). The seeds are also utilised in eradicating the unwanted wild fishes from aquaculture ponds (Jothivel and Paul, 2008b). Picrotoxin (cocculin) is the major reported toxic component of the seed and is composed of poisonous picrotoxinin and the bitter non-poisonous picrotin. Altogether five sesquiterpene lactones viz., picrotoxinin, methyl picrotoxate, picrotin, dihydroxypicrotoxinin and picrotoxic acid are isolated from the seeds of A. cocculus (Satya and Paridhavi, 2012). The possibility of the presence of other secondary metabolites in these seeds cannot be ruled out as it has been used in traditional medicine for treating various diseases (Mutheeswaran et al., 2011). Therefore, it is essential to conduct detailed phytochemical studies to ascertain the presence of more pharmaceutical as well as other compounds in the seeds of A. cocculus. As the problem of antibiotic resistance is growing among the microbes, the search for alternative antimicrobial drugs is absolutely essential.
Irrespective of the traditional usages, phytochemical analytical studies on A. cocculus are very much limited. In this context attempts have been made in the present investigation for the preliminary screening of the phytochemicals present in the seeds of A. cocculus using seven types of solvent extracts. The solvents were selected on the basis of their polarity indices and in the increasing order of polarity the selected solvents are petroleum ether, benzene, chloroform, acetone, methanol, ethanol, and a mixture of methanol and ethyl acetate (1:3). To the best of our knowledge, efforts have been made for the first time to compare the antimicrobial potencies of these extracts using five strains of bacteria viz., Staphylococcus aureus (S. aureus), Proteus vulgaris (P. vulgaris), Escherichia coli (E. coli), Salmonella typhi (S. typhi) and Klebsiella pneumoniae (K. pneumoniae).
All the bacterial strains used in the present study are disease causing species, particularly on humans. K. pneumoniae causes necrosis and inflammation in human beings, urinary tract infections, meningitis and other related problems. While S. aureus is a source of nosocomial infections as well as enterotoxins, P. vulgaris and E. coli cause urinary tract infections and S. typhi causes typhoid fever. Diseases due to these bacterial strains are prevalent in the Indian subcontinent and antibiotics are turning out to be quite expensive to a larger segment of the population. As a developing country, in India investigations on phytochemicals of ethnomedicinal origin are extremely needed for the development of affordable antibiotics and hence the present study finds the necessity.
2 Materials and methods
2.1 Plant material and chemicals
Seeds of A. cocculus were collected from the wild from Kerala state, India and the plant was identified and taxonomically authenticated by the Division of Botany, Kerala Forest Research Institute, Kerala, India as reported by Jothivel and Paul (2008a,b). The seeds were dried properly before the commencement of the experiment. Chemicals and solvents were procured from SD Fine (analytical grade), India. Other reagents were purchased from Hi-Media, India.
2.2 Preparation of seed extracts
Endosperms were collected from the dried seeds of A. cocculus. Five hundred grams each of the powdered endosperms were packed in the thimble of the Soxhlet apparatus and were extracted separately with petroleum ether, benzene, chloroform, acetone, methanol, ethanol and a mixture of methanol and ethyl acetate (1:3). Each extract was concentrated using a rotary vacuum evaporator and the percentage of yield was calculated following Maizura et al. (2011) using the formula, % yield = weight of crude extract/initial weight of sample × 100. The pH of all the extracts was determined using a digital pH metre and were subjected to physico-chemical characterisation. Various parameters such as loss during vacuum evaporation were checked following Bennerman et al. (1983) and Manalo et al. (1983).
2.3 Phytochemical tests
Preliminary phytochemical evaluation of all the extracts was performed by following standard methods of Sofowara (1993) and Trease and Evans (1989).
2.4 Bacterial strains
Pure bacterial strains used for the study were procured from the Department of Microbiology, Faculty of Agriculture, Annamalai University. They were S. aureus, S. typhi, K. pneumoniae, E. coli and P. vulgaris. Separate sterile nutrient agar slants were prepared and the bacterial strains were individually inoculated into separate slants under aseptic conditions and incubated at 37 °C for 24 h. Colonies were harvested separately under aseptic condition from the slants and individually inoculated into sterile nutrient broths in separate test tubes and kept in refrigerated condition.
2.4.1 Antimicrobial assay
Antimicrobial activities of the seed extracts and the standard (Rifampicin) were tested using disc diffusion method of Peach and Tracey (1956). Petri dishes and the Mueller–Hinton agar medium were sterilized for 20 min at 120 °C. Twenty-five millilitres of the medium was poured into sterile Petri dishes and allowed to get solidified under laminar airflow. From the respective nutrient broth subculture (bacterial concentration of 5 × 105 CFU/mL), bacteria were swabbed on the medium in Petri dishes separately using sterilized cotton swabs. Filter paper (Whatman No. 1) discs of 5 mm diameter were prepared and sterilized. Extracts to be tested were prepared by making five different concentrations of 20, 40, 60, 80, and 100 μg/mL in DMSO. Various concentrations were added to separate discs of holding capacity of 10 μL. The impregnated sterile discs with the seed extracts and standard were then placed carefully on the surface of the respective inoculated Petri dishes with separate sterilized forceps and gently pressed down to ensure complete contact of the disc with the agar surface. Filter paper discs soaked in DMSO alone were used as controls and the respective solvents as negative controls. After incubation for 24 h, the diameters of the inhibition zones were measured with micro scale. Each experiment was repeated at least three times and the mean of the diameter of the inhibition zones was calculated.
2.4.2 Determination of minimum inhibitory concentration (MIC)
The MICs (the lowest concentration that will inhibit the visible growth of the microorganism) of all the above extracts for all the five strains of bacteria were determined using broth dilution method of Forbes et al. (2007) and Talaro and Talaro (2002). To determine the MICs, the extracts were added in serial dilutions to a series of tubes containing Mueller–Hinton broth so that the concentrations ranged from 1 to 20 μg/mL. The bacterial concentration in each tube was 5 × 105 CFU/mL. Growth control and negative growth controls were also prepared. After 24 h of incubation at 37 °C, the lowest concentration of the respective extract that led to the inhibition of the growth of bacteria was considered as MIC. Data represent at least three replicated experiments per microorganism.
2.5 Statistical analysis
Experiments were replicated three times and the mean and standard deviation ( ± SD) of data were calculated statistically.
3 Results
3.1 Physico-chemical analysis of the seed extracts of A. cocculus
The preliminary examination of various extracts is summarised in the Table 1. Yield was maximum in the case of ethanolic extraction and minimum in the case of benzene. On the other hand, loss on evaporation was maximum in the case of the benzene extract. All the extracts were acidic in nature.
Particulars
Petroleum ether extract
Benzene extract
Chloroform extract
Acetone extract
Methanol extract
Ethanol extract
Methanol–ethyl acetate extract
Extractive value (yield)
22%
17.5%
19%
25%
25%
31%
25%
Colour
White
Yellowish white
Dark brown
Light brown
Dark brown
Brown
Grayish white
Consistency
Powder
Waxy solid
Waxy solid
Waxy solid
Waxy solid
Waxy solid
Waxy solid
Loss on evaporation
2.2%
5.5%
1%
1%
2%
1%
3.5%
pH
4.23
4.07
4.11
4.72
3.89
3.39
3.30
3.2 Phytochemical screening
Preliminary screening of all extracts (Table 2) showed the presence of a variety of phytochemicals including alkaloids, carbohydrates, glycosides, proteins, phytosterols, lipids, phenolic compounds, flavonoids, gums and mucilages, volatile oil, saponins, aldehydes etc. The respective tests for the various phytochemical moieties and their results are presented in Table 2. Note: 1 = petroleum ether extract; 2 = benzene extract; 3 = chloroform extract; 4 = acetone extract; 5 = methanol extract; 6 = Ethanol extract; 7 = methanol–ethyl acetate extract. + = present; ++ = strongly present; – = absent.
S. No.
Phytochemical tests
1
2
3
4
5
6
7
1
Molisch’s test (carbohydrates)
–
–
–
+
–
+
+
2
Fehling’s test (sugar)
–
–
++
++
+
–
–
3
Benedict’s test (sugar)
–
–
+
+
+
–
–
4
Borntrager’s test (glycosides)
–
–
–
+
–
+
–
5
Biuret test (proteins)
+
–
–
–
+
–
+
6
Ninhydrin test (amino acids)
–
–
–
–
+
–
–
7
Spot test (fixed oils)
+
+
+
+
+
+
+
8
Saponification test (fixed oils and fat)
+
+
–
–
–
+
–
9
Liberman Burchard’s test (phytosterols)
++
–
++
–
+
+
+
10
Mayer’s test (alkaloids)
+
+
−
−
+
−
−
11
Wagner’s test (alkaloids)
+
+
−
−
+
−
−
12
Hager’s test (alkaloids)
++
+
−
−
++
−
−
13
Foam test (saponins)
−
−
+
–
++
−
−
14
Ferric chloride test (phenolic compounds)
−
−
+
−
+
−
−
15
Gelatin test (phenolic compounds)
−
−
++
−
+
−
−
16
Lead acetate test (phenolic compounds)
−
−
+
–
++
−
–
17
Alkaline reagent test (flavonoids)
−
−
–
–
+
−
–
18
Volatile oil test (volatile oil)
–
–
−
+
+
+
−
19
Schiff’s reagent test (aldehydes)
−
−
+
−
−
−
+
20
Whistler and Miller’s test (gums and mucilages)
–
–
+
–
–
–
–
When compared to the benzene extract, the abundance of alkaloids in petroleum ether and methanol extracts was evidenced by the formation of strong yellow precipitate in the test tube by the Hager’s test (Table 2). Chloroform and methanol extracts showed the presence of higher amounts of phenolic compounds. They also showed the presence of saponins by the formation of 1.5 cm thick foam layer in the test tube. Methanol extract also confirmed the presence of flavonoids. While ethanol, acetone and methanol extracts confirmed the presence of volatile oil, chloroform extract showed the presence of aldehydes, gums and mucilage. Aldehydes were also associated with the mixture of methanol and ethyl acetate extract.
3.3 Antimicrobial assay
Results of the antimicrobial activity by the disc diffusion method of all the seven types of extracts of A. cocculus seeds along with the reference drug Rifampicin are presented in Fig. 1(a–g). It could be observed that the methanol extract showed the highest antimicrobial activity against S. typhi at 100 μg/mL with a 20 ± 0.31 mm zone of inhibition. Diameters of growth inhibition areas of extracts studied were in the range 5 ± 1.10 to 20 ± 0.31 mm and that of the standard drug (20 μg/mL) was in the range of 11 ± 0.10 to 13 ± 0.21. No inhibitory activities were seen against P. vulgaris and E. coli by ethanol extract and P. vulgaris, E. coli, S. typhi by benzene extract. Similarly chloroform and methanol–ethyl acetate extracts showed no activity on S. typhi. Even though zones of inhibitions were produced by acetone, methanol and petroleum ether extracts; methanol extract was most active followed by petroleum ether extract and acetone extract. When compared to the standard commercial antimicrobial agent (Rifampicin) used, all the extracts expressed potential antimicrobial activity against the respective sensitive strains used in the present study and their zones of inhibitions were also prominent (Fig. 1).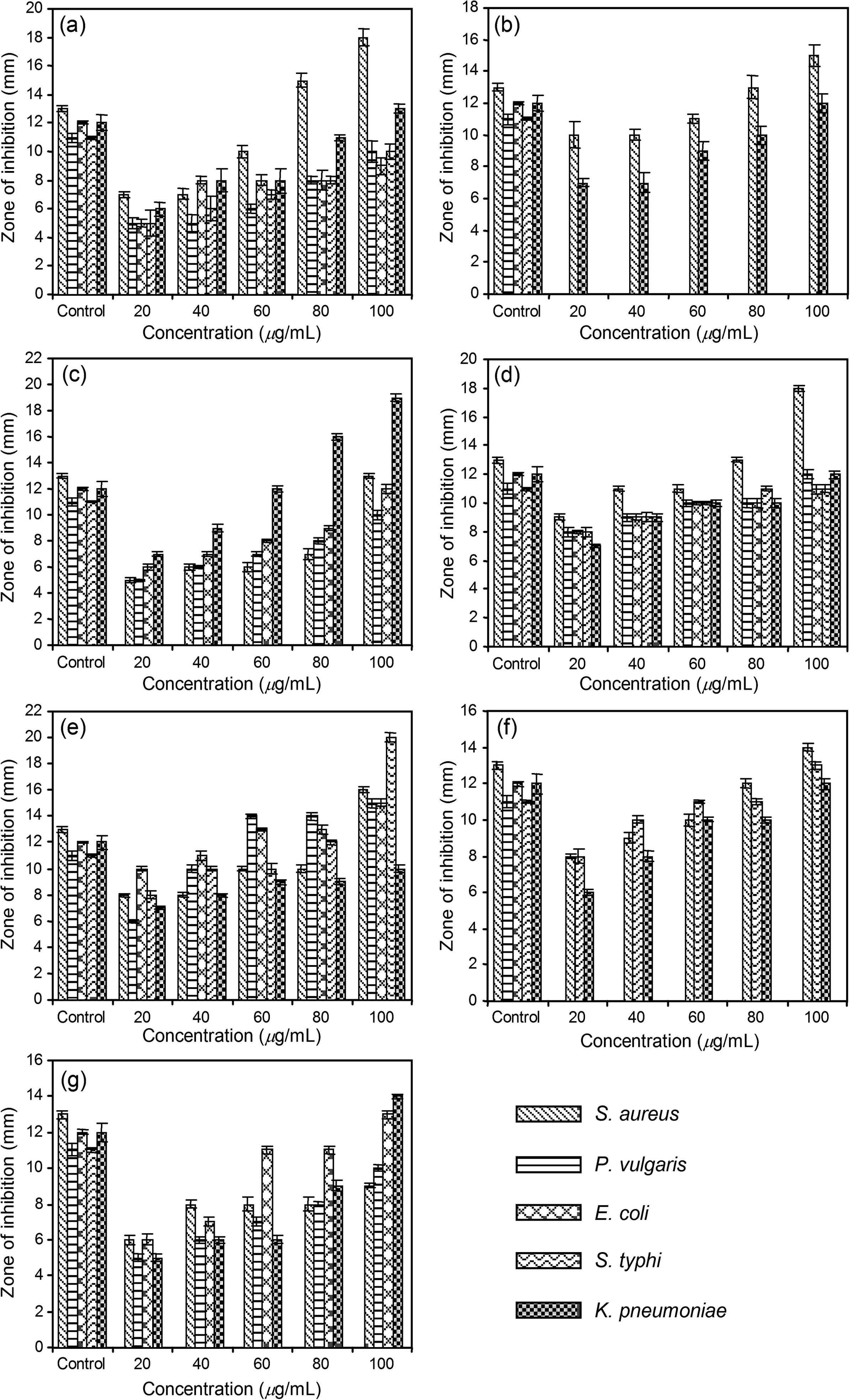
Antimicrobial activity of (a) petroleum ether, (b) benzene, (c) chloroform, (d) acetone, (e) methanol, (f) ethanol and (g) methanol–ethyl acetate (1:3) extracts of A. cocculus seeds at different concentrations against various tested bacterial strains.
3.3.1 Minimum inhibitory concentration
The obtained MIC values (Table 3) confirmed the significant antimicrobial properties of the screened A. cocculus extracts. MIC values of the extracts ranged from 3 to 15 μg/mL. Among the extracts, methanol fraction showed the lowest MIC value of 3 μg/mL against E. coli (Table 3) followed by S. aureus and S. typhi having 5 μg/mL each and P. vulgaris and K. pneumoniae with 7 μg/mL each. On the other hand, methanol–ethyl acetate fraction showed the highest MIC value of 15 μg/mL with P. vulgaris. NT = not tested as there was no activity.
A. cocculus extracts
MIC (μg/mL)
S. aureus
P. vulgaris
E. coli
S. typhi
K. pneumoniae
Petroleum ether extract
9
11
11
11
9
Benzene extract
7
NT
NT
NT
9
Chloroform extract
13
13
11
NT
9
Acetone extract
5
7
7
7
7
Methanol extract
5
7
3
5
7
Ethanol extract
7
NT
NT
7
11
Methanol–ethyl acetate extract
13
15
13
NT
13
4 Discussion
Variations in the extractive values of different solvents (Table 1) used might be due to the differential solubility of the constituents of the seed in these solvents. The rich presence of fats and fatty acids in the seeds of A. cocculus might be rendering the extracts a waxy-solid consistency as well as an acidic nature. The traditional use of A. cocculus as a remedy for barber’s itch, scald-head itch and other unyielding skin diseases indicates its antimicrobial role. Due to the presence of various bioactive compounds in A. cocculus, antibacterial, antifungal and anti-inflammatory properties have been attributed to it (Satya and Paridhavi, 2012). Phenolic compounds, alkaloids, and glycosides detected in the extracts (Table 2) are compounds that have been documented to possess medicinal properties including antibacterial activity (Okwu, 2004; Afolabi et al., 2007).
The toxicity of polyphenols in microorganisms is generally due to iron deprivation or hydrogen bonding with microbial enzymes affecting their activity or enzyme inhibition by the oxidation of phenolic compounds (Scalbert, 1991; Davidson and Naidu, 2000). Therefore the conspicuous antimicrobial activity exhibited by methanol and chloroform extracts in the present study may be attributed to the presence of phenolic compounds in it. Phenolic compounds are also reported to have anti-allergenic, anti-inflammatory and anti-thrombotic effects (Puupponen-Pimia et al., 2001; Williamson and Manach, 2005). In addition to the antimicrobial activity; alkaloids, glycosides and phenols present in plant extracts are so potent and are reported to cause mortality even in ticks also (Kumar et al., 2011). Similarly plant proteins (especially lectins and polypeptides) also possess antimicrobial activity and can block microbial infusion or adsorption by forming disulphide bridges (Tiwari et al., 2011). The observed antimicrobial activity in the present study may also be due to the presence of these metabolites that are present in the protein content of the seeds of A. cocculus (Table 2). Flavonoids are an important group of polyphenols and are known to have antimicrobial and anti-inflammatory properties (Miller, 1996). Due to these inherent properties, flavonoids are often referred to as nature’s biological response modifiers. Presence of flavonoids in the methanol extracts of A. cocculus seeds indeed present an opportunity for further exploration for the potential usage of these compounds for antimicrobial action. According to Cowan (1999) the antibacterial activity of flavonoids is due to their ability to complex with extra cellular and soluble proteins as well as with the bacterial cell wall.
Alkaloid derivatives of plant origin are widely used as antimicrobial agents. Presence of heterocyclic nitrogen containing alkaloids in benzene, petroleum ether and methanol extracts (Table 2) indicates the pharmacological significance of A. cocculus. Alkaloids isolated from the plants of the Ranunculaceae family as well as Solanum khasianum are also reported to have antimicrobial properties (McMahon et al., 1995; Omulokoli et al., 1997). Berberine is an important representative of the alkaloid group and is reported to be present in the seeds of A. cocculus (Jayasinghe et al., 1993; Satya and Paridhavi, 2012). It is potentially effective against trypanosomes and plasmodium (Freiburghaus et al., 1996; Omulokoli et al., 1997) also. The mechanism of action of this highly aromatic planar quaternary alkaloid is attributed to its ability to intercalate with DNA (Cowan, 1999). A. cocculus seeds are also sources for the nontoxic alkaloids menispermine and para-menispermine (Satya and Paridhavi, 2012). Given the rich presence of alkaloids in the seeds of A. cocculus and its traditional and folkloric uses as antimicrobial agent, further studies are essential for the optimum exploitation of this seed.
The presence of volatile oil in ethanol, acetone and methanol extracts of seeds of A. cocculus (Table 2) might also be imparting the antibacterial and anti-inflammatory activities as essential oils are reported to have excellent antibacterial and anti-inflammatory properties (Doughari, 2012). According to Sahoo et al. (2012), volatile oils can cause disruption of membranes in microbes by the action of the lipophilic compounds in these oils and thereby imparting antibacterial activity. They are also used as antiviral agents (Edris, 2007).
The present study has confirmed the presence of saponins in chloroform and methanol extracts (Table 2). Saponins are extremely poisonous, as they cause heamolysis of blood and are known to cause cattle poisoning (Kar, 2007). However, they are also having beneficial pharmacological effects including anti-inflammatory, anti-parasitic and anti-viral properties (Just et al., 1998; Traore et al., 2000). Therefore the presence of saponins in chloroform and methanol extracts might have contributed to their antimicrobial functions.
Glycosides are known to have antifungal properties (Abbassy et al., 2007). Some of the plant derived glycosides such as anthracene glycosides are used in the treatment of skin diseases (Doughari, 2012). Therefore the presence of glycosides in ethanol and acetone extracts of seeds of A. cocculus (Table 2) could be imparting antimicrobial property. The presence of phytosterols in petroleum ether, ethanol, chloroform, methanol and methanol–ethyl acetate extracts of seeds of A. cocculus (Table 2) is also worth mentioning because of their anti-inflammatory and immunomodulating activities that could support antimicrobial activity (Careri et al., 2001).
The importance of aldehydes as antimicrobial agents is widely accepted and the present study has also confirmed the presence of aldehydes in chloroform and methanol–ethyl acetate extracts of seeds of A. cocculus (Table 2). Recently it has been reported that cinnamic aldehyde could help in reducing oral bacterial growth by more than 50% and is especially effective against bacteria living at the back of the tongue and reduces anaerobic bacteria populations by about 43% (Cabello et al., 2009).
As far as the antimicrobial property of A. cocculus is concerned, the present study has witnessed the potential antimicrobial actions of seven types of extracts on five bacterial species (Fig. 1(a–g)) namely S. aureus, P. vulgaris, E. coli, S. typhi and K. pneumoniae. They are representatives of important pathogens in humans and many investigations related to antibacterial compounds have already been conducted using these species. Antimicrobial activities of herbal products are considered as major alternative measures for the control of harmful bacterial infestation. Basic forms of phytochemicals present in A. cocculus are found to be biologically effective and possess antimicrobial activities. In the present study, even though the antibacterial actions of the extracts are more pronounced on Gram-positive strains, they show remarkable antimicrobial activities against Gram-negative species also (P. vulgaris, E. coli, S. typhi and K. pneumoniae). However in many of the previous reports regarding the antibacterial activities of medicinal plants, most of the active components except a few show actions only against Gram-positive strains (Herrera et al., 1996; Kelmanson et al., 2000; Ali et al., 2001).
It is worth mentioning that all extracts in the present study exhibited antibacterial activity towards some or other (Fig. 1) bacterial strains studied in comparison to the standard commercial drug Rifampicin. This observation may justify the use of A. cocculus in traditional medicines for treating skin diseases and underscore the importance of the ethnobotanical approach in the discovery of new bioactive substances. The tested microorganisms can cause infections like necrosis in human lungs, urinary tract infections, meningitis, typhoid, food borne illness etc. Thus the results of the present study could have clinical importance also. MIC values of 3–15 μg/mL confirmed the bacteriostatic activity of all the tested extracts against the respective bacterial strains used. The results of the present study are also of interest since they have been obtained with crude fractions, and it is widely accepted that plant extracts that are active at 100 mg/mL dilution could be considered to have a good potency level (Rios et al., 1988).
Since all the extracts of A. cocculus seeds showed remarkable antimicrobial activity, several compounds of distinct nature as revealed in the present study (Table 2) might be acting as antimicrobial agents in these extracts. It is possible that these compounds act either singly or in combination to manifest the observed antibacterial properties. The outer cell membrane or cytoplasmic membrane of a bacterium is essentially composed of a phospholipid bilayer and proteins and is the major site of interaction with antimicrobial compounds. Damage to this vital membrane can result in death of the bacterium (Perumalla and Navam, 2011). In the light of the report by Gnanamani et al. (2003), the antibacterial activity of benzene, petroleum ether and methanol extracts as observed in this study may be due to the pore formation in the cell wall and the leakage of cytoplasmic constituents by the active components (alkaloids) present in these extracts. Phenolic constituents also alter the cell morphology by influencing the osmotic pressure of the cell, thus disrupting the cytoplasmic membrane and causing leakage of cell constituents (Sivarooban et al., 2008). Although further work is needed to precisely locate the active principles in various extracts, the methanol extract showed the lowest MIC values, suggesting that this fraction would contain the most active antimicrobial compounds.
5 Conclusion
In the present investigation, the overall results from the preliminary phytochemical screening of the seed extracts (petroleum ether, benzene, chloroform, acetone, methanol, ethanol and methanol–ethyl acetate) of A. cocculus revealed the potentials for developing antimicrobial pharmaceutical substances from them. The basic forms of phytochemicals present in these extracts are alkaloids, carbohydrates, glycosides, proteins, phytosterols, lipids, phenolic compounds, flavonoids, gums and mucilages, volatile oil, saponins and aldehydes. All the seven types of extracts of A. cocculus seeds were tested on five species of bacteriae (S. aureus, P. vulgaris, E. coli, S. typhi and K. pneumoniae) in order to prove their antimicrobial activities. Results indicated that all the seven extracts possessed good antimicrobial activity against some or other Gram-positive and Gram-negative bacteria tested. Our results suggest that A. cocculus seeds can serve as a potential source of pharmaceutically important bioactive compounds which could be useful in controlling the growth of various pathogenic bacteria. Further phytochemical analyses are required to determine and refine the types of compounds responsible for the antibacterial effects expressed by the seeds of A. cocculus.
Acknowledgments
The authors are thankful to the Department of Zoology, Annamalai University and the Department of Microbiology, Faculty of Agriculture, Annamalai University for providing necessary facilities.
References
- Insecticidal, antifeedant and antifungal activities of two glucosides isolated from the seeds of Simmondsia chinensis. Ind. Crop Prod.. 2007;26(3):345-350.
- [Google Scholar]
- Phytochemical constituents and antioxidant properties of extracts from the leaves of Chromolaena odorata. Sci. Res. Essays. 2007;2(6):191-194.
- [Google Scholar]
- Screening of Yemeni medicinal plants for antibacterial and cytotoxic activities. J. Ethnopharmacol.. 2001;74:173-179.
- [Google Scholar]
- Traditional Medicine and Health Care Coverage. Geneva, Switzerland: World Health Organization; 1983.
- The cinnamon-derived Michael acceptor cinnamic aldehyde impairs melanoma cell proliferation, invasiveness, and tumor growth. Free Radic. Biol. Med.. 2009;46(2):220-231.
- [Google Scholar]
- Liquid-chromatography – UV determination and liquid chromatography, atmospheric pressure, chemical ionization, mass spectrometric characterization of sitoterol and stimasterol in soy bean oil. J. Chromatogr.. 2001;935:249-257.
- [Google Scholar]
- Das, S., Pal, S., Mujib, A., Dey, S., 1999. Biotechnology of Medicinal Plants-Recent Advances and Potential, first ed. vol. 2. UK992 Publications, Hyderabad, pp. 126–139.
- Phyto-phenols. In: Naidu A.S., ed. Natural Food Antimicrobial Systems. Boca Raton, FL, USA: CRC Press; 2000. p. :265-294.
- [Google Scholar]
- Phytochemicals: extraction methods, basic structures and mode of action as potential chemotherapeutic agents. In: Rao V., ed. Phytochemicals – A Global Perspective of their Role in Nutrition and Health. Rijeka Croatia: InTech; 2012. p. :1-33.
- [Google Scholar]
- Pharmaceutical and therapeutic potentials of essential oils and their individual volatile constituents: a review. Phytother. Res.. 2007;21(4):308-323.
- [Google Scholar]
- Laboratory methods and strategies for antimicrobial susceptibility testing. In: Bailey & Scott’s Diagnostic Microbiology (12th ed.). St. Louis: Mosby Elsevier; 2007. p. :187-214.
- [Google Scholar]
- Evaluation of African medicinal plants for their in vitro trypanocidal activity. J. Ethnopharmacol.. 1996;55:1-11.
- [Google Scholar]
- Antibacterial activity of two plant extracts on eight burn pathogens. J. Ethnopharmacol.. 2003;86:59-61.
- [Google Scholar]
- Antimicrobial activity of extracts from plants endemic to the Canary Islands. Phytother. Res.. 1996;10:364-366.
- [Google Scholar]
- Evaluation of the acute toxicity of the seeds of Anamirta cocculus (Linn.) and its piscicidal effect on three species of freshwater fish. Internet J. Toxicol.. 2008;5:1-11.
- [Google Scholar]
- Exploitation of acute toxicity of the seeds of Anamirta cocculus (Linn.) as a potential aquaculture management tool to eradicate unwanted fish fauna. Asian Fish. Sci.. 2008;21(4):457-467.
- [Google Scholar]
- Anti-inflammatory activity of unusual lupane saponins from Bupleurum fruticescens. Planta Med.. 1998;64(5):404-407.
- [Google Scholar]
- Pharmacognosy and Pharmacobiotechnology (second ed.). New Delhi: New Age Int. Ltd.; 2007. p. :332-600.
- Zulu medicinal plants with antibacterial activity. J. Ethnopharmacol.. 2000;69:241-246.
- [Google Scholar]
- Phytochemical analysis of some indigenous plants potent against ectoparasite. Asian J. Exp. Biol. Sci.. 2011;2(3):506-509.
- [Google Scholar]
- Total phenolic content and antioxidant activity of kesum, ginger and turmeric extract. Int. Food Res. J.. 2011;18:529-534.
- [Google Scholar]
- Studies on ether soluble neutral compounds of Peperamia pellucida. Arch. Pharm. Res.. 1983;6:133-136.
- [Google Scholar]
- Michellamine B; a novel plant alkaloid, inhibits human immunodeficiency virus induced cell killing by at least two distinct mechanisms. Antimicrob. Agents Chemother.. 1995;39:484-488.
- [Google Scholar]
- Antioxidant flavonoids: structure function and clinical usage. Altern. Med. Rev.. 1996;1(2):103-111.
- [Google Scholar]
- Documentation and quantitative analysis of the local knowledge on medicinal plants among traditional siddha healers in Virudhunagar district of Tamilnadu, India. J. Ethnopharmacol.. 2011;137(1):523-533.
- [Google Scholar]
- Phytochemical and vitamin content of indigenous spices of South Eastern Nigeria. J. Sustain. Agric. Environ.. 2004;6:30-34.
- [Google Scholar]
- Antiplasmodial activity of four Kenyan medicinal plants. J. Ethnopharmacol.. 1997;56:133-137.
- [Google Scholar]
- Modern Methods of Plant Analysis. Berlin: Springer Verlag; 1956. p. :645-653.
- Green tea and grape seed extracts-potential applications in food safety and quality. Food Res. Int.. 2011;44:827-839.
- [Google Scholar]
- Antimicrobial properties of phenolic compounds from berries. J. Appl. Microbiol.. 2001;90:494-507.
- [Google Scholar]
- Screening methods for natural products with antimicrobial activity: a review of the literature. J. Ethnopharmacol.. 1988;23:127-149.
- [Google Scholar]
- Antibacterial activity of mangrove leaf extracts against human pathogens. Indian J. Pharm. Sci.. 2012;74(4):348-351.
- [Google Scholar]
- Ethano-botanical, phytochemical and pharmacological review of Anamirta cocculus (Linn.) Wight and Arn. Int. J. Rev Life Sci.. 2012;2(1):1-6.
- [Google Scholar]
- Transmission electron microscopy study of Listeria monocytogenes treated with nisin in combination with either grape seed or green tea extract. J. Food Protect.. 2008;71:2105-2109.
- [Google Scholar]
- Medicinal Plants and Traditional Medicine in Africa. Ibadan, Nigeria: Spectrum Books Ltd.; 1993. p. :191-289.
- Drugs, microbes, host – the elements of chemotherapy. In: Foundations in Microbiology (fourth ed.). New York: McGraw Hill; 2002. p. :348-379.
- [Google Scholar]
- Phytochemical screening and extraction: a review. Int. Pharm. Scientia. 2011;1(1):98-106.
- [Google Scholar]
- Structure and antiprotozoal activity of triterpenoid saponins from Glinus oppositifolius. Planta Med.. 2000;66(4):368-371.
- [Google Scholar]
- Pharmacognosy (11th ed.). London: Bailliere Tindall; 1989. p. :45-50.
- Bioavailability and bioefficacy of polyphenols in humans. II. Review of 93 intervention studies. Am. J. Clin. Nutr.. 2005;81(1):233S-255S.
- [Google Scholar]







