Translate this page into:
Preparation and characterization of novel CuBi2O4/SnO2 p–n heterojunction with enhanced photocatalytic performance under UVA light irradiation
*Corresponding author at: LCMIA, Laboratory, Faculty of Sciences, University of the Science and the Technology of Oran (USTO M.B), BP 1505 El M’naouar, 31000 Oran, Algeria elaziouti_a@yahoo.com (Elaziouti Abdelkader),
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Available online 7 July 2014
Peer review under responsibility of King Saud University.
Abstract
A novel p-CuBi2O4/n-SnO2 heterostructure photocatalyst with different mass ratios was synthesized by the solid state technique, and characterized by X-ray diffraction (XRD), scanning electron microscopy (SEM) and UV–Vis diffuse reflectance spectroscopy (DRS). The photocatalytic activities of p-CuBi2O4/n-SnO2 photocatalysts were assessed based on the photodegradation of Congo red (CR) dye as a probe reaction under UVA (365 nm) light irradiation. Experimental results showed that the phase composition, surface morphology of particles, and optical absorption of the sample were found to vary significantly with the mass ratios and pH medium. The p-CuBi2O4/n-SnO2 photocatalyst exhibited higher photocatalytic performance as compared with CuBi2O4 and SnO2. The photodegradation reactions were satisfactory correlated with the pseudo-first-order kinetic model. The optimum amount of doped CuBi2O4 was 5 wt% as a result of 58.06% of photoactivity of CR within 100 min under UVA light at pH = 8 and 25 °C, which is about 2 times higher than that of pure SnO2. On the basis of the calculated energy band positions and the active species during photocatalytic process, the mechanism of the enhanced photocatalytic activity was discussed by solid state z-scheme photocatalysis system.
Keywords
CuBi2O4/SnO2 heterostructure
z-scheme photocatalysis system
Congo red
Photocatalytic activity
Band theory
Synergy effect
1 Introduction
Advanced oxidation processes are of great scientific and practical interest in terms of ecology and sustainable development (Fujishima and Honda, 1972; Zhang and Zhu, 2005). The heterogeneous photocatalysis of organic pollutants on semiconductor surfaces has attracted much attention as a ‘green’ technique. Most researches consider heterogenic systems based on TiO2 (Degussa P25, Hombriat UV-100, Aldrich, etc.) owing to their high photocatalytic activity and stability as well as their widespread uses for large-scale water treatment (Wang et al., 2006a,b). However, the intrinsic band gap of TiO2 is 3.2 eV, which requires the excitation wavelength <387.5 nm. It is a major disadvantage of TiO2 using as a photocatalyst working under visible light (⩾420 nm). The high rate of electron–hole recombination often results in a low quantum yield and poor efficiency of photocatalytic reactions. In order tomeet the requirement of future environment and energy technologies, it is necessary to develop highly efficient, non toxic andchemically stable photocatalyst. Semiconductor catalysts such as TiO2 (Xiaoning et al., 2011), SnO2 (Sangami and Dharmaraj, 2012), ZrO2 (Karunakaran et al., 2009) CeO2 (Yongchuan et al., 2014), Fe2O3 (Seiji and Toshiyuki, 2009), Bi2O3 (Zhong et al., 2011), Sb2O3 (Arham et al., 2011), WO3 (Fumiaki et al., 2013) and ZnO (Vora et al., 2009) metal oxides and CdS (Chae et al., 2010;), CdSe (Frame et al., 2008) CdTe (Kovalenko et al., 2004), ZnS (Karunakaran et al., 2009), PbS (Wang et al., 2011) and HgS (Rengaraj et al., 2014) metal chalcogenides have long been investigated for environmental applications. However, their practical uses have been constrained by their low photocatalytic activity under solar light, short-term stability against photo- and chemical corrosion as well as potential toxicity.
SnO2, with a wide band gap of 3.6 eV at 300 K (Derbal et al., 2008), is known as n-type semiconductor and exhibits excellent optical, electrical and chemical properties and high thermal stability. Research has shown that the semiconductor SnO2 material has wide potential applicabilities such as solid-state gas sensors (Ying et al., 2004), transparent conducting electrodes (Chopra et al., 1983), rechargeable Li batteries and optical electronic devices (Aoki and Sasakura, 1970). The tin dioxide (SnO2) has low cost and toxicity, in addition to high availability. This oxide is among the few with the band gap energy near to visible light (Yang et al., 2006; Zhang et al., 2006). During the past decade, SnO2 nanostructures have been one of the most important oxide nanostructures due to their properties and potential application (Cheng et al., 2004). Recently its composites have been studied as promising semiconductors in the photocatalytic decoloration of wastewaters (Xi et al., 2008; Wang et al., 2006a,b; Nayral et al., 2000; Mukhpadhyay et al., 2000; Teeramongkonrasmee and Sriyudthsak, 2000). Although photocatalytic activity of SnO2 has intensively been investigated, the broad band gap energy and the electronic potential position in the conductance and valence bands of this material seriously limit its further application as a photocatalyst utilizing solar energy (Li et al., 2009).
Various strategies in the liquid-phase system have been adopted for size-controlled synthesis of various functional nanomaterials, including transition metal doping (Couselo et al., 2008), noble metal deposition (Sasahara et al., 2006) doping non-metallic elements (Geng et al., 2008), doping transition metal surface photosensitization (Mora-Sero et al., 2007) and coupled polycrystallites or colloidal semiconductors (Bian et al., 2008). Thus, the combination of semiconductor has become a hot topic among researchers in the last decade to improve the photostimulated electron–hole separation and effectively inhibit their recombination. The major characteristic of this technique is to assemble a heterojunction interface between wide and narrow band gap semiconductors with matching energy band potentials. In this way, electric field assisted transportation of charges from one particle to the other through interfaces is favorable for the electron–hole separations in the composite materials, and thus the electron and hole transfer from catalyst to adsorbed substrate can be obtained (Li and Yan, 2009; Liu et al., 2010a,b). The extensive search published on n–n type junction semiconductor systems were mostly focused on SnO2-based photocatalyst materials, such as ZnO/SnO2 (Wang et al., 2002), Fe2O3/SnO2 (Zhuang et al., 2008), SrNb2O6/SnO2 (Xinping et al., 2008), TiO2-SnO2/Fe+3 (Sikong et al., 2010), La2O3/SnO2 (Xia et al., 2006), Ag3PO4/SnO2 (Zhang et al., 2011), RGO/SnO2 (Zhang et al., 2012), Nb6O17/SnO2 (Wang et al., 2010a,b), TiO2/SnO2 (Hou et al., 2007; Sasikala et al., 2009), CeO2/SnO2 (Foletto et al., 2012), Cr2O3/SnO2 (Bhosale et al., 2013), and so on. The results showed that nearly all the n–n junction composite semiconductors exhibited better photocatalytic properties than single ones. However, to the best of our knowledge, the use of the p–n type composite semiconductors has been rarely reported in the literature and only few examples of the p–n junction photocatalysts, such as CuO/SnO2 (Xia et al., 2007), CuFeO2/SnO2 (Derbal et al., 2008), and NiO/SnO2 (n-SnO2 p-Si (Yang et al., 2009; Wang et al. 2010a,b; Mohamed and Aazam, 2012), have been studied. Theoretically, when p-type semiconductor and n-type semiconductor are connected to each other, the micro p–n heterojunction composite semiconductors will be formed; the inner electric field will also be produced in the interface. Once optical excitation occurs, a free electron (e−) and an electronic vacancy (a hole, h+) are formed, separated and migrated effectively in a semiconductor being partially localized on structural defective centers of its crystalline lattice, hence improving the electrical properties of semiconductor.
In this investigation, we have studied the photocatalytic efficiency of a p-CuBi2O4/n-SnO2 composite, in which SnO2 was associated with Bismuth Cuprites (CuBi2O4) to form p–n heterojunction composite semiconductors in different mass ratios. CuBi2O4 was chosen as a sensitizer semiconductor due to its narrow band gap energy of 1.5 eV (Arai et al., 2007; Liu et al., 2010a,b). CuBi2O4 is well-known as an excellent host matrix for luminescent materials due to its low phonon energy, high visible-light responsiveness and adequate thermal stability. It functions as a sensitizer by the absorption of UV light to yield an excited state in the heterojunction composite semiconductors of p-CuBi2O4/n-SnO2, which may increase the probability of light-generated carrier transfer and hence reduces the recombination of photogenerated electrons and holes substantially improving the photocatalytic properties.
So, the aim of this study is to clarify the photocatalytic efficiency of this novel p–n type composite semiconductor p-CuBi2O4/n-SnO2 prepared by a physical mixing process through doping CuBi2O4 into SnO2 matrix. The as-prepared p-CuBi2O4/n-SnO2 nanoparticles were characterized by a number of techniques such as X-ray diffraction (XRD), scanning electron microscopy (SEM) and UV–visible diffuse reflectance spectroscopy (DRS). The photocatalytic degradation of Congo red (CR) dye under UV light irradiation was investigated over nanosized photocatalyst p-CuBi2O4/n-SnO2 at different operating parameters such as, amount of doped CuBi2O4 and pH solution. The experimental data were quantified by applying the pseudo-first order kinetic model. Mechanisms of the increase in the photocatalytic activity were also investigated through a solid state z-scheme photocatalysis system, which mimics the z-scheme in the natural photosynthesis of green plants.
2 Experimental
2.1 Chemical reagents
The starting materials used in the synthesis: α-Bi2O3 (99.99%), CuO (99.99%) and SnO2 (99.99%) were all obtained from Aldrich chemical company Ltd. Congo red (C.I. 22020, MW = 696.67 g mol−1, C32H24N6O6S2·2Na, λmax = 497 nm and pKa = 4) and other chemicals used in the experiments (NH4OH and H2SO4) were purchased from C.I.S.A. Espagne. Distilled water was used for the preparation of various solutions.
2.2 Preparation of p-CuBi2O4
The p-CuBi2O4 powder was prepared according to the previously reported procedure (Arai et al., 2007; Liu et al., 2010a,b; Elaziouti et al., 2012). α-Bi2O3 and CuO were used as starting materials. The stoichiometric proportion mixture of Bi2O3 and CuO was previously ground for a period of time in an agate mortar, and then heated at the rate of 5 °C/min in a muffle oven (Linn High Therm) and thermally treated at 750 °C for 72 h in air. After the muffle oven was naturally cooled to room temperature, the black CuBi2O4 powder was ground in the agate mortar and then was collected as the precursor to prepare the composite photocatalyst p-CuBi2O4/n-SnO2.
2.3 Preparation of semiconductor p-CuBi2O4/n-SnO2
Combined semiconductors CuBi2O4/SnO2 were prepared by a physical milling technique. Different CuBi2O4/SnO2 powder samples were prepared in a ratio (mass concentration) of 5%, 10%, 20%, 30%, 40% and 50% respectively, by varying the amount of CuBi2O4 and subsequently milled in the agate mortar for 30 min. The final samples were used for the determination of characterization and photocatalytic activity.
2.4 Characterization
Phase evolution and crystalline of the resulting powders were characterized by X-ray diffraction (XRD) in an automatic D8 Bruker AXS diffractometer using CuKα radiation (λ = 1.5406 Å). XRD diffractogrammes were collected in 10–70° intervals with a scan speed of 10°/min. The crystallite average size (dDRX) calculation using the Scherrer (Pullar et al., 1988) equation is as follows (Eq. 1):
Scanning electron microscopy (SEM) (Hitachi S-4800 N) is used to characterize the morphology of the particles.
UV–Vis diffuse reflectance spectroscopy measurements were carried out using a Perkin Elmer Lambda 650 spectrophotometer equipped with an integrating sphere attachment. The analysis range was from 200 to 900 nm, and polytetrafluoroethylene (PTFE, Teflon) was used as a reflectance standard.
The residual RC concentrations during the course of degradation were monitored with UV mini-1240 Spectrophotometer (Shimadzu UV mini-1240) in the range 200–800 nm, using 1 cm optical pathway cells.
2.5 Photocatalytic study measurements
The photodegradation of CR catalyzed by the p-CuBi2O4/n-SnO2 samples was investigated under UVA light irradiation. 100 mg of catalyst was suspended in a CR solution (200 mL, 20 mg/L) in quartz cell tube. The suspension pH value was previously adjusted at 8 using NaOH/H2SO4 solutions using a (Hanna HI 210) pH meter. Prior to UV light irradiation, the suspension was stirred with magnetic stirrer (Speedsafe™ Hanna) for 30 min under dark conditions at 298 K to ensure the establishment of adsorption/desorption equilibrium between the catalyst and CR. The sample was then irradiated at 298 K using 6 W ultraviolet (λ = 365 nm, BLX-E365) photoreactor under continuous stirring. As the reaction proceeded, a 5 ml suspension was taken at 20 min intervals during the catalytic reaction and was centrifuged using centrifuge (EBA-Hetlich) at 3500 rpm for 15 min to completely remove photocatalyst particles. The residual RC concentrations during the course of degradation were monitored with a UV mini-1240 Spectrophotometer (Shimadzu UV mini-1240) in the range 200–800 nm, using 1 cm optical pathway cells.
The effect of initial pH on the photocatalytic degradation of CR only was conducted from pH 6–12 for avoiding dye aggregation. Data obtained from the photocatalytic degradation experiments were then used to calculate the degradation efficiency η′ (%) of the substrate (Eq. 2):
According to Planck’s Law and some further calculation, we can find that the absorption wavelength of the photoreactor can be calculated by determining its band gap value (Eq. 3):
From the calculation, in order to absorb a UVA-light wavelength, the band gap value of the photoreactor has to be below 3.49 eV and above 3.30 eV.
The photocatalytic degradation efficiency of catalyst for the degradation CR was quantified by the measurement of dye apparent first order rate constants under operating parameters. Surface catalyzed reactions can often be adequately described by a monomolecular Langmuir–Hinshelwood mechanism, in which an adsorbed substrate with fractional surface coverage θ is consumed at an initial rate given as follows (Eq. 4) (Vasanth Kumar et al., 2008):
The half-life of dye degradation at various process parameters was raised from Eq. (8):
3 Results and discussions
3.1 XRD analysis of (x wt%) CuBi2O4/SnO2 Nanocomposites
Fig. 1, shows the XRD patterns of the as-synthesized (5 wt%) CuBi2O4/SnO2 nanocomposites in comparison with those of CuBi2O4 precursor and pure SnO2. Diffraction peaks of pure SnO2 (Fig. 1a) at 2θ of 26.63°, 33.73°,37.94°, and 51.79° can be indexed as the (1 1 0), (1 0 1), (2 0 0), and (2 1 1) planes of tetragonal rutile structure of stannic oxide (lattice constant a = 4.7373(2) Å et b = 3.1865(3) Å), which is in good agreement with standard value (JCPDS file No. 41–1445). The crystallite sizes of pure SnO2, dXRD, deduced from the XRD patterns by calculation of the Scherrer equation, was found to be 50 nm. The diffraction peaks of the Cubi2O4 precursor (Fig. 1b) at 2θ of 28.03°, 29.73°, 30.73°, 32.54°, 33.36° and 46.71° were respectively indexed as (2 1 1), (2 2 0), (0 0 2), (1 0 2), (3 1 0), and (4 1 1) planes of pure tetragonal phase of crystalline Cubi2O4, according to the Joint Committee Powder Diffraction Standards (P42/mnm, JCPDS file No. 42–0334) with lattice constants (a = 8.5004 Å, c = 5.819 Å) calculated from their corresponding XRD pattern data obtained by Fullprof program. Both precursors CuBi2O4 and pure SnO2 show preferred (0 0 2) crystallographic orientation owing to the preparation route of the sample during the XRD analysis. On the other hand, the XRD patterns of (5 wt%) CuBi2O4/SnO2 nanocomposite exhibited characteristic diffraction peaks of both CuBi2O4 and SnO2 crystalline phases, suggesting that CuBi2O4 and SnO2 are coexistent in the composites as separate phases. It can be seen from Fig. 1c, that at 5% mass concentration of Cubi2O4, the diffraction pattern of the nanocomposite materials was approximately similar to that of pure SnO2. This is probably due to the high crystallinity of the SnO2 phase and the lowest amount of CuBi2O4 present in the composites (5 wt%), thus appearing as the dominant peaks in the XRD spectra of the nanocomposite sample. Compared with SnO2, the XRD patterns (Fig. 2) in the 2θ range from 20° to 37° of (5 wt%) CuBi2O4/SnO2 exhibits the same broader peaks, indicating a high solubility of CuBi2O4 in SnO2 matrix. Thus the presence of (5 wt%) Cubi2O4 has obviously no effect on the particle size of the (5 wt%) CuBi2O4/SnO2 nanocomposite.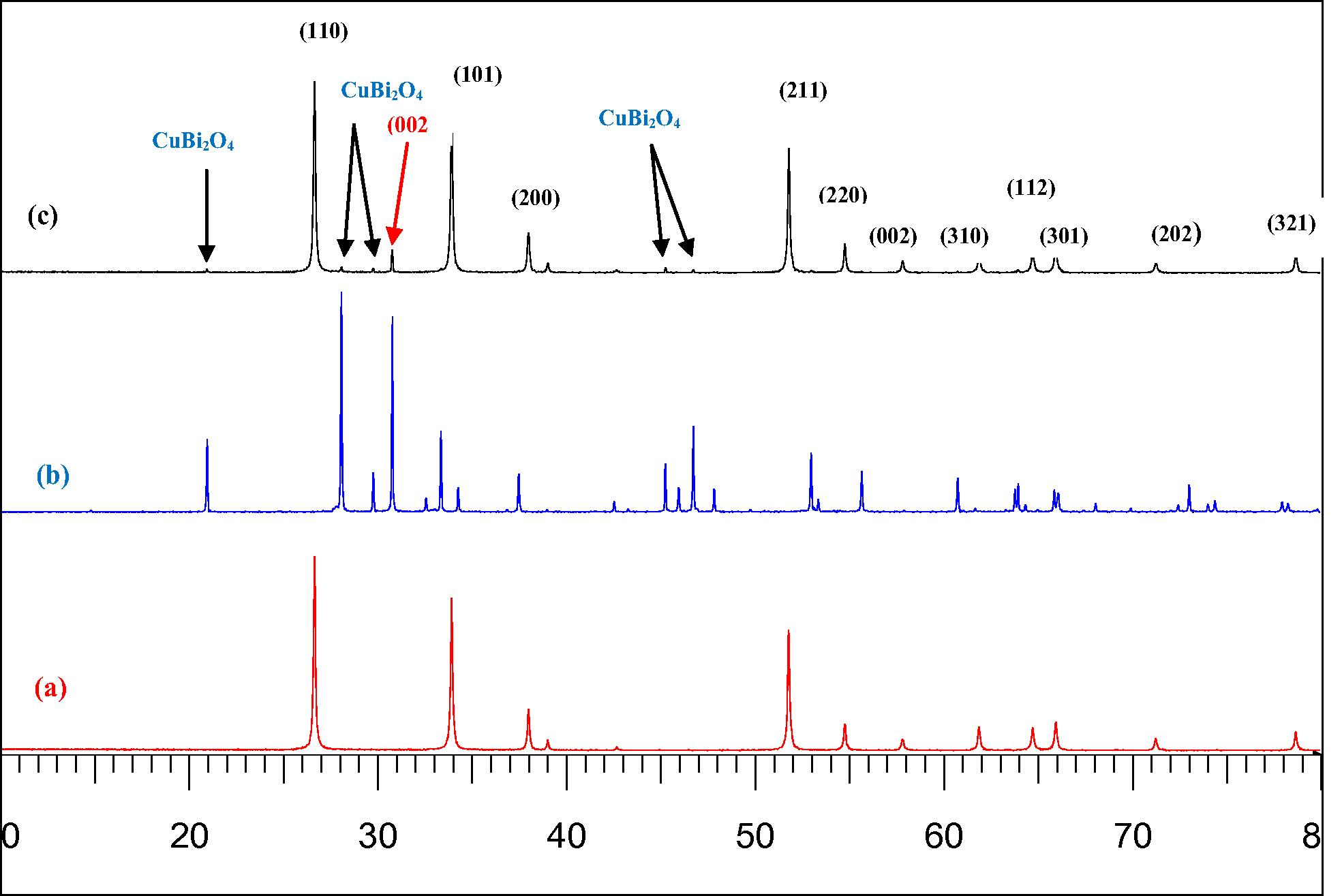
XRD patterns of pure SnO2 (a) precursor CuBi2O4 (b) and the synthesized (5 wt%) CuBi2O4/SnO2 (c).
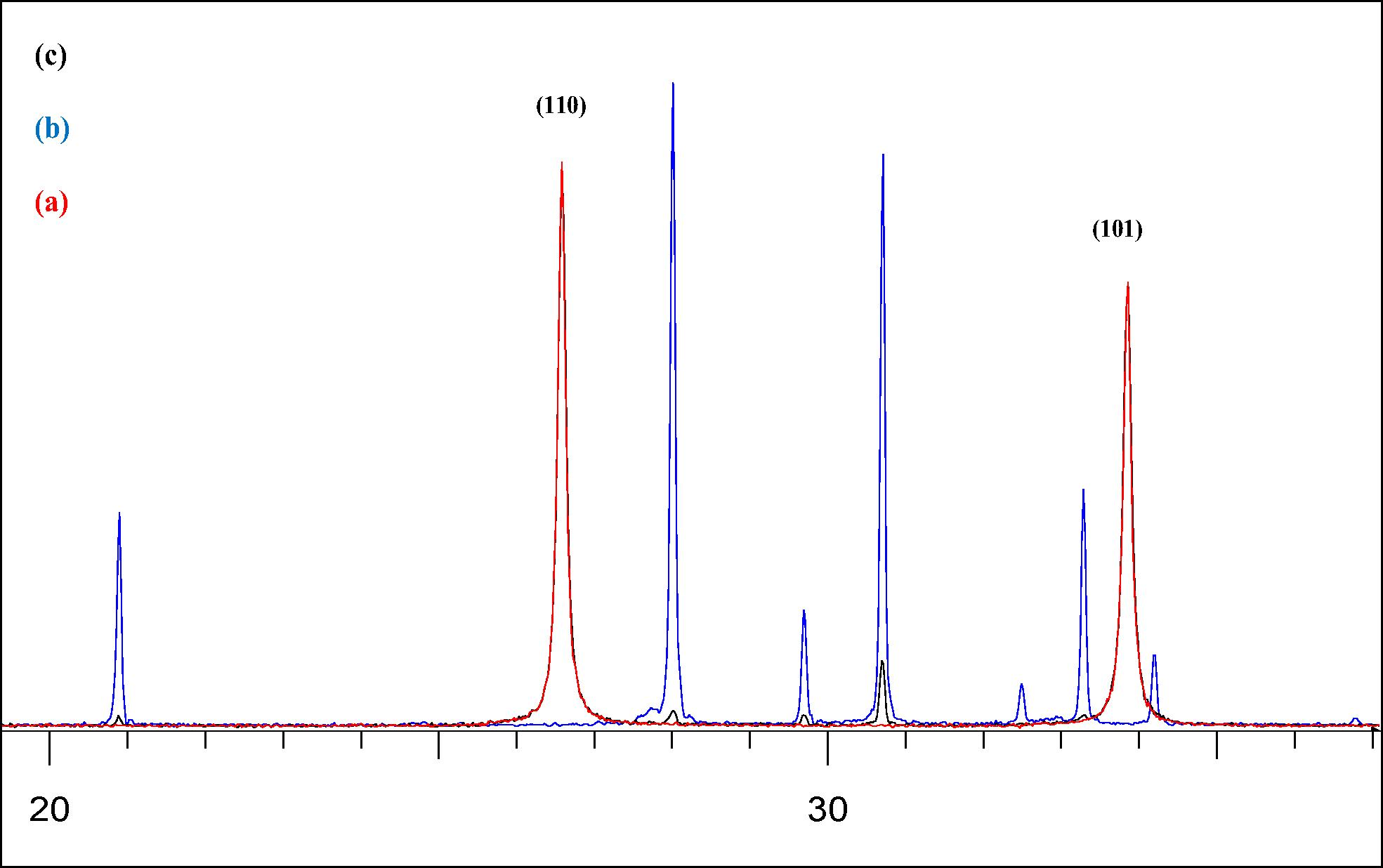
XRD patterns of pure SnO2 (a) precursor CuBi2O4 (b) and the synthesized (5 wt%) CuBi2O4/SnO2 (c) in the 2θ range from 20 to 37.
3.2 SEM analysis
Fig. 3a, illustrates typical SEM image of CuBi2O4 powder synthesized by solid-state reaction of CuO and α-Bi2O3 at 750 °C for 24 h, pure SnO2 and (5 wt%) CuBi2O4/SnO2 nanocomposites. It can be seen that, for the CuBi2O4, the appearance is a shapeless sheet, and the particle size of the CuBi2O4 is about 10–20 μm. Fig. 3b shows typical high-resolution SEM image of CuBi2O4 powder to further show the details of the nanoparticles. As shown in Fig. 3b, it clearly shows two different crystal shapes on the CuBi2O4 surface, corresponding to two different particle sizes of CuBi2O4. The appearance of CuBi2O4 is a shape sheet and a well-defined tetragonal phase with the crystallite diameter of the CuBi2O4 about 5 μm, whereas groups of smaller particles do not have any specific shape with size up to 500 nm tend to cover the bigger particles. However, pure SnO2 from SEM analysis (Fig. 3c) clearly shows two different tetragonal-shaped nanoparticle structures on the SnO2 surface, which can be assigned to SnO2 (ion radius Sn4+: rSn4+ = 0.071 nm) with a particle size in the range of 0.1 μm and SnO (ion radius Sn2+: rSn2+ = 0.112 nm) with approximately 0.2 μm dimensions, which agrees with the UV–Vis diffuse reflectance spectrum of SnO2 in Fig. 4. Both nanoparticles are close to each other in the form of chains.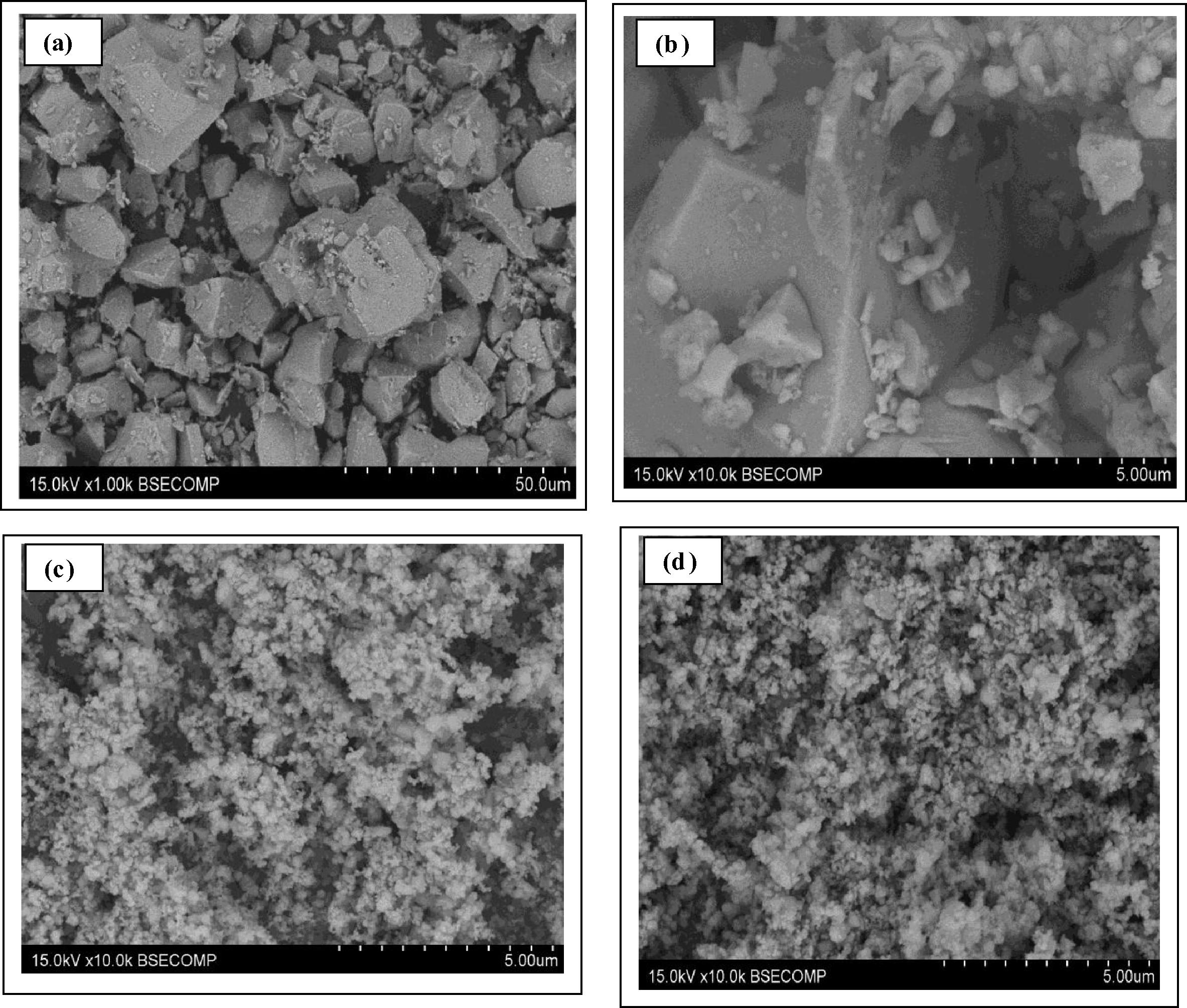
SEM images of (a) low-resolution of CuBi2O4 (b) high-resolution of precursor CuBi2O4 (c) pure SnO2 (d) (5 wt%) CuBi2O4/SnO2 nanocomposites.
UV–visible absorbance spectra of SnO2 and CuBi2O4 synthesized by solid-state reaction at 750 °C for 24 h.
The as synthesized (5 wt%) CuBi2O4/SnO2 nanocomposite via the physical milling synthesis method (Fig. 3d) clearly shows the presence of SnO2 nanoparticles deposited onto the CuBi2O4 surface, displaying a particle size of 0.1–0.2 μm and strong assembly of the nanoparticles measuring from 0.2 μm to 1 μm. Such aggregation can be explained by the solid-state synthesis route, which generally requires repeated mechanical mixing process and a high temperature process.
3.3 UV–Vis diffuse reflectance spectra and band gap energy
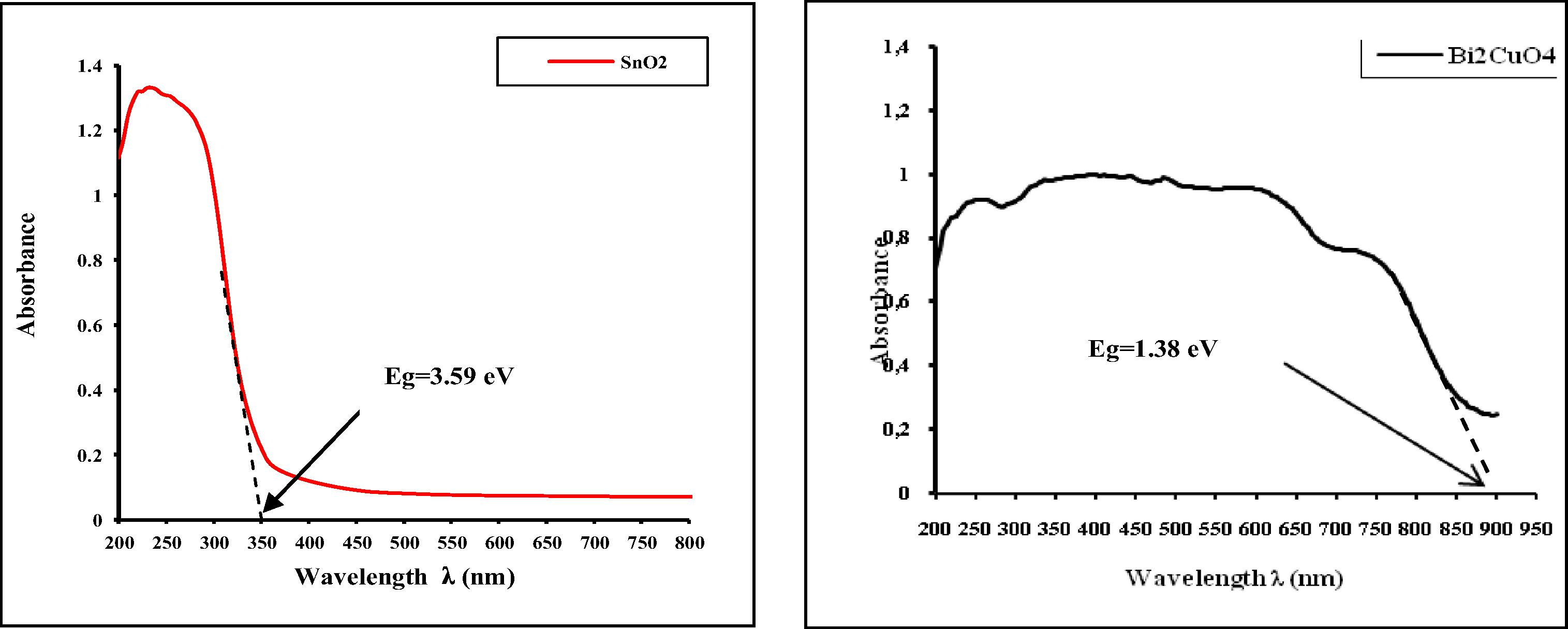
Fig. 4, shows the UV–Vis absorbance spectra of CuBi2O4 synthesized by solid-state reaction at 750 °C for 24 h and pure SnO2. It is clear from the recorded UV–visible spectrum of SnO2 that two absorption bands are observed in the UV region at 210–245 nm and at 270 nm. Generally, the absorption band at 210–245 nm of SnO2 in the UV region originates from the charge-transfer transition between the O 2p and Sn 4d states in O2− and Sn4+. The band at 270 nm is attributed, either to the inter-valence transition of Sn4+/Sn2+ (Shen et al., 1994), or to the charge-transfer transition s → p of Sn2+ ions (Teegarden, 1966).
The UV–visible spectrum of CuBi2O4 nanostructures synthesized by solid-state reaction at 750 °C for 24 h is presented in Fig. 4. It can be seen that it has strong and broad absorption in the range of 200–900 nm. This suggests that the prepared sample absorbs both UV and visible light. Obviously, for CuBi2O4 nanostructures, the broad absorption band observed in the UV–visible region was attributed to the charge-transfer transition between the O 2p and Cu 3dx2 − y2 states in O2− and Cu2+, respectively (Nathan et al., 2012). Fig. 5 shows UV–Vis diffuse reflectance spectra of a series of photocatalysts (x wt%) CuBi2O4/SnO2 for x = 0–20 wt%. A progressive red shift in the band gap absorption is observed with an increase in the amount of (x wt%) CuBi2O4 in the SnO2 matrix.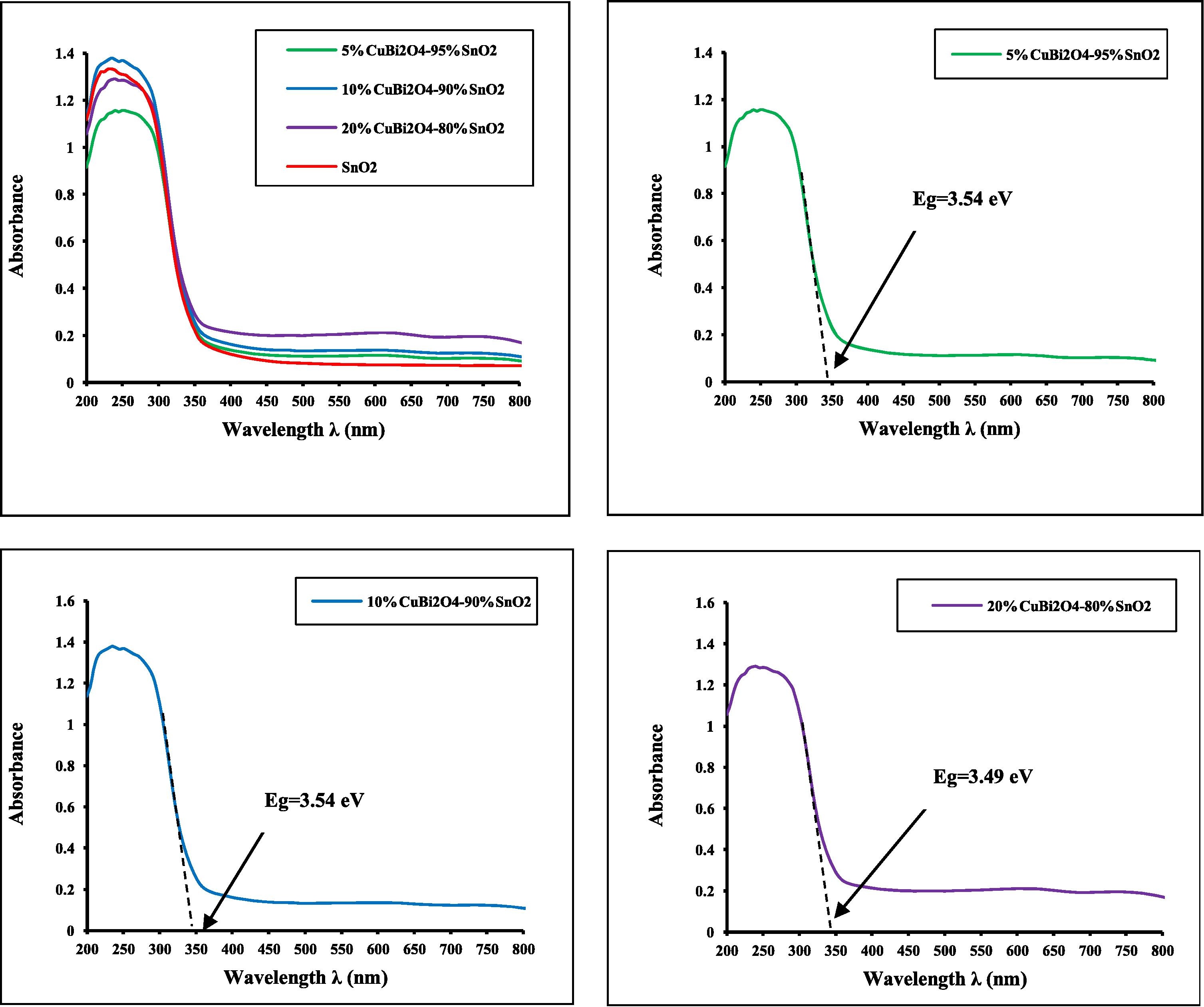
UV–visible absorbance spectra of a series of (x wt%) CuBi2O4/SnO2 (with x = 0–20 wt%) nanocomposites.
Pure SnO2 has a negligible absorption in the range of UVA light (355 nm ⩽ λ ⩽ 375 nm) because the catalyst is only effective under ultraviolet irradiation (λ ⩽ 345 nm). With an increase in the amount of x wt% of CuBi2O4, the absorption edge of the sample has some red shift. The red-shift observed in the nanocrystalline SnO2 would explain the formation of localized states within the band gap owing to oxygen vacancies and increase in Sn2+ ion concentration. This phenomenon is due to the shift of absorbance band toward the longer wavelength (Lu et al., 2009; Charitidis et al., 2005). The red shift is presumably ascribed to the homogeneous dispersion of CuBi2O4 particles within SnO2 matrix. The onset absorption edges and band gap energies of the as-synthesized CuBi2O4 particle, pure SnO2 nanoparticle and series of (x wt%) CuBi2O4/SnO2 nanocomposites are shown in Figs. 4 and 5, respectively.
The absorption onsets of crystalline semiconductor were determined by linear extrapolation from the infection point of the curve (Absorbance versus λAbsorp. Edge) to the baseline and Eg = 1240/λAbsorp. Edge as shown in Figs. 4 and 5. The as-synthesized CuBi2O4 exhibits an absorption onset at 900 nm, which corresponds to the band gap energy of 1.38 eV. This value is lower than that reported in the literature; 1.5 eV (Arai et al., 2007; Liu et al., 2010a,b). It is clear from the recorded spectrum (Fig. 4) that the pure nanocrystalline SnO2 has an absorption onset at 345 nm, which matches the band gap energy of 3.59 eV, attributing to stannic oxide (SnO2). These results are well in agreement with values reported in the literature (Casey and Stephenson, 1990). The optical properties of the as-synthesized CuBi2O4 and pure SnO2 nanoparticles are reported in Table 1. λ: Wavelength, Refs.: References.
Systems
λ (nm)
Charge-transfer transition
Band gap Eg (eV)
Experimental
Literature Refs.
CuBi2O4
900
2p6(O) → 3dx2-y2 (Cu)
1.38
1.5 (Arai et al., 2007; Liu et al., 2010a,b)
SnO2
345
2p6 (O) → 5s (Sn)
3 .59
3.5–4.1 (Casey and Stephenson, 1990)
05
350
2p6 (O) → 5s (Sn)
3.54
10
350
2p6 (O) → 5s (Sn)
3.54
20
355
2p6 (O) → 5s (Sn)
3.49
It is widely accepted that electronic transport properties depend on the physical and structural characteristics of photocatalyst, such as crystallite size, morphology, phase structure and amount of CuBi2O4 loaded (Yu et al., 2008). As reported from the UV–visible results in Fig. 5 and Table 1, for the series of (x wt%) CuBi2O4/SnO2 nanocomposite materials, the band gap energy slightly decreased from 3.59 to 3.49 eV as the amount of CuBi2O4 was increased up to 20 wt% on the SnO2 matrix, suggesting that a number of micro p–n heterostructures CuBi2O4/SnO2 will be formed after doping CuBi2O4 powder into SnO2 particles. Hence it was concluded that CuBi2O4 played a role in electron separation. The increase in photocatalytic activity of CuBi2O4/SnO2 would be attributed to the suppression of electron–hole recombination by the effective electron separation (Masami et al., 2013). So, the decrease in the band gap energy with an enhanced absorption intensity of the (5wt%) CuBi2O4/SnO2 nanocomposites upon loading the amount of CuBi2O4 could be ascribed to the homogeneous dispersion of CuBi2O4 within the SnO2 matrix in the bulk of the catalyst and the formation of conducting network at very low temperature.
Chun-Ming et al. (2007) have already reported band gap narrowing effect for doped SnO2 nanoparticles. However there is no clear understanding of this phenomenon. A direct–indirect transition has been proposed by Rakhshani et al. (1998). In order to explain the band gap narrowing effect, many groups have suggested that alloying effect of parent compound with some impurity phases may be responsible for the band gap narrowing (Park and Kim, 2003; Barreau et al., 2002). So we think that for the samples containing up to 5 wt% CuBi2O4 concentration, SnO2–SnO2-x alloying effect may be responsible for the band gap narrowing effect. For the SnO2 nanoparticles above 5 wt% CuBi2O4 concentration there is a huge drop in the band gap. This may be due to the formation of sub-bands in between the band gap and the conduction band and sub-bands are merging with the conduction band to form a continuous band. This is perfectly in agreement with the XRD analytical results and consistent with the previously reported work (Chun-Ming et al., 2007).
3.4 Photocatalytic activity tests
3.4.1 Effect of pH solution on the photocatalytic activity of (5 wt%) CuBi2O4/SnO2 nanocomposite
In order to study the effect of initial pH on the degradation efficiency of (5 wt%) CuBi2O4/SnO2 nanocomposite catalyst on photodecomposition of CR, experiments were carried out at various pH, ranging from 6 to12 for avoiding dye aggregation. Results showed that the pH significantly affected the photocatalytic degradation efficiency of both CR. As shown in Fig. 6 and Table 2, for CR, the degradation rate increased from 12.03 to 58.06% as the pH value was increased from 6 to 8, and then decreased to 12.27 at pH 12. The maximum degradation rate of CR (58.06%) was achieved at pH 8. For this reason, the pH 8 was selected for subsequent experiments.![Effect of the pH solution on the photocatalytic redox of CR under UVA light irradiation ([(5 wt% CuBi2O4/SnO2]=0.5 g/L, [CR]= 20 mg/L, T = 298 K, λmax = 365 nm, I = 90 J/cm2 and irradiation time = 100 min).](/content/185/2015/27/1/img/10.1016_j.jksus.2014.06.002-fig6.png)
Effect of the pH solution on the photocatalytic redox of CR under UVA light irradiation ([(5 wt% CuBi2O4/SnO2]=0.5 g/L, [CR]= 20 mg/L, T = 298 K, λmax = 365 nm, I = 90 J/cm2 and irradiation time = 100 min).
pH initial
Adsorption
activity η (%)Photocatalytic
activity η′ (%)
2
Dye aggregation
4
6
0.93
12.03
7
33.69
42.16
8
31.38
58.06
10
8.64
13.77
12
0.46
12.27
It is commonly accepted that in photocatalyst/aqueous systems, the potential of the surface charge is determined by the activity of ions (e.g. H+ or pH). A convenient index of the tendency of a surface to become either positively or negatively charged as a function of pH is the value of the pH required to give zero net charge (pH PZC) (Zhang et al., 1998; Yath, 1974). pH PZC is a critical value for determining the sign and magnitude of the net charge carried on the photocatalyst surface during adsorption and the photocatalytic degradation process. Most of the semiconductor oxides are amphoteric in nature, can associate Eq. (15) or dissociate Eq. (17) proton. To explain the relationship between the layer charge density and the adsorption, so-called Model of Surface Complexation (SCM) was developed (Fernandez et al., 2002), which consequently affects the sorption–desorption processes as well as the separation and transfer of the photogenerated electron–hole pairs at the surface of the semiconductor particles. In the 2-pK approach we assume two reactions for surface protonation.
The zero point charge pH PZC for SnO2 (about 5) is approximately identical to that of (5 wt%) CuBi2O4/SnO2 which is mainly composed of SnO2 nanopowders (shown in XRD patterns), since there is no adsorption of CR ions than the potential determining H+/OH− at the surface of CuBi2O4 particles. This is often the case for pure (“pristine surface”) oxides in water.
When the pH is lower than the pH PZC value, the system is said to be “below the PZC”. Below the PZC, the acidic water donates more protons than hydroxide groups, and so the adsorbent surface is positively charged (attracting anions/repelling cations), according to the following reaction Eqs. (9), (10):
At neutral and acidic media (pH < 8), lower photocatalytic efficiency of catalyst can be explained by the following possible reasons. First, the electrostatic repulsion forces between the negatively charged (5 wt%) CuBi2O4/SnO2 surface and CR anionic dye, mainly sulfonated groups (-SO−3), affecting strongly the accessibility of the surface reducing species to the CR photocatalytic oxidation/reduction kinetics. Second, the excess of H3O+ ions, especially at pH = 6, enters into electrostatic interactions with both the negatively charged (5 wt%) CuBi2O4/SnO2 surface and RC dye anions, leading to a minimum adsorption extent at pH 6. Furthermore we found that, where the adsorption of dye was strong, photodegradation remarkably occurred.
At pH higher than pH PZC value (i.e. pH > 8), excess of hydroxyl anions facilitates photogeneration of •OH radicals which are accepted as primary oxidizing species responsible for photocatalytic degradation, resulting in the enhancement of the efficiency of the process. However, a dramatic decrease in the degradation efficiency could be explained on the basis of amphoteric behaviors of (5 wt%) CuBi2O4/SnO2 catalyst. The negatively charged surface of (5 wt%) CuBi2O4/SnO2 catalyst (highly concentration of hydroxide ions) and the great negatively charged RC dye anions resulted in electrostatic repulsion, leading to the reduction in the efficiency of the photodegradation process. There were similar results in the previous reports (Liu et al., 2010a,b).
3.4.2 Effect of the amount of CuBi2O4 on the photocatalytic activity of (x wt%) CuBi2O4/SnO2
The effect of the amount of CuBi2O4 on photocatalytic degradation of CR was conducted over a range of catalyst amounts from x = 0 to x = 100 wt%. As observed in Fig. 7 and Table 3, it is evident that the photocatalytic redox of CR greatly depends on the amount of doped CuBi2O4. The photocatalytic activity increased drastically from 25.62 to 58.06% as the catalyst amount was raised from x = 0 to x = 5 wt%. Upon further increase in the CuBi2O4 amount beyond x = 5 wt%, the photocatalytic activity decreased gradually, almost reaching 3.13% at x = 100 wt%. The maximum photocatalytic activity of (x wt%) CuBi2O4/SnO2 (58.06%) under UVA light irradiation was achieved within 100 min of light illumination time when the amount of doped CuBi2O4 x was 5 wt%, which is obviously about 2.3 times higher than the value of 25.62% over pure SnO2. So there is an optimum CuBi2O4 contents for high dispersion morphology of nanoparticles CuBi2O4 on the SnO2 surface with high activity. The effective electron–hole separation both at the physically bonded interfaces and in the two semiconductors as well as charge defect during the physical mixing method was believed to be mainly responsible for the remarkably enhanced photocatalytic activity of (5 wt%) CuBi2O4/SnO2 in the course of the photocatalytic redox conversion of CR. But until now, there are no reports about synergistic effect between SnO2 and CuBi2O4 in the (5 wt%) CuBi2O4/SnO2 nanocomposite under visible light excitation. From Fig. 7, it is clear that the photocatalytic activity of SnO2 is significantly increased under the presence of an amount of CuBi2O4 (5 wt%) compared to pure SnO2 and the CuBi2O4 samples. These results strongly suggest the existence of a synergistic effect between SnO2 and the CuBi2O4 in the (5 wt%) CuBi2O4/SnO2 nanocomposite under UVA light excitation.![Effect of the amount of CuBi2O4 on the photocatalytic redox of CR under UVA light irradiation ([(x wt%) CuBi2O4/SnO2]=0.5 g/L, [CR]= 20 mg/L, pH = 8, T = 298 K, λmax = 365 nm, I = 90 J/cm2 and irradiation time = 100 min).](/content/185/2015/27/1/img/10.1016_j.jksus.2014.06.002-fig7.png)
Effect of the amount of CuBi2O4 on the photocatalytic redox of CR under UVA light irradiation ([(x wt%) CuBi2O4/SnO2]=0.5 g/L, [CR]= 20 mg/L, pH = 8, T = 298 K, λmax = 365 nm, I = 90 J/cm2 and irradiation time = 100 min).
Amount of CuBi2O4 x (%)
Adsorption activity η (%)
Photocatalytic activity η′ (%)
0
2.92
25.62
5
31.38
58.06
10
22.24
31.48
20
16.21
19.69
30
12.52
12.95
40
11.01
8.01
50
5.18
3.64
100
4.71
3.13
However, at higher amount of doped CuBi2O4 than 5 wt%, the photocatalytic redox activity of (x wt%) CuBi2O4/SnO2 photocatalyst was obviously decreased on further increase in the amount of CuBi2O4. Thus, such an above occurrence in the present experiment is primarily attributed to overlapping of adsorption sites of SnO2 particles as a result of overcrowding of the CuBi2O4 granule owing to the decrease in screening effect and interfering of light. Similar trends were reported in a series of p–n heterojunction photocatalysts p-CuBi2O4/n-TiO2 (Lin et al., 2008) and CuBi2O4/BiVO4 (Masami et al., 2013) with high photocatalytic activity under visible and UV light irradiation.
3.4.3 Effect of UV light and catalyst
The photocatalytic activities of all three CuBi2O4, SnO2 and (5 wt%) CuBi2O4/SnO2 photocatalysts were assessed by the photocatalytic redox reaction of Congo red (CR) aqueous solution under UVA light irradiation. Variations of CR reduced concentration (C/C0) versus visible-light irradiation time over different catalysts under different experimental conditions through alone (CR self-photolysis), CuBi2O4/UVA, SnO2/UVA and (5 wt%) CuBi2O4/SnO2/UVA are presented in Fig. 8. The synergistic effect between SnO2 and CuBi2O4 in the (5 wt%) CuBi2O4/SnO2 nanocomposite under UVA light excitation showed that (5 wt%) CuBi2O4/SnO2 exhibited higher photocatalytic efficiency, as compared to the single phases CuBi2O4 and SnO2. The highest efficiency was obtained, under UVA light irradiation over (5 wt%) CuBi2O4/SnO2, as a result of 58.06% degradation of CR for 100 min of irradiation time. However, the photocatalytic degradation of CR over single phases CuBi2O4 and SnO2 was only 3.13 and 25.62% respectively. With 20 mg/L of CR in the direct photolysis for the same optimum irradiation time, disappearance of dye was negligible (0.49%). On the basis of these results, the high decomposition of CR dye in the presence of (5 wt%) CuBi2O4/SnO2 catalyst is exclusively attributed to the photocatalytic reaction of the combined semiconductor particles under UVA light irradiation. As known, one of the basic requirements for the combined photocatalysts with a higher activity is the presence of the tightly physically bonded or close contact interfaces between the two materials, by which the photoinduced charge transfer from one particle to the other via interfaces spatially is available. Thus, such an above occurrence in the present experiment is primarily assigned to the charge defect during the physical mixing method, which is advantageous for the effective electron–hole separation and the suppression of the recombination rate of the photogenerated charge carriers; hence result in an improvement of the probability of light-generated carriers transfer via interfaces spatially available. A similar result was reported in the heterojunction semiconductor SnO2/SrNb2O6 with an enhanced photocatalytic activity (Gurlo, 2006).![Photocatalytic degradation kinetics of CR at different experimental conditions ([Catalyst]=0.5 g/L, [CR]= 20 mg/L, pH = 8, T = 298 K, λmax = 365 nm, I = 90 J/cm2 and irradiation time = 100 min).](/content/185/2015/27/1/img/10.1016_j.jksus.2014.06.002-fig8.png)
Photocatalytic degradation kinetics of CR at different experimental conditions ([Catalyst]=0.5 g/L, [CR]= 20 mg/L, pH = 8, T = 298 K, λmax = 365 nm, I = 90 J/cm2 and irradiation time = 100 min).
3.4.3.1 Kinetic modeling
The photocatalytic degradation of CR over different experimental conditions is displayed in Table 4. As it can be seen, the straight lines for the entire as-prepared samples of the plots of ln C/C0 versus t with high regression coefficients (R2 = 0.945–0.991), for the pseudo-first-order kinetic model strongly suggest that all the photodegradation systems were a pseudo-first-order model. Exception was observed in the cases of direct photolysis and photocatalysis reaction in the presence of the single phase CuBi2O4 respectively.
Systems
Adsorption activity η (%)
Photocatalytic activity η′ (%)
K1 (min−1)
t1/2 (min)
R2 (%)
CR/UV-A
–
0.49
–
–
–
CR/SnO2/UVA
2.92
25.62
0.0045
154.032
0.945
CR/CuBi2O4/UVA
0
3.130
0.0002
3465.736
0.203
CR/(5 wt.%)CuBi2O4-SnO2/UVA
31.38
58.06
0.052
13.329
0.991
3.5 Discussion of mechanism
The above analysis shows that the migration direction of the photogenerated charge carrier depends on the band edge positions of the two semiconductors. There are three methods to determine the band edge positions: experiments based on photoelectrochemical techniques, calculation according to the first principle, and predicting theoretically from the absolute (or Mulliken) electronegativity (Kim et al., 1993; Butler and Ginley, 1978; Xu and Schoonen, 2000). The first one is not always easy to handle, and the second one cannot obtain the absolute energy of band edges with respect to vacuum and always has large discrepancies between calculated and measured values. The third one is a simple approach with reasonable results for many oxide photocatalysts (Xu and Schoonen, 2000). The conduction band edge of a semiconductor at the point of zero charge (pHzpc) can be predicted by Eq. (13):
Systems
χ (eV)
λ (nm)
Eg (eV)
E0BC (eV)
E0BV (eV)
p-CuBi2O4
4.75
900
1.38
−0.44
+0.94
n-SnO2
6.25
345
3.59
−0.05
+3.55
Fig. 9 depicts reaction schemes of CuBi2O4 (a) and SnO2 (b) as the p and n type respectively for charge separation for the reductivity/oxidizability improvement model. CuBi2O4 is a p-type semiconductor, which always exhibits good stability under UVA visible illumination, and SnO2 is determined as an n-type material. It was reported previously that that the band gap of CuBi2O4 is 1.38 eV, which can be excited by photons with wavelengths below 900 nm, whereas SnO2 with band gap of about 3.59 eV can be excited by photons with wavelengths of 345 nm. Under UVA (λUVA = 355–375 nm → Eg_ = 3.3–3.49 eV) light irradiation, the energy of the excitation light is sufficient to directly excite the CuBi2O4 (λ = 900 nm → Eg = 1.38 eV) semiconductor and it is large enough to yield a higher energy level of SnO2 (λ = 345 nm → Eg = 3.59 eV) portion of the photocatalyst.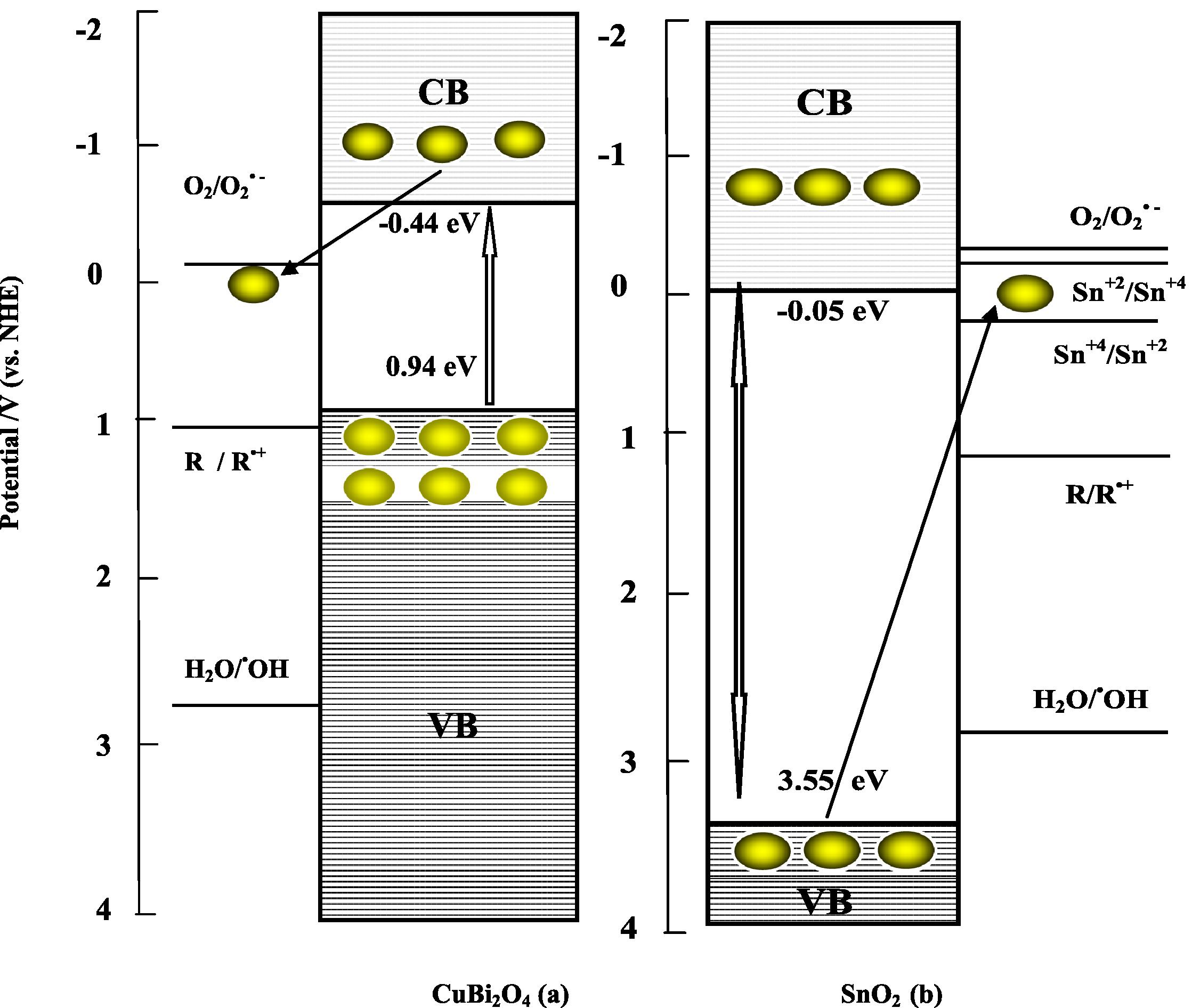
Reaction schemes of CuBi2O4 (a) and SnO2 (b) as the p- and n-type semi-conductor respectively for charge separation for the reductivity/oxidizability improvement model (electron
 and hole
and hole  ).
).
According to the band edge position (Table 5), for the p-CuBi2O4 alone (Fig. 9 a), the electronic potential of the conduction band of p-CuBi2O4 is around −0.44 eV/NHE at pH 7 which is more negative than that of superoxide radical (E0 (O2/O2•−) = −0.28 V/NHE at pH 7). This indicated that the electron photoproduced at the conduction band directly reduced O2 into O2•−. These reduced O2•− can subsequently transfer the charge to the species present in the reaction medium that is preferentially adsorbed onto the p-CuBi2O4 particles. Hence, the superoxide radical (O2•−) reduces the recombination of the charge carriers enhancing the activity in the UVA light. However, the p-CuBi2O4 valence band of +0.94 eV/NHE at pH 7, is too negative than that of hydroxyl radical (E0 (H2O/•OH)) = + 2.8 V/NHE at pH 7). The holes photogenerated in the p-CuBi2O4 are not able to oxidize H2O to •OH.
p-CuBi2O4 powder formed in our laboratory by the solid-state reaction of CuO and α-Bi2O3 at 750 °C for 24 h, exhibits a black color. The presence of non stoichiometric regions of the nominally p-CuBi2O4 particles or small domains of binary oxide phases of CuxO or BixO, undetected by XRD data, as unstable impurity phases which could be originated from a number of processes such as reduction of the p-CuBi2O4, could be responsible for higher recombination rates. Thus, the result is consistent with the previous study in the electrochemical synthesis and characterization of p-CuBi2O4 thin film photocathodes (Magesh et al., 2009). Therefore, CuBi2O4 alone shows negligible photocatalytic activity under UVA light. As a result, less efficient charge-carrier separation, and thus the increment of photocatalytic activity were restricted (Elaziouti et al., 2012).
For pure n-SnO2 (Fig. 9b) which is only effective under ultraviolet irradiation (λ = 345 nm), shows little photocatalytic activity under UVA light. These observations can be explained as follows: The reduction of Sn+4 to Sn+2 requires a potential of +0.15 V/NHE at pH 7 and the oxidation of Sn+2 to Sn+4 needs −0.15 V/NHE at pH 7. The SnO2 valence band of SnO2 is around +3.55 eV (vs. NHE at pH 7) which is more positive than Sn+2 to Sn+4 oxidation potential. The valence electron of SnO2 can hence oxidize Sn+2 to Sn+4. But the conduction band of SnO2 has a potential of −0.05 eV/NHE at pH 7, which is more negative than Sn+4 to Sn+2 reduction potential. Hence, the conduction band electrons of SnO2 may be able to reduce Sn+4 to Sn+2. These reduced Sn+2 and oxidized Sn+4 species can subsequently transfer the charge to the species present in the reaction medium. Hence, the Sn ions can suppress the recombination of the charge carriers, enhancing the activity in the UVA light.
In contrast, (5 wt%) p-CuBi2O4/n-SnO2 composite exhibits a higher photocatalytic activity compared to that of single photocatalyst (p-CuBi2O4 and n-SnO2). To overcome the recombination problem of composite photocatalyst while maintaining its high efficiency, an artificial z-scheme photocatalytic system, which mimics the z-scheme in the natural photosynthesis of green plants (Sasaki et al., 2008) has been suggested for p-CuBi2O4/n-SnO2 composite mediated photocatalytic degradation of Congo red as probe pollutant. The solid state z-scheme mechanism, in which two UVA light sensitive photocatalysts, p-CuBi2O4 and n-SnO2 in our case, with different levels of band gap energy Eg, is utilized without any intermediates. This system, however, cannot be considered to be a genuine z-scheme under irradiation by visible light because bare n-SnO2, can only generate electrons when irradiated with UV light (Hyeong et al., 2011). The z-scheme photocatalysis system diagram is depicted in Fig. 10.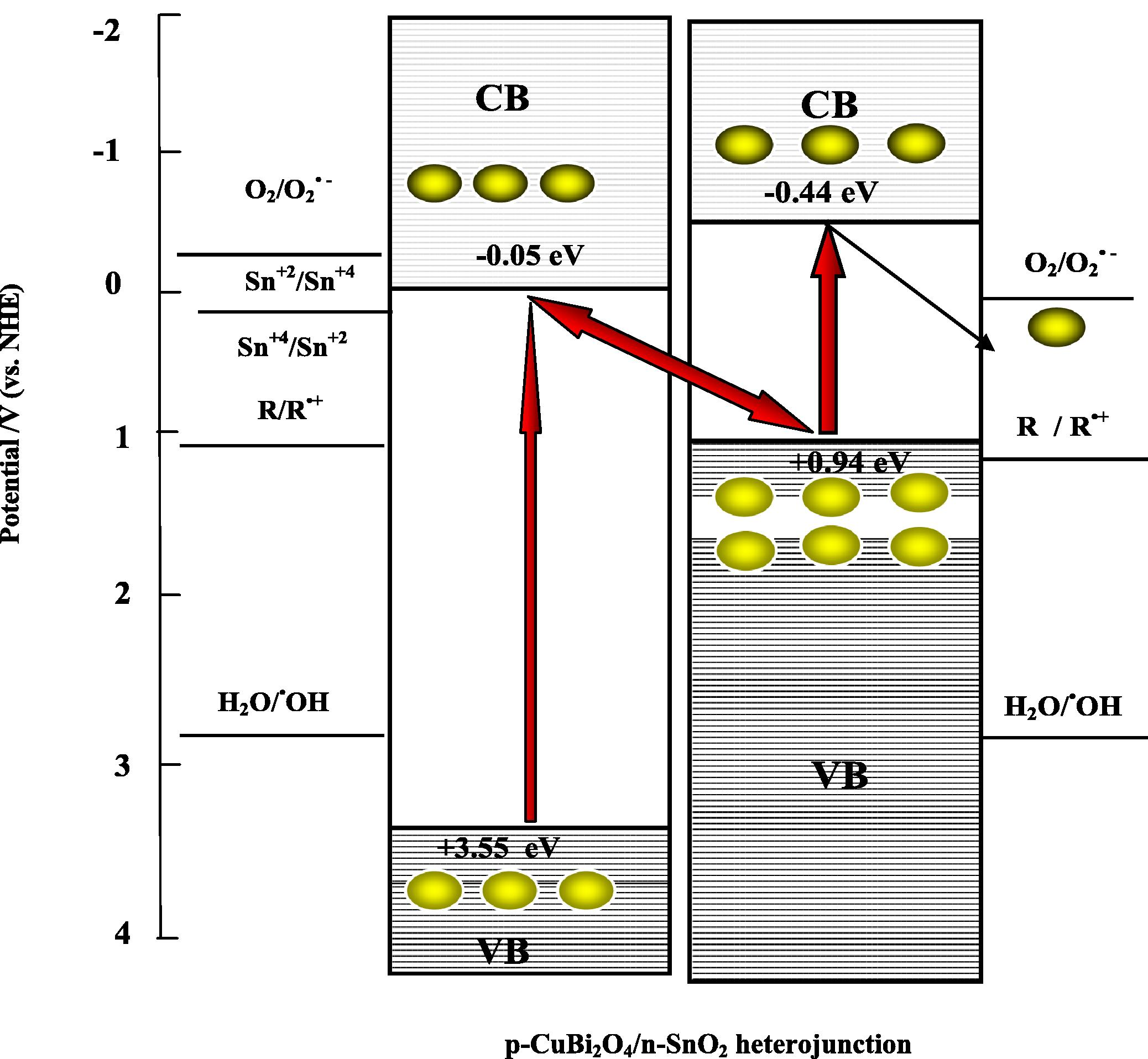
Mechanism of CR degradation in a z-scheme photocatalysis system consisting of CuBi2O4/SnO2 heterojunction under UVA-light irradiation. (electron
 and hole
and hole  ).
).
So, when p-type semiconductor CuBi2O4 and n-type semiconductor SnO2 were connected to each other, a number of micro p–n heterojunction CuBi2O4/SnO2 will be formed between p-CuBi2O4 powder and n-SnO2 granule at the interfaces of p-CuBi2O4 loaded n-SnO2 composite. According to the band edge position (Table 5), the electronic potential of the conduction band of n-SnO2 is slightly more anodic than that of p-CuBi2O4, whereas, the hole potential of the valence band top of n- SnO2, is more positive than that of p-CuBi2O4. The electron–hole pairs will be created under UVA light illumination. The electrons that are photogenerated by n-SnO2 are transferred to the valence band of p-CuBi2O4. These electrons then recombine with holes photogenerated at p-CuBi2O4. The electrons that are excited at p-CuBi2O4 then have a sufficiently high potential to participate in the reduction step of the reaction. By taking advantage of a combination of p-CuBi2O4 and n-SnO2, the probabilities of electron–hole recombination would be decreased significantly, thus resulting in more electrons available on CuBi2O4 surface and a larger amount of holes on SnO2 surface acting as powerful oxidants respectively (Eqs. 14, 15). The stepwise photocatalytic mechanism is illustrated below:
4 Conclusions
Novel p-CuBi2O4/n-SnO2 nanocomposite photocatalysts with different mass ratios were synthesized with the solid state method, and were characterized by a number of techniques such as X-ray diffraction (XRD), scanning electron microscopy (SEM) and UV–Vis diffuse reflectance spectroscopy (DRS) technique. The as-prepared p-CuBi2O4/n-SnO2 photocatalysts were assessed based on the photodegradation of Congo red (CR) dye as a probe reaction under UVA (365 nm) light irradiation. Experimental results indicated that the phase composition, surface morphology of particles, and optical absorption of the sample were found to vary significantly with the mass ratios and pH medium. The p-CuBi2O4/n-SnO2 photocatalyst exhibited a higher photocatalytic redox activity than single phases CuBi2O4 and SnO2. The photodegradation reactions were satisfactory correlated with the pseudo-first-order kinetic model. The highest efficiency was observed at 5 wt% p-CuBi2O4 content as a result of 58.06% of photoactivity for 100 min of exposure irradiation under UVA light at pH 8 and 25 °C. The effective electron–hole separation at the physically bonded interfaces was believed to be mainly responsible for the remarkably enhanced photocatalytic activity of 5 wt% CuBi2O4/SnO2 in the course of the photocatalytic redox conversion of CR. The presence of the tightly physically bonded or close contact interfaces largely performed via hydrophobic effect between guest molecules on the composite surface and/or H-bonding by which the photoinduced charge transfer from one particle to the other through interfaces spatially is available, leading to a strong photocatalytic redox of CR at pH = 8. The efficient electron–hole separation process in z-scheme mechanism under UVA light irradiation was considered to be mainly responsible for the obviously improved photocatalytic activity of (5 wt%) CuBi2O4/SnO2 catalyst in the course of the photocatalytic redox conversion of Congo red.
Acknowledgments
We are greatly indebted to the University of Science and Technology of Oran (Mohamed Boudiaf), and the University of Science and Technology of Saida (Moulay Tahar) for their material support. We gratefully acknowledge the support for X-ray diffraction (XRD), scanning electron microscopy (SEM) and UV–vis diffuse reflectance spectroscopy (DRS) by Mrs. Professor Rose-Noëlle Vannier of the Unit of Catalysis and Solid State Chemistry of Lille 1 University.
References
- Efficient complete oxidation of acetaldehyde into CO2 over CuBi2O4/WO3 composite photocatalyst under visible and UV light irradiation. J. Phys. Chem. C. 2007;111:7574-7577.
- [Google Scholar]
- Band gap narrowing and fluorescence properties of nickel doped SnO2 nanoparticles. J. Luminescence. 2011;131:1-6.
- [Google Scholar]
- Study of low temperature elaborated tailored optical band gap β-In2S3−3xO3x thin films. J. Cryst. Growth. 2002;235:439-449.
- [Google Scholar]
- Visible-light-activated nanocomposite photocatalyst of Cr2O3/SnO2. J. Nanostruct. Chem.. 2013;3:46.
- [Google Scholar]
- Self assembly of active Bi2O3/TiO2 visible photocatalyst with ordered mesoporous structure and highly crystallized anatase. J. Phys. Chem. C. 2008;112(2008):6258-6262.
- [Google Scholar]
- Prediction of flatband potentials at semiconductor-electrolyte interfaces from atomic electronegativities. J. Electrochem. Soc.. 1978;125:228-232.
- [Google Scholar]
- A study of undoped and molybdenum doped, polycrystalline, tin oxide thin films produced by a simple reactive evaporation technique. J. Phys. D Appl. Phys.. 1990;23:1212-1215.
- [Google Scholar]
- Photocatalytic efficiency analysis of CdS nanoparticles with modified electronic states. J. Anal. Sci. Technol.. 2010;1:25-29.
- [Google Scholar]
- Optical and mechanical performance of nanostructured cerium oxides for applications in optical devices. J. Phys.: Conf. Ser.. 2005;10:226-229.
- [Google Scholar]
- Large-scale, solution-phase growth of single-crystalline SnO2 nanorods. J. Am. Chem. Soc.. 2004;126:5972-5973.
- [Google Scholar]
- The influence of nickel dopant on the microstructure and optical properties of SnO2 nano-powders. Chin. Phys.. 2007;16:95-99.
- [Google Scholar]
- Tungsten-doped TiO2 vs pure TiO2 photocatalysts: effects on photobleaching kinetics and mechanism. J. Phys. Chem. C. 2008;112:1094-1100.
- [Google Scholar]
- Characterization of new heterosystem CuFeO2/SnO2 application to visible-light induced hydrogen evolution. Int. J. Hydrogen Energy. 2008;33:4274-4282.
- [Google Scholar]
- Synthesis, characterization and UV-A light photocatalytic activity of 20 wt% SrO–CuBi2O4 composite. Appl. Surf. Sci.. 2012;258:5010-5024.
- [Google Scholar]
- Factorial experimental design of Orange II photocatalytic decolouration. J. Photochem. Photobiol. A-Chem.. 2002;151:213-221.
- [Google Scholar]
- Degradation of leather dye using CeO2–SnO2 nanocomposite as photocatalyst under sunlight. Water Air Soil Pollut.. 2012;223:5773-5779.
- [Google Scholar]
- First demonstration of CdSe as a photocatalyst for hydrogen evolution from water under UV and visible light. Chem. Commun.. 2008;2008:2206-2208.
- [Google Scholar]
- Electrochemical photolysis of water at a semiconductor electrode. Nature. 1972;238:37-38.
- [Google Scholar]
- Effect of particle size on the photocatalytic activity of WO3 particles for water oxidation. J. Phys. Chem. C. 2013;117:22584-22590.
- [Google Scholar]
- Carbon-modified TiO2 nanotubes with enhanced photocatalytic activity synthesized by a facile wet chemistry method. Scr. Mater.. 2008;59:352-355.
- [Google Scholar]
- Interplay between O2 and SnO2: oxygen ionosorption and spectroscopic evidence for adsorbed oxygen. Chem. Phys. Chem.. 2006;7:2041-2052.
- [Google Scholar]
- Synthesis and photocatalytic property of SnO2/TiO2 nanotubes composites. J. Hazard. Mater. B. 2007;139:310-315.
- [Google Scholar]
- A combination of two visible-light responsive photocatalysts for achieving the z-scheme in the solid state. ACS Nano. 2011;5:4084-4090.
- [Google Scholar]
- Reduced graphene oxide as a solid-state electron mediator in z-scheme photocatalytic water splitting under visible light. J. Am. Chem. Soc.. 2011;133:11054-11057.
- [Google Scholar]
- Photoreduction of chromium(VI) on ZrO2 and ZnS surfaces. Monatsh. Chem.. 2009;140:1269-1274.
- [Google Scholar]
- Sensitized layered metal oxide semiconductor particles for photochemical hydrogen evolution from nonsacrificial electron donors. J. Phys. Chem.. 1993;97:11802-11810.
- [Google Scholar]
- Spectral, optical, and photocatalytic characteristics of quantum-sized particles of CdTe theo. Exp. Chem.. 2004;40:220-225.
- [Google Scholar]
- CeO2–Bi2O3 nanocomposite: two step synthesis, microstructure and photocatalytic activity. J. Non-Cryst. Solids. 2009;355:776-779.
- [Google Scholar]
- Monoclinic BiVO4 with regular morphologies: hydrothermal synthesis, characterization and photocatalytic properties. Mater. Chem. Phys.. 2009;115:9-13.
- [Google Scholar]
- Heterojunction semiconductor SnO2/SrNb2O6 with an enhanced photocatalytic activity: the significance of chemically bonded interface. Acta Mater.. 2008;56:2699-2705.
- [Google Scholar]
- Fabrication of TiO2/ZnO composite nanofibers by electrospinning and their photocatalytic property. Mater. Chem. Phys.. 2010;121:432-439.
- [Google Scholar]
- Preparation and characterization of p–n heterojunction photocatalyst p-CuBi2O4/n-TiO2 with high photocatalytic activity under visible and UV light irradiation. J. Nanopart. Res.. 2010;12:1355-1366.
- [Google Scholar]
- Hydrothermal synthesis of prism-like mesocrystal CeO2. J. Alloys Compd.. 2009;476:958-962.
- [Google Scholar]
- Photocatalytic behavior of CeO2–TiO2 system for degradation of methylene blue. Indian J. chem.. 2009;48A:480-488.
- [Google Scholar]
- Enhanced photocatalytic activity of BiVO4 by co-grafting of metal ions and combining with CuBi2O4. J. Photochem. Photobiol. A: Chem.. 2013;262:52-56.
- [Google Scholar]
- Photocatalytic oxidation of carbon monoxide over NiO/SnO2 nanocomposites under UV irradiation. J. Nanotechnol.. 2012;2012:1-9.
- [Google Scholar]
- Photosensitization of TiO2 layers with CdSe quantum dots: correlation between light absorption and photoinjection. J. Phys. Chem. C. 2007;111:14889-14892.
- [Google Scholar]
- Electrochemical synthesis and characterization of p-CuBi2O4 thin film photocathodes. J. Phy. Chem. C. 2012;116:6459-6466.
- [Google Scholar]
- Synthesis and use of a novel SnO2 nanomaterial for gas sensing. Appl. Surf. Sci.. 2000;2000(164):219-226.
- [Google Scholar]
- Sputtering growth and optical properties of [100]-oriented tetragonal SnO2 and its Mn alloy films. J. Appl. Phys.. 2003;94:6401.
- [Google Scholar]
- The manufacture of yttrium aluminium garnet (YAG) fibres by blow spinning from a sol-gel precursor. J. Europ. Cer. Soc.. 1988;18:1759-1764.
- [Google Scholar]
- Electronic and optical properties of fluorine- doped tin oxide films. J. Appl. Phys.. 1998;83:1049-1057.
- [Google Scholar]
- A simple hydrothermal route for the preparation of HgS nanoparticles and their photocatalytic activities. RSC. Adv.. 2014;4:15371-15376.
- [Google Scholar]
- UV–visible spectroscopic estimation of photodegradation of rhodamine-B dye using tin(IV) oxide nanoparticles. Spectr. Chim. Acta Part A: Mol. Biomol. Spect.. 2012;97:847-852.
- [Google Scholar]
- Local work function of Pt clusters vacuum-deposited on a TiO2 surface. J. Phys. Chem. B. 2006;110:17584-17588.
- [Google Scholar]
- The effect of co-catalyst for Z-scheme photocatalysis systems with an Fe3+/Fe2+ electron mediator on overall water splitting under visible light irradiation. J. Cat.. 2008;259:133-137.
- [Google Scholar]
- Solar water splitting using powdered photocatalysts driven by z-schematic inter-particle electron transfer without an electron mediator. J. Phys. Chem. C. 2009;113:17536-17542.
- [Google Scholar]
- Highly dispersed phase of SnO2 on TiO2 nanoparticles synthesized by polyol-mediated route: photocatalytic activity for hydrogen generation. Int. J. Hydrogen Energy. 2009;34:3621-3630.
- [Google Scholar]
- Photocatalysis for water oxidation by Fe2O3 nanoparticles embedded in clay compound: correlation between its polymorphs and their photocatalytic activities. J. Mater. Sci.. 2009;44:2890-2898.
- [Google Scholar]
- Microcalorimetric and infrared spectroscopic studies of γ-Al2O3 modified by tin oxides. Catal. Lett.. 1994;26(1994):247-257.
- [Google Scholar]
- Photocatalytic activity and antibacterial behavior of Fe3+-doped TiO2/SnO2 nanoparticles. Energy Res. J.. 2010;1:120-125.
- [Google Scholar]
- Methanol and ammonia sensing characteristics of sol–gel derived thin film gas sensor. Sens. Actuators B Chem.. 2000;66:256-259.
- [Google Scholar]
- Kinetic study of application of ZnO as a photocatalyst in heterogeneous medium. E-J. Chem.. 2009;6:531-536.
- [Google Scholar]
- Preparation, characterization and photocatalytic activity of nano-sized ZnO/SnO2 coupled photocatalysts. Appl. Cat. B-Environ.. 2002;39:269-279.
- [Google Scholar]
- Structure, preparation and photocatalytic activity of titaniumoxides on MCM-41. J. Catal.. 2006;238:13-20.
- [Google Scholar]
- Fast response thin film SnO”2 gas sensors operating at room temperature. Sens. Actuators B Chem.. 2006;119:380-383.
- [Google Scholar]
- Preparation and photocatalytic properties of mesoporous SnO2–hexaniobate layered nanocomposite. Microporous Mesoporous Mater.. 2010;130:344-351.
- [Google Scholar]
- Improved hydrogen monitoring properties based on p-NiO/n-SnO2 heterojunction composite nanofibers. J. Phys. Chem. C. 2010;114:6100-6105.
- [Google Scholar]
- Size-dependent photocatalytic reduction of CO2 with PbS quantum dot sensitized TiO2 heterostructured photocatalysts. J. Mater. Chem.. 2011;21:13452-13457.
- [Google Scholar]
- High surface area SnO2 nanoparticles: synthesis and gas sensing properties. Mater. Chem. Phys.. 2008;108:232-236.
- [Google Scholar]
- Degradation of C.I. Reactive Red 2 through photocatalysis coupled with water jet cavitation. J. Hazard. Mat.. 2011;185:315-321.
- [Google Scholar]
- Photosensitized and photocatalyzed degradation of azo dye using Lnn+-TiO2 sol in aqueous solution under visible light irradiation. Mater. Sci. Eng., B. 2005;117:325-333.
- [Google Scholar]
- The absolute energy positions of conduction and bands of selected semiconducting minerals. Amer. Mineral.. 2000;85:543-556.
- [Google Scholar]
- Eletrochemical synthesis and photocatalytic property of cuprous oxide nanoparticles. Mater. Res. Bull.. 2006;41:1310-1318.
- [Google Scholar]
- Randomly packed n-SnO2 nanorods/p-SiC heterojunction light-emitting diodes. Appl. Phys. Lett.. 2009;95:201104.
- [Google Scholar]
- Healy, site-binding model of the electrical double layer at the oxide/water interface. J. Chem. Soc., Faraday Trans.. 1974;70:1807-1818.
- [Google Scholar]
- SnO2 nanowhiskers and their ethanol sensing characteristics. Nanotechnology. 2004;15:1682.
- [Google Scholar]
- Improving the catalytic activity of CeO2/H2O2 system by sulfation pretreatment of CeO2. J. Mol. Cat. A: Chem.. 2014;381:38-45.
- [Google Scholar]
- Effects of pH on the microstructures and photocatalytic activity of mesoporous nanocrystalline titania powders prepared via hydrothermal method. J. Mol. Catal. A-Chem.. 2008;258:104-112.
- [Google Scholar]
- Synthesis of square Bi2WO6 nanoplates as high-activity visible-light-driven photocatalysts. Chem. Mater.. 2005;17:3537-3545.
- [Google Scholar]
- TiO2-assisted photodegradation of dye pollutants: II. Adsorption and degradation kinetics of eosin in TiO2, dispersions under visible light irradiation. Appl. Cat. B-Environ.. 1998;15:147-156.
- [Google Scholar]
- Preparation of Fenton reagent with H2O2 generated by solar light-illuminated nano- Cu2O/MWNTs composites. Appl. Catal. A. 2006;299:292-297.
- [Google Scholar]
- Graphene–metal–oxide composites for the degradation of dyes under visible light irradiation. J. Mater. Chem.. 2011;21:3634-3640.
- [Google Scholar]
- Ag3PO4/SnO2 Semiconductor Nanocomposites with Enhanced Photocatalytic Activity and Stability. The Royal Society of Chemistry and The Centre National de la Recherche Scientifique; 2012.
- Photocatalytic activity of Bi2O3 prepared by different precipitants. Adv. Mat. Res.. 2011;239–242:998-1001.
- [Google Scholar]
- Synthesis, characterization, and visible-light photocatalytic activity of Fe2O3/SnO2 nanocomposites. Mater. Sci-Poland. 2008;26:517-526.
- [Google Scholar]







