Translate this page into:
Green synthesis and biological evaluation of steroidal 2H-pyrans as anticancer and antioxidant agents
*Corresponding author. Tel.: +91 9411003465 shamsuzzaman9@gmail.com (Shamsuzzaman)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Available online 14 October 2013
Peer review under responsibility of King Saud University.

Abstract
In this study, we describe a green and simple procedure for the synthesis of steroidal 2H-pyrans 4–6 using chitosan as an eco-friendly heterogeneous catalyst. The synthesized compounds were characterized by IR, 1H NMR, 13C NMR, MS and analytical data. These compounds were tested in vitro against two cancer cell lines [HeLa (cervical) and Jurkat (leukemia)] and one normal cell line (PBMC). The compounds exhibited moderate to good activity against the two human cancer cell lines and were less toxic against the non-cancer cell line. In addition, the synthesized compounds were tested for their in vitro antioxidant activity by 1,1-diphenylpicrylhydrazyl method in which compounds 4 and 6 exhibited good antioxidant activity. This study provided a new molecular scaffold for the further development of anticancer as well as antioxidant agents.
Keywords
Anticancer
Antioxidant
Chitosan
Green chemistry
2H-pyran
1 Introduction
Green chemistry can be recognized as a pioneering research, which widely reports intrinsic atom economy, energy savings, waste reduction, easy work ups and the avoidance of hazardous chemicals (Kumar et al., 2012). The progress of a simple and eco-friendly reaction protocol for the synthesis of highly functionalized compound libraries of medicinal motifs is an attractive area of investigation in both academia and the pharmaceutical industry (Domling, 2006). Chitosan is readily prepared via aqueous alkali promoted hydrolysis of chitin. Being hydrophilic and possessing basic moieties (Bader and Birkholz, 1996), chitosan has been utilized as a heterogeneous eco-friendly basic catalyst for reactions carried out in protic medium (Gomha and Riyadh, 2009). Steroids are an important class of natural products which have high capability to penetrate cells and bind to nucleus and membrane receptors. They include great variations in structure and play a very important role in life (Festi et al., 2007; Ifere et al., 2009). The investigation of modified steroid derivatives condensed with different heterocyclic rings has drawn great attention (El-Far et al., 2009). Pyrans and their derivatives constitute an important class of organic compounds due to their attractive pharmacological and biological properties (Green et al., 1995; Nemouchi et al., 2012). They are widely used as anticoagulant, anticancer, diuretic, spasmolitic and antianaphylactin agents (Bonsignore et al., 1993) and can be used as cognitive enhancers for the treatment of neurodegenerative disease, including Alzheimer’s disease, Huntington’s disease, Parkinson’s disease and Down’s syndrome as well as for the treatment of schizophrenia and myoclonus (Konkoy et al., 2001). Our group has been involved in the study of the structural requirements of steroids to display biological activities, specifically, on heterosteroid studies involving in vitro anticancer and antimicrobial evaluation of newly synthesized steroids and analysis of structure–activity relationships. In light of our interest to develop environmentally benign processes to synthesize heterosteroids (Shamsuzzaman et al., 2013a), we here report green synthesis, in vitro anticancer and antioxidant activities of steroidal 2H-pyrans.
2 Experimental
2.1 Materials and methods
All chemicals were purchased from Sigma–Aldrich (India) and Merck (India) as analytical grade. Chitosan was supplied by Sigma–Aldrich (India) as practicle grade. The solvents were purified prior to use. Melting points were determined on a Kofler apparatus in degree Celsius. The IR spectra were recorded on KBr pellets with Interspec 2020 FT-IR Spectrometer SpectroLab and values are given in cm−1. 1H and 13C NMR spectra were run in CDCl3 on a JEOL Eclipse (400 MHz) instrument with TMS as internal standard and values are given in ppm (δ). Elemental analyses of all the new compounds were recorded on a Perkin Elmer 2400 CHN Elemental Analyzer. Mass spectra were recorded on a JEOL SX 102/DA- 6000 Mass Spectrometer. Thin layer chromatography (TLC) plates were coated with silica gel G and exposed to iodine vapors to check the homogeneity as well as the progress of reaction. Sodium sulfate (anhydrous) was used as a drying agent.
2.2 General method for the synthesis of steroidal 2H-pyran derivatives (4–6)
A mixture of steroidal α, β-unsaturated ketone 1, 2 or 3 (1 mmol) and ethyl acetoacetate (1 mmol) in 30 mL methanol was refluxed for 13–16 h in the presence of chitosan (20 mol%). The progress of the reaction was monitored by TLC. After completion of the reaction, chitosan was removed by filtration and the filtrate was taken in ether, washed with water, dried over anhydrous sodium sulfate and concentrated in vacuo giving a residue which was recrystallized from ethanol affording products 4, 5 or 6.
2.2.1 3β-Acetoxy-2′-oxo-3′-acetyl-3′,6′-dihydro-cholest-6-eno [5, 7- d e ] 2H-pyran (4)
White powder, Mol. Wt. 526.750, yield 73% (0.384 gm), m.p. 176–178 °C, IR (KBr) ν: 1743 (3β-AcO), 1715 (CHCOCH3), 1673 (OCOCH3), 1622 (C⚌C), 1245 (C–O). 1H NMR (400 MHz, CDCl3) δ: 5.21 (1H, s, C6H), 4.80 (1H, m, C3α-H, W ½ = 16 Hz), 3.21 (1H, s, C3′H), 2.03 (3H, s, OCOCH3), 2.01 (3H, s, CHCOCH3), 1.20 (3H, s, C13–CH3), 1.14 (3H, s, C10–CH3), 1.02 (3H, t, CH3), 0.97 & 0.82 (other methyl protons). 13C NMR (100 MHz, CDCl3) δ: 170.1 (3β-AcO), 168.3 (CHCOCH3), 163.5 (OCOCH), 157.2 (C7), 112.1 (C6), 72.2 (C3), 64.3 (C′3) 48.4 (C17), 41.4 (C12), 40.1 (C11), 39.4 (C24), 38.5 (C10), 35.3 (C22), 34.2 (C9), 32.3 (C5), 31.4 (C13), 30.1 (C1), 29.2 (C20), 28.3 (C25), 28.2 (C8), 28.1 (OCOCH3), 27.2 (C4), 25.4 (C23), 24.1 (C2), 22.3 (C″2), 22.2 (C26), 22.1 (C27), 21.3 (C14), 21.2 (C15), 21.1 (C16), 20.1 (C18), 18.4 (C21), 17.3 (C19). Anal. Calcd for C33H50O5: C, 75.25, H, 9.57. Found C, 75.21, H, 9.54. MS: m/z = (M+) 526, 365 (base peak).
2.2.2 3β-Chloro-2′-oxo-3′-acetyl-3′,6′-dihydro-cholest-6-eno [5, 7- d e ] 2H-pyran (5)
White powder, Mol. Wt. 503.160, yield 71% (0.357 gm), m.p. 165–167 °C, IR (KBr) ν: 1718 (CHCOCH3), 1675 (OCOCH), 1620 (C⚌C), 1243 (C–O), 745 (C3–Cl). 1H NMR (400 MHz, CDCl3) δ: 5.40 (1H, s, C6H), 3.81 (1H, m, C3α-H, W ½ = 17 Hz), 3.10 (1H, s, C3′H), 2.01 (3H, s, CHCOCH3), 1.20 (3H, s, C13–CH3), 1.14 (3H, s, C10–CH3), 1.02 (3H, t, CH3), 0.97 & 0.82 (other methyl protons). 13C NMR (100 MHz, CDCl3) δ: 167.1 (CHCOCH3), 161.3 (OCOCH), 159.4 (C7), 114.4 (C6), 64.5 (C′3), 52.1 (C3), 48.2 (C17), 41.3 (C12), 40.3 (C11), 39.1 (C24), 38.2 (C10), 35.3 (C22), 34.6 (C9), 32.4 (C5), 31.2 (C13), 30.3 (C1), 29.2 (C20), 28.2 (C25), 28.3 (C8), 27.1 (C4), 25.2 (C23), 24.3 (C2), 22.3 (C″2), 22.2 (C26), 22.1 (C27), 21.3 (C14), 21.2 (C15), 21.1 (C16), 20.3 (C18), 18.1 (C21), 17.2 (C19). Anal. Calcd for C31H47ClO3: C, 74.00, H, 9.42. Found C, 74.02, H, 9.44. MS: m/z = [M+] 502/ 504, 394 (base peak).
2.2.3 2′-Oxo-3′-acetyl-3′,6′-dihydro-cholest-6-eno [5, 7- d e ] 2H-pyran (6)
White powder, Mol. Wt. 468.710, yield 70% (0.327 gm), m.p. 170–172 °C, IR (KBr) ν: 1720 (CHCOCH3), 1677 (OCOCH), 1621 (C⚌C), 1246 (C–O). 1H NMR (400 MHz, CDCl3) δ: 5.31 (1H, s, C6H), 3.31 (1H, s, C3′H), 2.01 (3H, s, CHCOCH3), 1.20 (3H, s, C13–CH3), 1.14 (3H, s, C10–CH3), 1.02 (3H, t, CH3), 0.97 & 0.82 (other methyl protons). 13C NMR (100 MHz, CDCl3) δ: 165.1 (OCOCH3), 160.3 (OCOCH), 158.1 (C7), 116.3 (C6), 65.3 (C3′), 48.3 (C17), 41.4 (C12), 40.4 (C11), 39.1 (C24), 38.3 (C10), 35.3 (C22), 34.4 (C9), 32.3 (C5), 31.1 (C13), 30.3 (C1), 29.3 (C20), 28.2 (C25), 28.1 (C8), 27.3 (C4), 25.2 (C23), 25.1 (C3), 24.1 (C2), 22.3 (C″2), 22.2 (C26), 22.1 (C27), 21 (C14), 21 (C15), 21 (C16), 20 (C18), 18 (C21), 17 (C19). Anal. Calcd for C31H48O3: C, 79.44, H, 10.32. Found C, 79.42, H, 10.34. MS: m/z = (M+) 468, 400 (base peak).
2.3 In vitro anticancer activity
2.3.1 Cell lines and culture conditions
Human cancer cell lines HeLa (cervical) and Jurkat (leukemia) (obtained from NCCS Pune, Maharashtra) were taken for the study. The tumor cells and normal cells were maintained in RPMI 1640 culture medium supplemented with 10% heat-inactivated fetal calf serum (FCS).
2.3.2 Blood peripheral mononuclear cell isolation (PBMC)
Fresh blood (20–15 mL) was kindly provided by the Blood Bank Jawahar Lal Nehru Medical College, AMU Aligarh. The blood sample was diluted with the same volume of phosphate buffered saline (PBS) (1.5 KH2PO4, 6.5 Na2HPO4, 137 NaCl, and 2.7 mM KCl; pH 7.4). Then the diluted blood sample was carefully layered on Ficoll-Histopaque. The mixture was centrifuged at 400g for 30 min at 20–22 °C. The undisturbed lymphocyte layer was carefully transferred out. The lymphocyte was washed and pelleted down with 3 v of PBS for twice and resuspended RPMI-1640 media with antibiotic and antimycotic solution 10%, v/v FCS. Cell counting was performed to determine the PBMC cell number with equal volume of trypan blue (Yeap et al., 2007).
2.3.3 Cell viability assay (MTT)
The in vitro anticancer activity was measured using the 3-(4,5-dimethylthizao1-2-y1)-2,5-diphenyltetrazolium bromide (MTT) assay. The assay was carried out according to the known protocol (Shamsuzzaman et al., 2013b). Exponentially growing cells were harvested and plated in 96-well plates at a concentration of 1 × 104 cells/well. After 24 h incubation at 37 °C under a humidified 5% CO2 to allow cell attachment, the cells in the wells were respectively treated with target compounds at various concentrations for 48 h. The concentration of DMSO was always kept below 1.25%, which was found to be non-toxic to the cells. A solution of MTT, was prepared at 5 mg/mL in PBS. 20 μL of this solution was added to each well. After incubation for 4 h at 37 °C in a humidified incubator with 5% CO2, the medium/MTT mixtures were removed, and the formazan crystals formed by the mitochondrial dehydrogenase activity of vital cells were dissolved in 100 μL of DMSO per well. The absorbance of the wells was read with a microplate reader (Bio-Rad Instruments) at 570 nm. Effects of the drug cell viability were calculated using a cell treated with DMSO as control.
2.3.4 Data analysis
Cell survival was calculated by using the formula: Survival (%) = [(absorbance of treated cells − absorbance of culture medium)/(absorbance of untreated cells − absorbance of culture medium)] × 100 (Shamsuzzaman et al., 2013b). The experiment was done in triplicate and the inhibitory concentration (IC) values were calculated from a dose response curve. IC50 is the concentration in ‘μM’ required for 50% inhibition of cell growth as compared to that of control. IC50 values were determined from the linear portion of the curve by calculating the concentration of agent that reduced absorbance in treated cells, compared to control cells, by 50%. Evaluation is based on mean values from three independent experiments, each comprising at least six microcultures per concentration level.
2.4 Antioxidant activity
Steroidal 2H-pyran derivatives 4–6 were tested for their antioxidant property by 1,1-diphenylpicrylhydrazyl (DPPH) method (Lone et al., 2013). Drug stock solution (1 mg/mL) was diluted to final concentration of 2, 4, 6, 8, 10 and 12 mg/mL in methanol. Methanolic DPPH solution (1 mL, 0.3 mmol) was added to 3.0 mL of drug solution of different concentrations. The tube was kept at an ambient temperature for 30 min and the absorbance was measured at 517 nm. The scavenging activity was calculated by the following formula: where AControl is the absorbance of the l-ascorbic acid (Standard) and ASample is the absorbance of different compounds. The methanolic DPPH solution (1 mL, 0.3 mM) was used as control.
3 Results and discussion
3.1 Chemistry
The synthesis of steroidal 2H-pyran derivatives 4–6 involves the use of 3β-acetoxy-cholest-5-en-7-one (1), 3β-chloro-cholest-5-en-7-one (2) and cholest-5-en-7-one (3), as starting materials which have been synthesized by the literature method (Dauben and Takemura, 1953). The present strategy of synthesizing compounds 4–6 by green method involves the reaction of steroidal α, β-unsaturated ketones 1–3 with ethyl acetoacetate in the presence of chitosan as an eco-friendly heterogeneous catalyst. The catalytic system is influenced by various reaction parameters, such as amount of the catalyst employed, effect of catalyst and solvent system. Therefore, in our initial investigation to establish the optimum reaction conditions 3β-acetoxy-cholest-5-en-7-one (1) and ethyl acetoacetate in methanol were selected as model substrates. We have applied a wide range of basic catalysts such as piperidine, NaOMe, pyridine, triethylamine and chitosan to improve the yield for the specific synthesis of derivatives.
As shown in Table 1, chitosan is superior to other catalysts in terms of time and yield. We then tried to screen the reaction in various organic solvents in order to optimize the solvent using chitosan as catalyst. It is noteworthy to mention that the polar solvents (methanol and ethanol) afforded better yield than the non-polar ones and the best result was obtained in methanol as we can see in Table 2.
Entry
Catalysts
Time (h)
Yield of 4 (%)b
1
Piperidine
20
60
2
NaOMe
32
43
3
Pyridine
25
50
4
Triethylamine
36
34
5
Chitosan
16
73
We then tried to optimize the catalyst load for the formation of steroidal 2H-pyrans and we found that 20 mol% of the catalyst was sufficient to get optimum yields in less reaction time. Use of more than 20 mol% of the catalyst does not have any profound effect on the reaction rate as well as the yield. This result may be attributed to the coagulation of the catalyst which decreased the effective surface area of the catalyst (Bhattacharyya et al., 2012). Our study revealed that even after five cycles, the catalyst was able to carry out the reaction offering almost the same catalytic activity. In order to investigate the scope of this reaction, a variety of three steroidal compounds were subjected to this reaction and all the reactions proceeded smoothly and the reaction was completed within 13–16 h to afford the products 4–6 in good yields (70–73%). (Scheme 1).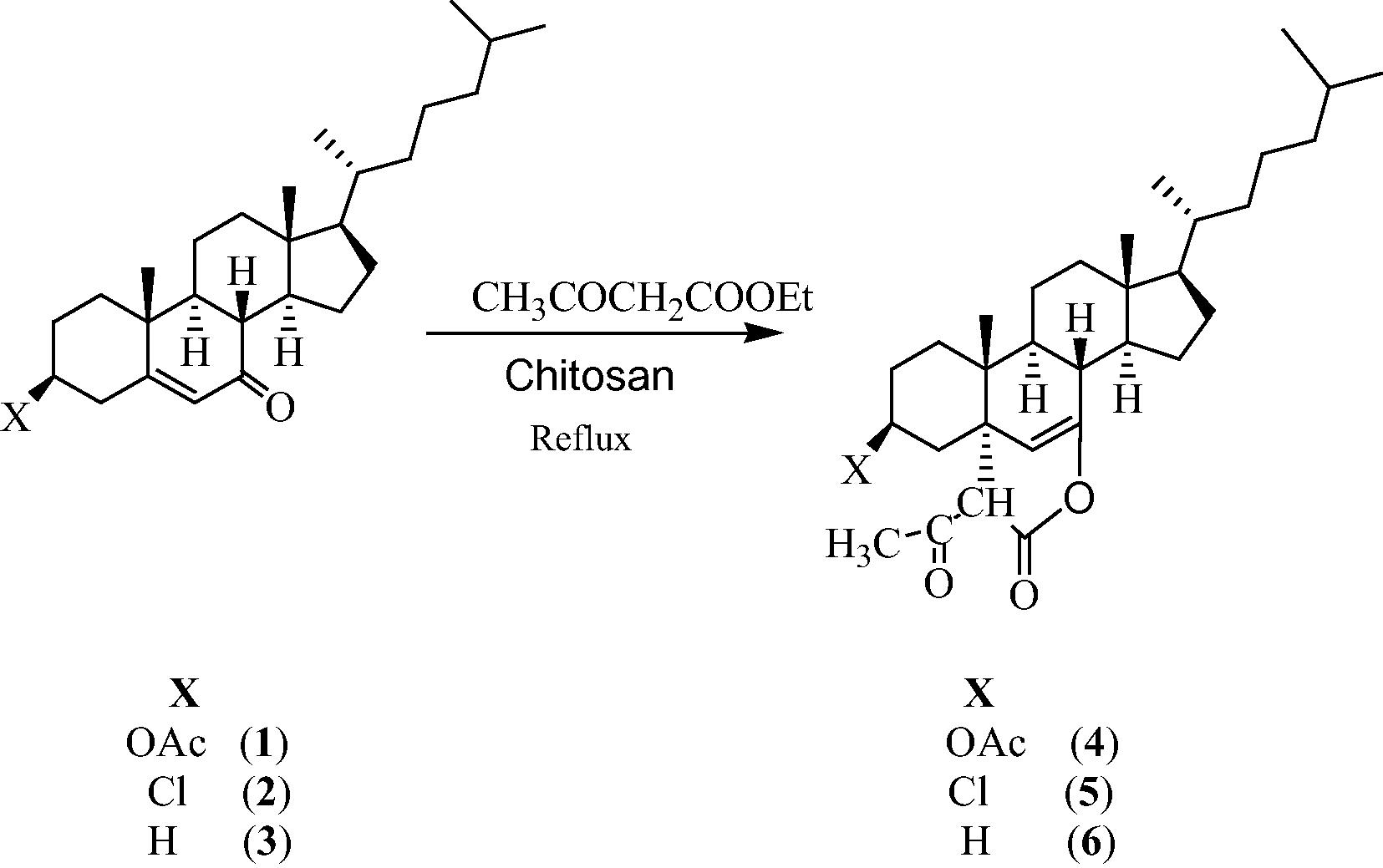
Showing the formation of steroidal 2H-pyrans 4–6.
The spectral studies of products were found in good agreement with the proposed structures for steroidal 2H-pyrans 4–6. In the IR spectra the absorption bands in the range 1715–1720 cm−1 show the presence of CHCOCH3 while the absorption bands at 1673–1677 cm−1 confirm the presence of OCOCH. The weak absorption band in the range 1620–1622 cm−1 confirms the presence of (C⚌C). In 1H NMR study of compounds 4–6 the presence of sharp singlet at δ 5.21–5.40 was assigned to olefinic proton (C6–H) while singlet at δ 3.10–3.31 integrated for one proton of C′3H. In 13C NMR study, the signals at δ 165.1–168.3 confirm the presence of OCOCH3 while the signals at δ 160.3–163.5 confirm the presence of OCOCH. Finally the mass spectra data of compounds showed molecular ion peaks [M+] at m/z: 526, 502/504 and 468 for 4, 5 and 6, respectively. All these data confirmed the formation of desired products. The stereochemical assignation of the C5–C bond has been established on the basis of half band width (W1/2) values of C3-axial proton in the 1H NMR spectra of compounds 4 and 5 which clearly suggested that A/B ring junction is trans (Shamsuzzaman et al., 2013c) and also on the basis of the fact that during the reaction, the attack of the reagent should be from (α) side which is less hindered, not from (β) side which is more hindered due to the presence of a methyl group at C10, hence the C5–C bond should be axial (α) and trans to C10 methyl group (Shamsuzzaman et al., 2010). C19 values in 13C NMR are strongly dependent on the ring fusion stereochemistry. In the synthesized compounds 4–6, C19 chemical shift values were observed in the range of 16–19 ppm, which is consistent with the values, obtained for trans steroids (Iida et al., 2007). The mechanism for the formation of steroidal 2H-pyran derivatives 4–6 involves the Michael addition of active methylene of ethyl acetoacetate to conjugated ketone at the electrophilic alkene C, in nucleophilic addition type process, which undergoes subsequent intramolecular cyclization to provide the desired product (Scheme 2).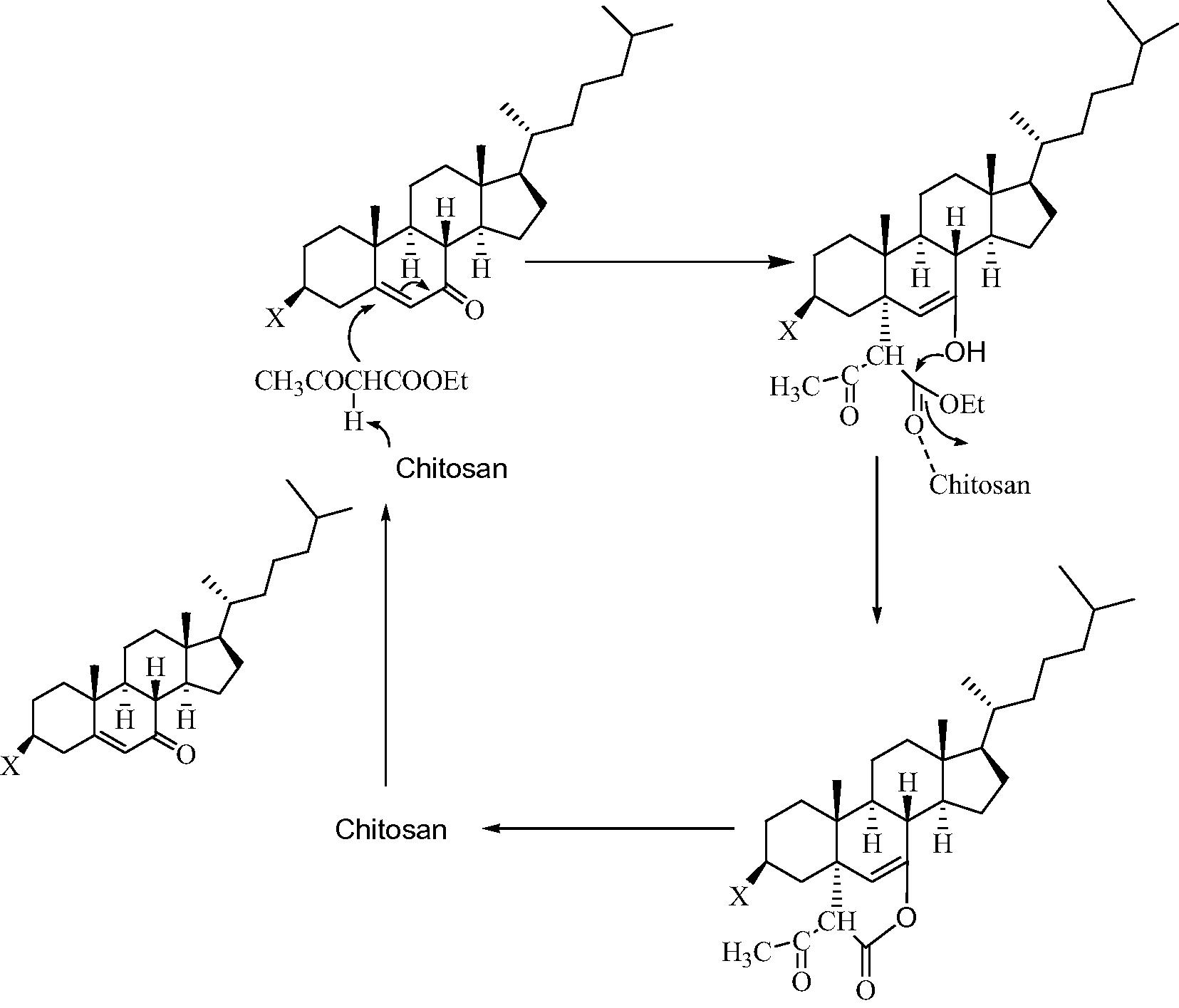
Plausible mechanism for the formation of steroidal 2H-pyrans 4–6.
3.2 In vitro anticancer activity
Steroids with α, β-unsaturated ketone core gave the potency against human cancer cell lines (Wang et al., 2011). With this intuition, an attempt of synthesizing steroidal 2H-pyran derivatives 4–6 from steroidal α, β-unsaturated ketones 1–3 was made. The synthesized compounds were evaluated for anticancer activity toward human HeLa (cervical) and Jurkat (leukemia) cancer cells. Doxorubicin (Dox) and 5-fluorouracil (5-Fu) were used as cytotoxic drugs of reference. The conversion of the soluble yellowish MTT to the insoluble purple formazan by active mitochondrial lactate dehydrogenase of living cells has been used to develop an assay system for the measurement of cell proliferation. Dose-dependent effects of steroidal 2H-pyranes 4–6 on cell viability of HeLa, Jurkat and PBMC cell lines are given in Fig. 1.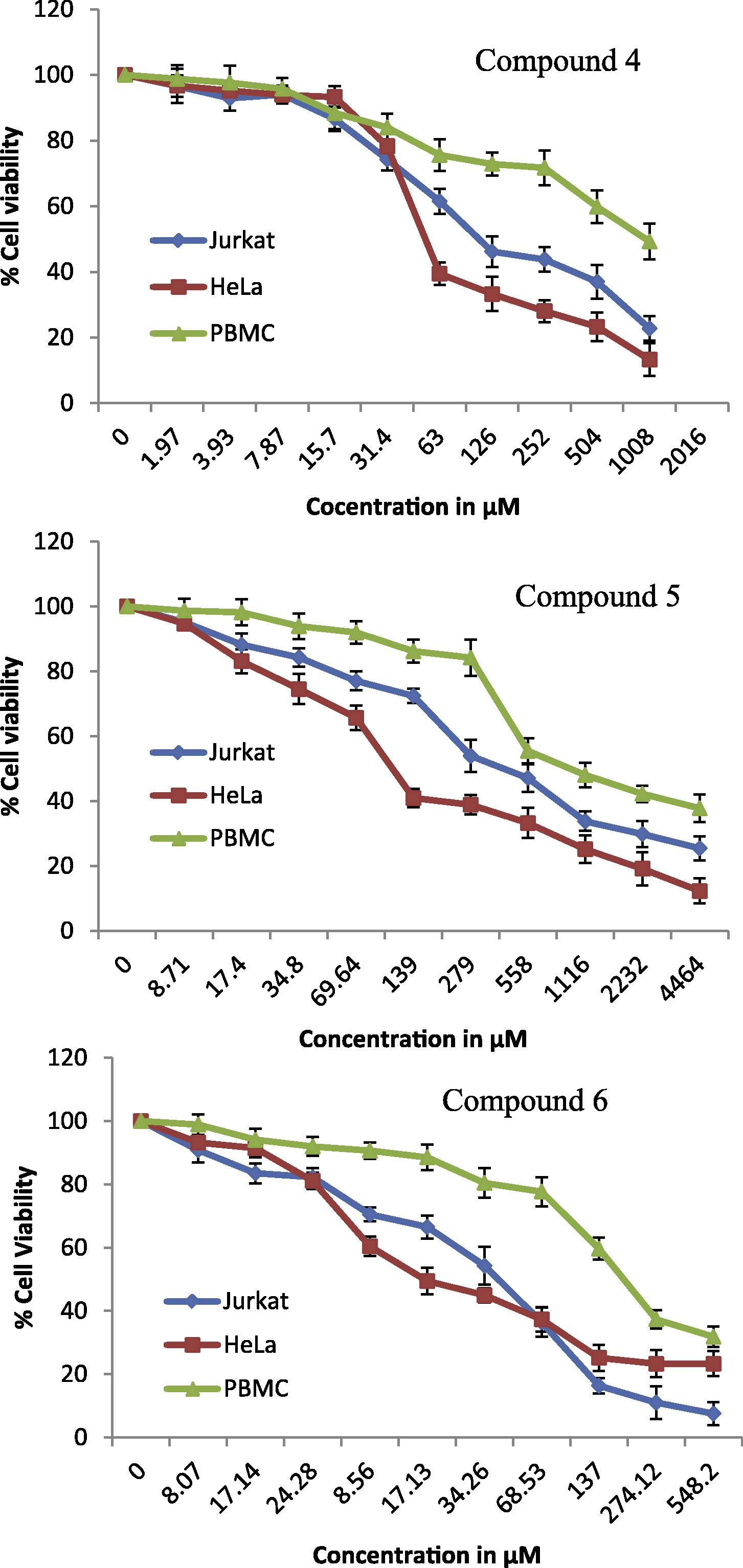
Dose-dependent effects of steroidal 2H-pyranes 4–6 on cell viability of HeLa, Jurkat and PBMC cell lines. Data shown are mean ± standard error of at least three independent experiments.
The anticancer data given in Table 3 show that compounds 4–6 exhibited moderate to good activity while compound 6 elicited the better inhibitory activity (IC50 < 19 μM) against both cell lines. Doxorubicin (Dox) and 5-fluorouracil (5-Fu) are drugs of reference.
Compounds
HeLa
IC50 μmol L−1 Jurkat
PBMC
4
18.5 ± 0.6
22.6 ± 0.1
67
5
20.2 ± 0.2
33.2 ± 0.4
65
6
14.21 ± 0.5
18.6 ± 0.6
64
Dox
4.1 ± 0.1
4.3 ± 0.4
-
5-Fu
8.1 ± 0.3
9.0 ± 0.6
-
Interestingly, the IC50 values of compounds 4 and 6 were close to each other, apparently indicating that the acetylation did not increase the anticancer activity. This might be due to the deacetylation of compound 4 by an esterase present indigenously in cells (Samanta et al., 2013). It is a noteworthy point that the synthesized compounds 4–6 were found to be slightly more active against HeLa. The synthesized compounds 4–6 were also tested with normal cell line (PBMC) during which they were found slightly toxic, all the compounds showed IC50 > 60 μM.
3.3 In vitro antioxidant activity
The in vitro antioxidant activity and scavenging effects of steroidal 2H-pyran derivatives 4–6 were evaluated by using different reactive species assay containing DPPH radical scavenging activity. The free radical scavenging activity of the synthesized compounds was evaluated through their ability to quench the DPPH• using ascorbic acid as a reference. The potencies for the antioxidant activity of compounds 4–6 to the reference drug are shown in Table 4.
Compounds
% inhibition
25 μg/mL
50 μg/mL
75 μg/mL
100 μg/mL
4
16.8 ± 0.2
23.7 ± 0.3
21.6 ± 0.6
24.5 ± 0.4
5
12.8 ± 0.4
13.6 ± 0.5
16.6 ± 0.8
21.9 ± 0.9
6
15.6 ± 0.2
19.7 ± 0.3
22.6 ± 0.2
25.5 ± 0.3
Standard
36.0 ± 0.3
37.0 ± 0.2
44.0 ± 0.3
50.0 ± 0.5
Controlb
-
-
-
-
In general, all the synthesized compounds were less potent than the reference. Among the synthesized compounds, compounds 4 and 6 exhibited a slightly better antioxidant activity than compound 5.
4 Conclusion
We have developed a green, facile, convenient and efficient approach for the synthesis of new steroidal 2H-pyrans 4–6 from steroidal α, β-unsaturated ketones 1–3 using chitosan as an eco-friendly heterogeneous catalyst. The in vitro anticancer screening showed the impressive behavior of compound 6 by being more potent anticancer agent. The antioxidant screening showed good antioxidant activity of compounds 4 and 6. The present study showed that steroidal 2H-pyrans 4–6 can be used for future development through modification and derivatization to design more potent and selective anticancer and antioxidant agents.
Acknowledgements
Authors thank the Chairman, Department of Chemistry, A.M.U., Aligarh, for providing necessary research facilities. One of the author (AS) acknowledges DBT for providing JRF (DBT-JRF/2011-12/160). Facilities provided by SAP (DRS-I) for their generous research support are also gratefully acknowledged.
References
- Chitin-Ein wertvolles polysaccharid aus krabbenpanzern. Prax. Nat. Chem.. 1996;45(6):24-30.
- [Google Scholar]
- Nano crystalline ZnO catalyzed one pot multicomponent reaction for an easy access of fully decorated 4H-pyran scaffolds and its rearrangement to 2-pyridone nucleus in aqueous media. Tetrahedron Lett.. 2012;53:4687-4691.
- [Google Scholar]
- Synthesis and pharmacological activity of 2-oxo-(2H) 1-benzopyran-3-carboxamide derivatives. Eur. J. Med. Chem.. 1993;28:517-520.
- [Google Scholar]
- A study of the mechanism of conversion of acetate to cholesterol via squalene. J. Am. Chem. Soc.. 1953;75:6302-6304.
- [Google Scholar]
- Recent developments in isocyanide based multicomponent reactions in applied chemistry. Chem. Rev.. 2006;106:17-89.
- [Google Scholar]
- Novel modified steroid derivatives of androstanolone as chemotherapeutic anti-cancer agents. Eur. J. Med. Chem.. 2009;44:3936-3946.
- [Google Scholar]
- Clinical efficacy and effectiveness of ursodeoxycholic acid in cholestatic liver diseases. Curr. Clin. Pharmacol.. 2007;2:155-177.
- [Google Scholar]
- Synthesis of triazolo[4,3-b][1,2,4,5]tetrazines and triazolo[3,4-b] [1,3,4]thiadiazines using chitosan as ecofriendly catalyst under microwave irradiation. Arkivoc. 2009;11:58-68.
- [Google Scholar]
- Ktritsky A.R., Rees C.W., Scriven E.F.V., eds. Comprehensive heterocyclic chemistry II, 5. Oxford: Permagon Press; 1995. p. :469.
- Differential effects of cholesterol and phytosterols on cell proliferation, apoptosis and expression of a prostate specific gene in prostate cancer cell lines. Cancer Detect. Prev.. 2009;32:319-328.
- [Google Scholar]
- Regioselective oxyfunctionalization of unactivated carbons in steroids by a model of cytochrome P-450: osmiumporphyrin complex/tert-butyl hydroperoxide system. J. Org. Chem.. 2007;72:823-830.
- [Google Scholar]
- Substituted 5-oxo-5,6,7,8-tetrahydro-4H-1-benzopyrans and benzothiopyrans and the use thereof as potentiators of AMPA. Chem. Abstr.. 2001;134:29313a.
- [Google Scholar]
- L-Proline catalysed multicomponent synthesis of 3-amino alkylated indoles via a mannich-type reaction under solvent-free conditions. Green Chem.. 2012;14:290-295.
- [Google Scholar]
- Synthesis antimicrobial and antioxidant studies of new oximes of steroidal chalcones. Steroids. 2013;78:945-950.
- [Google Scholar]
- Phenylboronic acid as an efficient and convenient catalyst for a three-component synthesis of tetrahydrobenzo[b]pyrans. C. R. Chimie. 2012;15:394-397.
- [Google Scholar]
- Mahanine, a DNA minor groove binding agent exerts cellular cytotoxicity with involvement of C-7-OH and -NH functional groups. J. Med. Chem.. 2013;56:5709-5721.
- [Google Scholar]
- Synthesis, antibacterial and antifungal activities of 6,5 fused steroidal oxazoles in cholestane series. Eur. J. Med. Chem.. 2010;45:1094-1097.
- [Google Scholar]
- Construction of novel steroidal isoxazolidinone derivatives under Vilsmeier–Haack conditions. Tetrahedron Lett.. 2013;54:874-877.
- [Google Scholar]
- Synthesis, evaluation and docking studies on steroidal pyrazolones as anticancer and antimicrobial agents. Med. Chem. Res. 2013
- [CrossRef] [Google Scholar]
- Shamsuzzaman, Mashrai, A., Khanam, H., Aljawfi, R.N., 2013c. Biological synthesis of ZnO nanoparticles using C. albicans and studying their catalytic performance in the synthesis of steroidal pyrazolines. Arab. J. Chem. doi.org/10.1016/j.arabjc.2013.05.004.
- Novel and efficient synthesis of22-alkynyl-13, 24(23)-cyclo-18,21-dinorchol-22-en-20(23)-one analogues. Steroids. 2011;76:491-496.
- [Google Scholar]
- Effect of rhaphidophora korthalsii methanol extract on human peripheral blood mononuclear cell (PBMC) proliferation and cytolytic activity toward HepG2. J. Ethnopharmacol.. 2007;114:406-411.
- [Google Scholar]







